Abstract
Background:
Few studies have examined the impact of lifestyle patterns on survival following breast cancer. We aimed to identify distinct lifestyle patterns based on five behavior/dietary exposures among a population-based sample of women diagnosed with breast cancer and to examine their association with subsequent survival.
Methods:
In the Carolina Breast Cancer Study Phases I/II, we interviewed 1,808 women 20–74 years of age following diagnosis of invasive breast cancer. We determined vital status using the National Death Index (717 deaths, 427 from breast cancer; median follow-up 13.56 years). We assessed lifestyle patterns using a latent class analysis based on five behavioral and dietary exposures: current versus never/former smokers; low versus high vegetable and fruit intake; high and low/moderate, versus no alcohol consumption; and no and low/moderate, versus high regular physical activity. We used Cox regression to estimate covariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality, and cause-specific and subdistribution HRs for breast cancer–specific mortality within 5 years and 13 years postdiagnosis conditional on 5-year survival.
Results:
We identified three distinct lifestyle patterns: healthy behavior and diet (n = 916); healthy behavior and unhealthy diet (n = 624); and unhealthy behavior and diet (n = 268). The unhealthy (vs. healthy) behavior and diet pattern was associated with a 13-year conditional all-cause mortality HR of 1.4 (95% CI = 1.1, 1.9) and with 13-year conditional breast cancer–specific and subdistribution HRs of 1.2 (95% CI = 0.79, 1.9) and 1.2 (95% CI = 0.77, 1.8), respectively.
Conclusions:
Behavioral and dietary patterns can be used to identify lifestyle patterns that influence survival patterns following breast cancer diagnosis.
Keywords: Alcohol, Breast cancer, Fruit and vegetable intake, Latent class analysis, Mortality, Physical activity, Smoking, Survival analysis
There are more than three million breast cancer survivors in the United States and an estimated 266,000 new cases will be diagnosed in 2018.1 By the year 2030, it is expected that the number of breast cancer survivors will increase by approximately 50%.2 Given the growing number of survivors and their high motivations to improve their overall health after diagnosis,3 it is important to understand whether modifiable lifestyle factors influence subsequent survival.4,5 Two well-established modifiable breast cancer prognostic factors include physical activity and smoking.6 Dietary factors, including high fruit and vegetable intake and limiting alcohol consumption, have been less consistently associated with breast cancer prognosis. The benefits of fruit and vegetable intake on overall mortality among cancer survivors are well known6; however, studies of fruit and vegetable intake and breast cancer survival suggest a modest protective effect on breast cancer prognosis.7,8 Additionally, while alcohol consumption is a risk factor for developing breast cancer,9 whether high intake impacts breast cancer–specific survival remains unclear.10 While it is important to understand how each of these modifiable factors impact breast cancer prognosis, these behaviors are also highly correlated.6 Therefore, studies focusing on individual factors may not capture interactions among these lifestyle factors. A better understanding of lifestyle patterns may help inform intervention strategies among women diagnosed with breast cancer, yet little work has been done that simultaneously examines these lifestyle patterns and their impact on survival following breast cancer.4,11
Using resources from the Carolina Breast Cancer Study (CBCS), a population-based study of breast cancer, the objectives of this study were to identify distinct lifestyle patterns based on five behavioral and dietary exposures: smoking, physical activity, vegetable intake, fruit intake, and alcohol consumption, and to examine their association with short- and long-term all-cause and breast cancer–specific survival.
METHODS
Study Population
This study used data from Phases I and II of the CBCS, a population-based case–control North Carolina study, with follow-up of survival. Details of the study design, including participant identification via rapid case ascertainment, recruitment, and eligibility are described elsewhere.12 Briefly, from 1993 to 1996 (Phase I) and 1996 to 2000 (Phase II), 1,808 women 20–74 years of age with a first diagnosis of invasive breast cancer in 24 counties were identified from the North Carolina Central Cancer Registry and recruited to participate in the CBCS. By design, the CBCS oversampled young (<50 years of age) and black women, so that sample sizes would be sufficient for stratified analyses. All procedures performed in the Carolina Breast Cancer Study involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of North Carolina at Chapel Hill and were in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
This study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
Lifestyle Behaviors
On average, within 5 months of breast cancer diagnosis (25th percentile = 2.4 months, 75th percentile = 5.66 months), the 1,808 CBCS participants included in this study completed an in-person, interviewer-administered questionnaire that assessed known and suspected breast cancer risk and prognostic factors including the following five behavioral and dietary exposures of interest in this study.
Smoking Status
Participants were asked about their current smoking status at the time of completion of the questionnaire.13 Former smokers were asked to report their age when they stopped smoking. Current smokers included women who had smoked at least 100 cigarettes during their lifetime and who reported smoking at the time of the interview, as well as women who quit smoking after diagnosis and those who quit within 1 year before their breast cancer diagnosis. Former smokers were women who had smoked at least 100 cigarettes during their lifetime, but who quit smoking at least 1 year before their breast cancer diagnosis. Never smokers were women who had smoked less than 100 cigarettes during their lifetime. We dichotomized smoking status as current versus never/former smokers.
Vegetable Intake and Fruit Intake
Participants were asked to report their usual weekly number of servings of fruits and vegetables during the winter and summer seasons over the past year before the questionnaire.14 The two vegetable items and the two fruit items were averaged to roughly approximate the number of cups of intake per week of vegetables and fruits, respectively. The continuous measures were dichotomized as low versus high intake of vegetables (<14 vs. ≥14 cups/week) and fruits (<10.5 vs. ≥10.5 cups/week) according to United States Department of Agriculture recommendations for vegetable15 and fruit16 intake.
