Highlights
-
•
Normally, action preparation involves a global suppression of motor activity.
-
•
Such preparatory suppression is deficient in alcohol-dependent patients.
-
•
Binge drinking may represent a first step toward alcohol-use disorder.
-
•
Here, we tested whether binge drinkers also display altered preparatory suppression.
-
•
Our results indicate abnormally high motor preparatory activity in binge drinkers.
Keywords: Binge drinking, Heavy drinking, Alcohol-use disorder, Transcranial magnetic stimulation, Motor-evoked potentials, Corticospinal excitability
Abstract
Binge drinking consists in a pattern of consumption characterised by the repeated alternation between massive alcohol intakes and abstinence periods. A continuum hypothesis suggests that this drinking endeavour represents an early stage of alcohol dependence rather than a separate phenomenon. Among the variety of alterations in alcohol-dependent individuals (ADIs), one has to do with the motor system, which does not show a normal pattern of activity during action preparation. In healthy controls (HCs), motor-evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) over primary motor cortex (M1) show both facilitation and suppression effects, depending on the time and setting of TMS during action preparation. A recent study focusing on the suppression component revealed that this aspect of preparatory activity is abnormally weak in ADIs and that this defect scales with the risk of relapse. In the present study, we tested whether binge drinkers (BDs) present a similar deficit. To do so, we recorded MEPs in a set of hand muscles applying TMS in 20 BDs and in 20 matched HCs while they were preparing index finger responses in an instructed-delay choice reaction time task. Consistent with past research, the MEP data in HCs revealed a strong MEP suppression in this task. This effect was evident in all hand muscles, regardless of whether they were relevant or irrelevant in the task. BDs also showed some preparatory suppression, yet this effect was less consistent, especially in the prime mover of the responding hand. These findings suggest abnormal preparatory activity in BDs, similar to alcohol-dependent patients, though some of the current results also raise new questions regarding the significance of these observations.
1. Introduction
Concision in style, precision in thought, decision in life (Victor Hugo); when meticulously selected, actions, just like words, are powerful and can lead to great accomplishments. By contrast, the inability to suppress prevailing tendencies that are not consistent with our internal goals – or to inhibit actions that are no longer appropriate – leads to improper and hazardous behaviours (Smith et al., 2014, van Velzen et al., 2014, Verdejo-García et al., 2008, Wilcox et al., 2014). So it is in the field of addictions where excessive and/or chronic alcohol consumption stands.
Ingesting alcohol occasionally – may it be through drinking a beer, a glass of wine or a cocktail - is widespread in the western society and occurs in many everyday life situations (Degenhardt et al., 2018). Indeed, people usually have a drink when they get a job, get married, celebrate their birthday, or watch football. For most of them, drinking alcohol is a recreational activity, and the amount consumed is controlled. For others, however, alcohol problems arise from drinking too much, too fast, or too often.
Unhealthy drinking habits are common characteristics of both binge-drinkers (BDs) and alcohol-dependent individuals (ADIs). BDs are people that display a pattern of consumption characterised by the repeated alternation between massive alcohol intakes and abstinence periods (i.e., binge drinking; (Courtney and Polich, 2009, Kuntsche et al., 2017, Lannoy et al., 2019a, Townshend and Duka, 2005)) whilst ADIs are individuals suffering from an alcohol use disorder (American Psychiatric Association, 2013). In these two populations, the results of excessive alcohol consumption are frequently appalling (alcohol is directly involved in ~4% of deaths worldwide (Peacock et al., 2018, Rehm et al., 2009)). For instance, in young adults, a population in which binge-drinking has become increasingly prominent (Degenhardt et al., 2013, Siqueira and Smith, 2015), regular heavy alcohol consumption is linked to poorer academic results, less adequate social integration, car accidents, sexual assaults, violence, health issues, etc. (Lannoy et al., 2019a, Maurage et al., 2012). For ADIs, the consequences are usually the same but exacerbated with many problems at personal (e.g., quarrels with partner), societal (e.g., bar brawls) and professional (e.g., being unfit to accomplish professional demands) levels (American Psychiatric Association, 2013, Degenhardt et al., 2018).
