Abstract
Wolfram syndrome (WS) is a rare, progressive disorder characterized by childhood-onset diabetes mellitus, optic nerve atrophy, hearing loss, diabetes insipidus, and neurodegeneration. Currently, there is no effective treatment for WS, and patients typically die between 30 and 40 years of age. WS is primarily caused by autosomal recessive mutations in the Wolfram syndrome 1 (WFS1) gene (OMIM 222300), which encodes for wolframin (WFS1). This disorder is therefore a valuable monogenic model for prevalent diseases, particularly diabetes mellitus and neurodegeneration. Whereas reduced survival and secretion are known cellular impairments causing WS, the underlying molecular pathways and the physiological function of WFS1 remain incompletely described. Here, we characterize WFS1 as a regulator of intracellular calcium homeostasis, review our current understanding of the disease mechanism of WS, and discuss candidate treatment approaches. These insights will facilitate identification of new therapeutic strategies not only for WS but also for diabetes mellitus and neurodegeneration.
Keywords: Orphan disease, WFS1, neurodevelopment, pancreatic β-cells, intracellular calcium signaling, ibudilast
Introduction: Ca2+ and Cell Function in Disease
Calcium (Ca2+) is a universal second messenger, and intracellular Ca2+ homeostasis is a key determinant of cell health and viability. Intracellular Ca2+ signaling is a highly versatile process that regulates numerous cell functions – including metabolism, gene transcription, and apoptosis – in all tissue types (for reviews see [1, 2]). As such, the Ca2+ concentration in different cellular compartments must be tightly regulated through a network of Ca2+ channels and transporters, which, in turn, are regulated by multiple signaling molecules and interacting partners. Generally, resting free cytosolic Ca2+ levels are low (approximately 100nM) compared to the concentration in organelles (endoplasmic reticulum (ER): approximately 1mM, mitochondria: approximately 0.1–500μM) or extracellular s ace (approximately 1mM) [2, 3]. It is well established that disruption of intracellular Ca2+ homeostasis underlies several pathologies, including Alzheimer’s disease [4], cancer progression [5], and diabetes mellitus [6]. In addition, emerging evidence suggests that intracellular Ca2+ dyshomeostasis contributes to the pathology of the rare disease call d Wolfram syndrome.
Wolfram Syndrome
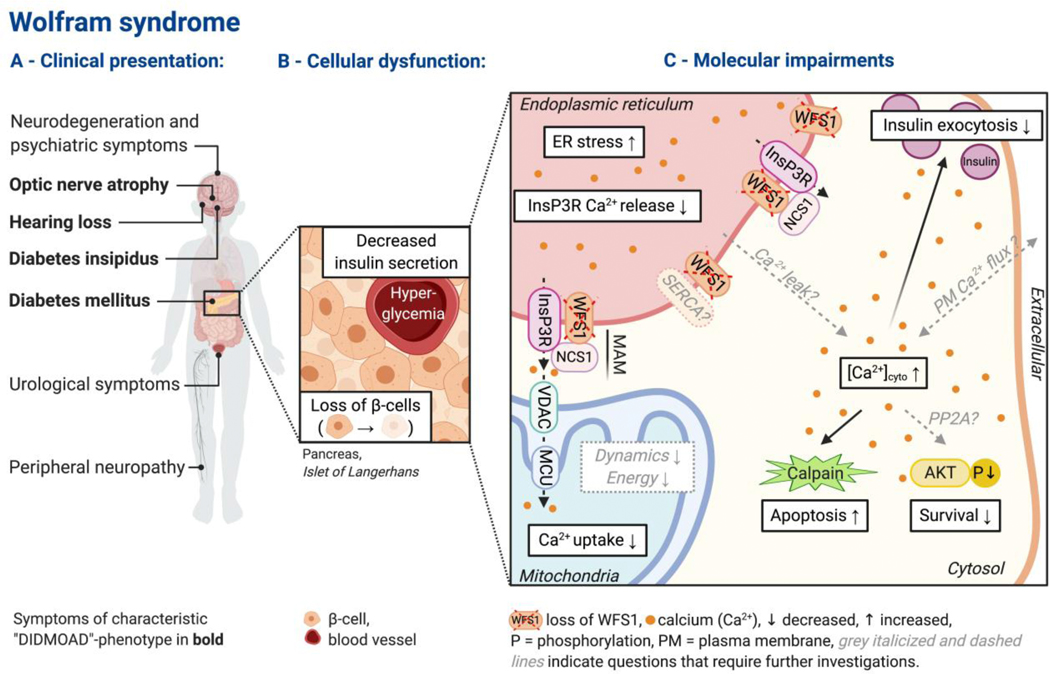
Wolfram syndrome (WS, OMIM 222300) is an orphan, autosomal recessive endocrinological and neurodegenerative disorder that affects 1:160,000 to 1:700,000 people worldwide [7]. In 1938, WS was first described by Wolfram and Wagener [8]. This disorder is characterized by the “DIDMOAD”-phenotype in the majority of patients: diabetes insipidus (average age of onset: 14 years), diabetes mellitus (6 years), optic nerve atrophy (11 years), and sensorineural deafness (12.5 yeas) (Figure 1A) [9]. Other common symptoms of WS include widespread neurodegeneration, cerebellar ataxia, autonomic symptoms, urological complications, and psychiatric illness (Figure 1A) [9]. At the moment, there is no effective treatment for this devastating disease, and patients typically die in their 30s, most often because of respiratory failure due to brain stem atrophy [10, 11].
