 Previously we identified a series of amidoalkylindoles as potent and selective CB2 partial agonists.
Previously we identified a series of amidoalkylindoles as potent and selective CB2 partial agonists.
Abstract
Previously we identified a series of amidoalkylindoles as potent and selective CB2 partial agonists. In the present study, we report our continuous effort to improve the aqueous solubility by introducing N atoms to the amidoalkylindole framework. Synthesis, characterization, and pharmacology evaluations were described. Bioisosteric replacements of the indole nucleus with an indazole, azaindole and benzimidazole were explored. Benzimidazole 43 (EC50,CB1 = NA, EC50,CB2 = 0.067 μM) and azaindole 24 (EC50,CB1 = NA, EC50,CB2 = 0.048 μM) were found to be potent and selective CB2 receptor partial agonists, both with improved aqueous solubility.
Introduction
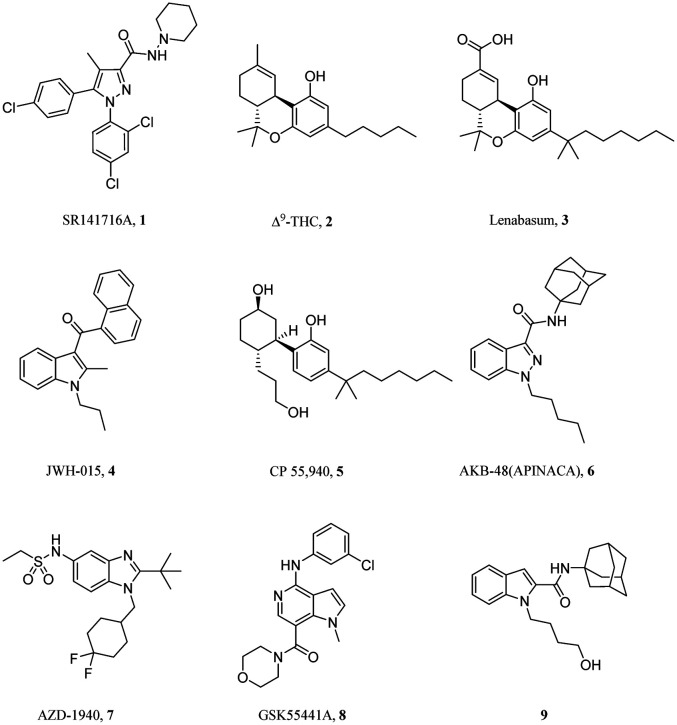
The endocannabinoid system (ECS) is composed of cannabinoid receptors, endogenous cannabinoids and enzymes that synthesize and degrade endocannabinoids. The two subtypes of cannabinoid receptors, namely CB1 and CB2, belong to the G protein-coupled receptor (GPCR) superfamily and are increasingly recognized as potential therapeutic targets for numerous diseases. The two receptors were characterized and cloned in mammalian tissues in the early 1990s.1,2 The CB1 receptor is primarily found in different areas of the brain, consistent with the known behavioral effects associated with cannabinoids.3 The CB1 receptor has been demonstrated to regulate the release of important neurotransmitters such as glutamate and GABA,4 thus in turn modulating appetite, learning and memory, mood, and pain perception. Drug discovery efforts directed towards this target resulted in the introduction of rimonabant (1, Fig. 1), a selective CB1 receptor inverse agonist developed by Sanofi-Aventis in 2006. Although initially approved in Europe as an anorectic antiobesity drug, it was later withdrawn worldwide due to serious psychiatric side effects, notably an increased risk of suicide attempts.5 Studies on other selective CB1 receptor antagonists, such as MK-0364 and CP-945598, were halted in the clinical phase due to similar risks of serious side effects.6,7 In contrast, the CB2 receptor was found primarily in the immune system and identified in macrophages from the spleen, B-cells, and natural killer cells.8,9 Recent drug discovery efforts by pharma and academia have been focused on the hypothesis that selective targeting of the CB2 receptor could confer therapeutic benefits for immune disorders while avoiding the CB1 receptor-mediated psychoactive side effects.
Fig. 1. Selected cannabinoid receptor ligands.
Δ9-Tetrahydrocannabinol (Δ9-THC, 2, Fig. 1) is the major psychoactive constituent isolated from marijuana. Δ9-THC possesses potent analgesic and anti-inflammatory properties as well as the ability to affect the activity of immune cells.10,11 It has been demonstrated that Δ9-THC treatment induced apoptosis of thymocytes and splenocytes in vivo,12 while in another study it was shown to suppress inflammatory responses associated with graft-versus-host disease (GVHD).13 Lenabasum (3, Fig. 1) is a Δ9-THC derivative and selective CB2 receptor agonist developed by Corbus Pharmaceuticals that recently received FDA Orphan Drug Designation for the treatment of dermatomyositis.14 The phase II studies report its clinical benefits on inflammatory and immunological markers in diffuse cutaneous systemic sclerosis, cystic fibrosis and dermatomyositis (DM).15 Acylindole JWH-015 (4, Fig. 1) is a selective CB2 agonist known to inhibit the proliferative response of T cells and thus exhibits potential as an anti-inflammatory and immunosuppressive agent.16 CP55,940 (5), a synthetic cannabinoid created by Pfizer in 1974, is a full agonist at both CB1 and CB2 receptors, currently a common choice for use as a positive control in cannabinoid receptor functional assays.17,18
A considerable amount of research was focused on the use of amidoalkylindole scaffolds as cannabinoid receptor ligands, as evidenced by the various 5,6-fused heteroaromatic replacements such as imidazopyridines, benzimidazoles, indazoles, and azaindoles.19,20 APINACA (6) is an indazole-3-carboxamide-based synthetic cannabinoid identified in Japan in 2012 as a designer drug. Various analytical techniques for the detection of this compound and its metabolites have been reported, but pharmacological and toxicological data on these compounds are still lacking.21 AZD1940 (7) is a benzimidazole-based CB1/CB2 agonist from AstraZeneca that was tested in phase II clinical trials for pain and hyperalgesia, but accompanied with reports of mild CNS and gastrointestinal side effects.22 GlaxoSmithKline has explored a series of azaindole scaffolds, exemplified by the morpholine GSK55441A (8) which has been identified as a potent and selective CB2 agonist (EC50,CB1 = 6.3 μM, EC50,CB2 = 0.005 μM) with efficacy in the chronic joint pain model without causing significant hypothermia.23
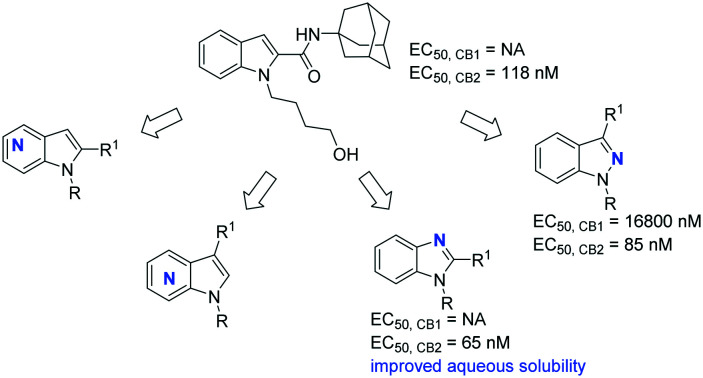
In our previous study,24 our group reported structural modifications of a mixed CB1R/CB2R agonist SDB-001 (EC50,CB1 = 0.034 μM, EC50,CB2 = 0.029 μM) to obtain a selective CB2R agonist 9 (Fig. 1; EC50,CB1 > 10 μM, EC50,CB2 = 0.118 μM). This compound was administered i.p. in a multiple sclerosis mouse model of experimental autoimmune encephalomyelitis, and resulted in significant alleviation of the clinical symptoms. Repositioning of the carboxamide moiety from the 3-position to the 2-position of the backbone indole ring has been considered as the main driving factor for improved CB2R selectivity. However, indole carboxamide 9 suffers from limited aqueous solubility and high lipophilicity as a consequence of the adamantyl amide fulfilling the structural requirements for the hydrophobic binding pockets of CB2R.25 We found that the oral exposure of indole carboxamide 9 was associated with very low bioavailability (unpublished data). With the aim to improve its drug-like physicochemical properties while maintaining CB2/CB1 selectivity, herein we report our ongoing efforts on a series of benzimidazoles, indazoles and 2- and 3-amidoalkylazaindoles as bioisosteric replacements of the indole nucleus. Their structure–activity relationship (SAR) data are presented herein, along with the initial assessment of aqueous solubility for selected compounds.
