Abstract
Background:
With the increase in endovascular treatment, reports of embolism other than thrombus are scattered, but intracranial tumorigenic embolism is rare and difficult to diagnose. Here, we describe a case of a tumorigenic embolism in a patient with lung cancer whose invasion into the vascular system was not detected on preoperative whole-body imaging.
Case Description:
A 66-year-old man who was hospitalized to undergo radiotherapy for pulmonary carcinoma suddenly developed left hemiplegia. He exhibited atrial fibrillation, and emergent radiographic examination revealed a right middle cerebral artery occlusion. Urgent mechanical embolectomy was performed, with successful revascularization. The excised embolus had a unique morphology and was pathologically diagnosed as a cerebral embolism caused by pleomorphic pulmonary carcinoma.
Conclusion:
Tumor-derived cerebral embolism is extremely rare, but it is necessary to consider it as a potential source of embolism during differential diagnosis in patients with malignant tumors.
Keywords: Cerebral embolism, Cerebral infarction, Hemiplegia, Pleomorphic carcinoma

INTRODUCTION
Intracranial tumorigenic embolism is rare and difficult to diagnose. Several authors reported that it can be suspected based on primary tumor biopsy or nonintracranial embolism and confirmed in autopsies.[3,8,12] Recent advances in intravascular interventions have facilitated embolism retrieval, but the prognosis of tumorigenic embolism remains unfavorable.[2] Herein, we report a case of sudden-onset left hemiplegia diagnosed as a right middle cerebral artery (MCA) occlusion by a tumorigenic embolus. Endovascular embolectomy was successfully performed and the outcome was favorable. The embolus exhibited a unique morphology but characteristic pathology.
MEDICAL HISTORY AND EXAMINATION
A 66-year-old man developed dyspnea and underwent bronchoscopy and lung biopsy at a local hospital. Lung carcinoma was suspected (pleomorphic lung carcinoma, cT4N3M0). He was referred to our institute and hospitalized to undergo palliative radiotherapy to prevent airway obstruction due to tumor invasion. Enhanced computed tomography (CT) performed on admission and after intervention did not reveal any emboli migrating into the pulmonary vein [Figure 1a and b]. On day 17 posthospitalization, he suddenly exhibited slightly impaired consciousness, severe left hemiplegia, and right conjugate deviation, resulting in a score of 14 points on the National Institutes of Health Stroke Scale. Electrocardiography revealed atrial fibrillation, and brain CT did not reveal any hemorrhagic lesions or hyperdense MCA signs. Magnetic resonance diffusion-weighted images showed high-intensity signals between white and gray matter areas in the right insular cortex, lateral lobe, and opercular part of the anterior lobe. Magnetic resonance angiography did not depict any arterial signals in the right MCA, and the diffusion- weighted imaging Alberta Stroke Program Early CT Score was 5 [Figure 2a].[1] A cardiogenic embolism due to atrial fibrillation was suspected, and an emergency thrombectomy was planned.
Figure 1:
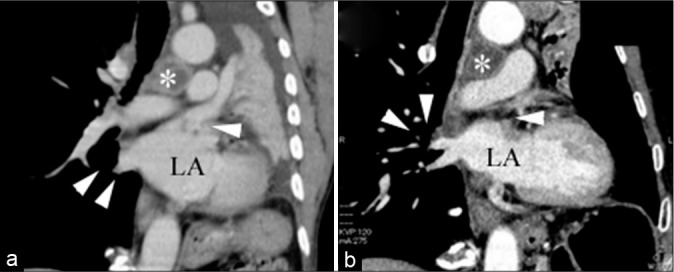
Coronal chest computed tomography (a) on admission (asterisk: tumor; LA: lung atrium; white arrows: pulmonary vein) and (b) after thrombectomy (asterisk: tumor; LA: lung atrium; white arrows: pulmonary vein). Tumor invasion of the pulmonary vein was not suspected.
