Abstract
Introduction
Angiosarcomas constitute approximately 2% to 3% of all soft tissue sarcomas, are characterised by an aggressive clinical behaviour and poor outcome. Optimal management of localised angiosarcomas consists of complete surgical resection with or without radiation. However, due to the infiltrating nature of this disease, complete resection is often not possible. Despite optimal management, the outcome of patients with localised disease remains poor. The role of (neo)adjuvant chemotherapy in angiosarcomas remains undefined. The aim of this study is to document the outcome of patients treated with (neo)adjuvant chemotherapy and assess the feasibility of performing a prospective trial by evaluating the number of patients treated at sarcoma referral centres.
Methods
A retrospective search within participating EORTC (European Organisation for Research and Treatment of Cancer) sites for patients treated with (neo)adjuvant chemotherapy was made. Patients treated between January 2007 and January 2016 were included.
Results
A total of 15 institutions participated and 86 patients were evaluable, 43 were treated with neoadjuvant, 27 with adjuvant chemotherapy and 16 with both. At the time of analysis, the median follow-up from diagnosis was 4.6 years. Median overall survival (OS) was 4.9 years (2.9 N) and the percentage alive at 4 years was 57.9 (45.5 to 68.4). The median disease-free survival was 1.4 years (0.9 to 1.7) and the percentage disease-free at 4 years was 26.8% (17.9 to 36.5).
Conclusion
The outcome of angiosarcoma patients treated with (neo)adjuvant chemotherapy in this case series compares favourably with previously published data. Due to the aggressive nature of angiosarcoma, a prospective trial of neoadjuvant chemotherapy should be considered.
Keywords: angiosarcoma, neoadjuvant, adjuvant, sarcoma chemotherapy, angiosarcoma surgery
Key questions.
What is already known about this subject?
Angiosarcomas comprise 2% to 3% of all soft tissue sarcomas, have aggressive clinical behaviour and poor outcome
Optimal management of localised disease consists of complete surgical resection with or without radiation; however, the outcome remains poor
The role of (neo)adjuvant chemotherapy in angiosarcomas remains undefined
What does this study add?
This is the largest retrospective series reporting on the outcomes of (neo)adjuvant therapy in angiosarcomas
Multimodality therapy of localised disease, including (neo)adjuvant chemotherapy, may be beneficial for angiosarcomas
There is variation in the systemic management of angiosarcoma in the neoadjuvant setting across Europe and no particular regimen(s) or dose(s) can be recommended
How might this impact on clinical practice?
Neoadjuvant chemotherapy should be further explored in the context of a prospective randomised controlled clinical trial
Introduction
Soft tissue sarcomas (STS) are rare tumours of mesenchymal origin accounting for about 1% of all adult cancers, and comprising over 50 different histological subtypes.1 Angiosarcomas account for approximately 2% to 3% of STS and are characterised by an aggressive clinical behaviour.2 They are malignant endothelial cell tumours of lymphatic or vascular origin and complex karyotype. They can affect any anatomical site, including the skin and viscera. Although they can arise spontaneously, angiosarcomas are commonly associated with predisposing factors, including chronic lymphoedema, radiation therapy and various occupational risk factors (arsenic, polyvinylchloride and thorium dioxide).3 They may also be linked to predisposing syndromes, including Recklinghausen’s disease, and Klippel-Trenaunay and Maffucci syndromes.