Results and discussion
Chemistry
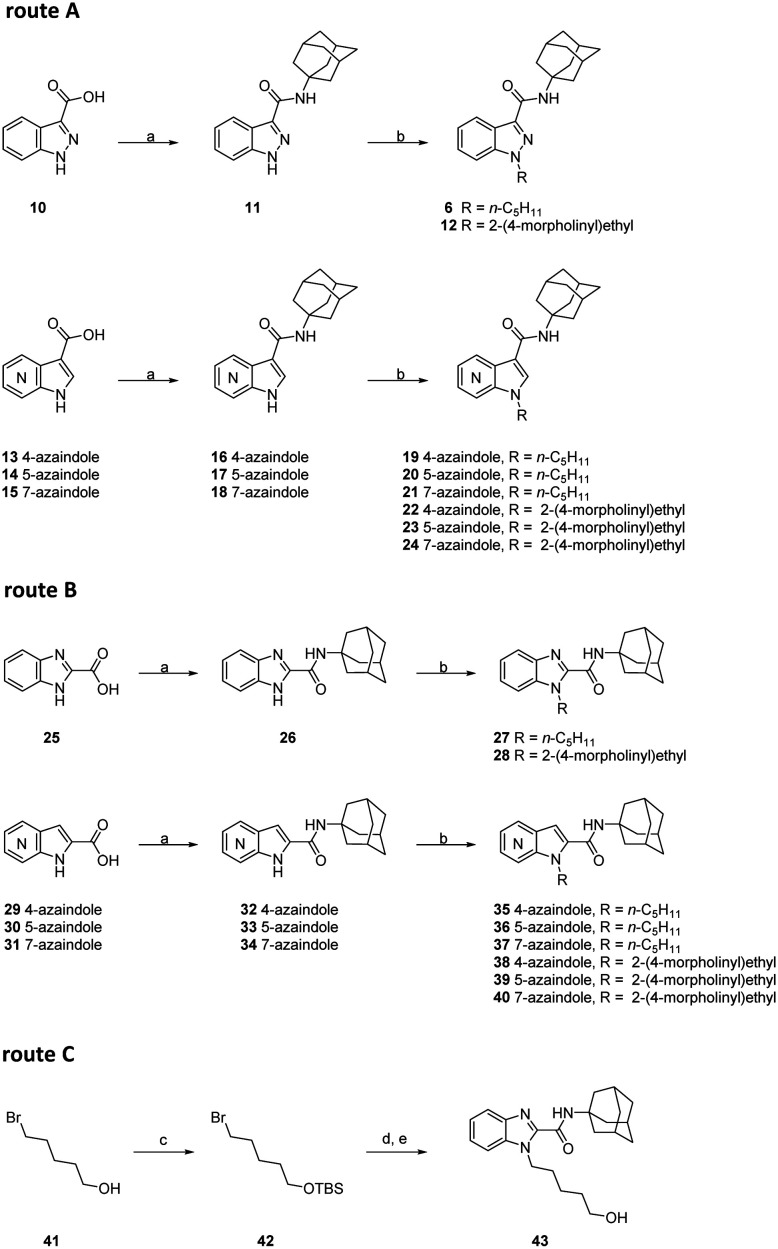
To access compound 6, commercially available indazole-3-carboxylic acid 10 was coupled with amantadine to generate intermediate 11 (route A, Scheme 1). Subsequent alkylation with 1-bromopentane or 4-(2-bromoethyl)morpholine using sodium hydride in anhydrous DMF afforded indazoles 6 and 12, respectively. Applying a similar procedure, 3-amidoalkylazaindole analogues 16–24 were synthesized according to route A shown in Scheme 1 utilising commercially available azaindole-3-carboxylic acids 13–15. In turn, benzimidazole-2-carboxylic acid 25 was chosen as the starting material to generate benzimidazole-2-carboxamide 26, which was then alkylated with either 1-bromopentane or 4-(2-bromoethyl)morpholine to give carboxamides 27 and 28. Applying a similar procedure to route A, 2-amidoalkylazaindole analogues 32–40 were synthesized according to route B. Silyl ether 42 was prepared through tert-butyldimethylsilyl (TBS) protection of commercially available 5-bromopentan-1-ol. Subsequent N-alkylation with benzimidazole-2-carboxamide 26 and deprotection of TBS in the presence of TBAF afforded the target compound 43.
Scheme 1. Reagents and conditions: a) N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, 1-hydroxybenzotriazole hydrate, Et3N, amantadine, DMF, rt; b) 1-bromopentane or 4-(2-bromoethyl)morpholine, NaH, DMF, 0 °C-rt, 5 h; c) TBSCl, imidazole, CH2Cl2, rt: d) 26, NaH, DMF, 0 °C-rt, 5 h; e) TBAF, THF, rt.
In vitro pharmacology and SAR
All final compounds were characterized using the calcium mobilization assay in Chinese hamster ovary (CHO) cells expressing CB1 or CB2 receptors as listed in Table 1. Compounds showing above 50% activation or below 50% inhibitory activity at 10 μM were assessed for their EC50 or IC50 values in a typical dose–response curve experiment. Removal of the n-pentyl group from APINACA 6 was favorable for CB2R selectivity as can be seen in the over 400-fold decrease in the CB1R potency for compound 11. Bioisosteric replacement of the indazole core with an indole,24 or 4-, 5-, 7-azaindole (16–18) was found to be highly detrimental to both CB1R and CB2R potency. Substitution of the n-pentyl chain with an ethylmorpholine (6vs.12) resulted in a 7-fold decrease in the CB1R potency without a considerable change of the CB2R activity. Intriguingly, among n-pentyl analogues 19–21, 7-azaindole 21 showed CB2R agonistic activity (EC50,CB2 = 0.032 μM), whereas 4- and 5-azaindoles 19 and 20 displayed good to moderate CB2R antagonistic activity with EC50 values of 0.012 and 0.26 μM. Among the 4-ethylmorpholine analogues (22–24), 7-azaindole 24 was found to be a potent and selective CB2R partial agonist (EC50,CB1 >10 μM, EC50,CB2 = 0.048 μM, Emax of 26%), while 4- and 5-azaindoles were found to be detrimental to CB2R potency (22–23), which is consistent with the trend observed with the n-pentyl analogues (19–21).
