Abstract
Objectives:
The objective of the study was to detect functional changes in the brain of cognitive impairment-temporal lobe epilepsy (CI-TLE) patient and to sort out the possible mechanism involved in CI in CI-TLE patients using resting-state functional magnetic resonance imaging (RS-fMRI).
Material and Methods:
Fifty-eight TLE cases were included, which was divided into 44 TLE patients without CI (cognitive not impairment [CNI]-TLE) and 14 TLE patients with CI (CI-TLE). The normal control (NC) group consisted of 40 participants. RS-fMRI data preprocessing was carried out in statistical parametric mapping (SPM) software. The data were realigned, coregistered, normalized, and finally smoothened and then were taken for amplitude of low-frequency fluctuation (ALFF) calculation in RS-fMRI data analysis toolkit (REST) software. For data analysis, voxel-wise two-sample t-test was carried out between TLE group and NC group; CI-TLE group and cognitive not impairment-TLE (CNI-TLE) group in SPM software, a cluster >10 voxels and P < 0.01 was considered to be significant.
Results:
Compared to NC, the TLE patients showed increased ALFF activation mostly in parahippocampal gyrus (PG), frontal lobe, midbrain, pons, insula, inferior temporal gyrus, and anterior cingulate gyrus (ACG) while decreased ALFF value was seen in posterior cingulate gyrus, cuneus, cerebellum posterior lobe, inferior parietal lobule (IPL), and superior temporal gyrus. Compared to CNI-TLE, CI-TLE patients showed increased ALFF in middle temporal gyrus (MTG), cuneus, ACG, IPL, middle frontal gyrus (MFG), superior frontal gyrus (SFG), cerebellum posterior lobe, and decreased ALFF cluster in the corpus callosum and MFG.
Conclusion:
Between TLE and NC, we found increased ALFF activation in PG, frontal lobe, thalamus, insula, midbrain, and pons in TLE patient. Between CI and CNI TLE, area of executive control network and default model network, especially in MTG, ACG, IPL, MFG, and SFG, had increased ALFF value in CI-TLE patient. Activation of these areas should be because of the decompensation mechanism.
Keywords: Resting-state functional magnetic resonance imaging, Amplitude of low-frequency fluctuation, Temporal lobe epilepsy, Cognitive impairment
INTRODUCTION
Temporal lobe epilepsy (TLE) belongs to focal (partial) epilepsy, which is a chronic neurological disorder characterized by abnormal synchronization of a group of neurons that result in a mesial or neocortical temporal lobe-induced seizure. It is refractory to the antiepileptic drug and often requires surgical intervention.[1,2] It represents about 2/3 of an intractable seizure coming for surgical intervention.[3]
The origin of the seizure from the hippocampus represents at least 80% of all in TLE.[4] In addition to seizures, TLE also has many different forms of well-known clinical features. Another worrisome feature of TLE is cognitive sequelae in the form of cognitive impairment (CI).[5] Several studies have shown that seizure due to TLE affects many aspects of cognitive functioning, which includes problem-solving skill, attention, executive functioning (Intelligence), language, praxis, and insight.[6] However, the mechanism of CI remains unclear.