The mainstay of management of localised disease is complete surgical resection with or without (neo)adjuvant radiation.4 Complete surgical resection can be difficult due to the anatomic location of these tumours and the diffuse (frequently multifocal) and infiltrating nature of the disease, particularly cutaneous angiosarcomas. Consequently, there may be a role for neoadjuvant approaches in treating this subtype, both in terms of downstaging primary disease and eradicating micrometastatic disease. Some retrospective studies have suggested that adjuvant radiation has benefit in the management of localised angiosarcomas. Mark and colleagues reported actuarial 2-year and 5-year disease-free survival (DFS) of 44% and 24%, respectively, in a series of 67 patients with localised angiosarcomas treated between 1955 and 1990.5 Pawlik and colleagues reported a median overall survival (OS) of 28.4 months in a series of 29 patients with cutaneous angiosarcomas treated between 1975 and 2002.6 In addition, the clinical behaviour of angiosarcomas can vary by primary site, and this heterogeneity in clinical behaviour can make the interpretation of different studies challenging.7 These data indicate that the outcome of patients with localised disease remains poor, with currently no conclusive data demonstrating a survival benefit for adjuvant chemotherapy in resected angiosarcomas.8
Several systemic therapies have shown activity in locally advanced and metastatic angiosarcoma. Retrospective and prospective studies of various conventional chemotherapy schedules have reported response rates between 18% and 25% and median progression-free survival (PFS) between 4 and 7 months.9–11 Similarly, for various tyrosine kinase inhibitors, median PFS between 3 and 6.6 months has been reported.12–15 There are also a number of prospective trials currently being conducted: NCT02048722 (Daily Oral Regorafenib for Chemotherapy-Refractory, Metastatic and Locally Advanced Angiosarcoma) is active and recruiting, while NCT01462630 (Pazopanib Hydrochloride in Treating Patients With Advanced Angiosarcoma) is active having completed recruitment. Recently, the randomised Phase III trial of pazopanib with or without the antiendoglin antibody demonstrated no difference in PFS and OS between the two arms.16
Responses in angiosarcoma are often of short duration and the 5-year OS remains poor, in the order of approximately 31% to 43%7 17 with some reports documenting 5-year disease-specific survival of 60% and a median OS in the advanced setting of 7 months.18 The results of a recent randomised trial of neoadjuvant chemotherapy have renewed interest in the use of this approach in specific STS subtypes.19 With a number of systemic agents showing a degree of activity in advanced angiosarcomas, there is a rationale for using systemic therapy in localised angiosarcoma, as the outcome of patients is poor. Therefore, the aim of this retrospective study was to document the outcome of patients with localised angiosarcoma treated with (neo)adjuvant chemotherapy at EORTC (European Organisation for Research and Treatment of Cancer) centres. A secondary aim was to record the number of patients with localised angiosarcoma treated to gauge the feasibility of a prospective randomised trial of neoadjuvant/adjuvant chemotherapy in this specific subtype.
Materials and methods
The EORTC Soft Tissue and Bone Sarcoma Group members were sent a questionnaire regarding interest in participating in the study. Institutional approval at each participating centre was obtained prior to data collection. Patients with histologically documented and non-metastatic angiosarcoma starting (neo)adjuvant treatment between January 2007 and January 2016 were included. All patients were 18 years or older. DFS and OS were investigated from both the date of diagnosis and the date of surgery (only for patients that underwent surgery).
The information retrieved included patient and tumour characteristics, treatment information and survival data. Patients with limited or no information in the required fields were excluded. Patients whose (neo)adjuvant treatment started before 1 January 2007 or after 1 January 2016 were also excluded, as were patients who did not receive (neo)adjuvant treatment.
Statistical analysis
Descriptive statistics were used to present patient and tumour characteristics as well as treatment-related information. Median and range were used for continuous variables and proportions (%) for categorical variables.
DFS from diagnosis was defined as the time, from the date of first diagnosis to the first documentation of relapse (local recurrence or metastasis) or death, whichever occurred first. Patients alive and relapse-free at the time of the clinical cut-off were censored at the date of the last follow-up. OS from diagnosis was defined as the time from the date of the first diagnosis to the date of death. Patients alive at the time of the clinical cut-off were censored at the date of last follow-up. Similar definition applies for DFS and OS from the date of surgery.
DFS and OS are displayed using Kaplan-Meier curves. Four-year survival estimates and median survival times are reported for DFS and OS (from both the date of diagnosis and the date of surgery), along with their two-sided 95% CI.
All statistical analysis was performed using SAS V.9.3.