Table 1. Agonist and antagonist activities of synthesized analogues 6, 11–12, 16–24, 26–28, 32–40, and 43 in CHO cells expressing the human CB1 or CB2 receptor by calcium mobilization assays a .
| Cmpd | Agonism |
Antagonism |
||||
| EC50,CB1 (μM) | E max, % | EC50,CB2 (μM) | E max, % | IC50,CB1 (μM) | IC50,CB2 (μM) | |
| 6 | 0.038 ± 0.003 | 99.6 ± 5.4 | 0.092 ± 0.014 | 58.5 ± 2.1 | NA b | NA |
| 11 | 17.1 ± 1.9 | 67.3 ± 1.2 | 0.086 ± 0.005 | 73.9 ± 1.6 | NA | NA |
| 12 | 0.30 ± 0.07 | 109 ± 8 | 0.058 ± 0.013 | 85.3 ± 5.0 | NA | NA |
| 16 | NA | ND c | NA | ND | NA | NA |
| 17 | NA | ND | NA | ND | NA | NA |
| 18 | NA | ND | NA | ND | NA | 6.5 ± 1.5 |
| 19 | 0. 18 ± 0.02 | 48.2 ± 5.5 | NA | ND | NA | 0.012 ± 0.004 |
| 20 | 2.1 ± 0.1 | 41.0 ± 3.4 | NA | ND | NA | 0.26 ± 0.08 |
| 21 | 0.74 ± 0.32 | 39.1 ± 3.3 | 0.032 ± 0.007 | 79.4 ± 5.7 | NA | NA |
| 22 | NA | ND c | NA | ND | NA | NA |
| 23 | NA | ND | NA | ND | NA | NA |
| 24 | NA | ND | 0.048 ± 0.024 | 25.7 ± 3.4 | NA | NA |
| 26 | NA | ND | 0.52 ± 0.05 | 85.3 ± 3.0 | NA | NA |
| 27 | NA | ND | 0.32 ± 0.07 | 62.2 ± 3.7 | NA | NA |
| 28 | NA | ND | NA | ND | NA | 0.036 ± 0.002 |
| 32 | NA | ND | 0.59 ± 0.12 | 38.0 ± 5.8 | NA | NA |
| 33 | NA | ND | 2.0 ± 0.2 | 121 ± 5 | NA | NA |
| 34 | NA | ND | 0.29 ± 0.06 | 40.7 ± 3.4 | NA | NA |
| 35 | NA | ND | 0.22 ± 0.03 | 50.0 ± 4.1 | NA | NA |
| 36 | NA | ND | NA | ND | NA | NA |
| 37 | NA | ND | 0.92 ± 0.02 | 46.2 ± 1.0 | NA | NA |
| 38 | NA | ND | NA | ND | NA | NA |
| 39 | NA | ND | NA | ND | NA | NA |
| 40 | 0.65 ± 0.15 | 88.4 ± 1.3 | NA | ND | NA | 0.078 ± 0.014 |
| 43 | NA | ND | 0.067 ± 0.011 | 64.0 ± 6.3 | NA | NA |
| CP55940 | 0.020 ± 0.004 | 100 ± 5 | 0.055 ± 0.003 | 100 ± 10 | — | — |
aSee the Experimental section. Data represent mean values of eight-point experiments each performed in triplicate. In the agonist mode, compound CP55940 was used as a positive control. In the antagonist mode, cells were pre-incubated with either the test compounds or SR141716A as a positive control for 10 min, followed by addition of agonist CP55940 at 100 nM.
bNA: not active, defined as <50% activation or <50% inhibitory activity at 10 μM in the primary assay.
cND: not determined, for compounds defined as not active, their maximal effects were not determined.
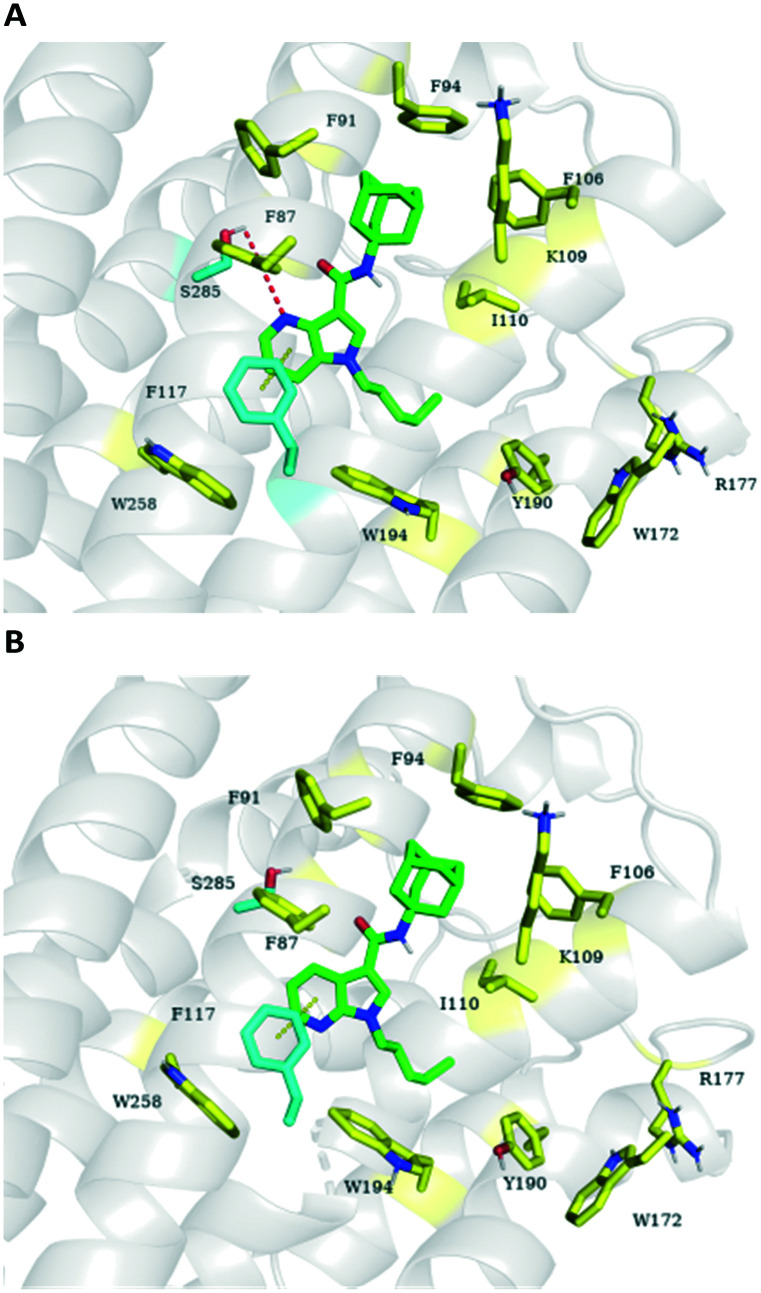
To address the agonist and antagonist behavior switch, we performed a molecular modeling study on compounds 19 and 21. The proposed binding modes are shown in Fig. 2 (PDBID: 5TGZ). Key amino acid residues S285 and F117 were previously demonstrated to affect the function of CB2R.31–33 For compound 19, the 4-azaindole behaving as a CB2R antagonist shares π–π interactions with F117 as well as hydrogen bonding interactions with S285 (Fig. 2A). Movement of the nitrogen atom from the 4-position to the 7-position of the azaindole moiety has been shown to switch the functional activity at the CB2 receptor possibly due do the lack of hydrogen bonding interaction with key residue S285 (Fig. 2B).
Fig. 2. Proposed binding modes of compounds (A) 19 (colored green) and (B) 21 (colored green). The key amino acid residues are colored cyan and yellow in the active site of CB2R (PDB ID ; 5ZTY). The key interactions are colored yellow (π–π) and red (hydrogen bond). Relative binding affinities were estimated by the Glide-XP scoring function. 19 (–9.2), 21 (–8.9).
Next, repositioning of the amidoadamantyl substituent from the 3-position to the 2-position (26–28, 32–40) essentially resulted in total removal of the CB1R activity, with the exception of 7-azaindole 40 with an EC50 value of 0.65 μM at CB1R. This trend is consistent with our observation for the indole scaffold in the previous series.24 On comparing the non-substituted analogues 26 and 32–34, it was found that the CB2R potency ranges from 0.29 to 2.0 μM. An n-pentyl substitution on the azaindole or the benzimidazole N1 (27 and 35–37) gave CB2R partial agonists with comparable activities to the non-substituted counterparts. It was observed that generally the 5-azaindole analogues had very weak or negligible activities at CB2R while the 7-azaindoles tend to be the most potent among the series. Replacement of the n-pentyl with an ethylmorpholine (27vs.28) for the benzimidazole resulted in an inactive compound. A similar trend was seen within the azaindoles (35–37vs.38–40). Applying the SAR obtained from our previous work on indolecarboxamides,24 installation of the pentanol side chain on benzimidazole 26 afforded a potent and selective CB2R partial agonist 43 (EC50,CB2 = 0.067 μM, EC50,CB1 >10 μM).