From a neural perspective also, excessive alcohol consumption has harmful consequences. For instance, several studies have revealed grey and white matter atrophy in ADIs in structures that are responsible for cognitive functions, such as attention, memory, problem solving, decision making, response inhibition, etc. with the underlined cognitive functions impaired (Brion et al., 2017, Sullivan and Harris, 2010, Xiao et al., 2015, Zahr and Pfefferbaum, 2017). Consequently, ADIs typically display a reduced ability to successfully regulate thoughts and behaviours, resulting in a tendency to make risky or thoughtless decisions through favouring immediate rewards without consideration for the delayed – potentially deleterious – consequences (Camchong et al., 2014). Critically, these cognitive deficits persist long after drinking cessation and are strongly involved in relapse (Stavro et al., 2013). In BDs also, the damages are substantial. For instance, BDs display a thinner cortex in prefrontal and cerebellar regions (Cservenka and Brumback, 2017, Heikkinen et al., 2017, Lisdahl et al., 2013, Pfefferbaum et al., 2016) and an overall decrease in white matter integrity (Cservenka and Brumback, 2017, Luciana et al., 2013). Behaviourally speaking, BDs show impairments in sustained attention, a lack of inhibitory control and an abnormally short post-error slowing indicating attenuated response monitoring (Bo et al., 2015, Cservenka and Brumback, 2017, Hartley et al., 2004). Importantly, to date, it is still difficult to know whether these shifts in norm arise from the neurotoxic effects of alcohol (Brust, 2010) or whether they represent – at least in part - a factor of vulnerability (Crabbe et al., 2011) as some of the structural and cognitive alterations reported above in BDs and ADIs are sometimes observed even before the engagement of excessive alcohol consumption. For instance, functional magnetic resonance imaging studies have shown reduced fronto-parietal activations in young adolescents prior to any alcohol consumption that may represent predictors of subsequent excessive drinking (Norman et al., 2011, Wetherill et al., 2013). Similarly, offspring of ADIs with limited or no past alcohol use display frontal and parietal structural alterations that might underlie cognitive and behavioural traits associated with a risk of alcohol use disorder (Henderson et al., 2018).
The similarities between the structural and functional alterations observed in ADIs and BDs (Brion et al., 2017, Stavro et al., 2013, Xiao et al., 2015) has led researchers to suggest that binge-drinking and alcohol use disorder represent two different stages of the same phenomenon rather than independent conditions (Bo et al., 2015, Maurage et al., 2013, Petit et al., 2014, Wagner, 2002). Based on this idea, called the continuum hypothesis, binge-drinking could be a first step towards alcohol use disorder and excessive drinking could lead individuals down the slippery slope of compulsive intake. The continuum hypothesis is further borne out by studies showing that binge-drinking patterns started during late adolescence frequently remain into early adulthood (Degenhardt et al., 2013) and that initiating heavy drinking at an early age significantly enhances the risk for subsequent alcohol use disorder (Hingson et al., 2006).
As a core cognitive function, inhibitory control is tightly related to the prefrontal cortex and its alteration seems to play a crucial role in the development and maintenance of addictions (Baler and Volkow, 2006, Billieux et al., 2010, Devos et al., 2015). Moreover, findings of recent work using transcranial magnetic stimulation (TMS) suggest that not only the prefrontal cortex, but the motor system as well, which is interconnected to prefrontal areas, may be involved in inhibitory control (Cos et al., 2014, Derosiere et al., 2017, Duque et al., 2017, Michelet et al., 2010). When applied over the primary motor cortex (M1), TMS elicits motor-evoked potentials (MEPs) in targeted contralateral hand or limb muscles. Their amplitude provides a temporally precise and muscle-specific measure of the net impact of facilitatory and inhibitory inputs to corticospinal (CS) cells (Bestmann and Duque, 2016, Bestmann and Krakauer, 2015, Derosiere and Duque, 2020, Dum and Strick, 2002). Inhibitory control is particularly strong in stop-signal tasks where the successful suppression of actions following stop signals relies on a decrease in the excitability of the CS tract (Duque et al., 2017, Wessel et al., 2013, Wessel and Aron, 2017). Interestingly, not only stopping but also preparing motor acts is associated with a robust CS suppression (Duque et al., 2017, Duque et al., 2012, Duque et al., 2010), a phenomenon referred to as preparatory suppression (or inhibition) (Derosiere, 2018, Duque et al., 2017, Greenhouse et al., 2015b, Hasbroucq et al., 1997, Labruna et al., 2019, Quoilin et al., 2019, Vassiliadis et al., 2018, Wilhelm et al.,2017). An advantage of the latter measure of inhibitory control is that it is obtained in a context that is quite representative of everyday life (we continuously need to prepare actions and avoid selecting inappropriate ones) while stop-signal tasks are more artificial: most situations require inhibitory control to be internally generated, in the absence of explicit stop-signals.