Results
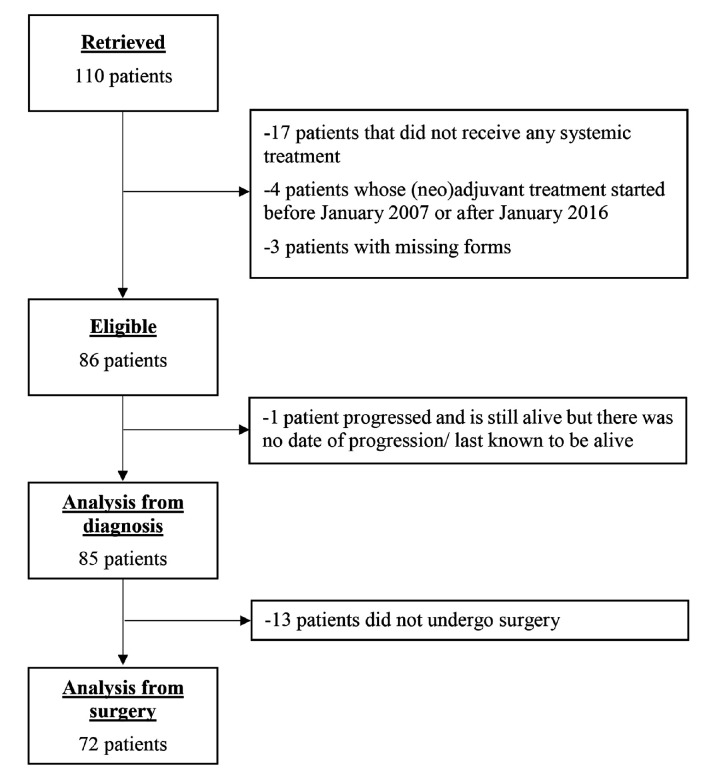
A total of 15 institutions from nine different countries contributed to the patient population (figure 1). Of the 110 patients considered for the study, three were excluded due to limited or no records available, four were excluded because their neoadjuvant treatment started before 1 January 2007 or after 1 January 2016 and 17 due to no systemic treatment.
Figure 1.
Flowchart.
The characteristics for the 86 eligible patients are shown in table 1.
Table 1.
Patient characteristics
| Total (n=86) |
|
| N (%) | |
| Age at diagnosis | |
| ≤40 | 21 (24.4) |
| 40 to ≤50 | 6 (7.0) |
| 50 to ≤70 | 38 (44.2) |
| >70 | 21 (24.4) |
| Age | |
| Median | 60.5 |
| Range | 18.0 to 84.0 |
| N obs | 86 |
| Gender | |
| Male | 38 (44.2) |
| Female | 48 (55.8) |
| ECOG PS at treatment start | |
| ECOG PS 0 | 44 (51.2) |
| ECOG PS 1 | 37 (43.0) |
| ECOG PS 2 | 3 (3.5) |
| Missing | 2 (2.3) |
ECOG, Eastern Cooperative Oncology Group; N obs, number of observations; PS, performance status.
The primary site was the breast in 35% of patients, and 69.4% of these primary breast angiosarcomas were associated with prior radiation. The median tumour size was 45 mm (range 1 to 170) and over 46% of patients had operable disease at presentation. All tumour characteristics are shown in table 2.
Table 2.
Tumour characteristics
| Radiation induced | Total (n=86) |
||
| No (n=62) |
Yes (n=24) |
||
| N (%) | N (%) | N (%) | |
| Site of the primary tumour | |||
| Breast | 8 (12.9) | 20 (83.3) | 28 (32.6) |
| Cutaneous/scalp | 15 (24.2) | 2 (8.3) | 17 (19.8) |
| Extremities | 13 (21.0) | 2 (8.3) | 15 (17.4) |
| Visceral | 13 (21.0) | 0 (0.0) | 13 (15.1) |
| Other | 13 (21.0) | 0 (0.0) | 13 (15.1) |
| Specification of other sites of tumour | |||
| Right atrium | 3 (4.8) | 0 (0.0) | 3 (3.5) |
| Maxilla | 2 (3.2) | 0 (0.0) | 2 (2.3) |
| Head and neck | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Iliacus muscle with pelvis infiltration | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Penis | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Bone (sacrum) | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Retroperitoneum | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Pelvis/perineum | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Bone (basin) | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Abdominal wall, subcutis | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| CNS (cerebellum) | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Tumour grade according to FNCLCC categorisation | 1 (1.6) | 0 (0.0) | 1 (1.2) |
| Grade 1 | 3 (6.5) | 0 (0.0) | 3 (3.5) |
| Grade 2 | 15 (32.6) | 6 (15.0) | 21 (24.4) |
| Grade 3 | 25 (54.3) | 32 (80.0) | 57 (66.3) |
| Missing | 3 (6.5) | 2 (5.0) | 5 (5.8) |
| Dimension | |||
| Median | 45.0 | 42.5 | 45.0 |
| Range | 8.0 to 170.0 | 1.0 to 100.0 | 1.0 to 170.0 |
| N obs | 52 | 18 | 70 |
| Tumour extent at presentation | |||
| Locally advanced | 34 (54.8) | 12 (50.0) | 46 (53.5) |
| Operable | 28 (45.2) | 12 (50.0) | 40 (46.5) |
CNS, central nervous system; FNCLCC, Fédération Nationale des Centers de Lutte Contre le Cancer.