Figure 1:
Overview of Wolfram syndrome pathology. (A) Clinical presentation. Symptoms most frequently observed in Wolfram syndrome patients are listed with labels indicating affected organs (schematic, list not exhaustive). The characteristic “DIDMOAD”-phenotype is shown in bold. (B-C) These two subpanels illustrate the pathomechanism of WS, focusing on pancreatic β-cells because diabetes mellitus is the first diagnosed symptom in WS and has been most frequently studied. (B) Cellular dysfunction due to the loss of WFS1 in the endocrine pancreas (Islet of Langerhans) is characterized by decreased insulin secretion and increased loss of β-cells. (C) Molecular impairments due to the loss of WFS1 are characterized by disrupted intracellular Ca2+ homeostasis, ER stress, and reduced mitochondrial functionality – resulting in reduced secretion, enhanced apoptosis, and reduced survival. Disruption of intracellular Ca2+ homeostasis, including elevation of resting free cytosolic Ca2+ [Ca2+]cyto and impaired ER-Ca2+ release into the cytosol and mitochondria, appears as a likely underlying cause for WS pathology.
Around 90% of WS cases are caused by inactivating mutations in the Wolfram syndrome 1 gene (WFS1) [9], which encodes for wolframin (WFS1), a transmembrane protein localized to the ER [12]. The remaining WS cases are attributed to sequence variants in the CISD2 gene (also known as WFS2) or other unknown genes [13]. The heterozygous carrier frequency of WFS1 variants is 0.3–1% [11], and these carriers show increased risks of psychiatric illnesses [14]. Moreover, some WFS1 variants can cause autosomal dominant low-frequency sensorial hearing loss (DFNA6/14) [15], autosomal dominant optic atrophy with hearing impairments [16, 17], and autosomal dominant diabetes mellitus [18]. Single nucleotide polymorphisms (SNPs) in the WFS1 gene locus can predispose patients to type 2 diabetes [19]. Still, despite the emerging importance of the WFS1 gene locus, the physiological functions of the protein WFS1 remain elusive.
This review summarizes our current understanding of the functions of WFS1 and the disease mechanism of WS. We focus on the role of intracellular Ca2+ signaling and describe candidate strategies to treat WS. Moreover, we propose future directions for studies on WS and discuss how a better understanding of WS may provide further insights into the pathology of diabetes mellitus and neurodegeneration.
The WFS1 Gene Product: Wolframin (WFS1)
The WFS1 gene is localized on chromosome 4p16.1 and encodes for wolframin (WFS1), an 890-amino-acid protein with nine putative transmembrane segments [20]. WFS1 is ubiquitously expressed, but particularly high expression levels are observed in the brain, heart, and pancreatic β-cells (β-cells) [21]. Intracellularly, WFS1 is predominantly localized to the ER with a Ncytosol/CER lumen topology [12, 21, 22] and appears to be enriched in mitochondria-associated ER-membranes (MAM) [23, 24]. WFS1 is also present in the membranes of secretory granules [25, 26]. The crystal structure of WFS1 has not been solved. Over 200 pathogenic variants in the WFS1 gene locus have been identified in WS patients, but genotype-phenotype correlations re not well established [9]. Typically, these genetic variants are loss-of-function mutations resulting in depletion of WFS1 mRNA or protein or expression of defective WFS1 protein [9]. Whereas in vivo and in vitro disease models of WFS1-deficiency may not reflect the numerous different mutations in patients, they recapitulate cardinal symptoms of WS [27–30].
WFS1 as a Regulator of Intracellular Ca2+ Homeostasis
The current body of evidence suggests an essential role for WFS1 in the regulation of ER homeostasis and intracellular Ca2+ signaling. In recent studies on β-cells, neurons, and patient-derived fibroblasts, loss of WFS1 function results in a global disruption of intracellular Ca2+ homeostasis, affecting Ca2+ signaling both at the resting state and under stimulation (Figure 1C) [23, 24, 29, 31, 32].
Elevation of cytosolic calcium.
Free resting cytosolic Ca2+ [Ca2+]cyto is elevated due to loss of WFS1 [24, 29, 31, 33]. Normally, [Ca2+]cyto is kept at low concentrations because sustained [Ca2+]cyto elevation results in cellular impairments and ultimately, apoptosis [34]. The molecular mechanisms that lead to elevation of [Ca2+]cyto in WS have yet to be determined. On one hand, dysfunction of the ER, the main intracellular Ca2+ store, may be an underlying cause. WFS1 likely regulates ER-Ca2+ sequestration through its interaction with the sarcoendoplasmic reticulum Ca2+ transport ATPase (SERCA), but it remains uncertain how WFS1-deficiency alters SERCA expression and activity [31, 35]. Additionally, some studies indicate an ER-Ca2+ leak in WFS1-deficient cells [31, 33, 36], although we and others did not see a difference in ER-Ca2+ loading [24, 29, 32]. On the other hand, the increase of [Ca2+]cyto due to loss of WFS1 may result from increased Ca2+ influx or decreased Ca2+ efflux across the plasma membrane. Mechanistically, it has been suggested that WFS1 positively regulates the expression of sodium/potassium ATPase β1 subunit (Na+/K+-ATPase β1), which would result in reduced Na+/K+-ATPase and sodium-calcium exchanger (NCX) activity in WFS1-deficient β-cells [37]. The effect of WFS1-deficiency on voltage-gated calcium channels and store-operated calcium channels should also be explored in future studies.
Reduction in stimulus-evoked ER-Ca2+ release.
Stimulus-evoked ER-Ca2+ release via the inositol 1,4,5-trisphosphate receptor (InsP3R) is impaired due to loss of WFS1 [23, 24, 29, 32]. The InsP3R can release Ca2+ from the ER into the cytosol and – at interorganellar signaling hubs called mitochondria-associated ER membranes (“MAMs”) – into mitochondria [38, 39]. Proper InsP3R activity, which is regulated by several interacting partners of the receptor, is integral to cellular signaling [38]. WFS1 appears to be a positive regulator of InsP3R-dependent ER-Ca2+ release into the cytosol and mitochondria [23, 24, 29, 32], but further studies on the underlying mechanisms are necessary. It has been described that the expression of InsP3R1 and InsP3R3 is not changed in WFS1-deficient β-cells [24]. Co-immunoprecipitation experiments suggest an interaction between InsP3R and WFS1, but it remains unclear whether they interact directly [32]. Alternatively, neuronal Ca2+ sensor-1 (NCS1) may bridge the two proteins as NCS1 interacts directly with InsP3R and WFS1 [32, 40]. Overexpression of NCS1 rescued Ca2+ signaling in WFS1-deficient fibroblasts and β-cells [24, 32], suggesting a WFS1-NCS1-InsP3R signaling complex which is disrupted in WS. Finally, InsP3R dysfunction may be a secondary result of other cellular signaling impairments due to loss of WFS1, such as unresolved cell stress [41].
