Abstract
Objectives
Whether and how delirium and sleep quality in the intensive care unit (ICU) are linked remains unclear. A recent randomised trial reported nocturnal low-dose dexmedetomidine (DEX) significantly reduces incident ICU delirium. Leeds Sleep Evaluation Questionnaire (LSEQ) scores were similar between intervention (DEX; n=50) and control (placebo (PLA); n=50) groups. We measured the association between morning LSEQ and delirium occurrence in the prior 24 hours (retrospective analysis) and the association between morning LSEQ and delirium occurrence in the following 24 hours (predictive analysis).
Design
Post hoc analysis of randomised controlled trial data.
Participants
Adult ICU patients (n=100) underwent delirium screening twice a day using the Intensive Care Delirium Screening Checklist (ICDSC) if Richmond Agitation Sedation Scale (RASS) was ≥−3 and patient-reported sleep quality evaluations at 09:00 daily with the LSEQ if RASS was ≥−1.
Outcomes
The analysis included all 24-hour study periods with LSEQ documentation and matched delirium screening in coma-free patients. Separate logistic regression models controlling for age, baseline Acute Physiology and Chronic Health Evaluation II score and DEX/PLA allocation evaluated the association between morning LSEQ and delirium occurrence for both retrospective and predictive analyses.
Results
The 100 patients spent 1115 24-hour periods in the ICU. Coma, delirium and no delirium occurred in 130 (11.7%), 114 (10.2%) and 871 (78.1%), respectively. In the retrospective analysis, when an LSEQ result was preceded by an ICDSC result (439/985 (44.6%) 24-hour periods), delirium occurred during 41/439 (9.3%) periods. On regression analysis, the LSEQ score had no relationship to prior delirium occurrence (OR (per every 1 point average LSEQ change) 0.97, 95% CI 0.72 to 1.31). For the predictive analysis, among the 387/985 (39.1%) 24-hour periods where an LSEQ result was followed by an ICDSC result, delirium occurred during 56/387 (14.5%) periods. On regression analysis, the LSEQ score did not predict subsequent delirium occurrence (OR (per 1 point LSEQ change) 1.02, 95% CI 0.99 to 1.05).
Conclusions
The sleep quality ICU patients perceive neither affects nor predicts delirium occurrence.
Trial registration number
Keywords: clinical epidemiology
Key messages.
The link between delirium and sleep quality in the intensive care unit (ICU) remains unclear.
ICU patient sleep quality perception appears to be neither affected by nor predictive of delirium occurrence.
Future studies evaluating the association between sleep and delirium should consider environmental disruption and pharmacological intervention confounders for delirium and sleep, objective sleep measurement quality limitations, what restorative sleep means to ICU patients, and explore correlations between sleep quality and meaningful patient-centred outcomes.
Introduction
Delirium occurs in up to 50% of critically ill adults, is associated with substantial burden to patients and their families and may lead to serious intensive care unit (ICU) and post-ICU complications.1 2 Sleep disruption and abnormal sleep architecture are common in the ICU.3 While several non-pharmacological sleep promotion efforts have been shown to reduce delirium, they have generally not been found to improve sleep, regardless of whether it is evaluated by objective or subjective means.4 Although clinicians assume poor sleep provokes delirium and delirium provokes poor sleep, whether delirium and sleep quality are associated in a complex ICU environment remains unclear.4 5
One 2018 systematic review of 12 studies evaluating patients admitted to the ICU after major surgery found a strong association between sleep disturbances and postoperative delirium occurrence.6 In contrast, a cohort analysis of medical ICU patients undergoing sleep protocol implementation found no association between daily perceived sleep quality ratings and transition to delirium.7 A recent trial comparing low-dose nocturnal dexmedetomidine to placebo in non-delirious ICU patients suggests dexmedetomidine significantly reduces delirium occurrence (relative risk 0.44; 95% CI 0.23 to 0.82).8 In this study, average Leeds Sleep Evaluation Questionnaire (LSEQ) scores were similar in both groups (mean difference, 0.02; 95% CI 0.42 to 1.92).8 9 It remains unclear from this study whether dexmedetomidine reduced delirium by improving sleep or reducing the exposure to gabaminergic sedatives like midazolam known to increase delirium and worsen sleep.4 10
The relationship between subjective sleep quality and delirium remains poorly explored; multiple investigative challenges exist in critically ill adults.3–5 Any study addressing whether a link between subjective sleep quality and delirium exists should pair delirium and sleep assessments in individual patients, and consider them over short time periods given the natural fluctuation of both of these outcomes over the course of the ICU stay.