Alcohol Consumption
Participants were asked to report on their consumption (drinks per day, week, or month) of beer wine, and hard liquor for three age periods: <25, 25–49, and ≥50 years of age.17 Alcohol consumption during the time period closest to diagnosis was the main exposure of interest. The continuous measure of drinks per week was categorized as high (≥7 drinks/week) and low/moderate (1–6 drinks/week) versus none (0 drinks/week), according to the American Cancer Society/American Society of Clinical Oncology breast cancer survivorship guidelines.18
Physical Activity
Participants were asked to report on their recreational physical activity in the three months before the interview.19 Women were asked if they engaged in any activities regularly, on a weekly basis, that helped them keep physically fit. Women who gave affirmative responses were asked to report on the number of times per week (once or twice, 3–4 times, 5–6 times, or 7 times per week) they engaged in those activities. The ordinal measure of physical activity was categorized as none (<1 time/week) versus low (1–4 times/week) and high (5–7 times/week) recreational physical activity, consistent with American Cancer Society/American Society of Clinical Oncology guidelines, assuming at least 30 minutes of physical activity on each occasion.18
Other Covariates
Potential confounders were selected from a directed acyclic graph and identified from the questionnaire and medical records. Covariates from the questionnaire included: self-reported race (black vs. white; 98% of white women were Caucasian), age at diagnosis (<50 vs. ≥50 years), education (<high school, high school or general equivalency diploma [GED], or ≥ college), marital status (unmarried vs. married), and body mass index (BMI; <25, 25–29, or ≥30 kg/m2). Covariates from the medical record included: stage (I/II vs. III/IV), grade (I/II vs. III) in Phase I only, tumor size (≤2.0 vs. >2.0 cm), node status (negative vs. positive), and estrogen receptor (ER) status (ER+ vs. ER−).
Outcome Assessment
Date of death and cause of death were identified by linkage to the National Death Index.20 All women diagnosed with breast cancer have been followed for vital status from diagnosis in 1993–1996 (Phase I) or 1996–2000 (Phase II) until date of death or 31 December 2011. Breast cancer–related deaths were classified as those that listed breast cancer (International Statistical Classification of Diseases codes 174.9 and C-50.9) as the underlying cause of death on the death certificate. By the end of follow-up (median = 13.56 years), we identified 717 deaths, 427 from breast cancer.
Statistical Analysis
Using logistic regression, we examined the associations between each of the dichotomous behavioral and dietary exposures. We examined these five exposures using a latent class analysis in Mplus version 7.4 (Muthén & Muthén, Los Angeles, CA). Latent class analysis is a statistical method used to identify a set of discrete, mutually exclusive, and exhaustive groups (i.e., latent classes) of individuals based on their responses to a set of observed categorical variables with the assumption that within a latent class the indicators are independent.21 Using maximum-likelihood estimation via the expectation-maximization algorithm, we generated estimates of all model parameters and posterior probabilities of class assignment.
We used several statistics to determine the best-fitting class solution, including the Akaike Information Criterion, the Bayesian Information Criterion, the Sample-Size adjusted Bayesian Information Criterion, the Lo-Mendell-Rubin adjusted likelihood ratio test, and entropy.21 In general, lower Akaike Information Criterion and Bayesian Information Criterion indicate a better fitting model and the latent class model with the smallest values on these three statistics is considered to be the best-fitting model.21 A nonsignificant Lo-Mendell-Rubin adjusted likelihood ratio test P value suggests that the model with one fewer class is a better explanation of the data. Higher entropy values reflect better classification of individuals. Once the best-fitting class solution was determined, individuals were assigned to the class in which they had the highest probability of membership. We then fit a series of bivariate logistic regression models to examine the associations between demographic and disease characteristics and the lifestyle patterns.
We conducted survival analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC). We used Kaplan-Meier survival curves stratified by lifestyle pattern to examine the unadjusted data over the median follow-up of 13 years and to assess the proportional hazards assumption. We observed an apparent divergence in the survival curves after 5 years of diagnosis for both all-cause survival and breast cancer–specific survival. Therefore, in multivariable analyses, we estimated from a single model with Heaviside function associations between lifestyle patterns and survival within 5 years of diagnosis, as well as 13 years after diagnosis conditional on 5-year survival. Other covariates were not examined for proportional hazards violations. We used Cox regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and breast cancer–specific mortality. For breast cancer–specific mortality, we conducted a competing risks analysis and estimated cause-specific HRs (csHRs) from Cox models with events censored at the time of non–breast cancer death, as well as subdistribution HRs (sHRs) using the Fine Gray model, as outlined by Austin et al.22 The csHR allows one to estimate the effect of covariates on the rate of occurrence of the outcome, in this case breast cancer–specific mortality, while the sHR allows one to estimate the effect of covariates on the cumulative incidence of the outcome over time. Covariates known to be associated with breast cancer mortality and associated with class assignment based on magnitude of association were included in the adjustment set in the multivariable Cox regression models, except for BMI, which we considered to be a potential mediator. All models were first adjusted for the study design variables (age and race). In subsequent models, we also included adjustment for demographic characteristics and disease characteristics. To examine effect measure modification by race (black vs. white), age at diagnosis (≥50 vs. <50 years), and estrogen receptor status (ER− vs. ER+), we included interaction terms in the fully adjusted models, which were evaluated using likelihood ratio tests, and also conducted stratified analyses.
RESULTS
Latent Class Analysis
We observed high correlations between low fruit intake and low vegetable intake (OR = 5.5, 95% CI = 4.5, 6.7), high alcohol consumption and current smoking (OR = 4.7, 95% CI = 3.4, 6.5), low physical activity and low fruit (OR = 1.6, 95% CI = 1.2, 2.0), and vegetable (OR = 1.4, 95% CI = 1.1, 1.8) intake (eTable 1; http://links.lww.com/EDE/B421). High (vs. no) alcohol consumption was also positively associated with low physical activity (OR = 1.3, 95% CI = 1.1, 1.7), but inversely associated with low vegetable (OR = 0.73, 95% CI = 0.54, 1.0) and fruit (OR = 0.82, 95% CI = 0.62, 1.1) intake.