Collectively, WFS1 has been characterized as a versatile regulator of intracellular Ca2+ homeostasis, likely through interaction with several key players of intracellular Ca2+ signaling, including InsP3R [32], SERCA2 [35], and NCS1 [32]. One study also suggested that WFS1 itself may act as a cation-selective ion channel [42]. This seems unlikely because in all recent studies, WFS1-deficiency results in elevated [Ca2+]cyto [24, 29, 31, 33], and we did not observe an increase in ER-Ca2+ release with WFS1 overexpression [24]. However, a better understanding of the WFS1 structure and tests for channel activity with purified WFS1 are necessary to clarify this matter. Additionally, we have posed several other questions that need future investigations in order to develop a global understanding of intracellular Ca2+ signaling in WS. Nevertheless, the impairments of intracellular Ca2+ signaling that have been described in WS thus far point to potential treatment targets as discussed in the section “Treatment Approaches for Wolfram Syndrome”.
Molecular Mechanisms of Wolfram Syndrome
Consistent with the neuroendocrinological symptoms in WS patients, in vivo and in vitro studies have established that WFS1-deficiency causes reduced cell viability and secretion primarily in β-cells and neurons (Figure 1B). This tissue selectivity is likely because β-cells and neurons are hypersusceptible to ER-dysregulations due to their high rates of protein synthesis as secretory cells [13, 43]. Here, we summarize current mechanistic insights and delineate the role of altered Ca2+ signaling in producing the WS phenotypes.
Secretion.
Impairments of secretion in WS have mostly been studied in β-cells. Glucose-stimulated insulin secretion (GSIS) from β-cells is required to facilitate glucose uptake into adipose tissue, muscle, and liver following a meal. Elevated plasma glucose concentrations stimulate ATP production in β-cells. Consequently, ATP-sensitive potassium channels close which causes depolarization of the plasma membrane. Voltage-gated calcium channels open and Ca2+ influx results in a rapid elevation in [Ca2+]cyto that ultimately triggers insulin exocytosis (for review see [44]). In contrast, in WFS1-deficient cells, this glucose-stimulated rise in [Ca2+]cyto is diminished, resulting in reduced GSIS [27]. Mechanistically, the rapid increase in [Ca2+]cyto is likely inhibited by the permanently elevated [Ca2+]cyto. This is supported by our observation that drug interventions that normalize [Ca2+]cyto in WFS1-KO cells also restore GSIS [24]. Moreover, ER Ca2+ release and mitochondrial Ca2+ transients, both of which are impaired in WFS1-deficient β-cells [24], are important for the fine-tuning of insulin exocytosis [45]. ER-Ca2+ release via the InsP3R – for example, stimulated by the parasympathetic neurotransmitter acetylcholine – potentiates insulin secretion by elevating [Ca2+]cyto [46]. Ca2+ uptake into mitochondria can stimulate ATP synthesis [47], which amplifies insulin release.
Overall, WFS1-dependent Ca2+ signaling appears to be crucial for GSIS (Figure 1C). Additionally, it was proposed that WFS1 positively regulates insulin expression and processing [25, 48]. Because proper Ca2+ signaling is as essential for secretion in neurons as for GSIS, and WFS1 was found to interact with vacuolar proton (H+)-ATPase in neuroblastoma cells [26], it can be expected that WFS1-deficient neurons show secretory impairments similar to β-cells. Consistently, dopamine release is decreased in the striatum of WFS1-deficient mice [49]. However, findings are sparse, most likely due to technical challenges and regional heterogeneity when studying secretion in the CNS.
Survival and Cell Death.
WFS1 has been characterized as a regulator of cellular homeostasis that promotes cell health [48] and inhibits ER stress [50]. Whereas overall cell viability appears to be similar between wild-type and WFS1-deficient β-cells in vitro [24], β-cells mass is reported to be reduced in WFS1-deficient animal models [28, 30, 48, 51]. This is consistent with studies showing that WFS1-deficiency renders β-cells and neurons hypersusceptible to cell death induced by stress, such as ER stress, age, and hyperglycemia [24, 27, 48, 52]. Decreased pro-survival and increased proapoptotic signaling are likely responsible for this phenotype.
We and others observed that the phosphorylation – and hence activity – of Akt, a well-known pro-survival effector [53], was decreased in WFS1-deficient β-cells [24, 48]. This seems to be a shared downstream consequence of decreased activity of the insulin receptor and increased expression of tribbles homolog 3 (Trib3) [24, 48]. Additionally, Akt activity may be downregulated by elevated [Ca2+]cyto either directly [54] or through Ca2+-dependent activation of protein phosphatase 2 A (PP2A) [55]. This hypothesis needs further elucidation. Mitochondrial energy metabolism and dynamics, which are also important for cell survival, are likely impaired due to loss of WFS1 in neurons and fibroblasts [29, 32]. Indeed, because WFS1 is enriched at mitochondria-associated ER-membranes (MAM) [23], it is well-positioned to regulate mitochondrial function. However, the nature of mitochondrial impairments in WFS1-deficient cells remains controversial [23], and reports about mitochondrial function in WFS1-deficient β-cells are lacking.