Neoadjuvant chemotherapy followed by surgery was the treatment paradigm followed in one-fourth of the patients (25.6%). In fewer patients, surgery was combined with adjuvant chemotherapy and radiotherapy (16.3%) or adjuvant chemotherapy alone (15.1%) or neoadjuvant and adjuvant chemotherapy (14%). Table 3 summarises the types of administered therapy in operable and locally advanced disease at presentation.
Table 3.
Type of treatment in operable and locally advanced disease
| Tumour extent | Total (n=86) |
||
| Locally advanced (n=46) |
Operable (n=40) |
||
| N (%) | N (%) | N (%) | |
| Type of treatment | |||
| Neoadjuvant chemotherapy alone | 10 (21.7) | 0 (0.0) | 10 (11.6) |
| Neoadjuvant chemotherapy+surgery | 13 (28.3) | 9 (22.5) | 22 (25.6) |
| Neoadjuvant chemotherapy+radiotherapy* | 4 (8.7) | 0 (0.0) | 4 (4.7) |
| Surgery+adjuvant chemotherapy | 2 (4.3) | 11 (27.5) | 13 (15.1) |
| Neoadjuvant chemotherapy+surgery+adjuvant chemotherapy | 6 (13.0) | 6 (15.0) | 12 (14.0) |
| Neoadjuvant chemotherapy+radiotherapy+surgery* | 3 (6.5) | 1 (2.5) | 4 (4.7) |
| Neoadjuvant chemotherapy+surgery+radiotherapy | 3 (6.5) | 0 (0.0) | 3 (3.5) |
| Surgery+radiotherapy+adjuvant chemotherapy* | 4 (8.7) | 10 (25.0) | 14 (16.3) |
| Neoadjuvant chemotherapy+radiotherapy+surgery+adjuvant chemotherapy* | 0 (0.0) | 3 (7.5) | 3 (3.5) |
| Neoadjuvant chemotherapy+surgery+radiotherapy+adjuvant chemotherapy* | 1 (2.2) | 0 (0.0) | 1 (1.2) |
*Information on whether radiotherapy was administered concomitantly with (neo)adjuvant chemotherapy was not consistently collected.
Surgery of the primary tumour was performed in 72 out of 86 patients (83.7%): in 53 patients (73%) with clear margins (R0), in 17 (23.6%) with R1 and in 1.4% with R2 resection. Radiotherapy was administered in 29 patients (33.7%); preoperatively in 11 patients (37.9%) and postoperatively in 18 patients (62.1%).
Neoadjuvant chemotherapy
In a total of 59 patients who received neoadjuvant chemotherapy, the median number of cycles administered was six (range 1 to 23) with seven patients receiving nine cycles or above. Of these seven patients, all received neoadjuvant chemotherapy until maximum response (defined by the individual investigators based on imaging (RECIST) or clinical response if clinically assessable disease), except for one patient, for whom paclitaxel was withdrawn due to disease progression (bone metastasis). The neoadjuvant chemotherapy regimens used in all 59 patients are shown in table 4.
Table 4.
Neoadjuvant treatment regimens administered
| Total (n=59) |
|
| N (%) | |
| Neoadjuvant treatment regimens | |
| Paclitaxel | 21 (35.6) |
| Doxorubicin+ifosfamide | 7 (11.9) |
| Gemcitabine+docetaxel | 6 (10.2) |
| Docetaxel | 5 (8.5) |
| Gemcitabine | 5 (8.5) |
| Doxorubicin+paclitaxel | 2 (3.4) |
| Etoposide+doxorubicin+ifosfamide | 2 (3.4) |
| Ifosfamide | 2 (3.4) |
| Sofarenib | 2 (3.4) |
| Cyclophosphamide+doxorubicin+dacarbazine | 1 (1.7) |
| Docetaxel+doxorubicin | 1 (1.7) |
| Doxorubicin | 1 (1.7) |
| Gemcitabine+docetaxel+doxorubicin+ifosfamide | 1 (1.7) |
| Gemcitabine+doxorubicin+ifosfamide | 1 (1.7) |
| Gemcitabine+paclitaxel | 1 (1.7) |
| Paclitaxel+bevacizumab | 1 (1.7) |
Of the 59 patients, 28 (47.5%) discontinued neoadjuvant chemotherapy when the scheduled treatment was completed, 20 (33.9%) discontinued chemotherapy on maximal response, and in the remainder, reasons for discontinuation were progressive disease (10.2%), physician choice (3.4%), adverse events (3.4%) and patient refusal (1.7%).