We identified three latent classes of lifestyle patterns, based on the lowest Akaike Information Criterion, the Bayesian Information Criterion, the Sample-Size adjusted Bayesian Information Criterion statistics and a statistically significant Lo-Mendell-Rubin likelihood ratio test (eTable 2; http://links.lww.com/EDE/B421). The posterior probabilities of exhibiting each of the five behavior/dietary exposures for each of the three classes identified from the latent class analysis are depicted in Figure 1. Class 1, the healthy behavior and diet pattern, comprised 916 (51%) never/former smokers, the majority of whom reported high vegetable and fruit intake, no or low/moderate alcohol consumption, and high and low/moderate engagement in regular physical activity. Class 2, the healthy behavior and unhealthy diet pattern, comprised 624 (34%) women, the majority of whom were never/former smokers, with no or low/moderate alcohol consumption, no regular physical activity and all of whom reported low vegetable and fruit intake. Class 3, the unhealthy behavior and diet pattern comprised 268 (15%) current smokers, 29% of whom reported low vegetable intake, 46% of whom reported low fruit intake, and the majority of whom reported low/moderate or high alcohol consumption, and no regular physical activity. In addition to comparing the healthy behavior and unhealthy diet and unhealthy behavior and diet groups separately, we also combined and compared them together with the healthy behavior and diet group. Compared with women in the healthy behavior and diet pattern, women in the healthy behavior and unhealthy diet/unhealthy behavior and diet patterns were less likely to be white, older, college educated, and married; and were more likely to be diagnosed with larger tumors (see Table 1 for participant characteristics, overall and by latent class assignment).
FIGURE 1.
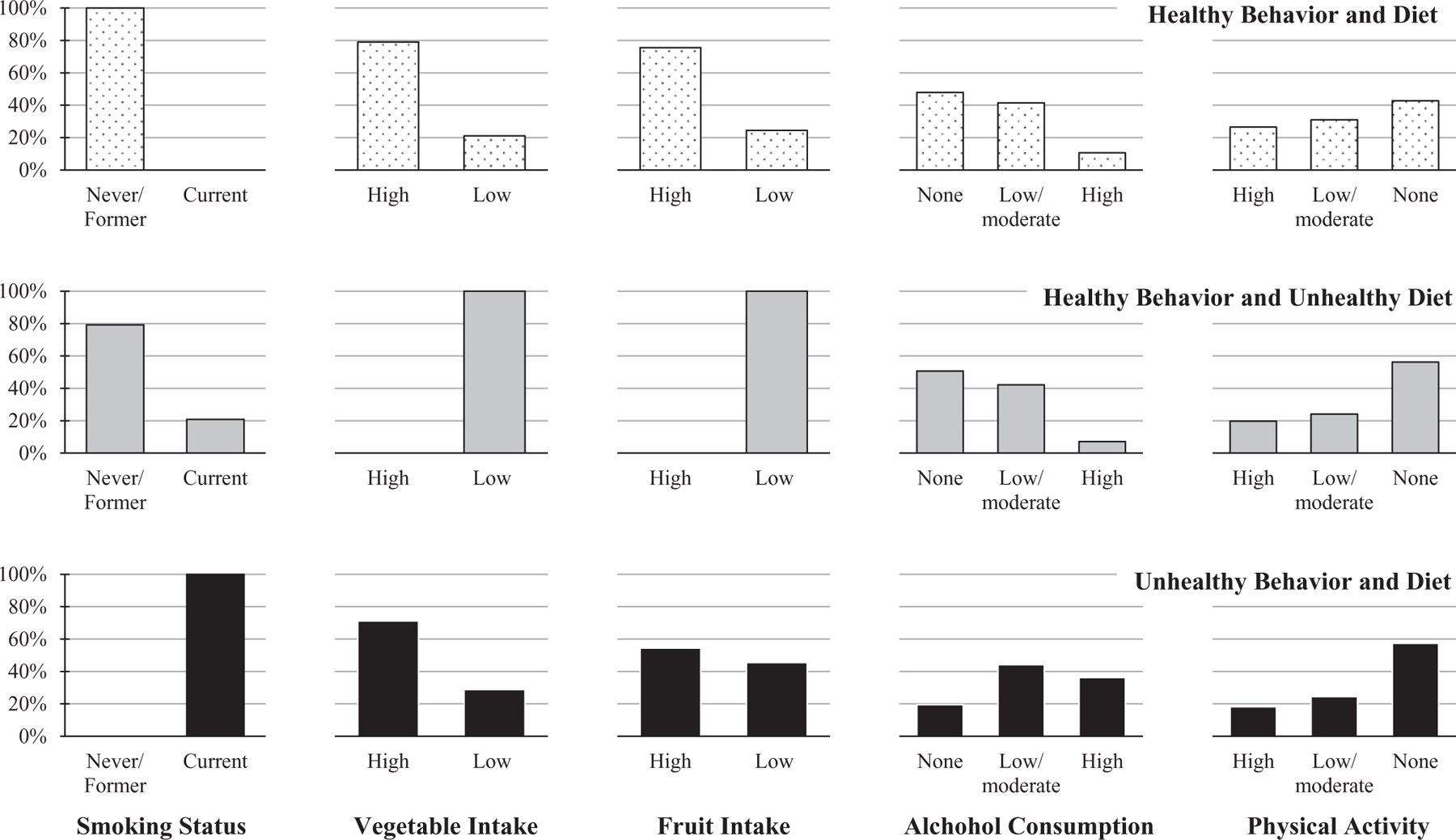
Posterior probabilities of each of five behavior/dietary exposures, for each of three lifestyle patterns: Healthy behavior and diet (n = 916, 51%); healthy behavior and unhealthy diet (n = 624, 34%); unhealthy behavior and diet (n = 268, 15%).
TABLE 1.
