Abstract
Introduction
Prescription opioid misuse is a risk factor for opioid use disorder (OUD). Patients who misuse prescribed opioids and those who misuse illicit opioids are demographically and medically distinct groups, and research has shown there is heterogeneity in treatment response between these groups. The objective of this study was to measure the adjusted odds of successful stabilization on buprenorphine in patients with baseline prescription opioid use compared to those not prescribed opioids.
Methods
A cohort of patients newly prescribed a buprenorphine product indicated for OUD between January 1 and November 30, 2018, were identified from the Texas prescription monitoring program. We excluded those under the age of 15 and those who filled an opioid prescription after initiating buprenorphine to limit misclassification. We then stratified the cohort based on type of prescription opioid use in the pre-index period. We defined chronic opioid use as being prescribed opioids for a period of 90 out of 120 days, ending no sooner than 90 days prior to treatment initiation. We defined acute opioid use as filling any opioid prescription in the 90 days prior to initiating buprenorphine. The outcome of interest—stabilization on buprenorphine—was met by filling two prescriptions totaling 30-days’ supply with no more than a six-day gap in therapy. We used multiple logistic regression to estimate the odds of stabilization in the prescription opioid use categories compared to those with no pre-index, opioid prescriptions.
Results
Among 6,756 eligible patients, 44.1% used prescription opioids in the 90 days prior to buprenorphine initiation. Of these, 62.2% met the criteria for acute prescription opioid use and 37.8% for chronic prescription opioid use. Patients with prescription opioid use at baseline were more likely to be older and insured compared to those with no prescription opioid use. After adjustment for covariates, both prescription opioid use groups were significantly more likely to be successfully stabilized on therapy (Acute: aOR=1.53, 95% CI=1.37–1.72; Chronic: aOR=2.43, 95% CI=2.08–2.85). In a second model, those with chronic prescription opioid use were significantly more likely than those with acute prescription opioid use to be successfully stabilized (aOR=1.60, 95% CI=1.31–1.90).
Conclusion
Persistence to buprenorphine treatment for OUD is, in part, dependent on baseline prescription opioid use. This study suggests that patients with chronic prescription opioid use may be more likely than nonprescription opioid users to be successfully stabilized on treatment and may thus benefit more from pharmacotherapy with buprenorphine than those with no prescription opioid use. Failing to account for this variation in future studies of buprenorphine treatment persistence may lead to significant residual confounding and biased results. Extending access to buprenorphine among those with prescription OUD may have a significant impact on opioid related morbidity and mortality.
1. Introduction
Patterns of opioid misuse in the United States have shifted dramatically over the last half century. In the 1960s, an estimated 80% of individuals entering treatment for opioid use disorder (OUD) had never taken prescription opioids.1 Aggressive marketing and prescribing of opioid analgesics from the 1980s through 1990s led to an epidemiologic shift in opioid use and by the 2010s, 75% of those who entered treatment for heroin use disorder misused prescription opioids before transitioning to heroin (Cicero, Ellis, Surratt, & Kurtz, 2014). Although not all of those with a history of prescription opioid use received their medication from a prescriber and the transition from prescribed opioid use to illicit opioid use remains uncommon (Muhuri, Gfroerer, & Davies, 2013), individuals prescribed opioids are at risk of OUD and opioid overdose (Wei, Chen, Fillingim, Schmidt, & Winterstein, 2019). Therefore, it is imperative that healthcare practitioners monitor patients prescribed opioids carefully and refer them to treatment for OUD, if necessary.
Buprenorphine is a partial opioid agonist used in pharmacotherapy-based opioid treatment models. Unlike patients treated with methadone, patients treated with buprenorphine are not required to make daily visits to a clinic for observed administration. Rather, treatment with buprenorphine requires patients to participate in a process of care similar to the routine of a patient receiving chronic opioid therapy: visits with a prescriber to obtain a prescription and subsequent visits to the pharmacy to fill the prescription. To remain adherent to OUD treatment, patients are recommended to repeat this routine every two weeks for a minimum of six months (Center for Substance Abuse Treatment, 2004). For patients with limited prior healthcare utilization or poor access to transportation, treatment with buprenorphine may be less effective (Waitzfelder, Engel, Gilbert, 1998; Davis, Davidov, Kristjansson, & Zullig, et al, 2018). Regardless of a patient’s history of prescription opioid use, all persons with OUD are at the highest risk of relapsed opioid misuse and treatment discontinuation in the initial thirty days of treatment with buprenorphine (Marcovitz, McHugh, Volpe, & Votaw, 2016). During this period, patients are required to make frequent visits to their provider and pharmacy as their dose is slowly titrated to an effective maintenance dose (Center for Substance Abuse Treatment, 2004).