Hyperactivation of calpains is one of the alterations that increase proapoptotic signaling in WFS1-deficient β-cells and neurons [24, 31]. As calpains are Ca2+-activated cysteine proteases, their hyperactivation is consistent with elevated [Ca2+]cyto in WS [24, 31]. Additionally, WFS1-deficient cells show increased levels of ER stress because WFS1 is a negative regulator of this pathway [29, 50]. Ultimately, unresolved, chronic ER stress in WS results in apoptosis (for review see [56]). Perturbations of ER-Ca2+ homeostasis are among stress signals that can trigger ER stress [57]. In turn, ER stress can also impair InsP3R-dependent Ca2+ signaling [41], suggesting a vicious cycle between disrupted Ca2+ signaling and ER stress. Lastly, increased levels of caspase activity, which can be triggered by Ca2+ dyshomeostasis [34], were observed following loss of WFS1 in β-cells [28, 48].
In sum, impaired Ca2+ signaling appears to be an underlying cause for the observed dysfunctions in WS, but further elucidation of the precise disease mechanism, especially in neurons, are required. Future studies should focus on recently developed patient-derived stem-cell models of WS for in-depth mechanistic studies [58, 59]. Thereby, distinct molecular consequences due to different disease-causing mutations in the human WFS1 gene may be identified.
Trajectories of Wolfram Syndrome
The natural history of WS is characterized by the childhood onset of endocrinological and neuronal symptoms associated with progressive degeneration. On a cellular level, this phenotype can be attributed to reduced secretion and survival – as described in the previous section. With non-autoimmune, insulin-dependent diabetes mellitus typically being the first diagnosed symptom of WS, hyperglycemia may accelerate the loss of neurons due to their hypersusceptibility to stress-induced cell death [27, 48, 52]. Therefore, strategies that aim to treat diabetes mellitus may delay the onset of other symptoms in WS. Furthermore, recent neuroimaging studies in WS patients suggest that impaired neurodevelopment precedes neurodegeneration (for review see [60]). Alterations in early brain development were primarily characterized by hypomyelination [60], necessitating studies on the function of WFS1 in oligodendrocytes. Interestingly, WFS1 expression is strongly upregulated in the human brain during development, with the highest levels occurring between 8 and 15 years of age [60]. Of note for further studies, reduced brain development and the trajectory of WFS1 expression are recapitulated in WFS1-deficie t mice [29, 61]. Future research endeavors should leverage functional neuroimaging and explore underlying molecular pathways, connecting impaired neurodevelopment and neurodegeneration.
Treatment Approaches for Wolfram Syndrome
Currently, there is no effective treatment for WS, and patients typically die in their 30s [9]. Disease management focuses on alleviating the symptoms in order to improve patients’ quality of life; for example, insulin treatment to control diabetes mellitus [13]. Candidate treatment strategies include the repurposing of existing drugs and development of regenerative medicine tools (Table 1). Most drug tests in WS animal models have focused on diabetes mellitus as an outcome measure. However, it is also essential to measure brain volume and assess neuronal functions because patients usually die due to brain stem atrophy [9].
Table 1:
List of candidate treatments for Wolfram syndrome (WS). In black are drugs that have been tested in in vivo or in vitro models of WS. In blue are proposed drugs and hypothesized mechanisms that should be assessed in future studies. FDA: U.S. Food and Drug Administration; NCT: Clinicaltrials.gov identifier; ER: endoplasmic reticulum; Ca2+: calcium; T2: type2; SERCA: sarcoendoplasmic reticulum Ca2+ transport ATPase; GLP1R: glucagon-like peptide-1 receptor; MANF: mesencephalic astrocyte-derived neurotrophic factor; Akt: protein kinase B.
| Drugs | Approved in humans? (FDA?; indication) | Clinical trial for WS | Proposed mechanism of action in WS |
|---|---|---|---|
| Targeting disrupted Ca2+ signaling (drug repurposing) | |||
| Dantrolene [31] | yes (FDA; malignant hyperthermia, muscle spasticity) | yes (NCT02829268) | Normalizing [Ca2+]cyto and calpain activity by inhibiting ER Ca2+efflux via ryanodine receptors [31, 62] |
| Ibudilast [24] | yes (Japan; asthma, post-stroke dizziness) | Normalizing [Ca2+]cyto [24] through interaction with NCS1 [64] or the cAMP-Ca2+ link [65] | |
| Calpain inhibitor [24] | no | Normalizing calpain activity and [Ca2+]cyto [24] | |
| Pioglitazone [51] | yes (FDA; diabetes mellitus T2) | Restoring ER Ca2+ h me stasis by enhancing SERCA levels [33, 69] | |
| Sitagliptin | yes (FDA; diabetes mellitus T2) | Potentially restoring ER Ca2+ homeostasis [70] | |
| Verapamil | yes (FDA; arrhythmia, angina, hypertension) | Potentially normalizing [Ca2+]cyto and calpain activity by blocking plasma-membrane Ca2+-influx and alleviating ER stress [29, 71, 72] | |
| Targeting ER stress (drug repurposing) | |||
| Valproate [77] | yes (FDA; seizures) | yes (NCT03717909) | Alleviating ER stress [75, 76] |
| GLP1R agonists [78, 79] | yes (FDA; diabetes mellitus T2) | Alleviating ER stress [78, 79] | |
| Stimulating proliferation ( egenerative medicine) | |||
| MANF [81] | no | Stimulating proliferation and alleviating ER stress [81] | |
| Akt activators | no | Potentially inducing proliferation and survival by stimulating Akt pathway [80] | |
Targeting Disrupted Ca2+ Signaling.