Aqueous-solubility determination
Aqueous solubility is an important factor to the absorption, penetration, and oral bioavailability of compounds, and more soluble compounds are often better suited for clinical development. CB2 receptor agonists in general are lipophilic due to the nature of the hydrophobic binding pocket and therefore tend to possess limited aqueous solubility. The kinetic solubility was measured based on the solution-precipitation method using DMSO stock solutions of compounds. The high performance liquid chromatography (HPLC) method is very applicable to the measurement of kinetic solubility.26 A wide range of concentrations can be measured with various pH values in solutions. Consequently, it became one of the more commonly used methods for aqueous solubility assessment of small drug-like molecules.27,28 In order to evaluate the kinetic aqueous solubility of CB2 receptor agonists, we used an analytical HPLC method, and the solubility of compounds was measured at pH 1.4 and pH 7.4. The calculated aqueous solubility using different methods and experimental aqueous solubility are shown in Table 2. In general, the predicted ACD Labs log S and the ALOGPS value trends are in agreement with the experimental data. Our previously reported indole 9 possesses a low-to-moderate aqueous solubility at around 24 μM L–1 at pH 7.4. Bioisosteric replacement of the indole with a benzimidazole (9 to 43) only brought about a modest improvement of 33 μM L–1. The unsubstituted indazole 11 had rather poor aqueous solubility at <10 μM L–1 at both pH 7.4 and 1.4, which contradicts the predicted values. However, as expected, the azaindole 24 and the benzimidazole 43 were significantly more soluble at pH 1.4 with values of around 87–96 μM L–1. 7-Azaindole 24 possesses an improved solubility of 69 μM L–1 at pH 7.4 due to the ethylmorpholine side chain.
Table 2. The calculated and experimental aqueous solubilities of compounds 9, 11, 20, 24, and 43.
| Cmpd | clog P a | Calculated aqueous solubility |
Experimental aqueous solubility |
|||||
| pH 7.4 |
pH 1.4 |
|||||||
| log S a | ACD Labs log S b | ALOGPS log S c | μM L–1 ± SD d | log S e | μM L–1 ± SD | log S e | ||
| SDB-001 | 6.37 | –6.71 | –5.54 | –5.31 | ND f | ND | ND | ND |
| 9 | 4.73 | –5.67 | –4.59 | –5.18 | 23.71 ± 0.49 | –4.63 | 16.77 ± 0.09 | –4.78 |
| 11 | 3.87 | –4.61 | –3.51 | –4.50 | <10 | <–5 | <10 | <–5 |
| 24 | 3.23 | –4.98 | –3.05 | –4.13 | 69.43 ± 0.27 | –4.15 | 86.6 ± 0.69 | –4.06 |
| 43 | 4.18 | –5.04 | –3.79 | –4.86 | 32.88 ± 0.12 | –4.48 | 96.00 ± 0.02 | –4.02 |
aCalculated with ChemDraw Ultra 14.0.
bACD calculated solubility values (ACD/Labs Percepta 2016, Build 2911).
cALOGPS 2.1 calculated solubility values (http://www.vcclab.org).
dStandard deviations (n = 3).
eCalculated with experimental aqueous solubility.
fNot determined.
Conclusions
In conclusion, we synthesized and evaluated a series of azaindole analogues including indazoles and benzimidazoles as indole bioisosteres of our previously reported amidoalkylindole-based selective CB2 receptor ligands. It is apparent from the 3-amidoalkylazaindole series that the position of the nitrogen atom plays an important role in determining the potency and selectivity to cannabinoid receptors. In particular, the 7-azaindole analogues were demonstrated to be the most potent and selective to the CB2 receptor, while all 4-azaindole and 5-azaindole analogues essentially lost CB2 agonistic activity. Consistent with our previous findings, the 2-amidoalkylazaindole series was generally highly selective for the CB2 receptor. In addition, we found that replacing the n-pentyl appendage of the benzimidazole (compound 27) with a morpholinoethyl substituent (compound 28) resulted in a selective CB2 receptor antagonist. Non-substituted indazole 11 was found to be a highly potent and selective CB2R agonist. Benzimidazole 43 exhibited the highest CB2 receptor agonist activity with no activity at the CB1 receptor. Moreover, the aqueous solubility of compound 43 (pH 1.4 = 96.1 μM L–1) was significantly improved compared to our previously reported compound 9 (pH 1.4 = 16.8 μM L–1). Overall, the SAR findings reported herein on 3-amidoalkylazaindoles and 2-amidoalkylazaindoles will guide further optimization of the drug-like physicochemical properties within the related series.
Experimental
General methods
Starting materials, reagents, and solvents were purchased from commercial suppliers and used without further purification, unless otherwise stated. All non-aqueous reactions were run under a nitrogen atmosphere with exclusion of moisture from reagents, and all reaction vessels were oven-dried. The progress of reactions was monitored by TLC on SiO2. Spots were visualized by their quenching of the fluorescence of an indicator admixed to the SiO2 layer, or by dipping into a phosphomolybdic acid ethanol solution followed by heating. SiO2 for flash chromatography was of 200–300 mesh particle size, and an EtOAc/hexane mixture or gradient was used unless stated otherwise. 1H NMR spectra were recorded at a spectrometer frequency of 400 MHz, and 13C NMR spectra at 101 MHz. Chemical shifts are reported in δ (ppm) using the δ 0 signal of tetramethylsilane (TMS) as an internal standard. High resolution mass spectra were obtained using a Bruker ESI-TOF high-resolution mass spectrometer.
Route A
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-indazole-3-carboxamide (6)
To a stirred solution of N-((3s,5s,7s)-adamantan-1-yl)-1H-indazole-3-carboxamide (41 mg, 0.14 mmol) in anhydrous DMF (1 mL) was added NaH (13 mg, 50% in mineral oil, 0.28 mmol) with ice cooling under N2. After stirring for 15 min at rt, 1-bromopentane (23 mg, 0.15 mmol) was added. Following stirring for 2 h at rt, the reaction was quenched with water (15 mL) with ice cooling. The resulting mixture was extracted with EtOAc (3 × 20 mL) and the combined organic layers were washed with H2O (3 × 40 mL) and then brine (40 mL), dried (Na2SO4) and concentrated in vacuo to give the white solid residue which was purified by flash chromatography on silica gel, eluted with hexane/EtOAc (5 : 1) to provide the title compound as a white solid; yield: 36 mg, 71%; 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 8.0 Hz, 1H), 7.38–7.37 (m, 2H), 7.26–7.22 (m, 1H), 6.81 (s, 1H), 4.34 (t, J = 7.2 Hz, 2H), 2.21–2.14 (m, 9H), 1.96–1.89 (m, 2H), 1.79–1.65 (m, 6H), 1.39–1.31 (m, 4H), 0.89 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.1, 140.9, 138.0, 126.5, 123.1, 122.8, 122.3, 109.1, 51.8, 49.3, 41.9 (3C), 36.5 (3C), 29.6 (3C), 29.5, 28.9, 22.2, 13.9. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2530.