Participant Characteristics—CBCS Phases I and II (n = 1,808)
| Latent Class Assignment |
||||
|---|---|---|---|---|
| Characteristic | Overall (n = 1,808) n (%) | HBD (n = 916) n (%) | HB-UD/UBD (n = 892) n (%) | HB-UD/UBD Versus HBD OR (95% CI)a |
| Race | ||||
| Black | 788 (44) | 367 (40) | 421 (47) | 1.00 |
| White | 1,020 (56) | 549 (60) | 471 (53) | 0.75 (0.62, 0.90) |
| Caucasian | 995 (98) | 537 (98) | 458 (97) | |
| Other | 25 (2.4) | 12 (2.2) | 13 (2.8) | |
| Age at diagnosis (years) | ||||
| <50 | 976 (54) | 443 (48) | 533 (60) | 1.00 |
| ≥50 | 832 (46) | 473 (52) | 359 (40) | 0.63 (0.52, 0.76) |
| Education | ||||
| <High school | 330 (18) | 140 (15) | 190 (21) | 1.00 |
| High school or GED | 669 (37) | 307 (34) | 362 (41) | 0.87 (0.67, 1.1) |
| ≥College | 809 (45) | 469 (51) | 340 (38) | 0.53 (0.41, 0.69) |
| Marital status | ||||
| Unmarried | 758 (42) | 338 (37) | 420 (47) | 1.00 |
| Married | 1,050 (58) | 578 (63) | 472 (53) | 0.66 (0.55, 0.79) |
| BMI at diagnosis (kg/m2) | ||||
| <25 | 515 (29) | 267 (30) | 248 (28) | 1.00 |
| 25–29 | 626 (35) | 324 (36) | 302 (35) | 1.0 (0.80, 1.3) |
| ≥30 | 627 (35) | 304 (34) | 323 (37) | 1.1 (0.91, 1.4) |
| Stage | ||||
| I/II | 1,478 (87) | 748 (87) | 730 (87) | 1.00 |
| III/IV | 219 (13) | 110 (13) | 109 (13) | 1.0 (0.76, 1.3) |
| Gradeb | ||||
| I/II | 427 (57) | 224 (58) | 203 (56) | 1.00 |
| III | 318 (43) | 161 (42) | 157 (44) | 1.1 (0.81, 1.4) |
| Tumor size (cm) | ||||
| ≤2.0 | 909 (54) | 479 (56) | 430 (51) | 1.00 |
| >2.0 | 786 (46) | 379 (44) | 407 (49) | 1.2 (0.99, 1.4) |
| Node status | ||||
| Negative | 1,123 (64) | 577 (65) | 546 (63) | 1.00 |
| Positive | 638 (36) | 315 (35) | 323 (37) | 1.1 (0.89, 1.3) |
| ER status | ||||
| ER− | 727 (43) | 358 (42) | 369 (45) | 1.00 |
| ER+ | 959 (57) | 499 (58) | 460 (55) | 0.89 (0.74, 1.1) |
CBCS participants were diagnosed with invasive breast cancer from 1993 to 1996 (Phase I) and 1996 to 2000 (Phase II) and followed-up for vital status through 31 December 2011.
ORs and 95% CIs were derived from bivariate logistic regression models.
Grade not available for CBCS Phase II.
GED indicates general equivalency diploma; HBD, healthy behavior and diet; HB-UD, healthy behavior and unhealthy diet; OR, odds ratios; UBD, unhealthy behavior and diet.
All-cause Mortality
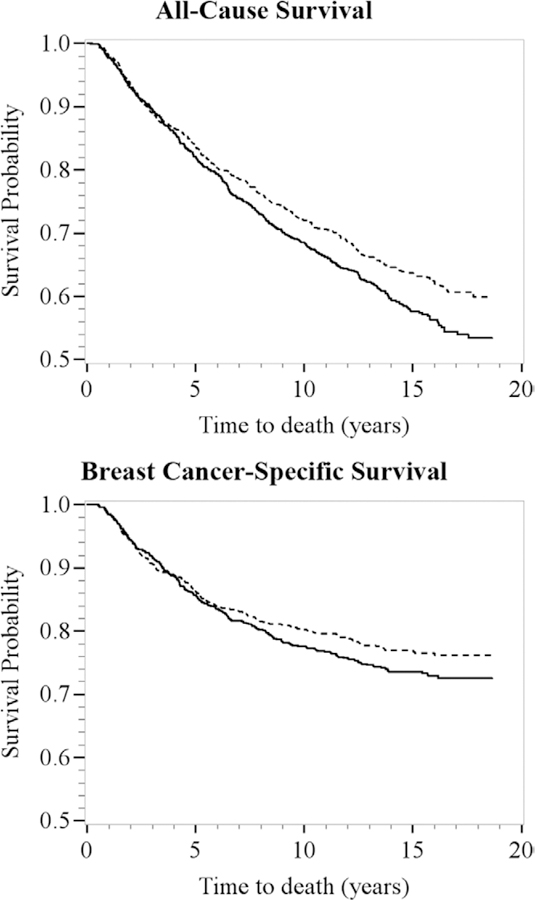
In the Kaplan-Meier survival curves, all-cause survival rates were similar between the healthy behavior and diet and healthy behavior and unhealthy diet/unhealthy behavior and diet groups during the first 5 years of follow-up. However, 5 years after diagnosis, the all-cause survival curves diverged with rates lower among women in the healthy behavior and unhealthy diet/unhealthy behavior and diet patterns compared with women in the healthy behavior and diet pattern (Figure 2).
FIGURE 2.
Kaplan-Meier survival curves for latent class assignment: healthy behavior and diet, dashed line (n = 916, 51%) versus healthy behavior and unhealthy diet (n = 624, 34%) and unhealthy behavior and diet (n = 268, 15%), solid line, for all-cause (top) and breast cancer–specific (bottom) survival. Carolina Breast Cancer Study participants were diagnosed with invasive breast cancer from 1993 to 1996 (Phase I) and 1996 to 2000 (Phase II) and followed-up for vital status through December 31, 2011 (n = 1,808). The x axes show time to death in years; the y axes show proportion of participants alive.