The drugs we summarized in this group likely act through normalization of [Ca2+]cyto or ER Ca2+ homeostasis. As such, dantrolene, which is used to treat muscle spasticity and malignant hyperthermia [62], restores [Ca2+]cyto and calpain activity in WFS1-deficient β-cells, neurons, and patient-derived neuronal progenitor cells [31]. This compound stabilizes ER-Ca2+ homeostasis through the inhibition of ryanodine receptors [62]. Currently, a clinical trial that assesses safety, tolerability, and efficacy in WS patients is ongoing (Clinical Trial Number: NCT02829268). Recently, we investigated the potential of ibudilast, a PDE4-inhibitor that is approved for treating asthma and post-stroke dizziness in Japan [63], in the context of WS [24]. We observed that this drug rescues [Ca2+]cyto, GSIS, and survival in WFS1-deficient β-cells [24]. Ibudilast may restore Ca2+ home stasis through its interaction with NCS1 [64] or cAMP-Ca2+ cross-talk [65], but further mechanistic studies are needed. This drug may also reduce neurodegeneration in WS, as it showed promising effects on neuropathy and multiple sclerosis [66, 67]. Because hyperactivation of calpain is observed in WFS1-deficient cells, we also tested the effect of calpain inhibitor XI (also known as AK295) [24]. Calpain inhibitor XI rescues [Ca2+]cyto, GSIS, and survival in WFS1-deficient β-cells [24]. Most calpain inhibitors are relatively toxic and produce broad effects, but new, more specific calpain inhibitors are under clinical trial [68] and could potentially be repurposed for the treatment of WS. The potential of oral antidiabetic drugs has also been investigated in the context of WS. For example, pioglitazone can restore ER-Ca2+ homeostasis, likely by enhancing SERCA levels [33, 69]. WFS1-deficient agouti (Avy) mice were partially protected against diabetes following pioglitazone treatment [51]. Another antidiabetic drug, sitagliptin, a dipeptidyl peptidase-4 inhibitor, can also restore ER Ca2+ signaling and may be tested in WS [70]. Furthermore, we suggest testing verapamil, a calcium channel blocker, because blocking Ca2+ influx may help to no malize [Ca2+]cyto and associated pathways in WS [29]. Similarly, verapamil reduced ne rodegeneration in a mouse model of ALS by inhibiting calpains, ER stress, and autophagy [71]. Additionally, a recent clinical trial found that this drug preserved β-cell function in patients with type 1 diabetes mellitus [72].
Targeting ER Stress.
Because ER stress is another established contributor to WS, associated pathways have been targeted for candidate treatments. Valproate (VPA) is a mood stabilizer [73]. that can inhibit histone deacetylase (HDAC) [74], reduce ER stress [75], and induce WFS1 expression [76]. In a mouse model of WS, valproate improved blood glucose tolerance, potentially through enhancement of insulin sensitivity [77]. Valproate is currently under assessment in a clinical trial in WS patients (Clinical Trial Number: NCT03717909). Exenid-4 and liraglutide, glucagon-like-peptide 1 (GLP1)-receptor agonists, can restore glucose tolerance in WFS1-deficient animal models likely by alleviating ER stress [78, 79]. Liraglutide also showed promising effects on glycemic control in a WS patient [79].
Regenerative Medicine.
Future advancements in the fields of gene therapy and replacement medicine may ultimately lead to a cure for WS [13]. Using gene editing, WFS1 mutations could be corrected in induced pluripotent stem cells (iPSCs) generated from patients’ skin cells. These iPSCs could then be differentiated and transplanted into patients [59]. Alternatively, wild-type WFS1 could be increased in affected organs with adeno-associated virus systems (AAV) [13]. Another approach involves stimulating the proliferation of remaining β-cells and neurons. For this purpose, restoring Akt activity in WS may be a promising new avenue [24, 53, 80]. Similarly, it has been reported that mesencephalic astrocyte-derived neurotrophic factor (MANF) can enhance the proliferation of remaining β cells in WS mice [81].
Overall, there are multiple promising avenues for the treatment of WS that should be further explored. Still, the multiorgan nature of this disorder presents significant challenges, and may necessitate a combination of treatment strategies.
Wolfram Syndrome as Monogenic Disease Model
WS represents a monogenic model for diabetes mellitus, neurodevelopment, and neurodegeneration. On one hand, monogenic models cannot replicate the complexity of multifactorial diseases. On the other hand, studies on single-gene forms of diabetes mellitus and neurodegeneration have provided many mechanistic insights (for reviews see [82, 83]). Furthermore, WS was recently used s diabetic disease model to improve approaches of gene editing and replacement medicine for the therapy of diabetes mellitus [59]. Mechanistically, diabetic and neurodegenerative pathology shares several molecular impairments with WS, particularly unresolved ER-stress, Akt dysregulation, and impaired Ca2+ signaling [6, 39, 56, 80, 84]. A recent review described WS as a prototype of ER stress-associated disorders [85]. We noted that several impairments of intracellular Ca2+ homeostasis in WS had been similarly observed in diabetes mellitus. First, [Ca2+]cyto is elevated in human and rat wild-type pancreatic islets following culture in hyperglycemia [86, 87]. Second, we observed that hyperglycemia reduced stimulus-evoked ER-Ca2+ release in wild-type cells [24]. Lastly, the MAM-signaling axis appears to be disrupted in diabetes mellitus [88, 89]. Therefore, drugs that restore Ca2+ homeostasis and reduce ER stress in WFS1-deficient cells may hold similar therapeutic potential for diabetes mellitus and neurodegeneration. This hypothesis is further supported by the recent observation that WFS1 was transcriptionally downregulated in pancreatic islets from type 2 diabetes mellitus patients, suggesting a role for WFS1 in the pathology of common forms of diabetes [48].
Concluding Remarks
Recent studies in vivo and in vitro have enriched our understanding of WS which represents a monogenic model for diabetes mellitus and neurodegeneration. WFS1 is an emerging regulator of cellular homeostasis and particularly intracellular Ca2+ signaling. Signaling impairments caused by the loss of WFS1 culminate in impaired secretion and survival. A more detailed view of how WFS1 regulates development will further elucidate the trajectories of WS. Future studies are necessary to determine the molecular mechanism of this rare disease and will simultaneously provide new insights into more complex diseases. We are optimistic that currently developed strategies will contribute to the treatment of WS and, eventually, diabetes mellitus and neurodegeneration.
Highlights.