Adjuvant chemotherapy
Out of the 86 patients, 43 (50%) received adjuvant chemotherapy with a median number of five cycles (1 to 26). One patient received a total of 26 cycles of docetaxel along with postoperative radiotherapy. Discontinuation of treatment was not due to progressive disease, but the decision of the treating physician.
The majority of patients (33, 76.7%) completed the scheduled treatment, three (7%) discontinued due to adverse events, two (4.7%) due to patient’s refusal and one (2.3%) due to physician’s choice. Chemotherapy comprised primarily taxanes (docetaxel and paclitaxel) and gemcitabine (table 5).
Table 5.
Adjuvant regimens administered
| Total (n=43) |
|
| N (%) | |
| Adjuvant treatment regimens | |
| Paclitaxel | 12 (27.9) |
| Doxorubicin+ifosfamide | 6 (14.0) |
| Gemcitabine+docetaxel | 6 (14.0) |
| Gemcitabine+docetaxel+doxorubicin+ifosfamide | 5 (11.6) |
| Doxorubicin | 3 (7.0) |
| Gemcitabine | 2 (4.7) |
| Cisplatin | 2 (4.6) |
| Docetaxel | 1 (2.3) |
| Ifosfamide | 1 (2.3) |
| Caelyx | 1 (2.3) |
| Epirubicine | 1 (2.3) |
| Temozolomide | 1 (2.3) |
| Vincristine+dacarbazine | 1 (2.3) |
| Vinorelbine | 1 (2.3) |
Due to missing data no analysis regarding duration of chemotherapy was performed.
Subsequent treatments following relapse
Fifty-eight patients eligible for DFS experienced a relapse, out of 85. For 27 patients (45.5%), subsequent treatment comprised of systemic therapy alone and for four (6.9%) surgery alone. Nineteen patients had different combinations of treatment while eight (13.8%) had no subsequent therapy at all.
A total of 13 (22.4%) patients had subsequent surgery and 10 (17.2%) had subsequent radiotherapy.
Efficacy
At the time of the analysis, the overall median follow-up from diagnosis was 4.6 years (IQR: 2.9 to 7.1) and from surgery was 4.3 years (IQR: 2.7 to 6.9). Over 55% were alive from diagnosis at their last follow-up date, while disease progression was the cause of death in 92.1% of patients (table 6A). Similar results were observed from the date of surgery, with 59.7% patients being alive from surgery at their last follow-up date, while disease progression was the cause of death in 89.7% (table 6B).
Table 6.
(A) Reason of death from diagnosis and (B) Reason of death from surgery
| A: Reason of death from diagnosis | Total (n=38) |
| N(%) | |
| Cause of death | |
| Progression of disease | 35 (92.1) |
| Both progression of disease and toxicity indistinguishable | 1 (2.6) |
| Wound complications | 1 (2.6) |
| Missing | 1 (2.6) |
| B: Reason of death from surgery | Total (n=29) |
| N (%) | |
| Cause of death | |
| Progression of disease | 34 (87.2) |
| Both progression of disease and toxicity indistinguishable | 1 (2.6) |
| Cardiac problem | 1 (2.6) |
| Wound complications | 1 (2.6) |
| Missing | 2 (5.1) |
Eighty-five patients were eligible for analyses of DFS and OS from the date of diagnosis. Seventy-two patients (who were event-free at the time of surgery) were eligible for analyses of DFS and OS from the date of surgery.
DFS and OS from date of diagnosis
The median DFS was 1.4 years (95% CI, 0.9 to 1.7) and 4-year DFS was 26.8% (95% CI, 17.9 to 36.5) figure 2A. The median OS was 4.9 years (95% CI, 2.9 n) and 4-year OS was 57.9% (95% CI, 45.5 to 68.4) figure 2B.
Figure 2.