Current (vs. never) smoking was associated with a HR of 1.2 (95% CI = 0.87, 1.5) for all-cause mortality within 5 years of diagnosis (eTable 3; http://links.lww.com/EDE/B421). Among women who survived at least 5 years after diagnosis, current (vs. never) smoking was associated with a HR of 1.4 (95% CI = 1.1, 1.8), after covariate adjustment (Table 2). The unhealthy (vs. healthy) behavior and diet pattern was associated with a HR of 1.4 (95% CI = 1.1, 1.9) for all-cause mortality, after covariate adjustment. Being in either of the healthy behavior and unhealthy diet or unhealthy behavior and diet patterns was associated with a HR of 1.2 (95% CI = 0.97, 1.5) for 13-year conditional all-cause mortality compared with the healthy behavior and diet pattern.
TABLE 2.
HRs and 95% CIs for Behavior/Dietary Exposures and 13-year Conditional All-cause Mortality—CBCS Phases I and II (n = 1,499)
| 13-year Conditional All-cause Mortality |
|||||
|---|---|---|---|---|---|
| Deaths (n = 408) | Censored (n = 1,091) | Model 1a HR (95% CI) | Model 2b HR (95% CI) | Model 3c HR (95% CI) | |
| Smoking status | |||||
| Never/former | 296 | 882 | 1.00 | 1.00 | 1.00 |
| Current | 112 | 209 | 1.5 (1.2, 1.9) | 1.4 (1.1, 1.8) | 1.4 (1.1, 1.8) |
| Vegetable intake | |||||
| ≥14 cups/week | 193 | 573 | 1.00 | 1.00 | 1.00 |
| <14 cups/week | 212 | 515 | 1.2 (1.0, 1.5) | 1.2 (0.96, 1.4) | 1.1 (0.91, 1.4) |
| Fruit intake | |||||
| ≥10.5 cups/week | 182 | 510 | 1.00 | 1.00 | 1.00 |
| <10.5 cups/week | 221 | 579 | 1.1 (0.91, 1.3) | 1.1 (0.87, 1.3) | 1.1 (0.89, 1.3) |
| Alcohol consumption | |||||
| None | 205 | 431 | 1.00 | 1.00 | 1.00 |
| 1–6 drinks/week | 142 | 507 | 0.78 (0.61, 1.0) | 0.79 (0.63, 0.99) | 0.79 (0.62, 1.0) |
| ≥7 drinks/week | 55 | 151 | 0.70 (0.48, 1.0) | 0.94 (0.69, 1.3) | 0.90 (0.65, 1.2) |
| Physical activity | |||||
| 5–7 times/week | 96 | 250 | 1.00 | 1.00 | 1.00 |
| 1–4 times/week | 90 | 331 | 0.75 (0.57, 1.0) | 0.77 (0.58, 1.0) | 0.82 (0.60, 1.1) |
| <1 time/week | 219 | 505 | 1.1 (0.88, 1.4) | 1.1 (0.83, 1.4) | 1.0 (0.80, 1.3) |
| Latent classes | |||||
| Healthy behavior and diet | 190 | 577 | 1.00 | 1.00 | 1.00 |
| Healthy behavior and unhealthy diet | 142 | 369 | 1.2 (0.98, 1.5) | 1.1 (0.9, 1.4) | 1.1 (0.88, 1.4) |
| Unhealthy behavior and diet | 76 | 145 | 1.5 (1.2, 2.0) | 1.4 (1.1, 1.9) | 1.4 (1.1, 1.9) |
| Healthy behavior and unhealthy diet and unhealthy behavior and diet | 218 | 514 | 1.3 (1.1, 1.6) | 1.2 (0.99, 1.5) | 1.2 (0.97, 1.5) |
CBCS participants were diagnosed with invasive breast cancer from 1993 to 1996 (Phase I) and 1996 to 2000 (Phase II) and followed-up for vital status through 31 December 2011. Conditional analyses are among women who survived >5 years.
Adjusted for age at diagnosis (≥50 vs. <50 years), race (black vs. white), study phase (Phase I vs. Phase II).
Adjusted for variables in model 1 and education (<high school, high school/GED, and ≥college), and marital status (unmarried vs. married).
Adjusted for variables in model 2 and tumor size (>2.0 vs. ≤2.0 cm), and estrogen receptor status (ER+ vs. ER−).
We observed little evidence of effect modification by race (white, HR = 1.2, 95% CI = 0.88, 1.6; black, HR = 1.2, 95% CI = 0.90, 1.6; eTable 4; http://links.lww.com/EDE/B421). However, the HR was elevated 38% (HR = 1.4, 95% CI = 1.1, 1.8) for the healthy behavior and unhealthy diet/unhealthy behavior and diet (vs. healthy behavior and diet) pattern among women ≥50 years old at diagnosis, but attenuated (HR = 1.1, 95% CI = 0.76, 1.5) among women <50 years old at diagnosis.
Breast Cancer Mortality
Similar to the patterns observed for all-cause survival, the breast cancer–specific survival curves diverged at 5 years postdiagnosis, with rates slightly lower among women in the healthy behavior and unhealthy diet/unhealthy behavior and diet patterns compared with women in the healthy behavior and diet pattern (Figure 2).
Among women who survived at least 5 years postdiagnosis, current (vs. never) smoking was associated with cause-specific and subdistribution HRs of 1.4 (95% CI = 1.0, 2.0) and 1.4 (95% CI = 1.0, 2.0), respectively, for breast cancer–specific mortality (eTable 5; http://links.lww.com/EDE/B421). The unhealthy (vs. healthy) behavior and diet pattern was associated with a csHR of 1.2 (95% CI = 0.79, 1.9) and with a sHR of 1.2 (95% CI = 0.77, 1.8), after covariate adjustment (eTable 5; http://links.lww.com/EDE/B421).
We observed little evidence of effect modification by race (eTable 6; http://links.lww.com/EDE/B421). However, the csHRs and sHRs were elevated for the healthy behavior and unhealthy diet/unhealthy behavior and diet (vs. healthy behavior and diet) pattern among women ≥50 years old at diagnosis and among women with ER− tumors, but attenuated among women <50 years old at diagnosis or with ER+ tumors.