Wolfram syndrome (WS) is a rare, incurable disease caused by mutations in the WFS1 gene
Clinical hallmarks of WS include childhood onset of diabetes mellitus and neurodegeneration
WFS1 protein is an emerging regulator of cellular homeostasis including calcium signaling
Loss of WFS1 function results in reduced secretion and survival primarily in pancreatic β-cells and neurons
Studies on WS will facilitate the development of therapeutics for diabetes mellitus and neurodegeneration
Acknowledgments
The authors thank Lien Nguyen for critical discussions throughout the process of writing this review. We also thank Allison Brill, Hannah Hauschild, Eiman Ibrahim, and Wei Mi for helpful comments. This laboratory was supported by NIH grant 5P01DK057751. T.T.F. was supported by a scholarship from the Germ n Academic Scholarship Foundation.
Footnotes
Competing interests
B.E.E is a founder of Osmol Therapeutics, a company that is targeting NCS1 for therapeutic purposes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:special interest
outstanding interest
- 1.Berridge MJ, Lipp P, and Bootman MD, The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol, 2000. 1(1): p. 11–21. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE, Calcium signaling. Cell, 2007. 131(6): p. 1047–58. [DOI] [PubMed] [Google Scholar]
- 3.Xu Z, et al. Transport of Calcium Ions into Mitochondria. Curr Genomics, 2016. 17(3): p. 215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magi S, et al. Intracellular Calcium Dysregulation: Implications for Alzheimer’s Disease. Biomed Res Int, 2016. 2016: p. 6701324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteith GR, Prevarskaya N, and Roberts-Thomson SJ, The calcium-cancer signalling nexus. Nat Rev Cancer, 2017. 17(6): p. 367–380. [DOI] [PubMed] [Google Scholar]
- 6.Gilon P, et al. Calcium signaling in pancreatic beta-cells in health and in Type 2 diabetes. Cell Calcium, 2014. 56(5): p. 340–61. [DOI] [PubMed] [Google Scholar]
- 7.Rigoli L, et al. Genetic and clinical aspects of Wolfram syndrome 1, a severe neurodegenerative disease. Pediatr Res, 2018. 83(5): p. 921–929. [DOI] [PubMed] [Google Scholar]
- 8.Wolfram DJ and Wagener HP, Diabetes mellitus and simple optic atrophy among siblings: report of four cases. Mayo Clin Proc., 1938. 1: p. 715–8. [Google Scholar]
- 9.de Heredia ML, Cleries R, and Nunes V, Genotypic classification of patients with Wolfram syndrome: insights into the natural history of the disease and correlation with phenotype. Genet Med, 2013. 15(7): p. 497–506. [DOI] [PubMed] [Google Scholar]
- 10.Kinsley BT, et al. Morbidity and mortality in the Wolfram syndrome. Diabetes Care, 1995. 18(12): p. 1566–70. [DOI] [PubMed] [Google Scholar]
- 11.Barrett TG, Bundey SE, and Macleod AF, Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet, 1995. 346(8988): p. 1458–63. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, et al. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet, 2001. 10(5): p. 477–84. [DOI] [PubMed] [Google Scholar]
- 13.Urano F, Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr Diab Rep, 2016. 16(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swift RG, et al. Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Mol Psychiatry, 1998. 3(1): p. 86–91. [DOI] [PubMed] [Google Scholar]
- 15.Bespalova IN, et al. Mutations in the Wolfram syndrome 1 gene (WFS1) are a common cause of low frequency sensorineural hearing loss. Hum Mol Genet, 2001. 10(22): p. 2501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiberg H, et al. Autosomal dominant optic atrophy associated with hearing impairment and impaired glucose regulation caused by a missense mutation in the WFS1 gene. J Med Genet, 2006. 43(5): p. 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rendtorff ND, et al. Identification of p.A684V missense mutation in the WFS1 gene as a frequent cause of autosomal dominant optic atrophy and hearing impairment. Am J Med Genet A, 2011. 155A(6): p. 1298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnycastle LL, et al. Autosomal dominant diabetes arising from a Wolfram syndrome 1 mutation. Diabetes, 2013. 62(11): p. 3943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandhu MS, et al. Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet, 2007. 39(8): p. 951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue H, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet, 1998. 20(2): p. 143–8. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann S, et al. Wolfram syndrome: structural and functional analyses of mutant and wild-type wolframin, the WFS1 gene product. Hum Mol Genet, 2003. 12(16): p. 2003–12. [DOI] [PubMed] [Google Scholar]
- 22.Philbrook C, Fritz E, and Weiher H, Expressional and functional studies of Wolframin, the gene function deficient in Wolfram syndrome, in mice and patient cells. Exp Gerontol, 2005. 40(8–9): p. 671–8. [DOI] [PubMed] [Google Scholar]
- 23.La Morgia C, et al. Calcium mishandling in absence of primary mitochondrial dysfunction drives cellular pathology in Wolfram Syndrome. Sci Rep, 2020. 10(1): p. 4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen LD, et al. Calpain Inhibitor and Ibudilast Rescue β-Cell Function 2 in a Cellular Model of Wolfram Syndrome. PNAS, 2020. in press.This is the first study providing a detailed characterization of intracellular Ca2+ dysregulation both under resting and stimulating conditions while comparing control and high glucose environment in WFS1-knockout and WFS1-wildtype β-cells. Additionally, this study investigates ibudilast and calpain inhibitor as novel drug candidates for the treatment of Wolfram syndrome. The authors show that these two drugs rescue glucose-stimulated insulin secretion, hyperglycemia-induced loss of cell viability, and free resting cytosolic Ca2+ in WFS1-knockout pancreatic β-cells, which will stimulate future in vivo studies.
