Abstract
Introduction
18F-FDOPA illustrates the properties of uptake and storage of catecholamines in pheochromocytomas (PHEOs). Until now, the relationship between 18F-FDOPA quantitative parameters and a PHEO secretory profile has not been specifically evaluated.
Materials and methods
Fifty-six patients (56% females, median age: 47.5 yrs) with non-metastatic PHEO, evaluated by 18F-FDOPA PET/CT, were included in this retrospective study. Forty-five patients had negative genetic testing(80.4%); five patients (8.9%) had RET, two patients (3.6%) had SDHB, two had SDHD (3.6%), one patient (1.8%) had NF1, and one patient had a VHL (1.8%) mutation. Correlation between 18F-FDOPA metabolic parameters (tumor SUVmax, tumor SUVmean, tumor SUVmax/liver SUVmax, MTV 42%, total lesion uptake), urinary metanephrines (MNs), and plasma chromogranin A (CgA) were evaluated.
Results
All patients had positive 18F-FDOPA PET/CT. On univariate analysis, there was a strong correlation between all metabolic parameters and urinary MNs and plasma chromogranin A (CgA). The highest correlations were observed between total lesion (TL) uptake and the value of urinary MNs regardless of their nature (p = 8.10−15 and r = 0.80) and between MTV 42% and plasma CgA levels (p = 2.10−9, r = 0.74). On multivariate analysis, the correlation of uptake parameters and CgA levels did not persist further due to the relation of CgA and tumor diameter. A correlation between TL uptake and the normetanephrine/metanephrine ratio (NMN/MN) was also found, a finding that was in accordance with in vitro studies, which were found to have a higher catecholamine content in epinephrine producing PHEOs.
Conclusion
This retrospective study shows a correlation between 18F-FDOPA uptake, especially using TL uptake, urinary MNs, and a PHEO biochemical phenotype. This illustrates that beyond its localization value, 18F-FDOPA PET further enables PHEO characterization at a specific metabolic level.
Keywords: Pheochromocytomas, 18F-FDOPA, Radionuclide imaging, Genetics
Introduction
Pheochromocytomas (PHEOs) are highly vascularized neural crest tumors that originate from the adrenal medulla. Most PHEOs secrete catecholamines and their metabolites with a secretory profile that may vary across tumors and genotypes. Thus, the diagnosis of PHEO relies on the presence of high levels of plasma or urinary metanephrines (MNs), methoxytyramine, and/or catecholamines in about 99% of patients [1, 2]. Furthermore, most PHEOs, as neuroendocrine tumors, secrete chromogranin A (CgA).
Radiologic features of anatomic and functional imaging are also suggestive of the diagnosis of PHEO. The role of functional imaging is to detect additional primary PHEOs (multifocality) that may coexist and potential metastases at sites where chromaffin cells are normally absent. An additional major advantage of functional imaging over other imaging modalities is to the ability provide better characterization of these tumors at the molecular level. This opportunity has recently been accelerated by a number of excellent radiopharmaceuticals which target different functional and molecular pathways that often reflect the diverse genetic landscape of PHEOs (e.g., catecholamine synthesis, somatostatin receptors, and amino acid transporter expression).
18F-FDOPA illustrates the properties of uptake and storage of catecholamines in PHEOs reflecting a catechol-aminergic imaging phenotype [3, 4]. 18F-FDOPA is taken up via the neutral amino acid transporter system L (LAT1/LAT2). PHEOs and paragangliomas (PPGLs) over-express LATs and are, therefore, targetable with 18F-FDOPA [5]. Previously, 18F-FDOPA PET/CT was found to have high sensitivity and specificity in patients with PPGLs [6]. Although the relation between a PHEO biochemical phenotype and 18F-FDOPA uptake is intuitive, this has not been specifically studied. Thus, the present study aimed to evaluate the relationship between the 18F-FDOPA uptake and PHEO catecholamine secretion.