Seizure due to TLE results in abnormal electric discharge in the brain, which consequently leads to functional changes in the brain.[7] Epileptic seizures can cause neurodegenerative changes creating an impact in the functional region of the brain that ultimately leads to CI.[5] Another possible mechanism for functional changes can be damaged to the synaptic compound caused by epileptic discharges, which is 5-hydroxytryptamine-1A receptor, which gets abnormal in TLE patient’s hippocampus. Besides, the adverse effect of antiepileptic drugs on neuronal excitability can also have a role in CI in TLE.[8]
Functional magnetic resonance imaging (fMRI) uses blood oxygenation level-dependent (BOLD) contrast and BOLD has better temporal and spatial resolutions. Nowadays, it is being broadly used in research related to epilepsy.[9] fMRI shows regional level changes in brain metabolism over time. This metabolic change may be due to changes in the cognitive status induced by the task or as a result of uncontrolled processes in the resting brain.[10] Therefore, it might be a helpful method to presume mechanisms related to the involvement of epileptic changes in different brain areas.[11]
In a task fMRI, only a high frequency of BOLD is analyzed while the low-frequency signal is filtered away and not used. However, low frequency is also related to spontaneous neuronal activity and has physiological significance and may represent energy consumption for maintaining the brain’s internal system at rest.[2] RS-fMRI shows the functional regions at the low-frequency level, which is <0.1 Hz in the BOLD signal.[12] Moreover, the RS-fMRI approach is a comfortable technique for the patient, as they do not need to perform any task but just need to rest. Meanwhile, the evaluation of spontaneous neuronal activity at rest occurs. Therefore, the technique is being more popular nowadays.[13]
The amplitude of low-frequency fluctuation (ALFF) is one of the methods of RS-fMRI, which detects the intensity of spontaneous fluctuations at the low-frequency level (0.01–0.08 Hz).[14] Therefore, in this study, we have applied the ALFF method of RS-fMRI in CI-TLE, cognitive not impairment-TLE (CNI-TLE) patient, and normal control (NC) individuals and have aimed to find the changes in the functional region of the whole brain in CI-TLE patient. We have also aimed to sort out the possible mechanism involved in CI in CI-TLE patients.
MATERIAL AND METHODS
Subjects
We recruited 98 subjects (male/female: 55/43, average age: 33.7 ± 11.23) between January 2017 and January 2019 and performed the study. Subjects were divided into two groups primarily, TLE group, which consisted of 58 patients (male/ female: 38/20, average age: 34.3 ± 11.8), and NC group, which consisted of 40 healthy controls (male/female: 17/23, average age: 33.17 ± 10.8). TLE was again subdivided into TLE patient without CI (CNI-TLE) which consisted of 44 patients (male/female: 29/15, average age: 32.29 ± 10.9) and TLE patient with CI (CI-TLE) which consisted of 14 patients (male/female: 9/5, average age: 39.8 ± 13.1). The demographic and neuropsychological data of these three groups (CI-TLE, CNI-TLE, and NC) are shown in Table 1. The patient was diagnosed as epileptic after fulfilling the criteria given by the International League against Epilepsy. Criteria’s included are as follows: (1) At least two unprovoked (or reflex) seizures occurring >24 h apart, (2) one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years, and (3) diagnosis of an epilepsy syndrome. Then, electroencephalogram (EEG) was done to confirm epilepsy, and finding included is interictal epileptic discharges in EEG. Then, epileptic classified patient underwent magnetic resonance imaging (MRI) to confirm TLE. There was no identifiable structural MRI abnormality other than hippocampal sclerosis in the patients’ brain.
Table 1:
Demographic and neuropsychological data of CI-TLE, CNI-TLE, and NC groups.
| Characteristics | CI-TLE (n=14) | CNI-TLE (n=44) | NC (n=40) | Pvalue |
|---|---|---|---|---|
| Age (years) | 39.8±13.1 | 32.29±10.9 | 33.17±10.8 | P=0.81† |
| Gender (M/F) | 9/5 | 29/15 | 17/23 | P=0.078ѱ |
| Education (years) | 12.85±2.28 | 13.93±2.5 | 14.6±2.37 | P=0.062† |
| MMSE | 24.7±1.05 | 29.5±0.87 | 29.6±0.5 | P<0.001*† |
| BDI | 9±7.2 | 6±5.2 | 5.8±2.57 | P=0.1† |
Values are given as mean±standard deviation (range). M: Male, F: Female, n means a total number of subjects, CI-TLE: Cognitively impaired-temporal lobe epilepsy, CNI-TLE: Cognitively not impaired-temporal lobe epilepsy, the value marked with “*” is statistically significant between CI-TLE, CNI-TLE, and NC (P<0.001), P-value was obtained by one-way ANOVA and Chi-square test. † signifies One-way ANOVA and ѱ signifies Chi-square test
CI-TLE was distinguished from CNI-TLE by mini-mental state examination (MMSE) score. Epileptic patient with MMSE score ≤26 was considered CI and was kept in the CI- TLE group. MMSE >26 was considered CNI and was kept in the CNI-TLE group.