Preparatory suppression is most pronounced in instructed-delay reaction time (RT) tasks that require postponing a pre-cued response until a delayed imperative signal (Bestmann and Duque, 2016, Duque et al., 2017, Grandjean et al., 2019, Greenhouse et al., 2015a, Lebon et al., 2016, Quoilin et al.,2016). In such circumstances, one prominent hypothesis is that the motor suppression observed during the delay period prevents the premature release of the prepared response (Duque et al., 2017, Duque and Ivry, 2009). Hence, preparatory suppression in this context may reflect the operation of processes ensuring some sort of impulse control (Grandjean et al., 2019, Quoilin et al., 2020). Consistent with this view, a recent study revealed that preparatory suppression is impaired in alcohol-dependent patients, a population typically lacking inhibitory control, and that this deficiency is linked to the risk of relapse (Quoilin et al., 2018).
In the present study, we assessed motor suppression during action preparation in 20 BDs and 20 healthy controls (HCs) who were asked to perform the same instructed-delay RT task as the ADIs in Quoilin et al. (2018). The goal here was to address the question of whether BDs share similar alterations as ADIs at the level of the motor system – i.e., the continuum hypothesis. Such an approach also allows extending the observations made in ADIs to another population associated with abnormal inhibitory control. Finally, it sheds new light on the neurophysiological correlates of a behaviour that remains ubiquitous in youths despite its deleterious consequences.
Overall, our data suggest abnormal preparatory activity in BDs, though some of the current results also raise new questions regarding the significance of these observations. Are inhibitory processes genuinely deficient in these patients during action preparation - as hypothesized here - or do these individuals (and maybe ADIs too) rather suffer from an excessive facilitation of prepotent motor representations? Perhaps they suffer from both, an issue for future investigations.
2. Materials and methods
2.1. Participants
Participants were recruited through a preliminary anonymous online survey posted on social media that assessed sociodemographic, psychological and medical variables as well as the pattern of alcohol consumption of the individuals. 192 answers were collected. To be selected for the study, participants needed to fulfil the following conditions: they had to be native or fluent French speakers, between 18 and 30 years old and right handed (according to the Edinburgh Questionnaire; subjects showing crossed laterality were not recruited (Oldfield, 1971)). Selection criteria also included an absence of psychological or neurological disorders, no current medication (i.e., except for oral contraception), no major medical problems, no history of concussion, normal or corrected-to-normal visual abilities, a total absence of past or current drug consumption (except for alcohol), no family history of alcohol use disorder, no >4 drinking occasions per week (to avoid any subject with chronic alcohol consumption) and no teetotalism (i.e., the practice of complete abstinence from alcoholic drinks (Lannoy et al., 2017b)). Based on this survey, some responders satisfying the aforementioned criteria were included. These individuals were then allocated to one of two groups (Table 1) based on the number of drinks they consumed in one occasion (i.e., alcohol consumption per se) and the specific pattern of alcohol consumption they exhibited (i.e., binge drinking score).
Table 1.
Results for demographic, psychological and alcohol consumption measures for binge drinkers (BDs) and healthy controls (HCs).
| BDs (N = 20) |
HCs (N = 20) |
||||
|---|---|---|---|---|---|
| mean (±SD) | min – max | mean (±SD) | min - max | ||
| AgeNS | 21.8 (±1.8) | 18–26 | 22.5 (±1.9) | 19–26 | |
| Gender (Female/Male)NA | 9/11 | NA | 10/10 | NA | |
| Educational Level NS | 15.8 (±2.1) | 12–19 | 15.9 (±1.6) | 12–19 | |
| Smoker | 0/20 | NA | 1/20 | NA | |
| BDI NS | 2 (±2.7) | 0–9 | 2.8 (±2.8) | 0–10 | |
| STAI-A NS | 31.5 (±6.8) | 20–44 | 32.2 (±7.7) | 20–46 | |
| STAI-B NS | 35.9 (±5.9) | 23–51 | 40.6 (±9.7) | 24–57 | |
| UPPS NS | 105.5 (±12.5) | 82–137 | 101.5 (±14.1) | 76–122 | |
| MCQ overall K NS | 8.10−3 (±0.01) | 10−4 − 0.04 | 0.016 (±0.03) | 10−4 − 0.11 | |
| AUDIT*** | 15.2 (±5.4) | 7–25 | 6.0 (±2.8) | 1–10 | |
| Binge drinking score*** | 37.4 (±23.2) | 16.4–110 | 7.2 (±3.2) | 2–12 | |
| Age at first alcohol consumption* | 14.6 (±1.1) | 12–17 | 15.3 (±1.2) | 13–17 | |
| Years of regulary alcohol consumptionNS | 4.8 (±1.8) | 1–8 | 4.2 (±2.3) | 0–7 | |
| Consumption speedab*** | 3 (±0.9) | 2–5 | 1.4 (±0.6) | 0.5–2 | |
| Number of alcohol drink consumed within 2 hoursb*** | 5.9 (±1.8) | 4–10 | 2.8 (±1.3) | 1–4 | |
| rMT left hemisphereNS | 41.7 (±5.1) | 34–52 | 41.5 (±5.8) | 33–57 | |
| rMT right hemisphereNS | 42.9 (±4.5) | 36–53 | 42.1 (±6.0) | 35–55 | |
BDI = Beck Depression Inventory (Beck et al., 1961, Collet and Cottraux, 1986); STAI = State Trait Anxiety Inventory (A = state anxiety; B = trait anxiety; Spielberger et al., 1983); UPPS = UPPS impulsive behaviour scale (Van der Linden et al., 2006); MCQ = Monetary Choice Questionnaire (Kirby et al., 1999); AUDIT = Alcohol Use Disorders Identification Test (Saunders et al., 1993); rMT = resting motor threshold; SD = standard deviation. a In doses per hour. b During the last six months. NA = not-applicable; NS = not-significant; * p < 0.05; *** p < 0.001.