- 25.Hatanaka M, et al. Wolfram syndrome 1 gene (WFS1) product localizes to secretory granules and determines granule acidification in pancreatic beta-cells. Hum Mol Genet, 2011. 20(7): p. 1274–84. [DOI] [PubMed] [Google Scholar]
- 26.Gharanei S, et al. Vacuolar-type H+-ATPase V1A subunit is a molecular partner of Wolfram syndrome 1 (WFS1) protein, which regulates its expression and stability. Hum Mol Genet, 2013. 22(2): p. 203–17. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara H, et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum M l Genet, 2004. 13(11): p. 1159–70. [DOI] [PubMed] [Google Scholar]
- 28.Riggs AC, et al. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic eticulum stress and apoptosis. Diabetologia, 2005. 48(11): p. 2313–21. [DOI] [PubMed] [Google Scholar]
- 29.Cagalinec M, et al. Role of Mitochondrial Dynamics in Neuronal Development: Mechanism for Wolfram Syndrome. PLoS Biol, 2016. 14(7): p. e1002511.This work currently offers the most in-depth mechanistic insights into neuronal dysfunction in Wolfram syndrome. Experiments in primary neurons are described showing that WFS1-deficiency causes ER stress, disrupted Ca2+ homeostasis, and mitochondrial impairments. Additionally, the authors provide evidence that these dysfunctions are causatively related, suggesting new pathomechanisms and drug targets for neuropsychiatric symptoms.
- 30.Plaas M, et al. Wfs1-deficient rats develop primary symptoms of Wolfram syndrome: insulin-dependent diabetes, optic nerve atrophy and medullary degeneration. Sci Rep, 2017. 7(1): p. 10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu S, et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc Natl Acad Sci U S A, 2014. 111(49): p. E5292–301.This is the first report describing the elevation of free resting cytosolic Ca2+ and calpain activity in Wolfram syndrome pathology. The authors hypothesized that these impairments are due to an ER-Ca2+ leak and consequently, tested the therapeutic potential of dantrolene, an inhibitor of ryanodine receptors. Because the results were promising, dantrolene is currently being evaluated in a clinical trial for Wolfram syndrome (NCT02829268).
- 32.Angebault C, et al. ER-mitochondria cross-talk is regulated by the Ca(2+) sensor NCS1 and is impaired in Wolfram syndrome. Sci Signal, 2018. 11(553).This study proposes that WFS1 forms a novel signaling complex with neuronal calcium sensor-1 (NCS1) and inositol 1,4,5-trisphosphate receptor (InsP3R). The authors observed that NCS1 protein levels and InsP3R-dependent ER-mitochondrial Ca2+ cross-talk are decreased in fibroblasts derived from Wolfram syndrome patients. Subsequent overexpression of NCS1 rescues Ca2+ signaling and mitochondrial functionality, which suggests NCS1 as a drug target in Wolfram syndrome.
- 33.Hara T, et al. Calcium efflux from the endoplasmic reticulum leads to beta-cell death. Endocrinology, 2014. 155(3): p. 758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orrenius S, Zhivotovsky B, and Nicotera P, Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol, 2003. 4(7): p. 552–65. [DOI] [PubMed] [Google Scholar]
- 35.Zatyka M, et al. Sarco(endo)plasmic reticulum ATPase is a molecular partner of Wolfram syndrome 1 protein, which negatively regulates its expression. Hum Mol Genet, 2015. 24(3): p. 814–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takei D, et al. WFS1 protein modulates the free Ca(2+) concentration in the endoplasmic reticulum. FEBS Lett, 2006. 580(24): p. 5635–40. [DOI] [PubMed] [Google Scholar]
- 37.Zatyka M, et al. Sodium-potassium ATPase 1 subunit is a molecular partner of Wolframin, an endoplasmic reticulum protein involved in ER stress. Hum Mol Genet, 2008. 17(2): p. 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge MJ, The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol Rev, 2016. 96(4): p. 1261–96. [DOI] [PubMed] [Google Scholar]
- 39.Joshi AU, Kornfeld OS, and Mochly-Rosen D, The entangled ER-mitochondrial axis as a potential therapeutic strategy in neurodegeneration: A tangled duo unchained. Cell Calcium, 2016. 60(3): p. 218–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen LD, et al. Characterization of NCS1-InsP3R1 interaction and its functional significance. J Biol Chem, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higo T, et al. Mechanism of ER stress-induced brain damage by IP(3) receptor. Neuron, 2010. 68(5): p. 865–78. [DOI] [PubMed] [Google Scholar]
- 42.Osman AA, et al. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. J Biol Chem, 2003. 278(52): p. 52755–62. [DOI] [PubMed] [Google Scholar]
- 43.Oyadomari S, Araki E, and Mori M, Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis, 2002. 7(4): p. 335–45. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu M, et al. Glucose-stimulated insulin secretion: A newer perspective. J Diabetes Investig, 2013. 4(6): p. 511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rieusset J, Mitochondria and endoplasmic reticulum: mitochondria-endoplasmic reticulum interplay in type 2 diabetes pathophysiology. Int J Biochem Cell Biol, 2011. 43(9): p. 1257–62. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz de Azua I, et al. Novel insights into the function of beta-cell M3 muscarinic acetylcholine receptors: therapeutic implications. Trends Endocrinol Metab, 2011. 22(2): p. 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarasov AI, et al. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic beta-cells. PLoS One, 2012. 7(7): p. e39722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abreu D, et al. Wolfram syndrome 1 gene regulates pathways maintaining beta-cell health and survival. Lab Invest, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matt V, et al. Impaired striatal dopamine output of homozygous Wfs1 mutant mice in response to [K+] challenge. J Physiol Biochem, 2011. 67(1): p. 53–60. [DOI] [PubMed] [Google Scholar]
- 50.Fonseca SG, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest, 2010. 120(3): p. 744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akiyama M, et al. Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia, 2009. 52(4): p. 653–63. [DOI] [PubMed] [Google Scholar]
- 52.Sakakibara Y, et al. Knockdown of wfs1, a fly homolog of Wolfram syndrome 1, in the nervous system increases susceptibility to age- and stress-induced neuronal dysfunction and degeneration in Drosophila. PLoS Genet, 2018. 14(1): p. e1007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song G, Ouyang G, and Bao S, The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med, 2005. 9(1): p. 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang JK, et al. Increased intracellular Ca(2+) concentrations prevent membrane localization of PH domains through the formation of Ca(2+)-phosphoinositides. Proc Natl Acad Sci U S A, 2017. 114(45): p. 11926–11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn JH, et al. The B″/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc Natl Acad Sci U S A, 2007. 104(23): p. 9876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fonseca SG, et al. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol, 2009. 9(6): p. 763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoyer-Hansen M and Jaattela M, Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ, 2007. 14(9): p. 1576–82. [DOI] [PubMed] [Google Scholar]
- 58.Shang L, et al. beta-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes, 2014. 63(3): p. 923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maxwell KG, et al. Gene-edited human stem cell-derived beta cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci Transl Med, 2020. 12(540). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samara A, et al. Developmental hypomyelination in Wolfram syndrome: new insights from neuroimaging and gene expression analyses. Orphanet J Rare Dis, 2019. 14(1): p. 279.Based on recent neuroimaging studies in Wolfram syndrome patients, this review proposes that Wolfram syndrome is also a neurodevelopmental disorder characterized by hypomyelination. Furthermore, the authors performed exploratory gene-expression analysis and found that WFS1 expression is strongly increased during early brain development, particularly in oligodendrocytes.