Beck’s depression inventory (BDI) score was used to rule out the depressive patient from TLE group as depressive patient can also present with the symptom of CI, which can mislead our findings in CI due to TLE. BDI >25 was considered depressive and was excluded from our study.
NC was selected under following inclusion criterion: Without any psychiatric disorders; not any contraindications to MRI; no any evidence of brain lesion in conventional MRI; and no CI, MMSE scores >26 for NC and BDI <25.
Data acquisition
Images were acquired using a 3.0-Tesla scanner (Siemens Magnetom Trio Tim, Siemens Medical Solutions, Erlangen, Germany) utilizing 32 channel head coil in Tianjin First Central Hospital, Tianjin, China. Before scanning, the patient was asked to lay in a supine position in the MRI table with head firmly fixed by straps and foam pads to minimize head movement.
For resting-state (RS) scanning, participants were asked to close the eyes and remain motionless and not to think of anything, in particular nor fall asleep. The RS functional data were acquired using an echo-planar imaging sequence with the following parameters: TR = 2000 ms, TE = 30 ms, flip angle = 90°, thickness/gap = 5.0/0.0 mm, field of view = 24 cm × 24 cm, matrix = 64 × 64, 300 volumes. A total of 300 slices of resting fMRI were acquired.
Structural images T1-weighted images were acquired using a magnetization-prepared rapid gradient-echo sequence with the following parameters: TR = 1900 ms, TE = 2.52 ms, TI = 900 ms, thickness = 1.0 mm, flip angle = 9°, matrix = 256 × 256, voxel size = 1 × 1×1 mm3.
Data processing
Data preprocessing was carried out in MATLAB (matrix laboratory) based statistical parametric mapping (SPM) v.8 software. First, DICOM data were converted to NIFTI images. Second, the first 10 volumes of each subject’s rest data were discarded to allow longitudinal magnetization to reach a steady state and for participants in getting used to the scanning environment. Third, both rest and T1 data underwent left to the right flip of the image using the matrix −1000; 0100; 0010; 0001. Fourth, flipped resting data were realigned so that the corresponding scans of the participant precisely fit one another. Participants with head motion larger than 2 mm or 2° in any of the six parameters (x, y, z, pitch, roll, and yaw) were excluded. Fifth, coregistration was done for alignment of resting functional image with a structural image. Sixth, normalization to Montreal Neurological Institute (MNI) template image and resampling of each voxel to 3 × 3 × 3 mm3 was done. Normalization fits the shape of functional images with the structural image to a canonical average template. Finally, the smoothening of the normalized functional image was done with full-width-at- half-maximum.[8]
ALFF calculation was carried out in REST v1.8 software (http://resting-fmri.sourceforge.net) based on MATLAB. Smoothened data were used, data of each voxel were linearly detrended, ideal bandpass filter 0.01~0.08 and TR; 2 s was used. The ALFF value of each voxel was standardized by dividing the full brain mean ALFF value.
Data analysis
Statistical analysis was conducted in MATLAB based SPM v.8 software.
ALFF between TLE and NC group
ALFF between TLE and NC was compared using voxel- wise two-sample t-test based on two group analysis in SPM software, a cluster >10 voxels, and P < 0.01 was considered to be significant.
ALFF between CI-TLE and CNI-TLE group
ALFF between CI-TLE and CNI-TLE was compared using voxel-wise two-sample t-test based on two groups analysis in SPM software, a cluster >10 voxels and P < 0.01 was considered to be significant.
RESULTS
Subject characteristics
Table 1 shows the demographic and neuropsychological data of CI-TLE, CNI-TLE, and NC group. Age, education, MMSE, and BDI were represented as mean ± standard deviation and statistical analysis was carried out with one-way ANOVA. Gender was represented as male-to-female ratio and statistical analysis was carried out by the Chi-square test. Of CI-TLE, CNI-TLE, and NC, age was, respectively, 39.8 ± 13.1, 32.29 ± 10.9, and 33.17 ± 10.8. Gender was, respectively, 9/5, 29/15, and 17/23. Education was, respectively, 12.85 ± 2.28, 13.93 ± 2.5, and 14.6 ± 2.37. MMSE was, respectively, 24.7 ± 1.05, 29.5 ± 0.87, and 29.6 ± 0.5, and BDI was, respectively, 9 ± 7.2, 6 ± 5.2, and 5.8 ± 2.57. Age, gender, education, and BDI were not statistically significant. However, MMSE appeared statistically significant (P < 0.001).