Materials and methods
Patient population
This retrospective study was conducted in the Department of Nuclear Medicine at La Timone Hospital in Marseille, France. We performed a comprehensive search in our database to identify all patients evaluated by 18F-DOPA PET/CT from 2007 to 2017 for a presumed non-metastatic PHEO (based on clinical, radiological, and functional imaging studies). Among these patients, only those who fulfilled the following criteria were included: (1) PHEO confirmed histopathologically, (2) 18F-DOPA PET/CT performed at initial diagnosis, (3) absence of multifocal disease or metastasis, (4) genetic testing for at least germline mutations (including large deletions) in SDHB/D, VHL, and RET (in the presence of hypercalcitoninemia). The diagnosis of NF1 was done on clinical grounds. Urinary total MN and normetanephrine (NMN) were measured by high-performance liquid chromatography (HPLC). Plasma CgA was measured using a commercially available radioimmunoassay (CgA-RIA CT, CIS Bio International, Gif-sur-Yvette Cedex, France).
18F-DOPA PET/CT imaging protocol
A combined PET/CT scanner was employed using Discovery ST or Discovery 710 GE Medical Systems. Patients fasted for at least 3 h prior to radiotracer injection. 18F-FDOPA was injected intravenously (3 MBq/kg). The 18F-FDOPA PET/CTacquisition protocol included a delayed whole-body acquisition (starting at approximately 60 min post-injection) from the top of the skull to the upper thigh (3 min/step).
Assessment of 18F-FDOPA uptake parameters
Volumetric regions of interest were placed over the areas of 18F-FDOPA uptake in the PHEO and liver. Initially, SUVmax (maximum voxel intensity within the volumetric region) was recorded for PHEO and liver. Mean SUV (SUVmean) that reflects the average 18F-FDOPA uptake in the tumor was also assessed. As an index of metabolic tumor burden, MTV 42%, was defined as the region enclosed by a 42% isocontour around the maximum PET voxel. Total lesion (TL) uptake that corresponds to the total lesion glycolysis on 18F-FDG PET/CT was calculated as the product of tumor SUVmean and MTV 42%. Tumor diameter was measured on the unenhanced axial CT slices.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 20.0 (IBM SPSS Inc., Chicago, IL, USA). Correlation between quantitative PET/CT parameters (uptake ratio and volumetric parameters) and catecholamine secretion was evaluated by Spearman’s rho test. Continuous variables are expressed as median with interquartile range (Q1, Q3). The Shapiro–Wilk test was used to test the normality of distribution. When data is non-normal, the log transformation is used to transform skewed data to approximately conform to normality; and multivariate analyze was performed using multiple linear regression. All tests were two-sided. The statistical significance was defined as p < 0.01.
Results
Patient and tumor characteristics
Fifty-six PHEO patients (56% female, median age: 47.5 yrs) were included in the study. Forty-five patients had negative genetic testing (80.4%) and, therefore, were considered sporadic cases. The remaining cases had MEN2A (n = 5), SDHB (n = 2), SDHD (n = 2), NF1 (n = 1), and VHL (n = 2) mutations. The median values for MN to the upper reference limit (URL) ratio, NM to URL ratio, highest value of MN or NMN to URL and plasma CgA were 2.9 (Q1 = 1.50; Q3 = 7.50), 2.45 (Q1 = 1.18; 4.05), 4.45 (Q1 = 2.33; Q3 = 7.73), and 2.5 (Q1 = 1.10; Q3 = 5.90), respectively. On CT imaging, the median tumor diameter was 25 mm (Q1 = 18; Q3 = 30).
18F-FDOPA uptake parameters
The median uptake values for PHEO-SUVmax, PHEO-SUVmax/liver SUVmax, MTV42%, and lesion (TL) uptake were 12 (Q1 = 6; Q3 = 15), 4.85 (Q1 = 2.81; Q3 = 7.18), 8.5 (Q1 = 4.9; Q3 = 13.2), and 57 (Q1 = 25.2; Q3 = 92.5), respectively.