To be classified as binge drinker (BD; n = 20: 9 women, 21.8 ± 1.8 years old), subjects needed to drink hebdomadally a minimum of 6 (for women) or 7 (for men) alcohol drinks overs a period of 2 h (i.e., BAC level = 0.08gr /dl) within the last 6 months (Courtney and Polich, 2009, National Institute of Alcohol Abuse and Alcoholism, 2004). We adapted the usual cutoff of 4/5 doses because the quantity of alcohol contained in one drink varies from one country to another (e.g., an alcohol drink contains 10 g of pure ethanol in Belgium whereas it only contains 8 g in the United Kingdom and 14 g in the United States). In addition, subjects needed to exhibit a binge drinking score higher than or equal to 16 (Lannoy et al., 2019b, Lannoy et al., 2018, Lannoy et al., 2017b). This score is crucial because - as mentioned above - it describes the drinking pattern exhibited in drinking occasions rather than alcohol consumption per se. It was measured as follows (Townshend and Duka, 2005):
| [(4 × consumption speed) + number of drunkenness episodes in the last six months + (0.2 × percentage of drunkenness episodes in the last six months)] |
The consumption speed was defined as the number of alcohol drinks usually consumed per hour; drunkenness was defined as an inability to speak clearly, a loss of coordination and nausea. The percentage of drunkenness episodes was the ratio between the number of drunkenness episodes and the total number of drinking episodes.
Other subjects were categorised as healthy controls (HCs; n = 20: 10 women, 22.5 ± 1.9 years old) if their binge drinking score was lower than or equal to 12 but higher than 0 (Lannoy et al., 2017b). They were matched to BDs for age, gender and education level (i.e., the number of education years completed since starting primary school (Maurage et al., 2013, Quoilin et al., 2018)). Some of the subjects who filled in the survey did not fall in neither group and were therefore not included in the study. Participants were naive to the purpose of the study and financially compensated. The protocol was approved by the institutional review board of Université catholique de Louvain (UCLouvain, Belgium) and required written informed consent.
2.2. Questionnaires
To be selected for the study, participants had to first fill in an online survey created specifically for this study. The online questionnaire assessed the specific pattern of alcohol consumption of the participants and was composed of the Alcohol Use Disorders Identification Test (AUDIT) and of other questions evaluating their drinking habits (e.g., consumption speed, number of drunkenness episodes in the last six months, etc.). It also ensured that every participant fitted with the conditions reported above. Some of the responders satisfying the aforementioned criteria were then invited to come to our laboratory where we ensured, once again, the total absence of exclusion criteria (e.g., history of concussion, history of epilepsy, etc.). Then, subjects performed the task. Once the task was finished, subjects filled in the Beck Depression Inventory (BDI; Beck et al., 1961, Collet and Cottraux, 1986), the State Trait Anxiety Inventory (STAI; A = state anxiety; B = trait anxiety; Spielberger et al., 1983), the UPPS impulsive behaviour scale (Van der Linden et al., 2006) and the Monetary Choice Questionnaire (MCQ; Kirby et al., 1999). The two first questionnaires were used to ensure that participants did not differ with regards to depression or anxiety. The UPPS and MCQ questionnaires were administrated to assess impulsivity and reward discounting, respectively.