Activation difference between TLE and NC
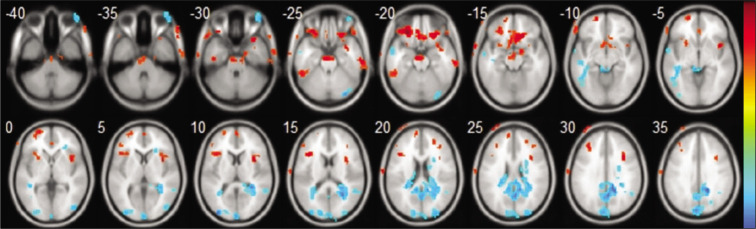
In Table 2 and Figure 1, warm colors show significantly increased ALFF between TLE and NC. Significantly, increased ALFF value was found in parahippocampal gyrus (PG), inferior frontal gyrus (IFG), superior frontal gyrus (SFG), middle frontal gyrus (MFG), midbrain and pons, insula, inferior temporal gyrus (ITG), anterior cingulate gyrus (ACG), and frontal lobe subgyral white matter.
Table 2:
Significant increased ALFF cluster between TLE and NC group, P<0.01, voxel >10.
| Brain Region | MNI space coordinates | Peak z-value | Voxels | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 | Inferior frontal gyrus-L | −27 | 36 | −21 | 4.84 | 560 |
| 2 | Frontal lobe subgyral WM-L | −39 | 18 | 18 | 3.749 | 109 |
| 3 | Midbrain and pons-L | −3 | −18 | −24 | 3.66 | 158 |
| 4 | Insula-R | 36 | 9 | 9 | 3.646 | 158 |
| 5 | Parahippocampal gyrus-R | 15 | −3 | −15 | 3.347 | 15 |
| 6 | Superior frontal gyrus-L | −18 | 57 | −12 | 3.237 | 93 |
| 7 | Inferior temporal gyrus-L | −51 | −45 | −24 | 3.13 | 38 |
| 8 | Frontal lobe subgyral WM-R | 36 | 33 | 21 | 3.00 | 18 |
| 9 | Anterior cingulate gyrus-R | 3 | 39 | −3 | 2.9503 | 15 |
| 10 | Middle frontal gyrus-L | −33 | 33 | 30 | 2.9402 | 46 |
| 12 | Superior frontal gyrus-R | 24 | 42 | 21 | 2.6359 | 16 |
R: Right hemisphere, L: Left hemisphere
Figure 1:
Axial structural image of the brain showing result of amplitude of low-frequency fluctuation (resting-state functional magnetic resonance imaging), statistic t-map with the difference between the temporal lobe epilepsy (TLE) group and normal control (NC) (two-sample t-test, P < 0.01, voxel >10). Warm colors indicate TLE >NC, while cool colors indicate NC >TLE.
In Table 3 and Figure 1, cool colors show significantly decreased ALFF between TLE and NC. Significantly, decreased ALFF value was found in posterior cingulate gyrus (PCG), cuneus, cerebellum posterior lobe, inferior parietal lobule (IPL), superior temporal gyrus (STG), and frontal lobe subgyral white matter.
Table 3:
Significant decreased ALFF cluster between TLE and NC group, P<0.01, voxel >10.