DISCUSSION
In this population-based study of women diagnosed with breast cancer, we detected three distinct lifestyle patterns, which reflected women with healthy behaviors and diets, healthy behaviors and unhealthy diets, and unhealthy behaviors and diets. Compared with the healthy behavior and diet lifestyle pattern, the unhealthy behavior and diet pattern was associated with worse long-term all-cause and breast cancer–specific survival.
Individually, prediagnosis physical activity and smoking are strong breast cancer prognostic factors.23,24 Engaging in at least 2.5 hours per week of moderate-intensity physical activity is associated with a 25% reduction in risk of breast cancer–specific mortality.24 Our results for physical activity and breast cancer–specific mortality unadjusted for disease characteristics are consistent with these reports; however, in our study, physical activity was not associated with either short- or long-term breast cancer survival after covariate adjustment, inconsistent with most previous studies assessing pre- and post-diagnosis physical activity.25–27 Our conflicting results may be due to our crude assessment of physical activity, which assessed frequency but not duration and intensity. Current cigarette smoking at the time of breast cancer diagnosis, on the other hand, is associated with a 33% increase in risk of breast cancer mortality compared with never smoking.23 Our results here and in a previous study28 are in agreement with this estimate. However, while the individual smoking measure was associated with a 44% increase in the hazard rate of breast cancer–specific mortality, we observed a moderate increase in the hazard rate of breast cancer–specific mortality among women in the lifestyle pattern comprised entirely of current smokers, suggesting that other behavioral or dietary patterns may mitigate the negative effects of smoking on breast cancer–specific survival. While several studies have found a positive association between the highest levels of intake of alcohol and breast cancer–specific mortality29–34 and an inverse association between moderate intake of alcohol and all-cause mortality,35–38 results of most studies have been null as summarized in a meta-analysis10 of 25 studies of alcohol use and breast cancer mortality. We observed inverse associations between alcohol consumption and breast cancer–specific mortality among women with low/moderate and high (vs. no) alcohol consumption, which may be due to reverse causation; women with poor breast cancer prognosis or comorbid conditions may avoid alcohol consumption.
Proposed mechanisms of each of the health behaviors assessed here are varied based on previous literature. Physical activity is hypothesized to provide a protective benefit on breast cancer prognosis by exerting positive effects on sex and metabolic hormones, including estrogen, sex-hormone–binding globulin, insulin, and insulin-like growth factors.39 Physical activity is also thought to result in positive anti-inflammatory effects mediated by a reduction in visceral fat mass, as well as an induction of an anti-inflammatory environment.39 Several biologic mechanisms have been proposed linking smoking and poor survival among women diagnosed with breast cancer. Nicotine, the main addictive constituent of cigarettes, has been shown in laboratory studies to suppress the immune system through loss of antibody responses and T-cell proliferation.40 Nicotine may also induce tumor growth and metastasis by promoting angiogenesis and epithelial-mesenchymal transition and by inhibiting apoptosis.41 Additionally, smoking is known to cause other chronic conditions including stroke, coronary heart disease, diabetes, and other cancers, which may impact overall survival.42 While dietary exposures have been less consistently associated with breast cancer survival,7,10 dietary components have demonstrated the ability to inhibit breast tumor cell proliferation in laboratory studies. Carotenoids, for example, have been shown to inhibit mammary cell proliferation43 and interfere with mammary cancer cell cycle progression.44 Alcohol is a well-known breast carcinogen45,46; it is hypothesized that alcohol consumption may also impact survival through similar mechanisms, including immunosuppression, induction of angiogenesis, DNA repair, oxidative stress, and altered methylation.47 How these exposures interact to impact breast cancer survival remains to be understood; however, oxidative stress leading to systemic inflammation, which has been associated with worse overall48 and cancer-specific49 mortality, including mortality from breast cancer,50 is one potential common biologic mechanism through which these exposures may synergistically impact prognosis.
Few studies have simultaneously examined multiple behavior and dietary exposures in association with breast cancer–specific mortality, though several51–53 have reported improved overall mortality among breast cancer survivors who adhere to cancer prevention guidelines. In the Women’s Health Initiative Observational Study (WHI-OS), a composite score of adherence to American Cancer Society Nutrition and Physical Activity Cancer Prevention Guidelines was associated with improved overall and breast cancer–specific survival.53 However, in the WHI-OS, follow-up time began at the time of enrollment into the study and not at the time of cancer diagnosis. When follow-up begins at cohort enrollment and when the exposure affects disease incidence, greater disease-specific mortality is expected even if there is no influence on survival.54 Our results may therefore not be comparable. A recent study among 7,195 participants of the Nurses’ Health Study who developed breast cancer examined a healthy life-style pattern consisting of meeting all of the criteria for smoking (<5 pack-years), alcohol consumption (≤1 drink/day), BMI (18.5≤ BMI ≤27.5 kg/m2), and aerobic physical activity (≥75 vigorous-intensity or 150 moderate-intensity minutes).11 In their study, the population-attributable risk, or the proportion of deaths that would be prevented if all individuals adopted the lifestyle of the low-risk population, was 12% for breast cancer mortality. While we observed modest increases in rate and risk of breast cancer–specific mortality, we did not consider obesity as lifestyle factor, but rather assumed that BMI is a result of these lifestyle factors and, therefore, did not include or adjust for BMI. Obesity is a well-established breast cancer prognostic factor; prediagnosis obesity is associated with up to a 35% increase in risk of breast cancer–specific mortality compared with ideal-weight women.55,56