2.3. Task
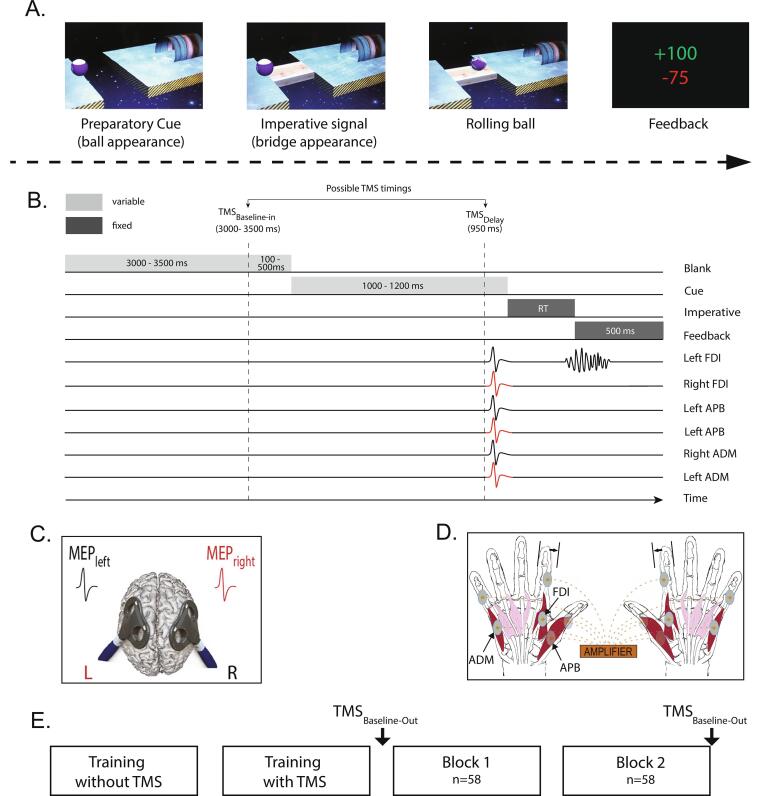
Participants performed an instructed-delay choice RT task, which was implemented with Matlab 7.5 (Mathworks, Natick, Massachusetts, USA) using the Psychophysics Toolbox extensions (Brainard, 1997, Pelli, 1997). It consisted in a virtual rolling ball game previously used in other studies (Grandjean et al., 2019, Quoilin et al., 2019, Quoilin et al., 2018, Quoilin et al.,2016, Vassiliadis et al.,2018b, Wilhelm et al.,2017) (Fig. 1A). In this task, a ball and a goal appear on a computer screen and participants must virtually “shoot the ball into the goal“, by performing an abduction movement with the left or right index finger which requires the activation of the left or right first dorsal interosseous (FDI) muscle, respectively.
Fig. 1.
A. Rolling ball task. Subjects performed an instructed-delay choice reaction time (RT) task, which required them to choose between an abduction movement of the left or right index finger (left in the current example) depending of the position of a preparatory cue (i.e., the ball). They had to withhold their response until the onset of an imperative signal (i.e., the bridge). Once the bridge appeared, they were required to release their prepared response as fast as possible. If they answered correctly, the ball then rolled on the bridge and reached the goal located on the other side of the gap. A feedback reflecting how fast and accurate subjects had been concluded each trial. B. Time course of a trial. Each trial started with the preparatory cue (random duration; 1000–1200 ms) followed by the imperative signal which remained visible until the subject responded (maximum duration of 500 ms). The feedback was presented at the end of each trial for 500 ms. A blank screen (inter-trial interval; between 3100 and 4000 ms) separated each trial. When TMS was applied, motor-evoked potentials (MEPs) were elicited in the first dorsal interosseous (FDI), in the abductor digiti minimi (ADM) and in the abductor pollicis brevis (APB) muscles of both hands at a near simultaneous time (1 ms delay interval between hands, see C). TMS pulses could occur during the inter-trial interval (between 3000 and 3500 ms after the blank screen onset; TMSbaseline−in), or during the delay period (950 ms after the preparatory cue onset; TMSDelay). This figure displays a left-hand trial with MEPs elicited at TMSDelay. C. TMS protocol. Two figure-of-eight coils were placed over the subject’s primary motor cortex (M1), eliciting near simultaneous MEPs (1 ms delay) in the left and right hands. D. Response device and EMG recording. Index finger responses were recorded using a home-made response device positioned under the left and right hands (graphic representation). The response device was composed of two pairs of metal edges fixed on a plastic support. Each response required to move one index finger from the outer to the inner metal edge. EMG activity was recorded from surface electrodes placed on the FDI, the ADM and the APB muscles of both hands. E. Time course of the experiment. After 2 training blocks, subjects performed 2 blocks of 58 trials during which MEPs were elicited either at TMSbaseline−in or TMSDelay, in a random order; MEPs were also elicited outside the context of the task (TMSbaseline−out), that is, before block 1 and after block 2.