| Brain Region | MNI space coordinates | Peak z-value | Voxels | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 | Posterior cingulate gyrus-R | 3 | −59 | 25 | −4.34 | 1043 |
| 2 | Cuneus-L | −36 | −90 | 9 | −4.004 | 381 |
| 3 | Midbrain-L | −6 | −36 | −6 | −3.625 | 30 |
| 4 | Cerebellum posterior lobe-R | 33 | −84 | −24 | −3.55 | 22 |
| 5 | Temporal lobe subgyral WM-L | −42 | −21 | −12 | −3.48 | 132 |
| 6 | Inferior parietal lobule-L | −45 | −60 | 57 | −3.32 | 33 |
| 7 | Superior temporal gyrus-R | 51 | −51 | 18 | −3.018 | 33 |
| 8 | Frontal lobe subgyral WM-R | 24 | 30 | 3 | −2.988 | 22 |
R: Right hemisphere, L: Left hemisphere
Activation difference between CI-TLE and CNI-TLE
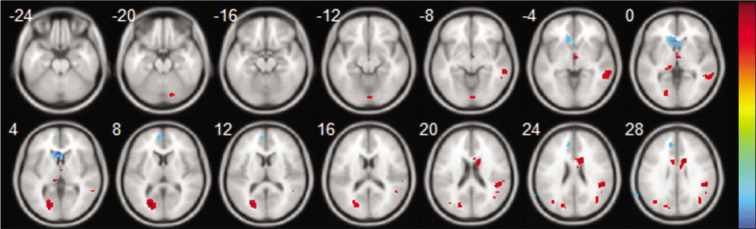
In Table 4 and Figure 2, warm colors show significantly increased ALFF between CI-TLE and CNI-TLE. Significantly, an increased ALFF cluster was found in middle temporal gyrus (MTG), cuneus, ACG, IPL, MFG, SFG, and cerebellum posterior lobe.
Table 4:
Significant increased ALFF cluster between CI-TLE and CNI-TLE group, P<0.01, voxel >10.
| Anatomical regions | MNI space coordinates | Peak z-value | Voxels | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 | Middle temporal gyrus-R | 60 | −36 | −6 | 4.06 | 72 |
| 2 | Cuneus-L | −21 | −75 | 9 | 3.69 | 124 |
| 3 | Anterior cingulate gyrus-R | 9 | −3 | 30 | 3.71 | 63 |
| 4 | Inferior parietal lobule-R | −6 | 0 | 30 | 3.1 | 12 |
| 5 | Middle frontal gyrus-R | 48 | −39 | 24 | 3.6 | 52 |
| 6 | alamus-B/L | 6 | −8 | 0 | 3.09 | 16 |
| 7 | Superior frontal gyrus-R | 24 | 27 | 48 | 2.89 | 15 |
| 8 | Cerebellum posterior lobe-R | 15 | −81 | −21 | 2.82 | 10 |
R: Right hemisphere; L: Left hemisphere; B/L: Bilateral, ALFF: Amplitude of low-frequency fluctuation, CI-TLE: Cognitive impairment-temporal lobe epilepsy, CNI-TLE: Cognitive not impairment-temporal lobe epilepsy
Figure 2:
Axial structural image of the brain showing result of amplitude of low-frequency fluctuation (resting-state functional magnetic resonance imaging), Statistic t-map with the difference between the cognitive impairment-temporal lobe epilepsy (CI-TLE) group and CNI-TLE (two-sample t-test, P < 0.01, voxel>10). Warm colors indicate CI-TLE >cognitive not impairment-temporal lobe epilepsy (CNI-TLE), while cool colors indicate CNI-TLE >CI-TLE.
In Table 5 and Figure 2, cool colors show significantly increased ALFF between CI-TLE and CNI-TLE. Significantly, a decreased ALFF cluster was seen in the corpus callosum and MFG.
Table 5:
Significant decreased ALFF cluster between CI-TLE and CNI-TLE group, P<0.01, voxel >10.
| Anatomical regions | MNI space coordinates | Peak z-value | Voxels | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 | Corpus callosum-L | −3 | 24 | 3 | −3.63 | 78 |
| 2 | Medial frontal gyrus-L | −9 | 39 | 30 | −3.04 | 26 |
R: Right hemisphere, L: Left hemisphere, MNI: Montreal Neurological Institute, ALFF: Amplitude of low-frequency fluctuation, CI-TLE: Cognitive impairment-temporal lobe epilepsy, CNI-TLE: Cognitive not impairment-temporal lobe epilepsy
DISCUSSION
In this study, we have investigated the difference in functional alteration of (i) TLE group with NC group and (ii) TLE with CI group from TLE without CI group by applying ALFF method of RS-fMRI. Our study showed widespread activation and deactivation in the brain which can be because of widespread pathological abnormalities by TLE and is consistent to a study by Moran et al. (2001) and Reyes et al. (2016).[2,15]
Between TLE and NC
Increased ALFF activation
Our result has demonstrated increased ALFF value in PG, IFG, SFG, MFG, midbrain and pons, insula, ITG, ACG, and frontal lobe subgyral white matter.