Figure 2:
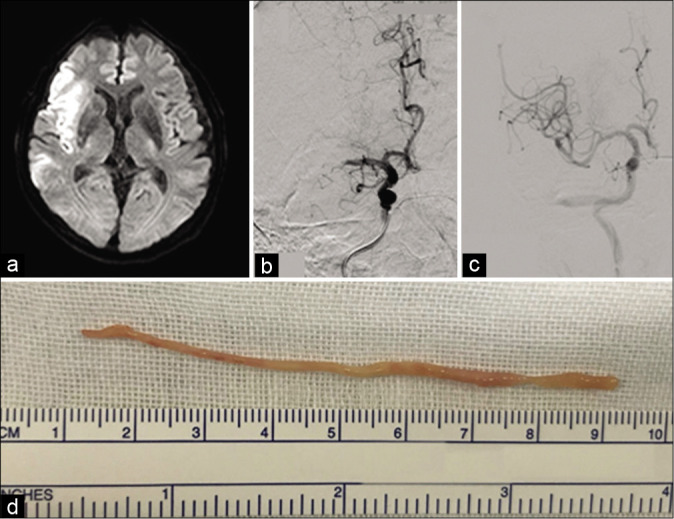
(a) Diffusion-weighted magnetic resonance image of the brain depicting high-intensity signals in the area of the right internal cerebral artery (Alberta Stroke Program Early Computed Tomography Score of 5). (b) Angiography image showing occlusion of the middle cerebral artery (posteroanterior view). (c) Postthrombectomy right internal carotid artery angiography image demonstrating recanalization of the middle cerebral artery. (d) Excised tumor tissue consisted of a single mass that was reddish- white in color, cylindrical in shape, and exhibited elasticity.
INTERVENTION
The patient did not receive intravenous tissue plasminogen activator therapy because he had terminal cancer. Emergency cerebral angiography revealed a right MCA occlusion at the M1 distal segment [Figure 2b]. A stent retriever (Trevo, Stryker; Kalamazoo, MI) was extended into the right MCA M1 segment.Under proximal flow control with a balloon- guided catheter, the Trevo was retrieved. Revascularization (thrombolysis incerebral infarction Grade 3) was confirmed through angiography performed 4 h 37 min after onset [Figure 2c]. The retrieved embolus differed substantially from a typical thrombus, as it was cylindrical, elastic, and whitish in color [Figure 2d].
POSTINTERVENTION COURSE
The day after the intervention, the patient’s neurological symptoms improved substantially. Only slight hemiplegia remained and he could walk unassisted. At 35 days after cerebral embolism onset, his Modified Rankin Scale score was 1 and he was discharged from the hospital. His performance status was maintained and chemotherapy for the lung carcinoma was initiated.
PATHOLOGY
The embolus excised from the intracranial vessel was 8 cm long and cylindrical, and pathological examination revealed central necrosis in the cross-sectional plane [Figure 3]. The tissue contained oval- and spindle-shaped nuclei with cornification. Immunohistochemical findings were concordant with the bronchoscopy-based diagnosis of pleomorphic carcinoma. Thus, a diagnosis of cerebrovascular tumorigenic embolism was confirmed.
Figure 3:
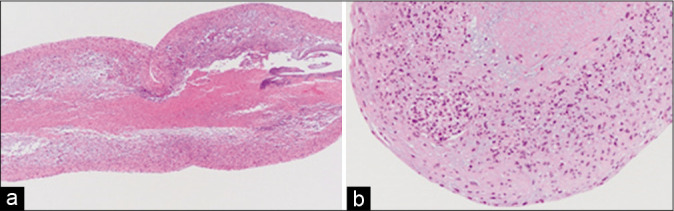
Longitudinal (a) and transverse (b) sections of the excised tumor. Necrosis was detected in the core of the tumor.
DISCUSSION
The most frequent cause of cerebrovascular malignant tumorigenic embolism is primary lung carcinoma, and the source of the embolus is often invasive carcinoma cells migrating into the pulmonary vein.[2,7,11,13] Alternatively, after surgical resection of lung carcinoma (especially left upper lobectomy), thrombosis in the intracranial arteries sometimes occurs. Several reports noted thrombus formation at the surgical stump, and the embolus source was identified in 13.5% of postsurgical resection cases.[4,5,7,9] In our patient, bronchial invasion of the lung carcinoma was present, and he underwent radiotherapy without prior surgery. We suspected the embolus migrated from an invasive lesion in the pulmonary vein, but no signs of this existed on the CT images acquired at admission or postthrombectomy.
A case of cerebral embolism caused by pleomorphic carcinoma (sarcoma-like malignant tumor) was reported previously, but the mechanisms causing the embolism were not investigated.[10] In that report, central necrosis identified through microscopy was detected within the embolus, which is similar to the findings herein. However, in that report, the macroscopic morphology and onset differed from those in the present case. The morphology appeared to be fragmented from the tumor, and tumor embolism occurred again. These findings suggest that the embolus was derived from cells torn from the proliferating tumor tissue.
Murai et al.[6] reported a case of tumorigenic embolism within the coronary arteries: the retrieved embolus exhibited an elongated morphology and central necrosis, similar to observations in our case. Tumor tissue was extracted from a coronary artery identified through angiography. An elongated embolic source with central necrosis was present in small arteries, which may have caused the embolism. That is, even though the tumor embolus did not invade the pulmonary vein, the mechanism was considered to be tumor- derived embolism in our case.