The pharmacologic differences between buprenorphine and other forms of pharmacotherapy for OUD, along with the demographic and medical variability between patients prescribed and not prescribed opioid medication (Fiellin, Schottenfeld, Cutter, & Moore, 2014), create the need to measure heterogeneous treatment effects between these groups. Given the risk of failure early in therapy, identifying those who are most likely to benefit from buprenorphine treatment and those who may benefit more from other pharmacologically and structurally different treatment modalities, such as outpatient treatment with methadone or extended release naltrexone, may potentially improve resource allocation and treatment outcomes in the treatment of OUD. The objective of the current study was to measure the adjusted odds of successful stabilization on buprenorphine in patients prescribed opioid medication compared to those not prescribed opioids at baseline.
2. Materials and methods
2.1. Data source
This retrospective, cohort study used data obtained from the Appriss Health® Texas Prescription Monitoring Program (PMP) Prescription Dispensation Data Set. In Texas, the Texas State Board of Pharmacy maintains the prescription monitoring program and requires that all noninstitutional pharmacies in the state report all dispensed DEA Schedule II-V controlled substance prescriptions within 24 hours of dispensation. This dataset, therefore, provides a complete record of all controlled substances dispensed in the state regardless of payer type. Patients, prescribers, and pharmacies are all represented by masked, unique identification numbers. Information in this dataset includes the national drug code (NDC), name, dose, and dosage form, quantity, and days’ supply of the dispensed medication as well as date written, and date dispensed for each prescription allowing for longitudinal studies. We used two calendar years of data, 2017 and 2018, in this study.
2.2. Study sample
We assembled a cohort of incident users of buprenorphine products indicated for the treatment of OUD (Department for Health and Human Services, 2019) who initiated therapy between January 1, 2018, the initial index date, and November 30, 2018. This included those prescribed the buprenorphine/naloxone oral tablet, buprenorphine/naloxone sublingual film, buprenorphine/naloxone buccal film, and buprenorphine/naloxone sublingual tablet. We included these products because they are only indicated for the treatment of OUD and are not indicated for the treatment of pain. Because this dataset does not provide diagnostic information, we excluded those who used any buprenorphine product, including products indicated for the treatment of pain, at any point between January 1, 2017, and December 31, 2018, to limit misclassification—an approach previously used in similar cohort studies (Lo-Ciganic, Donohue, Kim, et al., 2019; Williams, Samples, Crystal, & Olfson, 2019). We also excluded individuals under the age of 15, those who resided outside the state of Texas, and those who filled any opioid prescriptions after initiating buprenorphine. As diagnostic information is not available in the prescription monitoring program, the latter criterion was meant to exclude individuals using the selected buprenorphine products off-label for the treatment of pain.
2.3. Exposure and outcome of interest
2.3.1. Outcome
The outcome of interest was stabilization on buprenorphine. We considered a subject to be successfully stabilized if they filled at least two buprenorphine prescriptions totaling at least a 30-day’s supply within 30 days of treatment initiation (Center for Substance Abuse Treatment, 2004; Kimber, Larney, Hickman, Randall, et al, 2015; Baxter, Clark, Samnaliev, Aweh, et al, 2015). This definition is consistent with recommendations from the Substance Abuse and Mental Health Services Administration4 and has been used in other retrospective studies of buprenorphine treatment persistence (Kimber, Larney, Hickman, Randall, et al, 2015; Baxter, Clark, Samnaliev, Aweh, et al, 2015). Consistent with definitions from the National Quality Forum and Centers for Medicare and Medicaid Services’ definition of continuity of pharmacotherapy for OUD, we defined discontinuation by a six-day gap in buprenorphine possession (Department for Health and Human Services, 2019).
2.3.2. Exposure
We categorized subjects according to three distinct etiologies of OUD: patients with chronic prescription opioid use with an established history of filling prescriptions for opioid medication, patients with acute prescription opioid use who sporadically filled opioid prescriptions in the year prior to initiating treatment, and patients with no prior opioid prescriptions with no evidence of opioid dispensation prior to initiating therapy. We used RxNav, a database of all current and former national drug codes, to make a finder file of all opioid national drug codes. Next, we identified a subset of each subjects’ prescriptions from the prior year. We then merged the prescription file with the NDC finder to identify all opioid prescriptions that each subject used in the year prior to buprenorphine initiation. Next, we assigned each patient to one of the three prior prescription opioid use categories depending on their prior use. We classified subjects who filled no opioid prescriptions in the 90 days prior to initiating treatment as individuals with no prior opioid prescriptions. We classified individuals with prescription opioid possession for 90 days of a 120-day period ending no sooner than 90 days prior to buprenorphine initiation as patients with chronic prescribed opioid use (Inacio, Hansen, Pratt, Graves, et al, 2016; Thornton, Dwibedi, Scott, et al, 2018). Finally, we classified those possessing prescription opioid on at least one of the 90 days pre-index as patients with acute prescribed opioid use.