- 61.Tekko T, et al. Initiation and developmental dynamics of Wfs1 expression in the context of neural differentiation and ER stress in mouse forebrain. Int J Dev Neurosci, 2014. 35: p. 80–8. [DOI] [PubMed] [Google Scholar]
- 62.Krause T, et al. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesi, 2004. 59(4): p. 364–73. [DOI] [PubMed] [Google Scholar]
- 63.Rolan P, Hutchinson M, and Johnson K, Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother, 2009. 10(17): p. 2897–904. [DOI] [PubMed] [Google Scholar]
- 64.Benbow JH, et al. Inhibition of paclitaxel-induced decreases in calcium signaling. J Biol Chem, 2012. 287(45): p. 37907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landa LR Jr., et al. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem, 2005. 280(35): p. 31294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mo M, et al. Prevention of paclitaxel-induced peripheral neuropathy by lithium pretreatment. FASEB J, 2012. 26(11): p. 4696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox RJ, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med, 2018. 379(9): p. 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ono Y, Saido TC, and Sorimachi H, Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov, 2016. 15(12): p. 854–876. [DOI] [PubMed] [Google Scholar]
- 69.Kono T, et al. PPAR-gamma activation restores pancreatic islet SERCA2 levels and prevents beta-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol Endocrinol, 2012. 26(2): p. 257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark AL, et al. Targeting Cellular Calcium Homeostasis to Prevent Cytokine-Mediated Beta Cell Death. Sci Rep, 2017. 7(1): p. 5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, et al. Verapamil Ameliorates Motor Neuron Degeneration and Improves Lifespan in the SOD1(G93A) Mouse Model of ALS by Enhancing Autophagic Flux. Aging Dis, 2019. 10(6): p. 1159–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ovalle F, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med, 2018. 24(8): p. 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tunnicliff G, Actions of sodium valproate on the central nervous system. J Physiol Pharmacol, 1999. 50(3): p. 347–65. [PubMed] [Google Scholar]
- 74.Phiel CJ, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem, 2001. 276(39): p. 36734–41. [DOI] [PubMed] [Google Scholar]
- 75.Batjargal K, et al. Effect of 4-phenylbutyrate and valproate on dominant mutations of WFS1 gene in Wolfram syndrome. J Endocrinol Invest, 2020. [DOI] [PubMed] [Google Scholar]
- 76.Kakiuchi C, et al. Valproate, a mood stabilizer, induces WFS1 expression and modulates its interaction with ER stress protein GRP94. PLoS One, 2009. 4(1): p. e4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terasmaa A, et al. Wfs1 mutation makes mice sensitive to insulin-like effect f acute valproic acid and resistant to streptozocin. J Physiol Biochem, 2011. 67(3): p. 381–90. [DOI] [PubMed] [Google Scholar]
- 78.Toots M, et al. Preventive treatment with liraglutide protects against development of glucose intolerance in a rat model of Wolfram syndrome. Sci Rep, 2018. 8(1): p. 10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kondo M, et al. Activation of GLP-1 receptor signalling alleviates cellular stresses and improves beta cell function in a mouse model of Wolfram syndrome. Diabetologia, 2018. 61(10): p. 2189–2201. [DOI] [PubMed] [Google Scholar]
- 80.Huang X, et al. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci, 2018. 14(11): p. 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mahadevan J, et al. A soluble endoplasmic reticulum factor as regenerative therapy for Wolfram syndrome. Lab Invest, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peltonen L, et al. Lessons from studying monogenic disease for common disease. Hum Mol Genet, 2006. 15 Spec No 1 : p. R67–74. [DOI] [PubMed] [Google Scholar]
- 83.Klupa T, Skupien J, and Malecki MT, Monogenic models: what have the single gene disorders taught us? Curr Di b Rep, 2012. 12(6): p. 659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brini M, et al. Neuro al calcium signaling: function and dysfunction. Cell Mol Life Sci, 2014. 71(15): p. 2787–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stone SI, et al. Monogenic and syndromic diabetes due to endoplasmic reticulum stress. Journal of Diabetes and its Complications, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bjorklund A, Lansner A, and Grill VE, Glucose-induced [Ca2+]i abnormalities in human pancreatic islets: important role of overstimulation. Diabetes, 2000. 49(11): p. 1840–8. [DOI] [PubMed] [Google Scholar]
- 87.Khaldi MZ, et al. Increased glucose sensitivity of both triggering and amplifying pathways of insulin secretion in rat islets cultured for 1 wk in high glucose. Am J Physiol Endocrinol Metab, 2004. 287(2): p. E207–17. [DOI] [PubMed] [Google Scholar]
- 88.Thivolet C, et al. Reduction of endoplasmic reticulum-mitochondria interactions in beta cells from patients with type 2 diabetes. PLoS One, 2017. 12(7): p. e0182027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma JH, et al. Comparative Proteomic Analysis of the Mitochondria-associated ER Membrane (MAM) in a Long-term Type 2 Diabetic Rodent Model. Sci Rep, 2017. 7(1): p. 2062. [DOI] [PMC free article] [PubMed] [Google Scholar]