While controlling for the most common risk factors for delirium occurrence, we evaluated the association between the morning LSEQ assessment and delirium occurrence in the prior 24-hour period (ie, the retrospective analysis) and the association between the morning LSEQ assessment and delirium occurrence in the following 24-hour period (ie, the predictive analysis).
Methods
Study design and population
This retrospective, post hoc evaluation of a prospective randomised trial considered patients, interventions and outcome descriptors from the parent trial (ClinicalTrials.gov).8 Each 24-hour ICU nocturnal dexmedetomidine (or placebo) study day (for the 100 patients enrolled in the parent trial) was considered a distinct evaluation period regardless of study allocation. All 24-hour study periods where a morning LSEQ assessment was not or could not be completed (eg, Richmond Agitation Sedation Scale (RASS) ≤−1),9 11 coma was present (thus precluding delirium assessment) or a delirium assessment was not recorded were excluded from analysis.
Outcomes
Sleep assessments were conducted at approximately 09:00 daily using the LSEQ (figure 1), a 10-domain subjective sleep assessment testing four aspects of sleep: getting to sleep, quality of sleep, awakening from sleep and behaviour following wakefulness (online supplementary appendix).9 Each of the 10 domains is assessed using a 100 mm visual analogue scale. Delirium screening occurred at least once every 12 hours (at 07:00 and 19:00) with the Intensive Care Delirium Screening Checklist (ICDSC) scale and was validated clinically given delirium symptoms fluctuate (figure 1).12 Since fluctuation in symptoms is a cardinal feature of delirium, we did not consider delirium symptoms at 07:00 as a contraindication for the LSEQ assessment (2 hours later) at 09:00. Instead, nurses were instructed to truncate the LSEQ assessment if the patient was too inattentive to conduct it, even if the RASS was ≥−1.12 A patient was deemed to have delirium for either the retrospective or predictive analyses if at least one ICDSC score ≥4 was documented in the 24-hour study period and validated with a clinical assessment.
Figure 1.
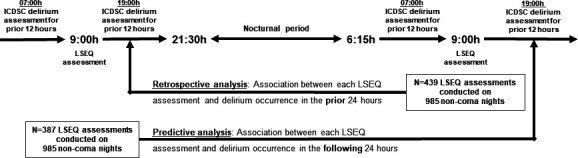
Description of the retrospective and predictive study cohort analyses in the context of the parent randomised controlled trial. ICDSC, Intensive Care Delirium Screening Checklist; LSEQ Leeds Sleep Evaluation Questionnaire.
bmjresp-2020-000576supp001.pdf (167.5KB, pdf)
Data analysis
A multivariable logistic regression model controlling for the average LSEQ score and the three risk factors most associated with delirium occurrence in the context of the study cohort (ie, age, baseline illness severity (Acute Physiology and Chronic Health Evaluation II score) and dexmedetomidine exposure) was constructed for both the retrospective and predictive analyses.4 13 The average LSEQ score was used for both analyses as these average scores were similar for all 24-hour periods, regardless of delirium occurrence. Secondary exploratory multivariable models were constructed for both the retrospective and predictive models for individual LSEQ score domain(s) if any domain differed between 24-hour periods, regardless of delirium, at a ≤0.1 threshold p value. The reported OR for the retrospective analysis represents the odds of having delirium in the prior 24 hours for every 1 point change in the average LSEQ score recorded at the end of the 24-hour period. The reported OR for the predictive analysis represents the odds of developing delirium in the following 24 hours for every 1 point change in the average LSEQ score recorded at the start of the 24-hour period. Statistical analyses were completed using SAS software V.9.4 for MS Windows (SAS).
Results
Coma precluded delirium reassessments in 130 (11.7%) of the overall 1115 24-hour study periods (figure 2). Delirium occurred in 114/985 (11.7%) of the remaining coma-free 24-hour periods. For the retrospective analysis, among the 439/985 (44.8%) 24-hour periods with a documented LSEQ assessment, delirium occurred in 41/439 (9.3%) periods. For the predictive analysis, among the 387/985 (39.3%) 24-hour periods with a documented LSEQ assessment, delirium occurred subsequently in 56/387 (15.5%) periods. Overall, 207 pre-LSEQ and post-LSEQ 24-hour periods were included in both of the separate retrospective and predictive analyses.