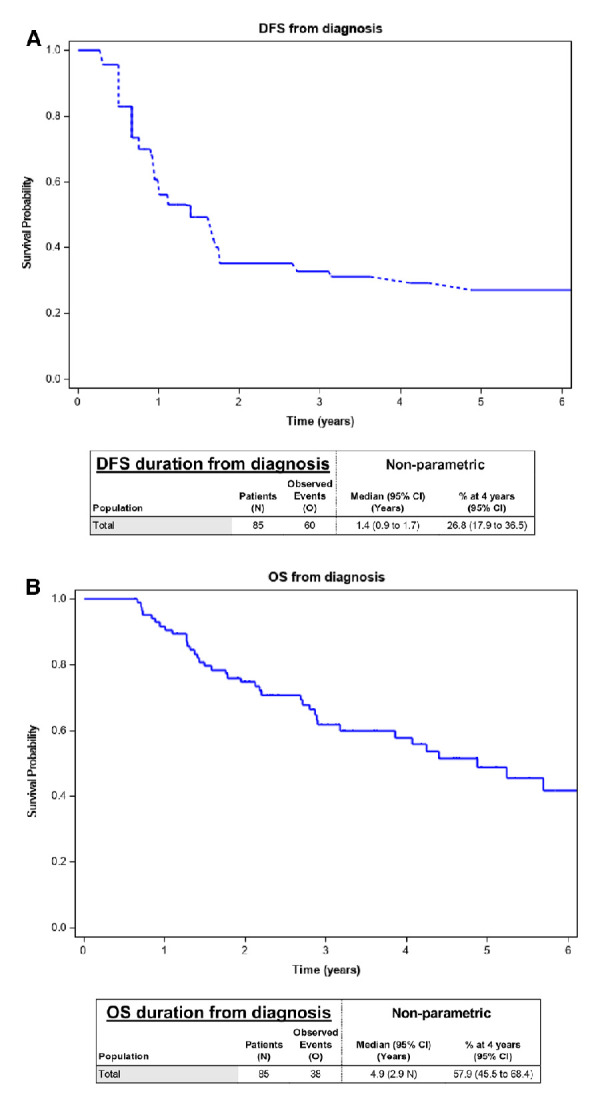
(A) DFS from diagnosis. (B) OS from diagnosis. DFS, disease-free survival; OS, overall survival.
DFS and OS from date of surgery
The median DFS was 1 year (95% CI, 0.7 to 1.6) and 4-year DFS was 29.9% (95% CI, 19.8 to 40.7) (figure 3A). The median OS was 5.1 years (95% CI, 3.2 n) and 4-year OS was 63% (95% CI, 49.3 to 17.9) (figure 3B).
Figure 3.
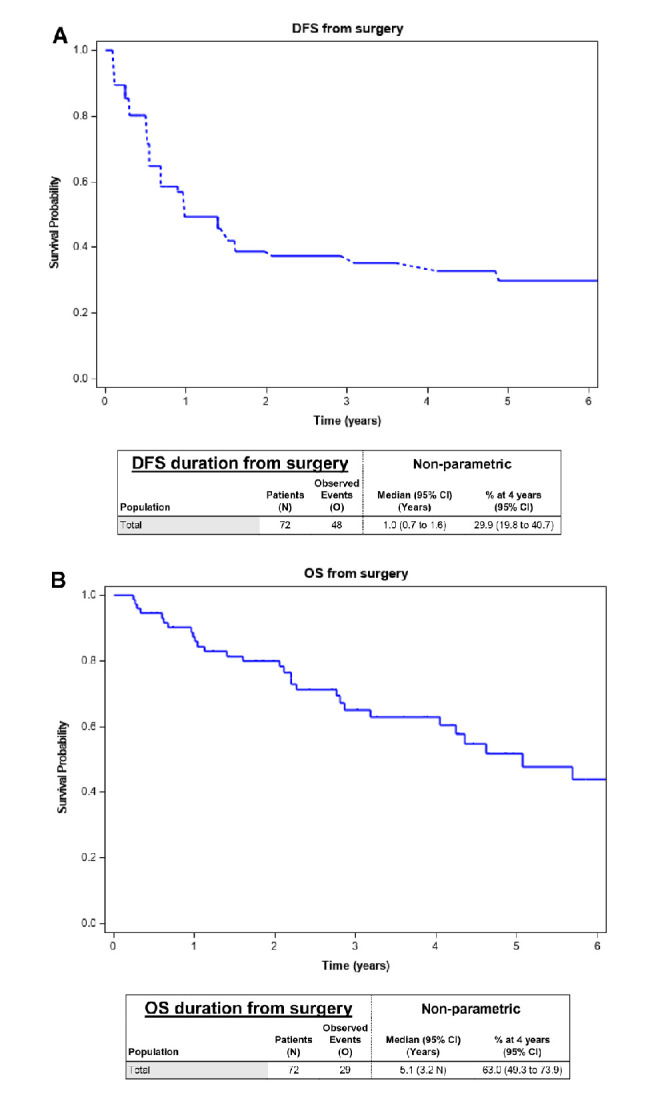
(A) DFS from surgery. (B) OS from surgery. DFS, disease-free survival; OS, overall survival.