2.4. Statistical analysis
We first used descriptive statistics to characterize the cohort’s demographics and prior controlled substance use. This included subject age, payment type (insurance or cash), rural/urban commuting area (RUCA) status, use of prescription benzodiazepines and amphetamines in the 90 days prior to treatment initiation, and buprenorphine dose at induction. We then used bivariate tests (χ2 for categorical variables and ANOVA for continuous) to define between-group differences among the three categories of prescription opioid use.
Next, among the individuals prescribed opioids chronically, we calculated average daily opioid doses over the 12-month pre-index period by first converting the prescribed daily dose to morphine milligram equivalents (MME) using the conversion factors that the Centers for Disease Control and Prevention recommends (Centers for Disease Control and Prevention, 2018). We then calculated the total dose in MMEs over each 30-day period and divided by 30 to provide an average daily dose in MMEs. We then used random intercept, quantile dependent slope quantile panel regression (Koenker, 2004) using the RQPD package in R (Bache, Dahl, & Kristensen, 2013) to model mean daily MMEs as a function of time in months in the pre-baseline period. We chose this method to account for the longitudinal structure of the data and to allow for the estimation of separate, pre-index opioid dose trajectories in the 25th, 50th, 75th, 90th, and 95th quintiles. Compared to traditional mixed models of the conditional mean, the chosen quantile regression technique provides a more complete characterization of baseline opioid use (Geraci, 2014). We initially fitted a simple model with a single effect for time-in-months as a continuous variable to verify that there was a significant change in mean MME in the pre-index period. Next, we specified a secondary model with linear splines at 60-day increments to provide a more detailed representation of the trend in mean daily MME in each of the quantiles over the pre-index period.
To test the hypothesis that baseline prescription opioid use is associated with stabilization on buprenorphine, we specified a multiple logistic model regressing the binary stabilization variable on a three-level, categorical prescription opioid use variable controlling for the baseline covariates described above. Further, we specified a second model excluding nonprescription opioid misusers to contrast the probability of successful stabilization on buprenorphine between chronic and acute opioid misusers. We adjusted this model for the same covariates as the primary model.
3. Results
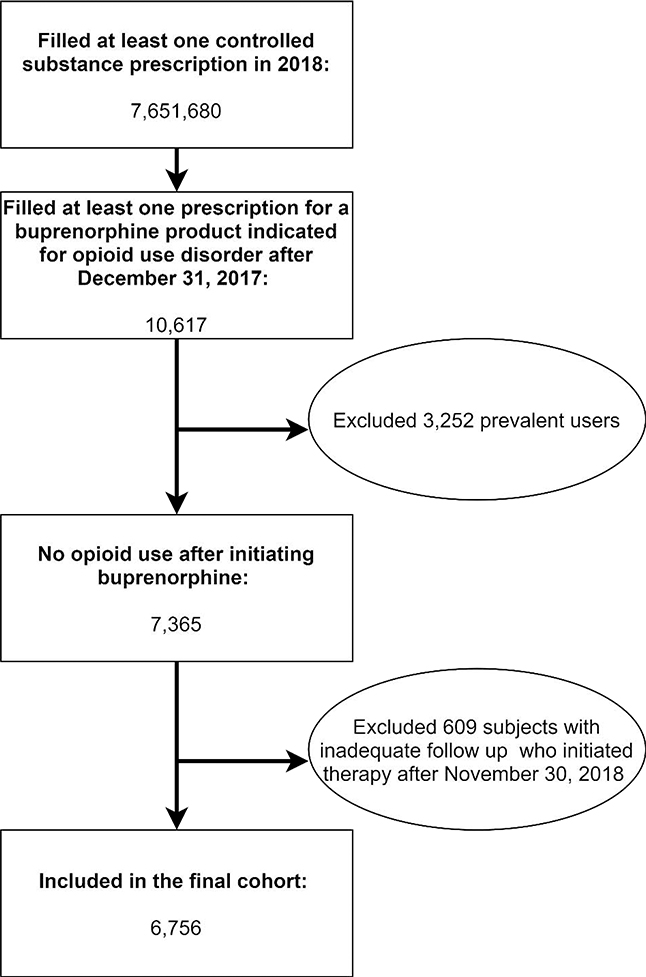
There were 31,208 distinct buprenorphine users in Texas in 2018. Among 10,617 incident buprenorphine users, 3,252 were prescribed an opioid after initiating a qualifying buprenorphine product and 609 initiated therapy after November 30, 2018. The final cohort included 6,756 patients with incident buprenorphine use with at least 30 days of follow-up and no overlapping opioid prescriptions (Figure 1). In this sample, 44.1% of subjects had some prescription opioid use in the 90-day period prior to buprenorphine initiation. A majority of these (62.2%) were prescribed opioids acutely and the remaining 37.8% were prescribed opioids chronically. Both classes of patients prescribed opioids were more likely to be insured, older, use benzodiazepines or prescription amphetamines at baseline, and live in an urban area than those with no prior opioid prescriptions (Table 1).