Figure 2.
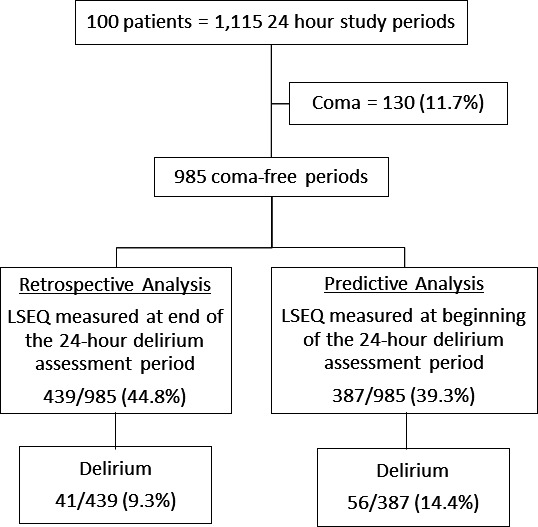
Allocation of the n=1115 24-hour intensive care unit (ICU) study periods into the retrospective and predictive cohort analyses. LSEQ Leeds Sleep Evaluation Questionnaire.
In the retrospective analysis cohort, after controlling for delirium risk factors, the average LSEQ score was not associated with delirium occurrence in the 24 hours prior to the LSEQ assessment (OR per 1 point average LSEQ change 1.01; 95% CI 0.71 to 1.30; p=0.79) (table 1). While LSEQ domain 7 (‘longer than usual time to awaken’) differed between the 24-hour periods with (vs without) delirium (OR 1.16; 95% CI 0.99 to 1.35; p=0.06), this difference was no longer significantly associated with delirium occurrence in the 24 hours prior to LSEQ assessment after controlling for delirium risk factors.
Table 1.
Association between average total LSEQ score and delirium occurrence in the prior 24 hours after controlling for age, baseline severity of illness and nocturnal dexmedetomidine exposure (retrospective analysis)
| Variable | 24-hour periods where both LSEQ score and delirium assessment documented (n=439) |
P value | Univariable analysis | Multivariable analysis | |||
| 24-hour periods with delirium (n=41) |
24-hour periods without delirium (n=398) |
OR (95% CI) | P value | OR (95% CI) | P value | ||
| LSEQ 10-domain average | 5.5±1.1 | 5.4±1.1 | 0.94 | 1.01 (0.76 to 1.35) | 0.94 | 0.97 (0.71 to 1.30) | 0.79 |
| Age (years) | 67.4±8.1 | 63.2±11.4 | 0.02* | 1.04 (1.01 to 1.07) | 0.02* | 1.03 (0.99 to 1.06) | 0.14 |
| APACHE-II score | 25.8±7.1 | 22.2±8.1 | 0.01* | 1.06 (1.02 to 1.10) | 0.01* | 1.04 (1.00 to 1.09) | 0.05* |
| Dexmedetomidine (vs placebo) | 17 (41.5%) | 213 (53.5%) | 0.19 | 0.62 (0.32 to 1.18) | 0.14 | 0.68 (0.35 to 1.33) | 0.26 |
*P≤0.05.
APACHE, Acute Physiology and Chronic Health Evaluation; LSEQ, Leeds Sleep Evaluation Questionnaire.
For the predictive analysis cohort, after controlling for delirium risk factors, the average LSEQ score was also not associated with delirium occurrence in the 24 hours prior to the LSEQ assessment (OR per 1 point average LSEQ change 1.20; 95% CI 0.92 to 1.58; p=0.18) (table 2). LSEQ domain 5 (‘more wakeful periods than usual’) scores differed between patients with, versus without, delirium, and were associated with higher delirium occurrence (OR 1.15; 95% CI 1.01 to 1.30; p=0.04); however, after controlling for delirium risk factors, domain 5 was no longer predictive of subsequent delirium (OR 1.13; 95% CI 0.99 to 1.29; p=0.08).
Table 2.