Discussion
Angiosarcomas constitute a rare but aggressive STS subtype. Complete surgical resection provides the best chance of cure for localised disease. However, despite optimal surgical resection, recurrence rates are high, and the outcome remains poor. The aggressive nature of angiosarcomas and the limited options available to treat metastatic disease indicate a potential role for (neo)adjuvant chemotherapy in this sarcoma subtype.
To our knowledge, this multi-institutional study represents the largest retrospective series published to date, presenting the outcomes of neoadjuvant and adjuvant chemotherapy in patients with angiosarcomas.20 21 Our study highlights the clinical heterogeneity of angiosarcomas and also the variation in management between high volume EORTC sarcoma centres. A variety of chemotherapy regimens were used in this cohort but the most commonly used were paclitaxel (36%) or the combination of gemcitabine with docetaxel (13.9%) in line with drug efficacy data demonstrated in prospective Phase II studies, although in advanced/metastatic disease.9 21 In addition brivanib, a selective inhibitor of vascular endothelial growth factor and fibroblast growth factor signalling, has shown some activity in angiosarcoma.22 The variation in therapeutic approaches used for localised (locally advanced or operable) disease is not surprising, given the absence of prospective randomised controlled data, and consequently, the lack of evidence-based recommendations on the neoadjuvant/adjuvant therapy of angiosarcoma. This presents an opportunity for research collaboration and also the development of consensus guidelines for the management of vascular sarcomas.
To our knowledge, this multi-institutional study represents the largest retrospective series published to date, presenting the outcomes of neoadjuvant and adjuvant chemotherapy in patients with angiosarcomas.20 21 Our study highlights the clinical heterogeneity of angiosarcomas and also the variation in management between high volume EORTC sarcoma centres. A variety of chemotherapy regimens were used in this cohort but the most commonly used were paclitaxel (36%) or the combination of gemcitabine with docetaxel (13.9%) in line with drug efficacy data demonstrated in prospective Phase II studies, although in advanced/metastatic disease.9 21 The variation in therapeutic approaches used for localised (locally advanced or operable) disease is not surprising, given the absence of prospective randomised controlled data, and consequently, the lack of evidence-based recommendations on the neoadjuvant/adjuvant therapy of angiosarcoma. This presents an opportunity for research collaboration and also the development of consensus guidelines for the management of vascular sarcomas.
The limitations of small size and retrospective studies are acknowledged. As the different treatment modalities are not allocated randomly, establishing causal effects between treatment allocation and outcome is not possible: it is likely that the association found may be explained by confounding factors (eg, patients with worse prognosis receiving more intensive regimens). Making unbiased treatment modality comparisons would require a larger sample size, as well as complete knowledge and collection of all factors linked to both treatment modalities and outcome. As a result, subgroup analyses were not performed, due to the heterogeneity of tumour characteristics and the variation of treatment approaches.
Conclusion
This is the largest retrospective series reporting on the outcomes of neoadjuvant/adjuvant therapy in angiosarcomas. The major challenges encountered in this study are the heterogeneity of angiosarcomas and consequently the variation in practice across sarcoma centres in Europe. This presents considerable challenges in the design of clinical trials in angiosarcomas, particularly in the localised disease setting. Given the aggressive clinical behaviour of this disease and poor outcome, collaborative efforts should focus on approaches and treatment modalities that can improve survival. Multimodality therapy of localised disease including neoadjuvant/adjuvant chemotherapy could be further explored in the context of a prospective randomised controlled clinical trial.
Footnotes
Correction notice: This paper has been updated since first publihsed to amend Reference 16.