N-((3s,5s,7s)-Adamantan-1-yl)-1H-indazole-3-carboxamide (11)
To a solution of 1H-indazole-3-carboxylic acid (300 mg, 1.85 mmol) in DMF (6 mL) was added 1-hydroxybenzotriazole (253 mg, 1.87 mmol), and triethylamine (749 mg, 7.4 mmol) was added followed by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (358 mg, 1.87 mmol) and finally amantadine (284 mg, 1.87 mmol) was added in a single portion. After stirring for 12 h at rt, the crude mixture was diluted with EtOAc (20 mL) and filtered off and the filter cake was washed with EtOAc (3 × 10 mL). The combined filtrates were washed with H2O (3 × 40 mL) and then brine (40 mL), dried (Na2SO4), and concentrated in vacuo to give the white solid residue which was purified by flash chromatography on silica gel, eluted with CH2Cl2/MeOH (10 : 1) to give the title compound. White solid; yield: 284 mg, 52%; 1H NMR (400 MHz, DMSO-d6) δ 13.48 (s, 1H), 8.16 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 8.4 Hz, 1H), 7.40 (t, J = 7.4 Hz, 1H), 7.24–7.22 (m, 2H), 2.11–2.07 (m, 9H), 1.76–1.61 (m, 6H). 13C NMR (101 MHz, DMSO-d6) δ 161.4, 141.2, 138.8, 126.4, 121.9, 121.6, 121.3, 110.6, 51.0, 41.1 (3C), 36.0 (3C), 28.9 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1587.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-indazole-3-carboxamide (12)
This compound was obtained from 11 and 4-(2-chloroethyl)morpholine via route A. White solid; yield: 61%; 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 8.0 Hz, 1H), 7.41–7.38 (m, 2H), 7.26–7.25 (m, 1H), 6.78 (s, 1H), 4.49 (t, J = 6.6 Hz, 2H), 3.67 (m, 4H), 2.87 (t, J = 6.0 Hz, 2H), 2.51 (m, 4H), 2.20–2.14 (m, 9H), 1.78–1.71 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 160.9, 140.1, 128.6, 125.7, 122.1, 121.8, 121.5, 108.1, 65.7, 56.5, 52.7 (2C), 50.9, 40.9 (3C), 35.4 (3C), 28.7 (2C), 28.5 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2614.
N-((3s,5s,7s)-Adamantan-1-yl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide (16)
This compound was obtained from 4-azaindole-3-carboxylic acid according to the methodology described for 11. White solid; yield 45%; 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 8.64 (s, 1H), 8.44 (d, J = 3.4 Hz, 1H), 8.08 (s, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.22 (dd, J = 7.3, 4.6 Hz, 1H), 2.18–2.02 (m, 9H), 1.68 (brs, 6H). 13C NMR (101 MHz, DMSO-d6) δ 162.2, 143.0, 142.2, 132.6, 129.1, 120.1, 117.1, 111.1, 50.5, 41.6 (3C), 36.0 (3C), 28.9 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1575.
N-((3s,5s,7s)-Adamantan-1-yl)-1H-pyrrolo[3,2-c]pyridine-3-carboxamide (17)
This compound was obtained from 1H-pyrrolo[3,2-c]pyridine-3-carboxylic acid according to the methodology described for 11. White solid; yield 75%; 1H NMR (400 MHz, MeOD-d6) δ 9.29 (s, 1H), 8.21 (d, J = 5.9 Hz, 1H), 8.03 (s, 1H), 7.49 (d, J = 5.8 Hz, 1H), 2.21–2.9 (m, 9H), 1.81–1.75 (m, 6H). 13C NMR (101 MHz, MeOD-d6) δ 166.2, 144.0, 142.3, 140.3, 130.5, 124.6, 114.1, 108.9, 53.4, 42.7 (3C), 37.6 (3C), 31.1 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1595.
N-((3s,5s,7s)-Adamantan-1-yl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (18)
This compound was obtained from 1H-pyrrolo[2,3-b]pyridine-3-carboxylic acid according to the methodology described for 11. White solid; yield 59%; 1H NMR (400 MHz, CDCl3) δ 10.21 (s, 1H), 8.42–8.32 (m, 2H), 7.78 (s, 1H), 7.22 (dd, J = 7.8, 4.8 Hz, 1H), 5.58 (s, 1H), 2.22–2.12 (m, 9H), 1.79–1.70 (m, 6H). 13C NMR (101 MHz, DMSO-d6) δ 163.6, 148.3, 143.2, 129.3, 127.9, 118.7, 116.6, 110.3, 51.0, 41.3 (3C), 36.1 (3C), 28.9 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1596.
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-pyrrolo[3,2-b]pyridine-3-carboxamide (19)
This compound was obtained from 16 according to the methodology described for 6. White solid; yield: 78%; 1H NMR (400 MHz, CDCl3) δ 8.70 (s, 1H), 8.47 (d, J = 4.3 Hz, 1H), 7.97 (s, 1H), 7.65 (d, J = 8.2 Hz, 1H), 7.15 (dd, J = 8.2, 4.7 Hz, 1H), 4.11 (t, J = 7.0 Hz, 2H), 2.29–2.12 (m, 9H), 1.89–1.71 (m, 8H), 1.34–1.24 (m, 4H), 0.87 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 163.3, 143.3, 143.3, 134.6, 129.6, 117.4, 116.7, 111.8, 51.6, 47.0, 42.0 (3C), 36.6 (3C), 29.8, 29.6 (3C), 28.9, 22.2, 13.8. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2541.
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-pyrrolo[3,2-c]pyridine-3-carboxamide (20)
This compound was obtained from 17 according to the methodology described for 6. White solid; yield: 75%; 1H NMR (400 MHz, CDCl3) δ 9.26 (s, 1H), 8.37 (d, J = 5.8 Hz, 1H), 7.63 (s, 1H), 7.25 (d, J = 5.8 Hz, 1H), 5.79 (s, 1H), 4.08 (t, J = 7.1 Hz, 2H), 2.25–2.09 (m, 9H), 1.87–1.70 (m, 8H), 1.39–1.22 (m, 4H), 0.87 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 163.3, 143.4, 141.4, 140.3, 131.5, 122.4, 112.8, 105.2, 52.3, 46.7, 42.1 (3C), 36.4 (3C), 29.7, 29.5 (3C), 28.8, 22.2, 13.8. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2560.
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (21)
This compound was obtained from 18 according to the methodology described for 6. White solid; yield: 77%; 1H NMR (400 MHz, CDCl3) δ 8.36 (d, J = 4.6 Hz, 1H), 8.26 (d, J = 7.9 Hz, 1H), 7.70 (s, 1H), 7.17 (dd, J = 7.2, 4.8 Hz, 1H), 5.56 (s, 1H), 4.29 (t, J = 7.2 Hz, 2H), 2.21–2.11 (m, 9H), 1.93–1.82 (m, 2H), 1.80–1.69 (m, 6H), 1.36–1.28 (m, 4H), 0.88 (t, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 163.8, 147.6, 143.5, 130.1, 128.7, 118.1, 117.2, 110.5, 52.2, 45.0, 42.2 (3C), 36.5 (3C), 29.9, 29.6 (3C), 28.9, 22.3, 13.9. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2558.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide (22)
This compound was obtained from 16 and 4-(2-chloroethyl)morpholine via route A. Yellow solid; yield: 67%; 1H NMR (400 MHz, CDCl3) δ 8.71 (s, 1H), 8.48 (d, J = 3.3 Hz, 1H), 8.04 (s, 1H), 7.69 (d, J = 8.1 Hz, 1H), 7.17 (dd, J = 7.3, 4.9 Hz, 1H), 4.23 (t, J = 6.1 Hz, 2H), 3.66 (brs, 4H), 2.74 (t, J = 6.1 Hz, 2H), 2.46 (brs, 4H), 2.28–2.11 (m, 9H), 1.81–1.69 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 163.2, 143.5, 143.2, 134.9, 129.7, 117.5, 116.8, 112.1, 66.8 (2C), 58.2, 53.8 (2C), 51.7, 44.4, 42.0 (3C), 36.6 (3C), 29.6 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2592.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-pyrrolo[3,2-c]pyridine-3-carboxamide (23)
This compound was obtained from 17 and 4-(2-chloroethyl)morpholine via route A. White solid; yield: 72%; 1H NMR (400 MHz, CDCl3) δ 9.22 (s, 1H), 8.39 (d, J = 5.6 Hz, 1H), 7.73 (s, 1H), 7.37–7.23 (m, 1H), 5.71 (s, 1H), 4.23 (t, J = 6.3 Hz, 2H), 3.72–3.66 (m, 4H), 2.74 (t, J = 6.3 Hz, 2H), 2.51–2.45 (m, 4H), 2.24–2.11 (m, 9H), 1.80–1.68 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 163.1, 143.1, 141.3, 140.5, 132.1, 122.2, 113.1, 105.2, 66.9 (2C), 58.0, 53.8 (2C), 52.4, 44.1, 42.1 (3C), 36.4 (3C), 29.5 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2618.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide (24)
This compound was obtained from 18 and 4-(2-chloroethyl)morpholine via route A. White solid; yield: 58%; 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 4.3 Hz, 1H), 8.26 (d, J = 7.8 Hz, 1H), 7.80 (s, 1H), 7.18 (dd, J = 7.7, 4.8 Hz, 1H), 5.55 (s, 1H), 4.43 (t, J = 6.5 Hz, 2H), 3.75–3.63 (m, 4H), 2.81 (t, J = 5.7 Hz, 2H), 2.53 (brs, 4H), 2.22–2.08 (m, 9H), 1.79–1.70 (m, 6H). 13C NMR (101 MHz, DMSO-d6) δ 163.3, 147.1, 142.9, 131.0, 129.5, 118.9, 116.9, 109.3, 66.1 (2C), 57.6, 53.1 (2C), 51.1, 41.3 (3C), 41.1, 36.1 (3C), 28.9 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2563.