Figure 1:
Paticipant flow with inclusion and exclusion criteria
Table 1:
Characteristics of a cohort of incident buprenorphine misusers stratified by baseline opioid use (N = 6,756).
| Entire Cohort | No Prescription Opioid Use | Acute Prescription Opioid use | Persistent Prescription Opioid use | Sig.1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| N=6,756 | N=3,778 | N=1,853 | N=1,125 | ||||||
| Freq | % | Freq | % | Freq | % | Freq | % | ||
| Benzodiazepine Use at Baseline | *** | ||||||||
| No | 5,692 | 84.3% | 3,369 | 89.2% | 1,524 | 82.3% | 799 | 71.0% | |
| Yes | 1,064 | 15.8% | 409 | 10.8% | 329 | 17.8% | 326 | 29.0% | |
| Amphetamine Use at Baseline | *** | ||||||||
| No | 6,274 | 92.9% | 3,598 | 95.2% | 1,683 | 90.8% | 993 | 88.3% | |
| Yes | 482 | 7.1% | 180 | 4.8% | 170 | 9.2% | 132 | 11.7% | |
| Patient Age | *** | ||||||||
| 15–34 | 2,615 | 38.7% | 1,830 | 48.4% | 693 | 37.4% | 92 | 8.2% | |
| 35–44 | 1,928 | 28.5% | 1,129 | 29.9% | 544 | 29.4% | 255 | 22.7% | |
| 45–54 | 968 | 14.3% | 416 | 11.0% | 276 | 14.9% | 276 | 24.5% | |
| 55–64 | 830 | 12.3% | 290 | 7.7% | 234 | 12.6% | 306 | 27.2% | |
| 65 and older | 415 | 6.1% | 113 | 3.0% | 106 | 5.7% | 196 | 17.4% | |
| Payment Type | *** | ||||||||
| Cash | 1,812 | 26.8% | 1,260 | 33.4% | 402 | 21.7% | 150 | 13.3% | |
| Insured | 4,944 | 73.2% | 2,518 | 66.7% | 1,451 | 78.3% | 975 | 86.7% | |
| Initial Buprenorphine Dose | *** | ||||||||
| < 12 mg/day | 3,713 | 55.0% | 2,140 | 56.6% | 923 | 49.8% | 650 | 57.8% | |
| ≥12 mg/day | 3,043 | 45.0% | 1,638 | 43.4% | 930 | 50.2% | 475 | 42.2% | |
| Rurality | ** | ||||||||
| Rural | 725 | 10.7% | 452 | 12.0% | 168 | 9.1% | 105 | 9.3% | |
| Urban | 6,031 | 89.3% | 3,326 | 88.0% | 1,685 | 90.9% | 1,020 | 90.7% | |
Results of chi square tests for covariate balance between treatment groups at baseline.
P<0.001,
P<0.01
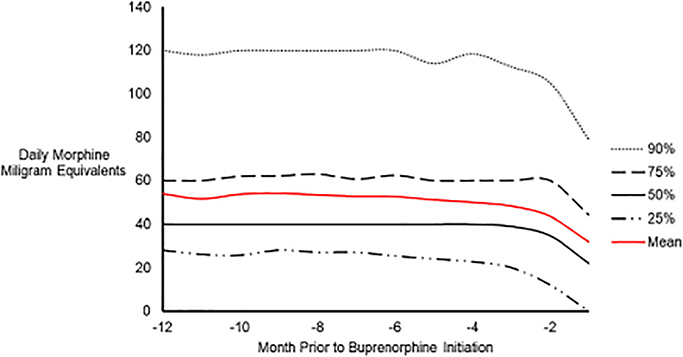
In the 1,125 subjects who had been prescribed opioids chronically, the median daily opioid dose 12-months prior to treatment initiation was 40 MMEs; although, this varied significantly from 28 MMEs in the lowest quartile to 60 in the highest. The median dose decreased in the three months prior to buprenorphine initiation before approaching 22 MMEs in month 12 (IQR: 0–44 MMEs). The random intercept quantile regression model with linear splines at two-month increments demonstrated no notable change in median daily MME until two months prior to buprenorphine initiation at which point the median daily MME began to decline significantly (β=7.5, P<0.001). We observed more volatility in the 25% quantile, where patients saw a statistically significant, gradual decline in MME from six months to two months prior to initiating buprenorphine. At two months prior to initiation, the decline became markedly more pronounced (β=11.24, P<0.001). Individuals in the upper, 75th and 90th quantiles saw a more gradual decline than those in the lower quantiles. A graphical presentation of the observed changes in MME may be found in Figure 2 and the quantile dependent effects from the quantile regression model may be found in Table 2.