Association between average total LSEQ score and delirium occurrence in the following 24 hours after controlling for age, baseline severity of illness and nocturnal dexmedetomidine exposure (predictive analysis)
| Variable | 24-hour periods where both LSEQ score and delirium assessment documented (n=387) |
P value | Univariable analysis | Multivariable analysis | |||
| 24-hour periods with delirium (n=56) | 24-hour periods without delirium (n=331) | OR (95% CI) | P value | OR (95% CI) | P value | ||
| LSEQ 10-domain average | 5.7±1.1 | 5.4±1.1 | 0.11 | 1.24 (0.95 to 1.62) | 0.110 | 1.20 (0.92 to 1.58) | 0.18 |
| Age (years) | 67.4±9.3 | 63.1±11.2 | 0.007* | 1.04 (1.01 to 1.07) | 0.008* | 1.03 (0.99 to 1.06) | 0.06 |
| APACHE-II score | 26.1±7.1 | 23.3±4.2 | 0.001* | 1.04 (1.01 to 1.08) | 0.017* | 1.02 (0.99 to 1.06) | 0.21 |
| Dexmedetomidine (vs placebo) | 18 (32%) | 183 (55%) | 0.001* | 0.38 (0.21 to 0.70) | 0.002* | 0.42 (0.23 to 0.77) | 0.005* |
*P≤0.05.
APACHE, Acute Physiology and Chronic Health Evaluation; LSEQ, Leeds Sleep Evaluation Questionnaire.
Discussion
This secondary analysis of our low-dose nocturnal dexmedetomidine delirium prevention trial is the first evaluation of a randomised controlled trial (RCT) to evaluate the relationship between subjective sleep quality and delirium occurrence in severely ill ICU patients.4 5 Our results suggest self-reported ICU patient sleep quality may be neither affected by, nor predictive of, delirium occurrence in critically ill adults.
Our analysis has important strengths. We controlled for delirium occurrence risk (ie, age, illness severity, dexmedetomidine exposure).4 13 All non-pharmacological delirium-reducing interventions were protocolised as part of the parent RCT, reducing potential confounders related to differential care of patients.8 A large number of 24-hour ICU periods with paired, sequential LSEQ/delirium and delirium/LSEQ assessments were analysed. The use of a 24-hour delirium evaluation window minimised chances that delirium symptoms might be missed, as they fluctuate over time. Only including data from patients with delirium evaluations documented throughout the 24 hours preceding, and 24 hours following the recorded LSEQ assessment limited our 24-hour periods with paired data.
Our report has limitations. We did not record why LSEQ evaluations were undocumented in patients with a RASS ≥−1 but speculate about whether 09:00 assessments are feasible in a busy ICU even within a clinical study. Nocturnal and daytime delirium ‘events’ were merged, preventing an exploration of delirium’s temporal manifestations on perceived sleep quality.14 Although the LSEQ has been validated as a method to evaluate sleep in the ICU, it has not been compared with polysomnography (PSG).9 The association between sleep quality and delirium remains unknown on ICU days where the patient was too sedated (RASS ≤−1) to conduct an LSEQ assessment. Subjective sleep assessment with the LSEQ may be affected by patients’ cognitive state which itself may be influenced by delirium.15 Patients whose baseline conditions may affect sleep quality (eg, baseline insomnia)4 or both sleep quality and delirium risk (eg, obstructive sleep apnoea)16 were not excluded. While patients were predominately managed with a volume control mode of mechanical ventilation at night, modes varied, and pressure support at night may have affected sleep quality.4 Our cohort was too small to consider other risk factors for delirium (eg, immobility) or nocturnal factors known to disrupt sleep (eg, patient interruption, environmental noise).4 13
The interplay between critical illness, delirium, sedatives and sleep is complex. While intensivists infer sleep and delirium are linked,17 evidence to support causality is lacking.4 5 7 18 19 Sleep abnormalities in ICU patients are highly variable.3 4 Our results highlight the challenges in measuring how sleep and delirium are linked, and suggest no association may exist. Future studies should consider environmental disruption and pharmacological intervention confounders for delirium and sleep, objective sleep measurement quality limitations, what restorative sleep means to ICU patients, and explore correlations between sleep quality and meaningful patient-centred outcomes.
Footnotes
Contributors: YS takes full responsibility for the content of the manuscript. YS and JWD were each involved in the conception, delineated the hypothesis and designed the study; acquired and analysed the data; and wrote the paper. MSD was involved in the study design, data analysis and in writing the paper.