Route B
N-((3s,5s,7s)-Adamantan-1-yl)-1H-benzo[d]imidazole-2-carboxamide (26)
This compound was obtained from 1H-benzimidazole-2-carboxylic acid according to the methodology described for 11. White solid; yield 52%; 1H NMR (400 MHz, DMSO-d6) δ 13.16 (s, 1H), 7.74 (s, 1H), 7.71 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 7.5 Hz, 1H), 7.36–7.20 (m, 2H), 2.19–2.06 (m, 9H), 1.68 (brs, 6H). 13C NMR (101 MHz, DMSO-d6) δ 157.7, 146.2, 142.3, 134.5, 124.0, 122.5, 119.8, 112.5, 51.6, 40.8 (3C), 35.9 (3C), 28.8 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1589.
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-benzo[d]imidazole-2-carboxamide (27)
This compound was obtained from 26 according to the methodology described for 6. White solid; yield 50%; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.2 Hz, 1H), 7.52 (s, 1H), 7.41 (d, J = 7.1 Hz, 1H), 7.36–7.30 (m, 1H), 4.69 (t, J = 6.5 Hz, 2H), 2.24–2.10 (m, 9H), 1.94–1.83 (m, 2H), 1.78–1.67 (m, 6H), 1.40–1.28 (m, 4H), 0.95–0.81 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 158.7, 144.1, 140.9, 136.5, 124.2, 123.2, 120.5, 110.7, 52.4, 45.4, 41.5 (3C), 36.4 (3C), 30.2, 29.5 (3C), 29.0, 22.4, 14.0. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2525.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-benzo[d]imidazole-2-carboxamide (28)
This compound was obtained from 26 and 4-(2-chloroethyl)morpholine via route B. Yellow solid; yield: 69%; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.6 Hz, 1H), 7.52 (s, 1H), 7.43 (d, J = 7.8 Hz, 1H), 7.39–7.29 (m, 2H), 4.85 (t, J = 6.6 Hz, 2H), 3.70–3.59 (m, 4H), 2.77 (t, J = 6.6 Hz, 2H), 2.58–2.47 (m, 4H), 2.24–2.10 (m, 9H), 1.81–1.68 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 158.7, 144.4, 140.9, 136.5, 124.3, 123.3, 120.5, 110.7, 67.0 (2C), 58.1, 53.9 (2C), 52.4, 42.8, 41.5 (3C), 36.3 (3C), 29.5 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2620.
N-((3s,5s,7s)-Adamantan-1-yl)-1H-pyrrolo[3,2-b]pyridine-2-carboxamide (32)
This compound was obtained from 1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid according to the methodology described for 11. White solid; yield 58%; 1H NMR (400 MHz, DMSO-d6) δ 11.69 (s, 1H), 8.37 (s, 1H), 7.84–7.70 (m, 2H), 7.32 (s, 1H), 7.25–7.09 (m, 1H), 2.18–2.01 (m, 9H), 1.68 (brs, 6H). 13C NMR (101 MHz, DMSO-d6) δ 160.0, 145.1, 143.6, 135.0, 129.1, 119.4, 118.1, 102.7, 51.7, 40.9 (3C), 36.0 (3C), 28.9 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1591.
N-((3s,5s,7s)-Adamantan-1-yl)-1H-pyrrolo[3,2-c]pyridine-2-carboxamide (33)
This compound was obtained from 5-azaindole-2-carboxylic acid according to the methodology described for 11. White solid; yield 41%; 1H NMR (400 MHz, DMSO-d6) δ 8.90 (s, 1H), 8.20 (d, J = 5.7 Hz, 1H), 7.76 (s, 1H), 7.36 (d, J = 5.6 Hz, 1H), 7.32 (s, 1H), 2.14–2.05 (m, 9H), 1.67 (brs, 6H). 13C NMR (101 MHz, DMSO-d6) δ 159.9, 144.7, 141.4, 139.2, 133.8, 124.4, 107.2, 101.9, 51.7, 40.9 (3C), 36.0 (3C), 28.9 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1597.
N-((3s,5s,7s)-Adamantan-1-yl)-1H-pyrrolo[2,3-b]pyridine-2-carboxamide (34)
This compound was obtained from 1H-pyrrolo[2,3-b]pyridine-2-carboxylic acid according to the methodology described for 11. White solid; yield 43%; 1H NMR (400 MHz, DMSO-d6) δ 12.10 (s, 1H), 8.31 (d, J = 3.9 Hz, 1H), 8.03 (d, J = 7.7 Hz, 1H), 7.73 (s, 1H), 7.15–7.06 (m, 2H), 2.19–2.04 (m, 9H), 1.67 (brs, 6H). 13C NMR (101 MHz, DMSO-d6) δ 159.7, 148.2, 145.0, 133.1, 129.7, 119.2, 116.2, 102.3, 51.7, 40.9 (3C), 36.0 (3C), 28.9 (3C). HRMS (ESI): calcd for C18H21N3NaO [M + Na]+, 318.1577; found 318.1594.
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-pyrrolo[3,2-b]pyridine-2-carboxamide (35)
This compound was obtained from 32 according to the methodology described for 6. Yellow solid; yield: 51%; 1H NMR (400 MHz, CDCl3) δ 8.23 (d, J = 7.8 Hz, 1H), 7.69 (d, J = 6.0 Hz, 1H), 7.60 (brs, 1H), 7.09 (s, 1H), 7.08–7.03 (m, 1H), 4.42 (t, J = 7.4 Hz, 2H), 2.24–2.12 (m, 9H), 2.08–2.01 (m, 2H), 1.78–1.68 (m, 8H), 1.36–1.33 (m, 2H), 0.89 (t, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 163.6, 155.4, 143.7, 140.1, 131.2, 129.8, 111.8, 92.9, 57.4, 51.9, 41.6 (3C), 36.5 (3C), 29.6 (3C), 28.9, 28.8, 22.2, 13.8. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2528.
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-pyrrolo[3,2-c]pyridine-2-carboxamide (36)
This compound was obtained from 33 according to the methodology described for 6. Yellow solid; yield: 65%; 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.74–7.61 (m, 2H), 7.54 (d, J = 4.9 Hz, 1H), 7.38 (s, 1H), 4.27 (t, J = 6.8 Hz, 2H), 2.26–2.09 (m, 9H), 2.02–1.90 (m, 2H), 1.81–1.67 (m, 6H), 1.39–1.25 (m, 4H), 0.90 (t, J = 6.7 Hz, 3H). 13C NMR (101 MHz, MeOD-d6) δ 163.7, 147.7, 147.3, 139.7, 133.1, 128.1, 113.3, 105.7, 61.0, 53.5, 42.4 (3C), 37.5 (3C), 32.4, 31.0 (3C), 29.5, 23.3, 14.2. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2546.