Our study has several strengths, including the large population-based design and long-term follow-up. We also pursued a data-driven approach to consider health behaviors, and interestingly found that the latent class analysis approach produced groupings of individuals largely similar to expectation based on number of unhealthy behaviors. Several limitations should be noted. First, our study relied on a single assessment of the exposures shortly after diagnosis, and we did not consider the impact of postdiagnosis changes in behaviors on breast cancer prognosis. Furthermore, these single measures were asked with reference to varying time points, either before (i.e., alcohol consumption) or at the time of diagnosis (i.e., smoking), or immediately before the questionnaire (i.e., fruit and vegetable intake and physical activity). Observational studies suggest that women may alter their diets, physical activity, and smoking behaviors after breast cancer diagnosis, which may impact prognosis.57–61 However, the women in our study completed the questionnaire shortly after diagnosis which limits the impact of long-term postdiagnosis changes in behavioral exposures on breast cancer mortality. Second, we relied on crude assessments of self-reported diet and physical activity; fruit and vegetable intake were each based on two items rather than on a comprehensive dietary questionnaire and physical activity was assessed using one item with ordinal responses. Our exposure assessment may have resulted in exposure misclassification biasing our results toward the null. However, these assessments may be sufficient to rank and categorize respondents into various lifestyle patterns. Furthermore, the averages for fruit and vegetable intake reported in the CBCS are similar to other studies of North Carolina women that did use a more detailed food frequency questionnaire.14,62 Although our results may not directly inform postdiagnosis intervention strategies, they may inform our understanding of the associations with lifestyle patterns around the time of diagnosis in relation to survival. Third, our results may be confounded by other unmeasured lifestyle and dietary factors; however, we focused on the five major behavioral and dietary breast cancer risk and prognostic factors.6 We were also unable to control for all tumor characteristics; however, we observed little correlation between the lifestyle patterns and most of the disease characteristics available. Fourth, we were unable to include treatment factors or to account for screening and other health behaviors that likely affect mortality. Fifth, as with any observational case–control study, there is a potential for selection bias; however, the CBCS was a population-based study and previous analyses comparing participants and nonparticipants in the CBCS suggest that selection bias is unlikely.63 Last, we did not assess overall survivorship according to the same characteristics among controls in the CBCS, so it is unclear whether the differences observed here reflect those in the general population or are specific to cancer survivors. Future study should assess how overall survival varies in both breast cancer patients and the source population from which they were derived.
In conclusion, in the present study, we identified three distinct lifestyle patterns based on five behavior and dietary exposures. Compared with women with a lifestyle pattern that reflected engaging in healthy behaviors and having healthy diets, women with unhealthy behaviors and unhealthy diets had worse overall and breast cancer–specific survival. Given the large number of breast cancer survivors in the United States, it is important to understand how health behaviors affect survivorship and whether this differs from unaffected women. It is possible that women who are diagnosed with breast cancer would experience higher overall mortality due to side effects of treatment, but it is also possible that breast cancer survivors have more regular interaction with health care providers and heightened awareness of health behavior.64 Future studies should continue to examine how breast cancer diagnosis affects lifestyle and how these behaviors in turn impact subsequent survival.
Supplementary Material
Acknowledgments
This research was funded in part by the University Cancer Research Fund of North Carolina and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223) and by a grant from the National Institute of Environmental Health Sciences (T32 ES007018).
Footnotes
The authors report no conflicts of interest.
The data and computer code for replicating these results would require a collaboration with the senior author, a formal data use agreement, and institutional review board approval from the participating institutions.
Compliance with Ethical Standards.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.AACR. U.S. Breast Cancer Cases Expected to Increase by as Much as 50 Percent by 2030. Available at: http://mb.cision.com/Public/3069/9755232/81b414b4ec298479.pdf. Published 2015. Accessed May 6, 2015.
- 3.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control. 2003;10:325–333. [DOI] [PubMed] [Google Scholar]
- 4.Kushi LH, Kwan ML, Lee MM, Ambrosone CB. Lifestyle factors and survival in women with breast cancer. J Nutr. 2007;137(1 suppl): 236S–242S. [DOI] [PubMed] [Google Scholar]
- 5.Ellsworth RE, Valente AL, Shriver CD, Bittman B, Ellsworth DL. Impact of lifestyle factors on prognosis among breast cancer survivors in the USA. Expert Rev Pharmacoecon Outcomes Res. 2012;12:451–464. [DOI] [PubMed] [Google Scholar]
- 6.Vijayvergia N, Denlinger CS. Lifestyle factors in cancer survivorship: where we are and where we are headed. J Pers Med. 2015;5:243–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink BN, Gaudet MM, Britton JA, et al. Fruits, vegetables, and micronutrient intake in relation to breast cancer survival. Breast Cancer Res Treat. 2006;98:199–208. [DOI] [PubMed] [Google Scholar]
- 9.Key J, Hodgson S, Omar RZ, et al. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control. 2006;17:759–770. [DOI] [PubMed] [Google Scholar]
- 10.Gou YJ, Xie DX, Yang KH, et al. Alcohol consumption and breast cancer survival: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:4785–4790. [DOI] [PubMed] [Google Scholar]
- 11.Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol. 2016;2:1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35:51–60. [DOI] [PubMed] [Google Scholar]
- 13.Millikan RC, Pittman GS, Newman B, et al. Cigarette smoking, N-acetyltransferases 1 and 2, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:371–378. [PubMed] [Google Scholar]
- 14.Moorman PG, Ricciuti MF, Millikan RC, Newman B. Vitamin supplement use and breast cancer in a North Carolina population. Public Health Nutr. 2001;4:821–827. [DOI] [PubMed] [Google Scholar]
- 15.USDA. All about the vegetable group. Available at: https://www.choosemyplate.gov/vegetables. Published 2016. Accessed February 1, 2017.
- 16.USDA. All about the fruit group. Available at: https://www.choosemyplate.gov/fruit. Published 2016. Accessed February 1, 2017.
- 17.Williams LA, Olshan AF, Tse CK, Bell ME, Troester MA. Alcohol intake and invasive breast cancer risk by molecular subtype and race in the Carolina Breast Cancer Study. Cancer Causes Control. 2016;27:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66:43–73. [DOI] [PubMed] [Google Scholar]
- 19.Marcus PM, Newman B, Moorman PG, et al. Physical activity at age 12 and adult breast cancer risk (United States). Cancer Causes Control. 1999;10:293–302. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National Death Index. Available at: http://www.cdc.gov/nchs/ndi.htm. Published 2017. Accessed August 11, 2017.