PG is the mesial temporal lobe (MTL) structure. Ji et al.[16] have described an increased in ALFF in the MTL in their study. They have mentioned MTL as the area of the genesis of epileptic activity. MTL also includes amygdala and hippocampus in addition to the PG.[17] Zeng et al.[14] by their regional homogeneity finding have also described PG as the area of epilepsy genesis. In our study, we found that PG had increased ALFF.
We have also found the widespread frontal lobe increased ALFF value. IFG, SFG, frontal white matter, and MFG were all involved. Frontal lobe impairment either should be a consequence of temporal lobe involvement by frontotemporal propagation or is secondary to the propagation of epileptic activity from the epileptogenic zone of similar functioning integrated cortex elsewhere with frontal lobe.[18] Reyes et al. described widespread frontotemporal functional abnormality in TLE patients through their FALFF study in TLE and Guedj et al. through their FDG- PET study in TLE.[2,19] We also had an area of lateral temporal lobe with increased ALFF value, especially in ITG.
The insula is a complex structure. It is said that it has a role in several higher-order cognitive functions such as in saliency, switching, attention, and control network. Several studies have already talked about insula having the role of seizure propagation in TLE.[14,20] Because of its role in seizure propagation, we could have found increased ALFF value in the insula in TLE patient.
Moreover, ALFF was also increased in midbrain and pons. Mueller et al. in their study have concluded the involvement of brainstem in TLE and can be associated with its atrophic changes.[21] In addition to this, findings by Blumenfeld and the team, that is, increased CBF in the midbrain of TLE patients in their study in SPECT also supports increase ALFF in the midbrain.[22]
To conclude, increased ALFF value in PG, frontal lobe, thalamus, insula, midbrain, and pons in TLE and NC comparison should be related to the seizure initiation and propagation in TLE.
Decreased ALFF activation
Decreased ALFF value was found in PCG, cuneus, cerebellum posterior lobe, IPL, STG, and frontal lobe subgyral white matter.
Decreased ALFF in PCG, IPL, and STG is moreover related to the default model network (DMN). DMN includes the posterior cingulate cortex, medial prefrontal cortex, precuneus, IPL, and temporoparietal junction.[23]
DMN exhibits higher metabolic activity at rest than while performing cognitive tasks,[24] Therefore, DMN is activated in the state of passive relaxation or internal mental processes such as autobiographical memory, the theory of mind, self-referral processing, and future thinking with a high degree of connectivity across the brain regions, whereas the DMN is disabled during a wide range of attention-seeking operations.[25] DMN should be unaltered, activated in healthy subjects, and altered in TLE patients, which is consistent with our findings.
Reduced activation in the cerebellum can be a sign of disconnection of the glutamatergic corticopontocerebellar tracts and may denote decreased motor coordination.[14,26-28].