N-((3s,5s,7s)-Adamantan-1-yl)-1-pentyl-1H-pyrrolo[2,3-b]pyridine-2-carboxamide (37)
This compound was obtained from 34 according to the methodology described for 6. White solid; yield: 57%; 1H NMR (400 MHz, CDCl3) δ 8.41 (d, J = 4.0 Hz, 1H), 7.90 (d, J = 7.7 Hz, 1H), 7.07 (dd, J = 7.7, 4.7 Hz, 1H), 6.66 (s, 1H), 5.89 (s, 1H), 4.68 (t, J = 7.3 Hz, 2H), 2.14 (brs, 9H), 1.82–1.69 (m, 8H), 1.37–1.23 (m, 4H), 0.86 (t, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 161.7, 148.6, 145.1, 133.8, 129.6, 118.7, 116.6, 100.9, 52.6, 42.9, 41.7 (3C), 36.3 (3C), 30.5, 29.5 (3C), 29.0, 22.5, 14.0. HRMS (ESI): calcd for C23H32N3O [M + H]+, 366.2540; found 366.2544.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-pyrrolo[3,2-b]pyridine-2-carboxamide (38)
This compound was obtained from 32 and 4-(2-chloroethyl)morpholine via route B. Yellow solid; yield: 60%; 1H NMR (400 MHz, CDCl3) δ 8.22 (d, J = 7.2 Hz, 1H), 7.75 (d, J = 5.0 Hz, 1H), 7.57 (brs, 1H), 7.13–6.97 (m, 2H), 4.50 (t, J = 4.9 Hz, 2H), 3.73–3.56 (m, 4H), 2.94 (t, J = 4.9 Hz, 2H), 2.52–2.40 (m, 4H), 2.24–2.10 (m, 9H), 1.78–1.69 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 162.7, 154.6, 142.7, 138.9, 131.2, 129.1, 110.4, 91.4, 65.8 (2C), 55.3, 53.3, 52.6 (2C), 50.8, 40.6 (3C), 35.5 (3C), 28.5 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2581.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-pyrrolo[3,2-c]pyridine-2-carboxamide (39)
This compound was obtained from 33 and 4-(2-chloroethyl)morpholine via route B. Yellow solid; yield: 52%; 1H NMR (400 MHz, CDCl3) δ 8.55 (s, 1H), 7.77–7.67 (m, 2H), 7.64 (d, J = 6.7 Hz, 1H), 7.33 (s, 1H), 4.41 (t, J = 5.0 Hz, 2H), 3.67–3.54 (m, 4H), 2.83 (t, J = 5.3 Hz, 2H), 2.51–2.41 (m, 4H), 2.21–2.00 (m, 9H), 1.71–1.60 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 162.1, 150.0, 147.0, 136.4, 129.8, 127.1, 113.1, 104.8, 66.8 (2C), 58.9, 56.9, 53.6 (2C), 52.3, 41.6 (3C), 36.5 (3C), 29.5 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2588.
N-((3s,5s,7s)-Adamantan-1-yl)-1-(2-morpholinoethyl)-1H-pyrrolo[2,3-b]pyridine-2-carboxamide (40)
This compound was obtained from 34 and 4-(2-chloroethyl)morpholine via route B. White solid; yield: 48%; 1H NMR (400 MHz, CDCl3) δ 8.18 (d, J = 7.3 Hz, 1H), 7.77 (d, J = 5.8 Hz, 1H), 7.49 (s, 1H), 6.87 (t, J = 6.5 Hz, 1H), 4.75 (t, J = 5.5 Hz, 2H), 3.73–3.61 (m, 4H), 2.97 (t, J = 5.8 Hz, 2H), 2.61–2.51 (m, 4H), 2.23–2.11 (m, 9H), 1.80–1.68 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 163.9, 149.4, 147.1, 133.5, 132.0, 130.7, 109.5, 102.7, 66.9 (2C), 57.3, 53.7 (2C), 51.4, 49.8, 41.7 (3C), 36.5 (3C), 29.6 (3C). HRMS (ESI): calcd for C24H33N4O2 [M + H]+, 409.2598; found 409.2603.
Route C
N-((3s,5s,7s)-Adamantan-1-yl)-1-(5-hydroxypentyl)-1H-benzo[d]imidazole-2-carboxamide (43)
To a solution of 5-bromopentan-1-ol (1.67 g, 10.0 mmol) in CH2Cl2 (15 mL) was added tert-butyldimethylsilyl chloride (1.8 g, 12.0 mmol) and imidazole (0.83 g, 12.1 mmol) at rt. After stirring for 3 h, the reaction mixture was filtered off and the filtrate was concentrated to provide crude silyl ether 42 as a colorless oil. To a solution of 26 (40 mg, 0.14 mmol) in anhydrous DMF (1 mL) was added NaH (13 mg, 50% in mineral oil, 0.27 mmol) with ice cooling. After stirring for 15 min, the crude silyl ether 42 (61 mg, 0.27 mmol) was added to the reaction mixture. After stirring for 1 h at rt, the reaction was quenched with water (10 mL) and extracted with EtOAc (3 × 20 mL). The combined organic layers were washed with water (3 × 10 mL) and brine (10 mL) and concentrated under vacuum. The residue was dissolved in a solution of TBAF in THF (2 mL, 0.6 mmol). After stirring overnight at rt, the mixture was concentrated under vacuum and the residue was purified by flash chromatography on silica gel, eluted with hexane/ethyl acetate (5 : 1) to provide 43. Yellow solid; yield 34 mg, 65%; 1H NMR (400 MHz, CDCl3) δ 9.10 (brs, 1H), 8.03 (d, J = 4.0 Hz, 1H), 7.72–7.52 (m, 3H), 4.89 (t, J = 7.3 Hz, 2H), 3.67 (t, J = 6.1 Hz, 2H), 2.30–2.12 (m, 9H), 2.03–1.94 (m, 2H), 1.78–1.64 (m, 8H), 1.56–1.49 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 152.8, 139.2, 132.6, 130.6, 127.9, 127.6, 116.8, 112.1, 62.2, 55.3, 46.8, 40.7 (3C), 36.2 (3C), 31.9, 29.8, 29.4 (3C), 23.1. HRMS (ESI): calcd for C23H31N3NaO2 [M + Na]+, 404.2308; found 404.2334.
Calcium mobilization assay29
Chinese hamster ovarian (CHO) cells (CHO-K1 cells were obtained from American Type Culture Collection) were stably transfected with the plasmid encoding hCB1 or hCB2 receptors cloned by our laboratory as reported previously.29 CHO cells stably expressing Gα16 with either the CB1 or CB2 receptor were seeded onto 96-well plates and incubated for 24 h. Cells were loaded with 2 μM fluo-4 AM in Hanks' balanced salt solution (HBSS, containing 5.4 mM KCl, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 4.2 mM NaHCO3, 1.3 mM CaCl2, 0.5 mM MgCl2, 0.6 mM MgSO4, 137.0 mM NaCl, 5.6 mM d-glucose and 250 μM sulfinpyrazone, pH 7.4) at 37 °C for 45 min. In the agonist mode, the excess dye was removed and 50 μL HBSS was added; 25 μL HBSS containing variable concentrations of test compounds, CP55940 (positive control), or DMSO (negative control) was added using a FlexStation microplate reader. Meanwhile, the intracellular calcium change was recorded at an excitation wavelength of 485 nm and an emission wavelength of 525 nm. In the antagonist mode, the excess dye was removed and 50 μL HBSS containing variable concentrations of test compounds, rimonabant (positive control), or DMSO (negative control) was added. After incubation at room temperature for 10 min, 25 μL HBSS containing CP55940 was dispensed into the wells using a FlexStation microplate reader. Florescence measurement data were continuously recorded for 60 s and the maximum intracellular calcium peak change was recorded at an excitation wavelength of 485 nm and an emission wavelength of 525 nm. All experiments were performed in triplicate. EC50 and IC50 values were analyzed with sigmoidal dose–response curve fitting using the GraphPad Prism program.