Determinants of 18F-FDOPA uptake
On univariate analysis, there was a strong correlation between all quantitative PET/CT parameters and tumor secretion (p < 0.01, Fig. 1). Tumor SUVmax, SUVmean, and tumor SUVmax to liver SUVmax ratio showed a statistically significant correlation with urinary MNs (the highest value between urinary MN and NMN was taken): p = 10−5 (r = 0.542), p = 5.10−10 (r = 0.716), and p = 10−4 (r = 0.72), respectively (Fig. 1a). Statistically significant correlations were also observed between the value of urinary MNs and MTV 42% (p = 10−4 and r = 0.478) and TL uptake (p = 8.10−15 and r = 0.80; Fig. 1b). There was also a correlation between plasma CgA and SUVmax (p = 10−5 et r = 0.593), SUVmean (p = 0.008 et r = 0.380), MTV 42% (p = 2.10−9 et r = 0.744), and TL uptake (p = 10−4 and r = 0.70; Fig. 1c). On a multivariate analysis including age, gender, tumor diameter, and genetic features (sporadic cases vs. hereditary ones), the correlation between TL uptake and urinary MNs remained statistically significant (p = 0.003). By contrast, on multivariate analysis, the correlation of uptake parameters and CgA did not persist further due to the relation of CgA and tumor diameter (Table 1).
Fig. 1.
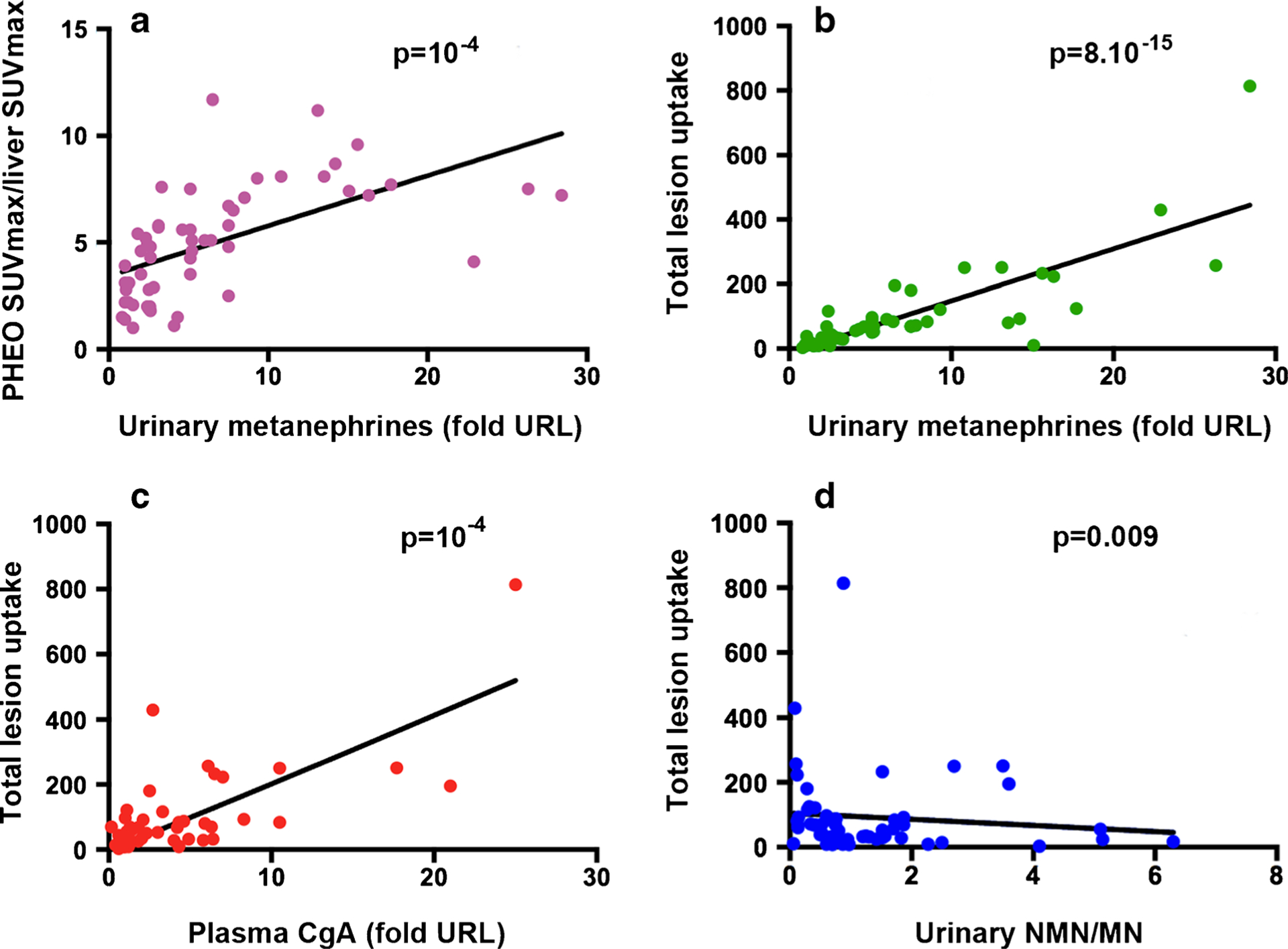
Relationship between 18F-FDOPA uptake and biochemical phenotype. There is strong positive correlation between PHEO-SUVmax/liver SUVmax and urinary metanephrines (a) and between TL uptake and urinary metanephrines (b), chromogranin A, (c) and urinary normetanephrine/metanephrine (NMN/MN) ratio (d)
Table 1.