Between CI-TLE and CNI-TLE
Increased ALFF activation
The previous studies have shown that areas in frontal, temporal, parietal, and limbic lobe are related to cognitive functioning.[29] Executive control network (ECN) and default mode network (DMN) are believed to play an important role in cognition.[30,31] The executive network includes the dorsolateral prefrontal cortex and the anterior cingulate cortex. The DMN includes the posterior cingulate cortex, medial prefrontal cortex, precuneus, and inferior parietal lobes, including the angular and supramarginal gyrus and temporoparietal junction. Executive functions include important cognitive activities, which include planning, decision-making, knowledge, and understanding. The frontal lobes support a high level of cognitive processes which include talent and working memory.[18] Precuneus and cuneus have common divergent thinking behaviors and are backed by proven basic connectivity for the dorsal precuneus and ventral cuneus.[32] In our study, we found the area of ECN and DMN, especially in MTG, cuneus, ACG, IPL, MFG, and SFG, had increased ALFF value. DMN and ECN are a potential cognitive area and can be found activated even in a RS. Activation of these areas more in CI-TLE than CNI-TLE can be because of the decompensation mechanism. Repeated epilepsy could have arrested restoration of the original function of these areas effectively but instead could have been improperly reorganized by some unsuitable compensation mechanism resulting in activation of these areas in CI-TLE patients in an impaired way but not in CNI-TLE patient, which is a decompensation mechanism.[8]
Studies have shown that the cerebellum is involved in the motor domain as well as higher-level cognitive and affective functions. Sensorimotor tasks activate the anterior lobe, whereas the posterior lobe is involved in higher-level tasks, including language and verbal working memory, executive functions, spatial tasks, and emotional processing. In particular, language and executive tasks activate the posterior lobe and are involved in prefrontal-cerebellar loops.[33]
Decreased ALFF activation
Interestingly, decreased ALFF was seen in corpus callosum and medial frontal gyrus (MeFG). Moreover, MeFG had decreased ALFF, which is the component of DMN. The epilepsy-related deactivation in the DMN has already been documented in a few fMRI studies in the previous studies.[34,35] Deactivation of DMN and ECN area in TLE should signify CI.[8,9]
We acknowledge that our study has several limitations. First, comparatively, CI-TLE group contained relatively a smaller number of patients than the CNI-TLE group and NC. Second, it is difficult to apply our study to a single patient as this study is moreover two-group analysis and third, the potential effect of an antiepileptic drug in TLE patients was not considered.
CONCLUSION
In this paper, we have applied the ALFF method of RS- fMRI to trace the possible pathophysiology in TLE patients with CI. Between TLE and NC, we found increased ALFF activation in PG, frontal lobe, thalamus, insula, midbrain, and pons, increased activation in these areas should be because of seizure initiation and propagation.
Decreased ALFF was found in PCG, cuneus, cerebellum posterior lobe, IPL, STG, and frontal lobe subgyral white matter, these areas are related to DMN.
Between CI-TLE and CNI-TLE, especially in MTG, ACG, IPL, MFG, and SFG, we found increased ALFF value. These areas are the component of ECN and DMN. Activation of these areas should be because of the decompensation mechanism.
Acknowledgments
The authors thank the Natural Science Foundation of Tianjin (18JCZDJC44800) and Tianjin 131 Innovative Talent Team Project in 2016 for financial support” .
Footnotes
How to cite this article: Singh TB, Aisikaer A, He C, Wu Y, Chen H, Ni H, Song Y, Yin J. The Assessment of Brain Functional Changes in the Temporal Lobe Epilepsy Patient with Cognitive Impairment by Resting-state Functional Magnetic Resonance Imaging. J Clin Imaging Sci 2020;10:50.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Chinese National Science and Technology Support Project (2011BAI08B10) and Tianjin Science and Technology Support Project (15ZCZDSY00520).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nugent AC, Martinez A, D'Alfonso A, Zarate CA, Theodore WH. The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: A preliminary study in healthy subjects and those with temporal lobe epilepsy. J Cereb Blood Flow Metab. 2015;35:583–91. doi: 10.1038/jcbfm.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes A, Thesen T, Wang X, Hahn D, Yoo D, Kuzniecky R, et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57:1475–84. doi: 10.1111/epi.13456. [DOI] [PubMed] [Google Scholar]