- 21.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14: 671–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bérubé S, Lemieux J, Moore L, Maunsell E, Brisson J. Smoking at time of diagnosis and breast cancer-specific survival: new findings and systematic review with meta-analysis. Breast Cancer Res. 2014;16:R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beasley JM, Kwan ML, Chen WY, et al. Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat. 2012;131:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. [DOI] [PubMed] [Google Scholar]
- 26.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. [DOI] [PubMed] [Google Scholar]
- 27.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54:635–654. [DOI] [PubMed] [Google Scholar]
- 28.Parada H Jr, Sun X, Tse CK, Olshan AF, Troester MA, Conway K. Active smoking and survival following breast cancer among African American and non-African American women in the Carolina Breast Cancer Study. Cancer Causes Control. 2017;28:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebert JR, Hurley TG, Ma Y. The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat. 1998;51:17–28. [DOI] [PubMed] [Google Scholar]
- 30.Kwan ML, Kushi LH, Weltzien E, et al. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol. 2010;28:4410–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald PA, Williams R, Dawkins F, Adams-Campbell LL. Breast cancer survival in African American women: is alcohol consumption a prognostic indicator? Cancer Causes Control. 2002;13:543–549. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs CS, Stampfer MJ, Colditz GA, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. [DOI] [PubMed] [Google Scholar]
- 33.Allemani C, Berrino F, Krogh V, et al. Do pre-diagnostic drinking habits influence breast cancer survival? Tumori. 2011;97:142–148. [DOI] [PubMed] [Google Scholar]
- 34.Vrieling A, Buck K, Heinz J, et al. Pre-diagnostic alcohol consumption and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res Treat. 2012;136:195–207. [DOI] [PubMed] [Google Scholar]
- 35.Reding KW, Daling JR, Doody DR, O’Brien CA, Porter PL, Malone KE. Effect of prediagnostic alcohol consumption on survival after breast cancer in young women. Cancer Epidemiol Biomarkers Prev. 2008;17: 1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxe GA, Rock CL, Wicha MS, Schottenfeld D. Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat. 1999;53:241–253. [DOI] [PubMed] [Google Scholar]
- 37.Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–3316. [DOI] [PubMed] [Google Scholar]
- 38.Flatt SW, Thomson CA, Gold EB, et al. Low to moderate alcohol intake is not associated with increased mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Boer MC, Wörner EA, Verlaan D, van Leeuwen PAM. The mechanisms and effects of physical activity on breast cancer. Clin Breast Cancer. 2017;17:272–278. [DOI] [PubMed] [Google Scholar]
- 40.Sopori M Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Department of Health and Human Services. The Health Consequences of smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA; 2014. [Google Scholar]
- 43.Levy J, Bosin E, Feldman B, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995;24:257–266. [DOI] [PubMed] [Google Scholar]
- 44.Karas M, Amir H, Fishman D, et al. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer. 2000;36:101–111. [DOI] [PubMed] [Google Scholar]
- 45.Hamajima N, Hirose K, Tajima K, et al. ; Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer– collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longnecker MP. Alcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and review. Cancer Causes Control. 1994;5:73–82. [DOI] [PubMed] [Google Scholar]
- 47.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. [DOI] [PubMed] [Google Scholar]
- 48.Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. Systemic inflammation predicts all-cause mortality: a Glasgow inflammation outcome study. PLoS One. 2015;10:e0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 50.Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer. 2006;94:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cloud AJ, Thai A, Liao Y, Terry MB. The impact of cancer prevention guideline adherence on overall mortality in a high-risk cohort of women from the New York site of the Breast Cancer Family Registry. Breast Cancer Res Treat. 2015;149:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomson CA, McCullough ML, Wertheim BC, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women’s health initiative. Cancer Prev Res (Phila). 2014;7:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cespedes Feliciano EM, Prentice RL, Aragaki AK, et al. Methodological considerations for disentangling a risk factor’s influence on disease incidence versus postdiagnosis survival: the example of obesity and breast and colorectal cancer mortality in the Women’s Health Initiative. Int J Cancer. 2017;141:2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. [DOI] [PubMed] [Google Scholar]
- 57.Salminen EK, Lagström HK, Heikkilä S, Salminen S. Does breast cancer change patients’ dietary habits? Eur J Clin Nutr. 2000;54:844–848. [DOI] [PubMed] [Google Scholar]
- 58.Thomson CA, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J Am Diet Assoc. 2002;102:801–808. [DOI] [PubMed] [Google Scholar]
- 59.Borch KB, Braaten T, Lund E, Weiderpass E. Physical activity before and after breast cancer diagnosis and survival - the Norwegian women and cancer cohort study. BMC Cancer. 2015;15:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parada H Jr, Bradshaw PT, Steck SE, et al. Postdiagnosis changes in cigarette smoking and survival following breast cancer. JNCI Cancer Spectr. 2017;1. doi: 10.1093/jncics/pkx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passarelli MN, Newcomb PA, Hampton JM, et al. Cigarette smoking before and after breast cancer diagnosis: mortality from breast cancer and smoking-related diseases. J Clin Oncol. 2016;34:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McClelland JW, Demark-Wahnefried W, Mustian RD, Cowan AT, Campbell MK. Fruit and vegetable consumption of rural African Americans: baseline survey results of the Black Churches United for Better Health 5 A Day Project. Nutr Cancer. 1998;30:148–157. [DOI] [PubMed] [Google Scholar]
- 63.Moorman PG, Newman B, Millikan RC, Tse CK, Sandler DP. Participation rates in a case-control study: the impact of age, race, and race of interviewer. Ann Epidemiol. 1999;9:188–195. [DOI] [PubMed] [Google Scholar]
- 64.Nord C, Mykletun A, Thorsen L, Bjøro T, Fosså SD. Self-reported health and use of health care services in long-term cancer survivors. Int J Cancer. 2005;114:307–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.