Each trial started with the presentation of a preparatory cue, consisting of a ball and a goal separated by a gap. Participants had to prepare an abduction of the left index finger when the ball was presented on the left side of the screen, and an abduction of the right index finger when it appeared on the right side. Subjects were explicitly told to withhold their prepared response until the onset of the imperative signal (i.e., the bridge) which appeared 1000 to 1200 ms later (Fig. 1B). We purposely varied the duration of the delay period to decrease the subjects' tendency to respond prematurely (i.e., before the imperative signal). For the same reason, each block involved a few trials in which the bridge did not appear (i.e., catch trials − 2 per block). In these trials, subjects were required not to respond and were penalised if they did so. Once the bridge was on the screen, subjects had to respond as fast as possible to allow the ball to roll on it and to quickly reach the goal. Each trial ended with a feedback displaying a score for 500 ms, followed by a blank screen lasting between 3100 and 4000 ms (inter-trial interval).
The feedback score depended on how fast and accurate subjects had been on the previous trial. That is, on correct trials, scores were inversely proportional to the RT (i.e., the faster the subjects, the higher the score); they were displayed in green and ranged from 1 to 100. The score was individualised based on the following equation (Vassiliadis et al.,2018b, Vassiliadis et al., 2018), with α = 0.8 × median RT measured at the end of the training session (please refer to the 2.4 experimental procedure section for more information):
Incorrect responses were penalised by a negative score (−75 points) displayed in red. They involved (1) responses occurring too early, referred to as anticipated responses, (2) responses occurring too late, referred to as time-out responses, (3) responses provided with the incorrect hand, referred as the choice errors and (4) responses provided on catch trials, referred to as catch errors. Anticipated responses consisted in responses provided either before the bridge onset or after its onset but with a RT < 100 ms (Grandjean et al., 2019, Vassiliadis et al., 2018, Wilhelm et al., 2016): below this time, we consider that the response was released prematurely (i.e., before detection of the bridge) given the fixed time required for visuo-motor conversion to occur (Valls-Solé et al., 1999). Time-out responses consisted in responses that were longer than 500 ms (i.e., provided after the bridge offset) or that were never provided (Grandjean et al., 2019, Quoilin et al., 2019, Vassiliadis et al.,2018b, Wilhelm et al., 2016). Note that when subjects succeeded not to respond on a catch trial, they received + 75 points.
2.4. TMS protocol
TMS was always delivered using a double-coil TMS method recently developed in our laboratory (Grandjean et al., 2018, Grandjean et al., 2017, Grandjean et al., 2017b, Vassiliadis et al.,2018b, Wilhelm et al., 2016). This method consists in a near-simultaneous stimulation of the two M1 (1 ms inter-pulse interval), eliciting MEPs in both hands at once (Fig. 1C). The short interval allows to avoid direct electromagnetic interference between the two coils, while preventing transcallosal interactions to occur between motor areas (Hanajima et al., 2001, Reis et al., 2008). The MEPs obtained using this double-coil1ms approach are comparable to those elicited using single-coil TMS, regardless of the pulse order or the intensity of stimulation (Derosiere et al., 2020, Grandjean et al., 2018, Vassiliadis et al.,2018b). In the present study the order of stimulation was pseudo-randomised. That is, 50% of the participants (50% of the HCs and 50% of the BDs) received the first TMS pulse over the right M1, eliciting the first MEP in the left hand whilst the other 50% received the first TMS pulse over the left M1 (i.e., first MEP in the right hand).
We applied TMS pulses through small figure-of-eight coils (wing internal diameter, 35 mm), each connected to a stimulator delivering monophasic pulses. The coils were placed tangentially on the scalp of the participant with the handle oriented toward the back of the head and laterally at a 45° angle away from the midline, approximately perpendicular to the central sulcus (Fig. 1C) (Duque and Ivry, 2009, Klein et al., 2016, Quoilin et al., 2019). For each M1, we identified the optimal scalp position for eliciting consistent MEPs in 3 different muscles of the contralateral hand, including the FDI, the abductor digiti minimi (ADM) and the abductor pollicis brevis (APB). This multi-muscle hotspot was marked on a cap placed on the subject’s head to provide a reference mark throughout the experiment (Grandjean et al., 2019, Grandjean et al., 2018, Vandermeeren et al., 2002, Vassiliadis et al., 2018, Wilhelm et al., 2016). Such a procedure allowed us to obtain MEPs from several effectors (bilaterally), including a muscle that was involved in the task (i.e., FDI), another muscle that was irrelevant to the task but with the same innervation as the FDI (i.e., ADM, ulnar nerve) and finally, an irrelevant muscle with a different innervation compared to the FDI (i.e., APB, median nerve) (Fig. 1D). Obtaining MEPs in all these finger muscles was critical as recent studies suggest that preparatory suppression can be underestimated in task relevant effectors, due to concurrent facilitatory influences producing an opposite boost in MEP amplitudes, especially in the prime mover (Quoilin et al.,2016, Wilhelm et al., 2016). In contrast, because preparatory suppression is a rather global effect surpassing the relevant muscles (Duque et al., 2017, Greenhouse et al., 2015b), MEPs in surrounding effectors, which are irrelevant in the task, provide a particularly pure measure of preparatory suppression (preserved from any facilitatory input), probably even more dependable when the innervation diverges. Notably, when finding the hotspots on both M1, we always checked that both coils could be positioned simultaneously on the head without touching each other (Grandjean et al., 2018, Vassiliadis et al.,2018b, Wilhelm et al., 2016). Minor adjustments were sometimes necessary without precluding the acquisition of reliable MEPs in these 3 muscles.