- 3.Blair RD. Temporal lobe epilepsy semiology. Epilepsy Res Treat. 2012;2012:751510. doi: 10.1155/2012/751510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:5. doi: 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao F, Kang H, You L, Rastogi P, Venkatesh D, Chandra M. Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Ann Indian Acad Neurol. 2014;17:374–82. doi: 10.4103/0972-2327.144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Titiz AS, Mahoney JM, Testorf ME, Holmes GL, Scott RC. Cognitive impairment in temporal lobe epilepsy: Role of online and offline processing of single cell information. Hippocampus. 2014;24:1129–45. doi: 10.1002/hipo.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: Perceptual and behavioral phenomena reported in the old and new literature. Front Hum Neurosci. 2010;4:46. doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Zhang C, Liu C, Yu T, Zhang G, Chen N, et al. Brain network alteration in patients with temporal lobe epilepsy with cognitive impairment. Epilepsy Behav. 2018;81:41–8. doi: 10.1016/j.yebeh.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp. 2010;31:1851–61. doi: 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin North Am. 2011;22:133–9. doi: 10.1016/j.nec.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huettel SA. Functional MRI (fMRI) In: Lindon JC, Tranter GE, Koppenaal DW, editors. Encyclopedia of Spectroscopy and Spectrometry. 3rd ed. Oxford: Academic Press; 2017. pp. 778–84. [DOI] [Google Scholar]
- 12.Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: A review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34:1866–72. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Wang J, Zhang J, Wen H, Zhang Y, Kang H, et al. Altered spontaneous brain activity in children with early Tourette syndrome: A resting-state fMRI study. Sci Rep. 2017;7:4808. doi: 10.1038/s41598-017-04148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng H, Pizarro R, Nair VA, La C, Prabhakaran V. Alterations in regional homogeneity of resting-state brain activity in mesial temporal lobe epilepsy. Epilepsia. 2013;54:658–66. doi: 10.1111/epi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran NF, Lemieux L, Kitchen ND, Fish DR, Shorvon SD. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain. 2001;124:167–75. doi: 10.1093/brain/124.1.167. [DOI] [PubMed] [Google Scholar]
- 16.Ji GJ, Zhang Z, Zhang H, Wang J, Liu DQ, Zang YF, et al. Disrupted causal connectivity in mesial temporal lobe epilepsy. PLoS One. 2013;8:e63183. doi: 10.1371/journal.pone.0063183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hoesen GW. Anatomy of the medial temporal lobe. Magn Reson Imaging. 1995;13:1047–55. doi: 10.1016/0730-725X(95)02012-I. [DOI] [PubMed] [Google Scholar]
- 18.Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res. 2012;98:1–13. doi: 10.1016/j.eplepsyres.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guedj E, Bonini F, Gavaret M, Trebuchon A, Aubert S, Boucekine M, et al. 18FDG-PET in different subtypes of temporal lobe epilepsy: SEEG validation and predictive value. Epilepsia. 2015;56:414–21. doi: 10.1111/epi.12917. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz TH. Insular seizures: Have we been missing the boat? Epilepsy Curr. 2005;5:147–8. doi: 10.1111/j.1535-7511.2005.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin. 2014;5:208–16. doi: 10.1016/j.nicl.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- 23.Molnar-Szakacs I, Uddin L. Self-processing and the default mode network: Interactions with the mirror neuron system. Front Hum Neurosci. 2013;7:571. doi: 10.3389/fnhum.2013.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–37. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Yuan H, Lei X. Activation and connectivity within the default mode network contribute independently to future-oriented thought. Sci Rep. 2016;6:21001. doi: 10.1038/srep21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, Tervonen O, et al. Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res. 2011;1373:221–9. doi: 10.1016/j.brainres.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini A, Van Billoen H, et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2006;32:684–95. doi: 10.1016/j.neuroimage.2006.04.185. [DOI] [PubMed] [Google Scholar]
- 28.Zhong Y, Lu G, Zhang Z, Jiao Q, Li K, Liu Y. Altered regional synchronization in epileptic patients with generalized tonic-clonic seizures. Epilepsy Res. 2011;97:83–91. doi: 10.1016/j.eplepsyres.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Arden R, Chavez RS, Grazioplene R, Jung RE. Neuroimaging creativity: A psychometric view. Behav Brain Res. 2010;214:143–56. doi: 10.1016/j.bbr.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. Neuroimage. 2012;59:1783–94. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Geary D. United States: American Psychological Association; 2006. The Origin of Mind: Evolution of Brain, Cognition, and General Intelligence. [DOI] [Google Scholar]
- 32.Heinonen J, Numminen J, Hlushchuk Y, Antell H, Taatila V, Suomala J. Default mode and executive networks areas: Association with the serial order in divergent thinking. PLoS One. 2016;11:e0162234. doi: 10.1371/journal.pone.0162234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su J, Wang M, Ban S, Wang L, Cheng X, Hua F, et al. Relationship between changes in resting-state spontaneous brain activity and cognitive impairment in patients with CADASIL. J Headache Pain. 2019;20:36. doi: 10.1186/s10194-019-0982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI deactivation of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–22. doi: 10.1016/S1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 35.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. PNAS. 2005;102:15236–40. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]