Factors influencing total lesion uptake. Univariate and multivariate analyses
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Spearman’s rho | P | B° | SE# | P | |
| Urinary metanephrines (fold URL) | 0.826 | 8.10−15 | 0.493 | 0.021 | 3.10−4 |
| Age | 0.188 | 0.180 | 0.06 | 0.077 | 0.340 |
| Tumor diameter | 0.662 | 3.10−8 | 0.051 | 0.453 | 2.10−4 |
| Chromogranin A (fold URL) | 0.538 | 10−4 | 0.003 | 0.013 | 0.92 |
| NMN/MN | −0.289 | 0.009 | −0.115 | −0.113 | 0.045 |
| Genetic status (sporadic vs. mutated) | 0.184 | 0.45 | 0.077 | 0.029 | 0.739 |
On univariate analysis, TL uptake is correlated with urinary metanephrines, tumor diameter, chromogranin A, and urinary normetanephrine/metanephrine (NMN/MN) ratio. On multivariate analysis, TL uptake remains correlated with all the variables, except for chromogranin A
Relationship to biochemical phenotype
Moreover, in univariate and multivariate analysis, we established the correlation between TL uptake and the urinary NMN/MN ratio, showing that the more a PHEO secretes MN over NMMN, the more TL uptake is elevated (p = 0.009), regardless of the tumor diameter (Fig. 1d).
Discussion
To the best of our knowledge, this is the first study that evaluates the relationship between 18F-FDOPA uptake parameters and a PHEO biochemical phenotype. The principal conclusion of our study is that there is a strong correlation between 18F-FDOPA-derived metabolic parameters, especially between TL uptake and total urinary MN levels. There is also a correlation between high 18F-FDOPA uptake and a predominant epinephrine secretion. Finally, no correlation was found between 18F-FDOPA uptake parameters and plasma CgA levels on multivariate analysis.
To date, one study has evaluated the relationship between 18F-FDOPA uptake and hormone secretion in 77 consecutive carcinoid patients of which 74 had metastatic disease [7]. A correlation was found between whole-body metabolic burden (WBMTB) and urinary serotonin, and urinary/plasma 5-HIAA [7].
In the current study, we have only included patients with single, non-metastatic PHEO in order to accurately evaluate the relationship between 18F-FDOPA uptake and a PHEO biochemical phenotype, based on the measurement of urinary MNs. Parasympathetic PGLs were excluded since, in the majority of cases, they do not produce catecholamines.
The impact of a specific PHEO biochemical phenotype on functional imaging is of a particular interest. Secretory profiles and expression of key enzymes involved in catecholamine metabolism have been well studied. Based on the evaluation of bovine adrenal glands, two populations of adrenomedullary cells have been described: one with low density which are mainly norepinephrine-producing and one with high density which are mainly epinephrine-producing. This study also showed that norepinephrine-producing cells released a higher percentage of their catecholamine content than epinephrine-producing cells [8]. Another analysis revealed differences in secretory profiles in VHL vs RET tumors, a finding related to differences in expression of numerous genes encoding multiple components of the regulated secretory pathway [9]. Finally, divergent lower tissue concentrations but higher rates of catecholamine secretion were found in noradrenergic than adrenergic PPGLs [10]. Norepinephrine-producing tumors were found to release 57% of their tumor tissue content of catecholamines into the bloodstream per day (34%, 46%, and 15% for VHL-, SDHB-, and SDHD-related PPGLs, respectively) compared to only 2–5% for epinephrine-secreting and hereditary tumors. Similarly, only between 0.1 and 0.4% of the catecholamine content of epinephrine-secreting sporadic PHEOs were excreted into urine per day, compared to 3% for sporadic norepinephrine-secreting ones (between 1.2% and 4.9% for VHL-, SDHB-, and SDHD-related PPGLs) [10].