Fig. 2.
Graph depicting the observed change in daily average opioid dose in morphine milligram equivalents (MMEs) among quantiles of chronic opioid users in the year prior to buprenorphine initiation.
Table 2:
Quantile dependent effects from a random intercept repeated measures, quantile regression model analyzing the trend in average daily MMEs among chronic opioid users in the year prior to buprenorphine initiation.
| Quantile and Effect | Std. Error | Pr(>|t|) | Quantile andEffect | Std. Error | Pr(>|t|) | ||
|---|---|---|---|---|---|---|---|
| 0.25 | 0.75 | ||||||
| 12–10 Months1 | −2.33 | 0.68 | *** | 12–10 Months | −1.65 | 0.49 | *** |
| 10–8 Months | 1.33 | 0.33 | *** | 10–8 Months | 0.33 | 0.26 | |
| 8–6 Months | −0.67 | 0.32 | * | 8–6 Months | −1.01 | 0.35 | *** |
| 6–4 Months | −1.00 | 0.45 | * | 6–4 Months | −0.15 | 0.20 | |
| 4–2 Months | −2.26 | 0.54 | *** | 4–2 Months | −0.36 | 0.22 | |
| 2–0 Months | −11.24 | 0.66 | *** | 2–0 Months | −1.61 | 0.35 | *** |
| 0.5 | 0.9 | ||||||
| 12–10 Months | 0.00 | 0.15 | 12–10 Months | −0.65 | 1.46 | ||
| 10–8 Months | 0.00 | 0.01 | 10–8 Months | −1.42 | 0.84 | * | |
| 8–6 Months | 0.00 | 0.01 | 8–6 Months | −2.63 | 0.81 | *** | |
| 6–4 Months | 0.00 | 0.00 | 6–4 Months | 0.00 | 0.67 | ||
| 4–2 Months | −0.50 | 0.18 | ** | 4–2 Months | 0.13 | 0.65 | |
| 2–0 Months | −7.03 | 0.37 | *** | 2–0 Months | −4.05 | 0.65 | *** |
P<0.05,
P<0.01,
P<0.001
Months prior to buprenorphine induction
In this cohort of patients with incident buprenorphine treatment, 51.2% were successfully stabilized on buprenorphine therapy. When stratified by opioid prescription history at baseline, 43.7% of patients with no prior opioid prescriptions, 56.3% of patients prescribed opioids acutely, and 67.9% of patients prescribed opioids chronically were successfully stabilized. Multiple logistic regression confirmed that patients prescribed opioids acutely (aOR 1.53, 95% CI: 1.37–1.72) and chronically (aOR: 2.43, 95% CI: 2.08–2.85) were significantly more likely to be successfully stabilized on buprenorphine compared to patients with no prior opioid prescriptions. The model contrasting those with prescribed opioids acutely and chronically revealed that patients prescribed opioids chronically were significantly more likely (aOR: 1.60, 95% CI: 1.35–1.90) to be successfully stabilized on buprenorphine than patients prescribed opioids acutely after adjustment for the same set of covariates (Table 3).