The resting motor threshold (rMT) was determined as the minimal TMS intensity required to evoke MEPs of at least 50 µV peak-to-peak in all 3 muscles for at least 5 out of 10 consecutive trials (Derosiere et al., 2020, Derosiere et al., 2019, Duque et al., 2016, Labruna et al., 2019, Vassiliadis et al., 2019). In practice, most of the time it consisted in finding the rMT for the ADM, which was usually a bit higher than the rMT of the 2 other muscles. Across participants, the rMT corresponded to 41.6 ± 5.4% and 42.5 ± 5.2% of the maximum stimulator output for the left and the right M1, respectively (Table 1). For each hand, the TMS intensity was set at 120% of the individual rMT. Such an intensity allowed us to elicit reliable MEPs in the 3 different hand muscles on both sides.
TMS pulses were delivered during the blocks at one of 2 possible timings (Fig. 1B). To obtain a baseline measure of CS excitability, TMS pulses fell during the inter-trial interval, between 3000 and 3500 ms after the blank screen onset (20 trials per block), eliciting MEPs at rest but in the context of the task (TMSBaseline-In). In other trials (32 trials per block – including catch trials), TMS pulses were delivered 950 ms after the preparatory cue onset (TMSDelay) (Fig. 1B). Based on previous studies, we assumed that at TMSDelay, MEPs would be strongly suppressed – at least for HCs –, reflecting preparatory suppression when subjects are withholding a motor response (Bestmann and Duque, 2016, Duque et al., 2017, Lebon et al., 2016, Vassiliadis et al., 2018). The remaining trials (6 per block), did not include any TMS pulse, preventing participants from anticipating TMS pulses at TMSDelay when it had not occurred at TMSBaseline-In. As a result, each block was composed of 58 trials. In addition to these probes of CS excitability within the blocks, MEPs were also elicited out of the blocks to obtain a baseline measure of the CS excitability at rest and outside the context of the task (TMSBaseline-Out). This involved applying 20 TMS pulses (every 5100 to 6200 ms) at both the beginning and the end of the experiment (Fig. 1E).
2.5. Experimental procedure
All participants were asked to abstain from any alcohol consumption for at least 3 days before the session (Maurage et al., 2013). Experiments were conducted in a quiet and dimly lit room. Participants sat in front of a 21-inch monitor screen positioned about 60 cm in front of them. Their arms were semi-flexed with both hands resting palm-down on a home-made response device developed in our laboratory to detect any horizontal movement of the index fingers (Fig. 1D). This setup provides us with a very precise measure of the RT (precision = 1 ms) and allows us to control the initial index finger position at the beginning of each trial (for more details regarding this device, please refer to Quoilin et al., 2016). Before starting the experiment, participants were presented with some slides containing all the instructions required to understand and perform the task properly. This was done to standardise the information we gave. After presentation of the slides, subjects were asked if they had any questions regarding the task.
The experiment always started with 2 training blocks (Fig. 1E). The first one was composed of 20 trials without TMS and served to familiarize the subjects with the task. The second one involved TMS pulses and was composed of 58 trials, as in the main experiment. Thereby, the subjects could first practice the task without being disturbed by the TMS pulses and then get used to the stimulations while performing the task in the second training block. The latter block also served to obtain the median RT for each individual. As mentioned above, this RT was used to individualise the feedback score (cf. section 2.2 Task). Then, during the main phase of the experiment, subjects performed 2 blocks of 58 trials that were preceded and followed by 20 MEP measurements at TMSBaseline-Out. Using these numbers, for each FDI, ADM and APB muscles, we obtained in each hand, a total of 40 MEPs at TMSBaseline-Out, 40 MEPs at TMSBaseline-In and 64 MEPs at TMSDelay (with half elicited in left hand trials and the other half in right hand trials). Each block lasted approximately 5 min; 2-minute breaks were made between them. After completion of the experiment, participants were asked to fill in psychopathological questionnaires such as the STAI (Spielberger et al., 1983), the BDI (Beck et al., 1961, Collet and Cottraux, 1986), the AUDIT (Saunders et al., 1993), etc. Results of these surveys are reported in Table 1.