Funding: Dr. Duprey's efforts are supported by NIA 1F31AG066460-01. The parent student was suppported by an unrestricte, investigator-initiated grant from Hospira Canada.
Disclaimer: Hospira Canada had no role in the conception, design, or conduct of this study (or the parent study); collection, management, analysis, interpretation, or presentation of data; or preparation, review, or approval of this manuscript (or the parent study).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.Zaal IJ, Slooter AJC. Delirium in critically ill patients: epidemiology, pathophysiology, diagnosis and management. Drugs 2012;72:1457–71. 10.2165/11635520-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 2.Wolters AE, Slooter AJC, van der Kooi AW, et al. Cognitive impairment after intensive care unit admission: a systematic review. Intensive Care Med 2013;39:376–86. 10.1007/s00134-012-2784-9 [DOI] [PubMed] [Google Scholar]
- 3.Pisani MA, Friese RS, Gehlbach BK, et al. Sleep in the intensive care unit. Am J Respir Crit Care Med 2015;191:731–8. 10.1164/rccm.201411-2099CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devlin JW, Skrobik Y, Gélinas C, et al. Executive summary: clinical practice guidelines for the prevention and management of pain, Agitation/Sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:1532–48. 10.1097/CCM.0000000000003259 [DOI] [PubMed] [Google Scholar]
- 5.Pisani MA, D'Ambrosio C. Sleep and delirium in adults who are critically ill: a contemporary review. Chest 2020;157:977–84. 10.1016/j.chest.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Fadayomi AB, Ibala R, Bilotta F, et al. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med 2018;46:e1204–12. 10.1097/CCM.0000000000003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamdar BB, Niessen T, Colantuoni E, et al. Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Crit Care Med 2015;43:135–41. 10.1097/CCM.0000000000000610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skrobik Y, Duprey MS, Hill NS, et al. Low-Dose nocturnal dexmedetomidine prevents ICU delirium. A randomized, placebo-controlled trial. Am J Respir Crit Care Med 2018;197:1147–56. 10.1164/rccm.201710-1995OC [DOI] [PubMed] [Google Scholar]
- 9.Parrott AC, Hindmarch I. The leeds sleep evaluation questionnaire in psychopharmacological investigations - a review. Psychopharmacology 1980;71:173–9. 10.1007/BF00434408 [DOI] [PubMed] [Google Scholar]
- 10.Nuzzo E, Girard TD. The Sandman in the ICU: a novel use of dexmedetomidine? Am J Respir Crit Care Med 2018;197:1098–9. 10.1164/rccm.201802-0359ED [DOI] [PubMed] [Google Scholar]
- 11.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–44. 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 12.Bergeron N, Dubois MJ, Dumont M, et al. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 2001;27:859–64. 10.1007/s001340100909 [DOI] [PubMed] [Google Scholar]
- 13.Zaal IJ, Devlin JW, Peelen LM, et al. A systematic review of risk factors for delirium in the ICU. Crit Care Med 2015;43:40–7. 10.1097/CCM.0000000000000625 [DOI] [PubMed] [Google Scholar]
- 14.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007;33:66–73. 10.1007/s00134-006-0399-8 [DOI] [PubMed] [Google Scholar]
- 15.Wilcox ME, Brummel NE, Archer K, et al. Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Crit Care Med 2013;41:S81–98. 10.1097/CCM.0b013e3182a16946 [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Sigua NL, Manchanda S, et al. Preoperative Stop-Bang scores and postoperative delirium and coma in thoracic surgery patients. Ann Thorac Surg 2018;106:966–72. 10.1016/j.athoracsur.2018.05.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamdar BB, Knauert MP, Jones SF, et al. Perceptions and practices regarding sleep in the intensive care unit. A survey of 1,223 critical care providers. Ann Am Thorac Soc 2016;13:1370–7. 10.1513/AnnalsATS.201601-087OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drouot X, Roche-Campo F, Thille AW, et al. A new classification for sleep analysis in critically ill patients. Sleep Med 2012;13:7–14. 10.1016/j.sleep.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 19.Boesen HC, Andersen JH, Bendtsen AO, et al. Sleep and delirium in unsedated patients in the intensive care unit. Acta Anaesthesiol Scand 2016;60:59–68. 10.1111/aas.12582 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000576supp001.pdf (167.5KB, pdf)