Table 3:
Adjusted association between baseline opioid use and the odds of successful stabilization on buprenorphine and a post-hoc model contrasting subjects with acute prescription opioid use and persistent prescription opioid use at baseline.
| Odds of successful stabilization in full cohort N=6,756 | Odds of successful stabilization in prescription opioid misusers N=2,978 | |||
|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | |
| Baseline Opioid Use | ||||
| No Prescription Opioid Use | 1 | - | N/A | N/A |
| Acute Prescription Opioid Use | 1.53 | (1.37–1.72) | 1 | - |
| Persistent Prescription Opioid | ||||
| Use | 2.43 | (2.08–2.85) | 1.60 | (1.35–1.90) |
| Baseline Controlled Substance Use | ||||
| Benzodiazepines | 0.9 | (0.78–1.04) | 1.13 | (0.94–1.36) |
| Amphetamines | 1.29 | (1.06–1.58) | 1.01 | (0.79–1.30) |
| Patient Age | ||||
| 15–34 | 1 | - | 1 | - |
| 35–44 | 1.33 | (1.18–1.50) | 1.13 | (0.92–1.39) |
| 45–54 | 1.35 | (1.16–1.58) | 1.34 | (1.06–1.70) |
| 55–64 | 1.22 | (1.03–1.44) | 1.06 | (0.84–1.35) |
| 65 and older | 0.95 | (0.76–1.19) | 0.87 | (0.65–1.16) |
| Payment Type | ||||
| Cash | 1 | - | 1 | - |
| Insured | 1.51 | (1.35–1.69) | 1.18 | (0.98–1.43) |
| Initial Buprenorphine Dose | ||||
| < 12 mg/day | 1 | - | 1 | - |
| ≥12 mg/day | 1.31 | (1.19–1.45) | 1.15 | (0.99–1.33) |
| Rurality | ||||
| Rural | 1 | - | 1 | - |
| Urban | 1.22 | (1.04–1.43) | 0.87 | (0.67–1.13) |
4. Discussion
This study demonstrates that patients prescribed opioids prior to initiating treatment with buprenorphine for OUD are significantly more likely than those with no history of opioid prescription to be successfully stabilized on buprenorphine pharmacotherapy for the treatment of OUD. We found significant demographic differences between these groups, as those prescribed opioids prior to treatment initiation were significantly more likely to be insured and to live in urban areas, two factors previously found to improve adherence to treatment for OUD (Andrilla, Moore, Patterson, & Larson, 2019). Pharmacotherapy with buprenorphine is affordable for patients with private insurance who paid a median of $10 per month for treatment in 2015 (Roberts, Saloner, & Dusetzina, 2018). This is significantly different from the median total cost of $376 per month from the same study (Roberts, Saloner, & Dusetzina, 2018). Assuming uninsured patients are required to bear the full cost of pharmacotherapy, remaining in treatment with buprenorphine becomes a gargantuan task. Extending access to buprenorphine for the 27% of patients in this study who purchased their prescription with cash may significantly improve adherence early in treatment.
Even after adjusting for insurance status, our study showed that individuals prescribed opioids were still significantly more likely than those not prescribed opioids to be successfully stabilized on pharmacotherapy for OUD. We also found that those who received prescription opioids consistently in the four months prior to initiating treatment were more likely than those who were prescribed opioids sporadically in the same period to be successfully stabilized. Consistently filling a prescription for an opioid medication prior to initiating buprenorphine contributes to a successful stabilization on pharmacotherapy. While unmeasured differences may exist among these groups, prescription opioid use at baseline stands as a significant predictor of successful stabilization on buprenorphine treatment.
Data comparing patients with and without a history of prior opioid prescription remain sparse; however, a small clinical trial demonstrated that patients prescribed opioids at baseline receiving treatment for OUD were significantly more likely to complete the trial, stayed in treatment longer, and had a higher proportion of opioid negative urine samples than patients with no prescription opioid use or those who used prescription and illicit opioids concomitantly (Moore, Fiellin, Barry, et al, 2007).
Not only are patients prescribed opioids more likely to remain adherent to treatment, they are also less likely to benefit from drug abuse counseling added to medical management and pharmacotherapy while those with a history of heroin use were shown to benefit from additional counseling (Weiss, & Rao, 2017). This may, in part, explain why patients with chronic prescription opioid use benefit more from the traditional medical model of office-based buprenorphine administration than those with no opioid prescriptions and adds valuable context to the findings of this study. Although all patients should be provided access to psychosocial treatment for OUD (American Society of Addiction Medicine, 2015), evaluating a patient’s history of prescription opioid use may help clinicians to tailor therapy to the patient and thus increase the patient centeredness of treatment for OUD. Our findings seem to suggest that individuals who routinely access care and are prescribed opioids are more likely to be successfully stabilized on buprenorphine. We cannot conclude that other pharmacotherapeutic options may be more beneficial in individuals with limited prior healthcare utilization or no prior opioid prescriptions; however, this is a worthwhile topic for future research.