Molecular modeling
For the molecular docking of compounds 19 and 21, we used the solved crystal structures of the CB2 receptor containing antagonist AM10257 (PDB ID ; 5ZTY). The protein structure was prepared by Protein Preparation Wizard. All the compounds were flexibly docked into the binding pocket defined by residues F87, F91, F94, F106, K109, I110, F117, W172, R177, Y190, W194, W258, and S285 using Glide with the XP scoring function. Top-scoring poses were used for visualization analysis. CB2 interactions with each compound were pictured using Pymol. All of the molecular docking work was performed using the Maestro software.
Aqueous solubility Testing30
For these tests, the compound dissolved in DMSO was configured to a concentration of 10 mM, then diluted with 10 mM phosphate buffer at pH 7.4 or 1.4, and finally, the concentration of DMSO was 1%. Then, the final solution was shaken by ultrasonication for ten minutes at room temperature. After that, it was filtered through a 0.22 μm PTFE needle filter. Then, the compound was analyzed by HPLC and the concentration was determined by its standard curve which was obtained by analytical HPLC at different concentrations. Concentrations of compounds were determined by analytical HPLC that was performed on a Waters e2695 HPLC system. This system has a ZORBAX SB-C18 column, and detects at 275 nm and 290 nm by a 2998 PDA which is a variable wavelength detector. Compounds 3 and 9 were tested using gradient I: 80–95% methanol in water (both containing 0.5 ‰ of trifluoroacetic acid) in 19 min and its flow rate is 1.2 mL min–1; compound 11 was tested using gradient II: 60–95% methanol in water (both containing 0.5‰ v of trifluoroacetic acid) in 19 min and its flow rate is 1.0 mL min–1; compounds 20 and 24 were tested using gradient III: 50–95% methanol in water (both containing 0.5‰ of trifluoroacetic acid) in 23 min and its flow rate is 1.0 mL min–1; these reported data were presented as the average of three determinations.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by grants from the Innovation Program of Shanghai Municipal Education Commission (15ZZ027), the National Natural Science Foundation of China (81425024 and 81730099), and the Open Program of State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences (SIMM1501KF-05).
Footnotes
†Electronic supplementary information (ESI) available: NMR and HRMS spectra of all the synthetic compounds. See DOI: 10.1039/c9md00411d
References
- Lisac A. M., Stephen J. L., Michael J. B., Alice C. Y., Tom I. B. Nature. 1990;346:561–563. [Google Scholar]
- Munro S., Thomas L. K., Abu-Shaar M. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Panagis G., Mackey B., Vlachou S. Front. Psychiatry. 2014;5:1–20. doi: 10.3389/fpsyt.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. I., Nicoll R. A. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Christensen R., Kristensen P. K., Bartels E. M., Bliddal H., Astrup A. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Kim M-a., Yun H., Kwak H., Kim J., Lee J. Tetrahedron. 2008;64:10802–10809. [Google Scholar]
- Presley C. S., Abidi A. H., Moore B. M. Anal. Biochem. 2016;498:8–28. doi: 10.1016/j.ab.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Ferrini M. E., Hong S., Stierle A., Stierle D., Stella N., Roberts K., Jaffar Z. Allergy. 2017;72:937–947. doi: 10.1111/all.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J. P., An J., Xu Y., Huang Y., Cyster J. G. Nat. Immunol. 2009;10:403–411. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. G. Int. J. Obes. 2006;30:S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Olah A., Szekanecz Z., Biro T. Front. Immunol. 2017;8:1487. doi: 10.3389/fimmu.2017.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip R. J., Lombard C., Martin B. R., Nagarkatti M., Nagarkatti P. S. J. Pharmacol. Exp. Ther. 2002;302:451–465. doi: 10.1124/jpet.102.033506. [DOI] [PubMed] [Google Scholar]
- Pandey R., Hegde V. L., Nagarkatti M., Nagarkatti P. S. J. Pharmacol. Exp. Ther. 2011;338:819–828. doi: 10.1124/jpet.111.182717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S. H. Pharmacol. Res. Perspect. 2018;6:e00394. doi: 10.1002/prp2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Zeidi M., Bonciani D., Pena S. M., Tiao J., Sahu S., Werth V. P. Br. J. Dermatol. 2018;179:669–678. doi: 10.1111/bjd.16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard C., Nagarkatti M., Nagarkatti P. Clin. Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A., Zhao P., Sharir H., Bai Y., Caron M. G., Barak L. S. J. Biol. Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svizenska I., Dubovy P., Sulcova A. Pharmacol., Biochem. Behav. 2008;90:501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Han S., Thatte J., Buzard D. J., Jones R. M. J. Med. Chem. 2013;56:8224–8256. doi: 10.1021/jm4005626. [DOI] [PubMed] [Google Scholar]
- Blaazer A. R., Lange J. H., van der Neut M. A., Mulder A., den Boon F. S., Werkman T. R., Kruse C. G., Wadman W. J. Eur. J. Med. Chem. 2011;46:5086–5098. doi: 10.1016/j.ejmech.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Uchiyama N., Kawamura M., Kikura-Hanajiri R., Goda Y. Forensic Toxicol. 2012;30:114–125. [Google Scholar]
- Kalliomaki J., Annas P., Huizar K., Clarke C., Zettergren A., Karlsten R., Segerdahl M. Clin. Exp. Pharmacol. Physiol. 2013;40:212–218. doi: 10.1111/1440-1681.12051. [DOI] [PubMed] [Google Scholar]
- Giblin G. M., Billinton A., Briggs M., Brown A. J., Chessell I. P., Clayton N. M., Eatherton A. J., Goldsmith P., Haslam C., Johnson M. R., Mitchell W. L., Naylor A., Perboni A., Slingsby B. P., Wilson A. W. J. Med. Chem. 2009;52:5785–5788. doi: 10.1021/jm9009857. [DOI] [PubMed] [Google Scholar]
- Shi Y., Duan Y. H., Ji Y. Y., Wang Z. L., Wu Y. R., Gunosewoyo H., Xie X. Y., Chen J. Z., Yang F., Li J., Tang J., Xie X., Yu L. F. J. Med. Chem. 2017;60:7067–7083. doi: 10.1021/acs.jmedchem.7b00724. [DOI] [PubMed] [Google Scholar]
- Tuccinardi T., Ferrarini P. L., Manera C., Ortore G., Saccomanni G., Martinelli A. J. Med. Chem. 2006;49:984–994. doi: 10.1021/jm050875u. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ikeda Y. J. Drug Delivery Sci. Technol. 2016;33:13–18. [Google Scholar]
- Ohta H., Ishizaka T., Tatsuzuki M., Yoshinaga M., Iida I., Yamaguchi T., Tomishima Y., Futaki N., Toda Y., Saito S. Bioorg. Med. Chem. 2008;16:1111–1124. doi: 10.1016/j.bmc.2007.10.087. [DOI] [PubMed] [Google Scholar]
- Yrjölä S., Sarparanta M., Airaksinen A. J., Hytti M., Kauppinen A., Pasonen-Seppänen S., Adinolfi B., Nieri P., Manera C., Keinänen O., Poso A., Nevalainen T. J., Parkkari T. Eur. J. Pharm. Sci. 2015;67:85–96. doi: 10.1016/j.ejps.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Zhu T., Fang L. Y., Xie X. Acta Pharmacol. Sin. 2008;29:507–516. doi: 10.1111/j.1745-7254.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- Amato G. S., Manke A., Harris D. L., Wiethe R. W., Vasukuttan V., Snyder R. W., Lefever T. W., Cortes R., Zhang Y., Wang S., Runyon S. P., Maitra R. J. Med. Chem. 2018;61:4370–4385. doi: 10.1021/acs.jmedchem.7b01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee M. H. J. Vet. Sci. 2002;3:185–191. [PubMed] [Google Scholar]
- Alqarni M., Myint K. Z., Tong Q., Yang P., Bartlow P., Wang L., Feng R., Xie X. Q. Biochem. Biophys. Res. Commun. 2014;452:334–339. doi: 10.1016/j.bbrc.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir M., Lane S., Lai F., Connor M., Hibbs D. E., Kassiou M. Eur. J. Med. Chem. 2019;180:291–309. doi: 10.1016/j.ejmech.2019.07.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.