The few reports that exist on the treatment of intracranial vascular tumorigenic embolism suggest that the prognosis is poor. Fortunately, in our patient, revascularization was achieved during the early phase of disease onset, resulting in a favorable clinical outcome. Elucidating the embolism’s source is essential for preventing recurrence. The central necrosis we noted through pathologic examination implies the involvement of a highly proliferative entity, which may cause frequent repeated embolism.[12] Although pre- and post-surgical thoracic CT did not reveal the source of the embolism, pathological evaluation of the thrombus was useful for establishing a precise diagnosis. We believe early and aggressive intravascular intervention may be beneficial for patients in whom a tumorigenic embolus is suspected, especially patients with comorbid lung carcinoma.[2]
CONCLUSION
Herein, we described a case of tumorigenic cerebral embolism associated with a rare form of lung carcinoma and pleomorphic carcinoma. Excised tumor tissue exhibited a long cylindrical shape, and central necrosis was detected through pathological assessment. Early and aggressive intravascular intervention may be effective in cases of tumorigenic embolus.
Footnotes
How to cite this article: Yoshikawa S, Kamide T, Kasakura S, Arai N, Osada T, Mouri A, et al. A case of cerebral infarction due to pleomorphic carcinoma of the lung. Surg Neurol Int 2020;11:217.
Contributor Information
Shinichiro Yoshikawa, Email: yskw@saitama-med.ac.jp.
Tomoya Kamide, Email: kamide@med.kanazawa-u.ac.jp.
Shigen Kasakura, Email: yskw@saitama-med.ac.jp.
Noriko Arai, Email: yskw@saitama-med.ac.jp.
Takashi Osada, Email: yskw@saitama-med.ac.jp.
Atsuto Mouri, Email: yskw@saitama-med.ac.jp.
Mei Hamada, Email: yskw@saitama-med.ac.jp.
Tomonori Kawasaki, Email: yskw@saitama-med.ac.jp.
Masaki Takao, Email: yskw@saitama-med.ac.jp.
Shinya Kohyama, Email: yskw@saitama-med.ac.jp.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–4. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Ku A, Pu C, Wright DG, Tayal AH. Endovascular mechanical retrieval of a terminal internal carotid artery breast tumor embolus. J Neurosurg. 2009;112:572–4. doi: 10.3171/2009.6.JNS09221. [DOI] [PubMed] [Google Scholar]
- 3.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64:16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hughes SE, Hunter A, Campbell J, Brady A, Herron B, Smyth G, et al. Extraction of tumour embolus following perioperative stroke. J Neurol Sci. 2015;353:172–4. doi: 10.1016/j.jns.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Imaizumi K, Murate T, Ohno J, Shimokata K. Cerebral infarction due to a spontaneous tumor embolus from lung cancer. Respiration. 1995;62:155–6. doi: 10.1159/000196412. [DOI] [PubMed] [Google Scholar]
- 6.Murai T, Yonetsu T, Isobe M, Kakuta T. Coronary embolization caused by pleomorphic lung carcinoma. Intern Med. 2016;55:3607–9. doi: 10.2169/internalmedicine.55.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano T, Inaba M, Kaneda H. Recurrent cerebral attack caused by thrombosis in the pulmonary vein stump in a patient with left upper lobectomy on anticoagulant therapy: Case report and literature review. Surg Case Rep. 2017;3:4–8. doi: 10.1186/s40792-017-0376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neill BP, Dinapoli RP, Okazaki H. Cerebral infarction as a result of tumor emboli. Cancer. 1987;60:90–5. doi: 10.1002/1097-0142(19870701)60:1<90::aid-cncr2820600116>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Ohtaka K, Hida Y, Kaga K, Kato T, Muto J, Nakada-Kubota R, et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg. 2013;95:1924–8. doi: 10.1016/j.athoracsur.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Pop R, Mihoc D, Manisor M, Richter JS, Lindner V, Janssen-Langenstein R, et al. Mechanical thrombectomy for repeated cerebral tumor embolism from a thoracic sarcomatoid carcinoma. BMJ Case Rep. 2017;2017:013092. doi: 10.1136/bcr-2017-013092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts TE, Hasleton PS, Musgrove C, Swindell R, Lawson RA. Vascular invasion in non-small cell lung carcinoma. J Clin Pathol. 1992;45:591–3. doi: 10.1136/jcp.45.7.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers LR. Cerebrovascular complications in cancer patients. Neurol Clin. 2003;21:167–92. doi: 10.1016/s0733-8619(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 13.Woodring JH, Bognar B, van Wyk CS. Metastatic chondrosarcoma to the lung with extension into the left atrium via invasion of the pulmonary veins. Clin Imaging. 2002;26:338–41. doi: 10.1016/s0899-7071(02)00445-x. [DOI] [PubMed] [Google Scholar]