The term chronic prescription opioid use is not adequate to describe this complex group of patients. The quantile regression model presented here draws attention to several distinct trajectories of chronic prescription opioid use in the year prior to buprenorphine initiation. After controlling for clustering within subjects, the average daily opioid dose declined significantly across all quantiles of individuals who were prescribed opioids chronically in the year prior to initiating treatment. This was most pronounced in the lowest quantile of subjects who discontinued in the month prior to treatment and far less pronounced in the 90th quantile who essentially remained on a stable dose prior to treatment initiation. These results are difficult to reconcile without a history of illicit opioid use in the peri-initiation period; however, the variability in prescribed opioid dose in the peri-initiation period should be considered at the time of induction. This also highlights the difficulty in correlating baseline opioid dose with an effective initial buprenorphine dose. As a partial opioid agonist, buprenorphine demonstrates significantly different pharmacokinetic and pharmacodynamic properties compared to full opioid agonists. With a lower maximum concentration (CMax), longer time to reach that concentration (TMax), a long half-life of elimination, and a large volume of distribution, induction requires careful titration and a patient centered approach to care (Elkader & Sproule, 2005).
This study does have some limitations. While the PMP offers a robust and complete record of prescribed controlled substances, it contains no diagnostic information. Limiting our sample to patients receiving buprenorphine products only indicated for OUD was intended to limit the potential for misclassification; however, it is possible that some individuals were prescribed these products off-label and we, therefore, misclassified them. There is also a possibility that some patients were provided verbal instructions from the prescriber to modify their dose after a certain time period (i.e., four mg daily in week one and then increase in week two). Our adherence measure was based on cumulative day’s supply. If verbal instructions were provided to the contrary, this may have had some effect on our results. The group of patients with no prior prescription opioid use remain somewhat nondescript without a history of illicit opioid use; although, it is highly unlikely that an individual would initiate treatment with a buprenorphine product indicated for OUD with no prior opioid exposure. The problem exists in the acute use category to a lesser degree. We are certain these individuals filled opioid prescriptions; however, nonprescription illicit opioid use remains unmeasurable. Prescription opioid use at baseline may actually mediate the association between other demographic and medical factors and the odds of stabilization on therapy. While this more complex causal path may exist, our results show that there is a significant difference in the odds of stabilization among those who were prescribed opioid medication at baseline and those who were not. In no way can we fully explain why this is, and further research is required to truly understand the differences between these two groups. Finally, this dataset is under the management of the Texas State Board of Pharmacy and some variables are highly masked to prevent incidental identification. For this reason, we were unable to exclude patients between the ages of 15 and 18 as age was provided as a categorical variable. Information on patient sex was also unavailable.
Finally, because our study was a single-state study, our findings may not generalize to other geographic areas. Nevertheless, trends in opioid prescribing in Texas between 2006 and 2017 were similar to those nationwide over the same time period (Schieber, Guy, Seth, et al., 2019). Between 2006 and 2010, average MMEs per person per day increased by 3.7% per year in Texas and between 2010 and 2017, the same measure declined 5% per year. Nationwide, average MMEs per person increased by 6.9% per year between 2006 and 2010 before falling 5.8% per year between 2010 and 2017. Although Texas had the largest decrease in the rate of high-dose opioid prescriptions between 2010 and 2017, prescription durations in Texas were similar to those nationwide (Schieber, Guy, Seth, et al., 2019). The similarities in opioid prescribing between Texas and other states may improve the external validity of our findings.
5. Conclusion
Not all patients entering treatment for OUD are going to benefit equally from a standard treatment. A patient’s opioid use history is critical in selecting appropriate treatment. Patients prescribed opioids chronically may be particularly more likely to be successfully stabilized on treatment with buprenorphine when compared to those not prescribed opioids at baseline. Although previous randomized, controlled trials have examined the varying efficacy of buprenorphine in those with and without prior prescription opioid use, this study demonstrates that even in an uncontrolled setting, this subgroup of patients with OUD is particularly likely to benefit from treatment with buprenorphine. Policy intended to extend buprenorphine coverage and to promote buprenorphine therapy to patients chronically prescribed opioids may have a significant, positive impact on patient safety and contribute to a welcome reduction in healthcare utilization and costs in this high-risk patient population. Finally, researchers must consider how they define and measure baseline prescription opioid use when modeling the probability of early-stage retention in buprenorphine treatment. Failing to do so means ignoring a readily measurable and significant confounder, an error that may drastically limit the utility of future models in this area.
Highlights.
Persons taking buprenorphine for OUD have varying baseline prescription opioid use.
Patients with prior opioid prescriptions vary demographically than those without.
Prior prescription opioid use predicts stabilization on buprenorphine.
Patients with chronic prescription opioid use are most likely to be stabilized.
Acknowledgement of Funding
Dr. Thornton and Dr. Shen were both partially supported by a grant from the National Institute of Health, National Institute on Drug Abuse (1R03DA047597-01). Data for this project were purchased by the Prescription Drug Misuse Education and Research (PREMIER) Center at the University of Houston College of Pharmacy.