Furthermore, our results suggest that a predominant MN secretion is associated with a higher rate of 18F-FDOPA uptake that could potentially be explained by higher tumor catecholamine content [9]. However, the precise mechanism of how this is happening is currently unknown. The relationship between uptake and genotype, especially SDHx mutation status, has not been evaluated in this study due to the small number of hereditary PHEOs.
Recently, it has been shown that metabolic parameters have emerged as promising diagnostic and prognostic biomarkers. 18F-FDOPA-derived quantitative parameters can provide useful information in the evaluation of PHEO when MN concentrations are not reliable or normal, especially in the presence of an indeterminate adrenal mass on conventional imaging. These parameters are also valuable in monitoring PHEO therapeutic responses since it is expected that any medication that would decrease or deplete catecholamines should be associated with decreased 18F-FDOPA uptake. Finally, the potential value of TL uptake as a biomarker of PHEO catecholamine concentrations should be evaluated for a prediction of catecholamine release associated with cardiovascular changes during adrenalectomy.
We acknowledge several limitations to our study: the retrospective nature of the study, the limited sample size, the absence of respiratory gating, and the lack of partial volume effect correction.
In conclusion, the present study exemplifies that, beyond its localization value, 18F-FDOPA PET/CT enables the in vivo assessment of a PHEO metabolic profile reflected by its catecholamines and the production and secretion of their metabolites.
Funding
The review did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Footnotes
Disclosure of potential conflicts of interest The authors have nothing to disclose.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenhofer G, Siegert G, Kotzerke J, Bornstein SR, Pacak K. Current progress and future challenges in the biochemical diagnosis and treatment of pheochromocytomas and paragangliomas. Horm Metab Res. 2008;40:329–37. 10.1055/s-2008-1073156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-Fluoro-L-DOPA, 18F-Fluoro-Deoxyglucose, and 18F-Fluorodopamine PET and 123I-MIBG Scintigraphy in the localization of Pheochromocytoma and Paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havekes B, King K, Lai EW, Romijn JA, Corssmit EP, Pacak K. New imaging approaches to phaeochromocytomas and paragangliomas. Clin Endocrinol. 2010;72:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barollo S, Bertazza L, Watutantrige-Fernando S, Censi S, Cavedon E, Galuppini F, et al. Overexpression of L-type amino acid transporter 1 (LAT1) and 2 (LAT2): novel markers of Neuroendocrine Tumors. PLoS One. 2016;11:e0156044 10.1371/journal.pone.0156044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treglia G, Cocciolillo F, de Waure C, Di Nardo F, Gualano MR, Castaldi P, et al. Diagnostic performance of 18Fdihydroxyphenylalanine positron emission tomography in patients with paraganglioma: a meta-analysis. Eur J Nucl Med Mol Imaging. 2012;39:1144–53. 10.1007/s00259-012-2087-y. [DOI] [PubMed] [Google Scholar]
- 7.Fiebrich HB, de Jong JR, Kema IP, Koopmans KP, Sluiter W, Dierckx RA, et al. Total 18F-dopa PET tumour uptake reflects metabolic endocrine tumour activity in patients with a carcinoid tumour. Eur J Nucl Med Mol Imaging. 2011;38:1854–61. 10.1007/s00259-011-1862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause W, Michael N, Lubke C, Livett BG, Oehme P. Catecholamine release from fractionated chromaffin cells. Eur J Pharmacol. 1996;302:223–8. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhofer G, Huynh TT, Elkahloun A, Morris JC, Bratslavsky G, Linehan WM, et al. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am J Physiol Endocrinol Metab. 2008;295: E1223–33. 10.1152/ajpendo.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