Contributors: All authors have contributed sufficiently to be included in the authorship list. They have all reviewed and approved the final version of the article.
Funding: NS work as Fellow at EORTC Headquarters was supported by a grant from the EORTC Soft Tissue and Bone Tumour Group.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. EORTC data sharing policy is described on EORTC website via the following link: https://www.eortc.org/data-sharing/. Access to data can be requested at the same location.
References
- 1.Fletcher CDM. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology 2014;64:2–11. 10.1111/his.12267 [DOI] [PubMed] [Google Scholar]
- 2.Young RJ, Woll PJ, Staton CA, et al. Vascular-targeted agents for the treatment of angiosarcoma. Cancer Chemother Pharmacol 2014;73:259–70. 10.1007/s00280-013-2345-0 [DOI] [PubMed] [Google Scholar]
- 3.Depla AL, Scharloo-Karels CH, de Jong MAA, et al. Treatment and prognostic factors of radiation-associated angiosarcoma (RaaS) after primary breast cancer: a systematic review. Eur J Cancer 2014;50:1779–88. 10.1016/j.ejca.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Sinnamon AJ, Neuwirth MG, McMillan MT, et al. A prognostic model for resectable soft tissue and cutaneous angiosarcoma. J Surg Oncol 2016;114:557–63. 10.1002/jso.24352 [DOI] [PubMed] [Google Scholar]
- 5.Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 1996;77:2400–6. [DOI] [PubMed] [Google Scholar]
- 6.Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer 2003;98:1716–26. 10.1002/cncr.11667 [DOI] [PubMed] [Google Scholar]
- 7.Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol 2007;18:2030–6. 10.1093/annonc/mdm381 [DOI] [PubMed] [Google Scholar]
- 8.Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol 2014;37:473–9. 10.1097/COC.0b013e31827e4e7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penel N, Bui BN, Bay J-O, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol 2008;26:5269–74. 10.1200/JCO.2008.17.3146 [DOI] [PubMed] [Google Scholar]
- 10.Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European organisation for research and treatment of cancer soft tissue and bone sarcoma group trials. Eur J Cancer 2014;50:3178–86. 10.1016/j.ejca.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Stacchiotti S, Palassini E, Sanfilippo R, et al. Gemcitabine in advanced angiosarcoma: a retrospective case series analysis from the Italian rare cancer network. Ann Oncol 2012;23:501–8. 10.1093/annonc/mdr066 [DOI] [PubMed] [Google Scholar]
- 12.Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French sarcoma group (GSF/GETO). Oncologist 2012;17:260–6. 10.1634/theoncologist.2011-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 2013;24:257–63. 10.1093/annonc/mds237 [DOI] [PubMed] [Google Scholar]
- 14.Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol 2015;33:2797–802. 10.1200/JCO.2015.60.8505 [DOI] [PubMed] [Google Scholar]
- 15.Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC soft tissue and bone sarcoma group (STBSG) retrospective analysis. Acta Oncol 2017;56:88–92. 10.1080/0284186X.2016.1234068 [DOI] [PubMed] [Google Scholar]
- 16.Jones RL, Ravi V, Brohl AS, et al. Results of the TAPPAS trial: an adaptive enrichment phase III trial of TRC105 and pazopanib (P) versus pazopanib alone in patients with advanced angiosarcoma (as). Annals of Oncology 2019;30:v683–709. 10.1093/annonc/mdz283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005;11:241–7. 10.1097/00130404-200505000-00011 [DOI] [PubMed] [Google Scholar]
- 18.Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol 2007;14:1953–67. 10.1245/s10434-006-9335-y [DOI] [PubMed] [Google Scholar]
- 19.Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812–22. 10.1016/S1470-2045(17)30334-0 [DOI] [PubMed] [Google Scholar]
- 20.DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck 2008;30:639–46. 10.1002/hed.20757 [DOI] [PubMed] [Google Scholar]
- 21.Oxenberg J, Khushalani NI, Salerno KE, et al. Neoadjuvant chemotherapy for primary cutaneous/soft tissue angiosarcoma: determining tumor behavior prior to surgical resection. J Surg Oncol 2015;111:829–33. 10.1002/jso.23891 [DOI] [PubMed] [Google Scholar]
- 22.Jones RL, Ratain MJ, O'Dwyer PJ, et al. Phase II randomised discontinuation trial of brivanib in patients with advanced solid tumours. Eur J Cancer 2019;120:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]