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Society of Addiction Medicine. (2015). The National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Chevy Chase, MD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrilla CHA, Moore TE, Patterson DG, & Larson EH. (2019). Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: A 5-year update. The Journal of Rural Health: Official Journal of the American Rural Health Association and the National Rural Health Care Association, 35(1), 108–112. [DOI] [PubMed] [Google Scholar]
- Bache SHM, Dahl CM, Kristensen JT. (2013). Headlights on tobacco road to low birthweight outcomes. Empirical Economics, 44(3), 1593–1633. [Google Scholar]
- Baxter JD, Clark RE, Samnaliev M, Aweh G, & O’Connell E. Adherence to Buprenorphine Treatment Guidelines in a Medicaid Program. Subst Abuse, 36(2), 174–182. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. (2004). Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2018). Analyzing Prescription Data and Morphine Milligram Equivalents (MME). Bethesda, MA. [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. (2014). The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry, 71(7), 821–826. [DOI] [PubMed] [Google Scholar]
- Davis SM, Davidov D, Kristjansson AL, Zullig K, Baus A, Fisher M. (2018). Qualitative case study of needle exchange programs in the Central Appalachian region of the United States. PLoS One, 13(10), e0205466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department for Health and Human Services. Centers for Medicare and Medicaid Services. (2019). Quality ID #468 (NQF 3175): Continuity of Pharmacotherapy for Opioid Use Disorder. Baltiore, MD. [Google Scholar]
- Elkader A, Sproule B. (2005). Buprenorphine: Clinical pharmacokinetics in the treatment of opioid dependence. Clinical Pharmacokinetics, 44(7), 661–680. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O’Connor PG. (2014). Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Internal Medicine, 174(12), 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci M (2014). Linear quantile mixed models: The lqmm package for laplace quantile regression. Journal of Statistical Software, 1(13). [Google Scholar]
- Inacio MCS, Hansen C, Pratt NL, Graves SE, Roughead EE. (2016). Risk factors for persistent and new chronic opioid use in patients undergoing total hip arthroplasty: A retrospective cohort study. BMJ Open, 6(4), e010664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. (2015). Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. The Lancet Psychiatry, 2(10), 901–908. [DOI] [PubMed] [Google Scholar]
- Koenker R (2004). Quantile regression for longitudinal data. Journal of Multivariate Analysis, 91(1), 74–89. [Google Scholar]
- Lo-Ciganic WH, Donohue JM, Kim JY, et al. (2019). Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidemiology and Drug Safety, 28(1), 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovitz DE, McHugh RK, Volpe J, Votaw V, Connery HS. (2016). Predictors of early dropout in outpatient buprenorphine/naloxone treatment. The American Journal on Addictions, 25(6), 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, et al. (2007). Primary care office-based buprenorphine treatment: Comparison of heroin and prescription opioid dependent patients. Journal of General Internal Medicine, 22(4), 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhuri PK, Gfroerer JC, Davies MC. (2013). CBHSQ data review. Center for Behavioral Health Statistics and Quality, SAMHSA, 1, 17. [Google Scholar]
- Roberts AW, Saloner B, Dusetzina SB. (2018). Buprenorphine use and spending for opioid use disorder treatment: Trends From 2003 to 2015. Psychiatric Services (Washington, DC), 69(7), 832–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber LZ, Guy GP Jr, Seth P, et al. (2019). Trends and patterns of geographic variation in opioid prescribing practices by state, United States, 2006–2017. JAMA Network Open, 2(3), e190665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JD, Dwibedi N, Scott V, et al. (2018). Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. American Health & Drug Benefits, 11(1), 12–21. [PMC free article] [PubMed] [Google Scholar]
- Waitzfelder BE, Engel CC Jr., Gilbert FI Jr. (1998). Substance abuse in Hawaii: Perspectives of key local human service organizations. Substance Abuse, 19(1), 7–22. [DOI] [PubMed] [Google Scholar]
- Wei Y-JJ, Chen C, Fillingim R, Schmidt SO, Winterstein AG. (2019). Trends in prescription opioid use and dose trajectories before opioid use disorder or overdose in US adults from 2006 to 2016: A cross-sectional study. PLoS Med, 16(11), e1002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Samples H, Crystal S, Olfson M. (2019). Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. American Journal of Psychiatry, appiajp201919060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, & Rao V. (2017). The Prescription Opioid Addiction Treatment Study: What have we learned. Drug and Alcohol Dependence, 173(Suppl 1), S48–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]