2.6. Electromyography (EMG) recording
EMG activity was recorded from surface electrodes (Neuroline, Medicotest, Oelstykke, Denmark) disposed on the FDI, ADM and APB muscles of both hands (Fig. 1D). The electrodes (Ambu Blue Sensor NF-50-K/EU/12) have a skin contact size of 28 × 20 mm. The raw EMG signals were amplified (gain, 1 K), bandpass filtered online (10–500 Hz; NeuroLog; Digitimer) and digitised at 2000 Hz for offline analysis. EMG data were collected for 3000 ms on each trial, starting always 200 ms before the TMS pulse. Trials with any background EMG activity (root mean square computed in the 200 ms windows preceding the TMS artefact) exceeding 3 standard deviations (SD) above the mean were removed; this was made for each muscle to prevent contamination of the MEP measurements by significant fluctuations in background EMG (Grandjean et al., 2019, Vassiliadis et al., 2018). The remaining MEPs were then classified according to the muscle and the experimental condition within which they were elicited. For the analysis of MEPs at TMSDelay, trials in which the subjects made an error were also discarded (Grandjean et al., 2019, Quoilin et al., 2019, Vassiliadis et al.,2018b, Wilhelm et al., 2016). For each condition, we excluded trials with peak-to-peak MEP amplitudes exceeding 3 SD above the mean (Vassiliadis et al., 2020). Following data cleaning, a minimum of 13 trials remained in each condition for each subject. The means of the remaining MEPs in TMSDelay and TMSBaseline-In trials were 27.5 ± 3.1 SD and 38.6 ± 1.6 SD respectively. Importantly, data processing led to the same rate of trial rejection in the two groups.
2.7. Statistical analysis
2.7.1. Demographic and psychopathological measures
Comparisons between groups were performed on demographic, psychological and alcohol consumption characteristics using independent-samples two-sided t-tests (for all variables but gender and smoker). Statistical significance was set at p < 0.05.
2.7.2. Behaviour
Behaviour was assessed by considering RTs as well as the percentage of anticipated and time-out errors for each subject; choice errors and catch errors were rare and thus not analysed. RTs were analysed using a mixed-design analysis of variance (ANOVA) with TMS TIMING (TMSBaseline-In, TMSDelay) and RESPONDING-HAND (left, right) as within-subject factors and GROUP (BDs, HCs) as between-subject factor. Trials without TMS were not analysed because of their limited number (i.e., 12 trials in total; all conditions together). For the analysis of the anticipated and time-out errors, 2 two-sided t-tests were used to compare the percentage values between either group (BDs, HCs). For this analysis, all trials were pooled together, regardless of the responding hand and the TMS setting, to increase the number of observations in each condition. When appropriate, ANOVAs were followed by post-hoc tests using the Fishers least significant difference (LSD) procedure. This procedure was the same as that used in Quoilin et al. (2018). Statistical significance was always set at p < 0.05 for all the analyses. All data are expressed as mean ± standard error (SE).
2.7.3. MEP amplitudes
First, we considered MEPs elicited at baseline. The raw amplitudes of FDI, ADM and APB MEPs (mV) were analysed using three separate mixed-design ANOVAs (one for each muscle) with MEP-SIDE (left, right) and TMS TIMING (TMSBaseline-Out, TMSBaseline-In) as within-subject factors and GROUP (BDs, HCs) as between-subject factor. The three muscles were analysed separately consistent with past research on preparatory suppression, as the multi-muscle hotspot procedure provides MEP amplitudes that vary a lot from one muscle to the other, which makes comparisons between them meaningless. Second, MEPs elicited at TMSDelay were expressed as percentage of MEPs at TMSBaseline-In. To assess the presence of preparatory suppression in each condition, multiple one-sided t-tests (the critical area of the distribution that represents preparatory suppression is one-sided) were carried out to compare these values to 100 (i.e., standing for MEPs elicited at TMSBaseline-In) (Grandjean et al., 2019, Greenhouse et al., 2015a, Quoilin et al., 2019, Vassiliadis et al., 2018). Then, to contrast the amount of preparatory suppression between conditions and groups, we used three mixed-design ANOVAs (one for each muscle) with MEP-SIDE (left, right) and RESPONDING-HAND (left, right) as within-subject factors and GROUP (BDs, HCs) as between-subject factors. Finally, similar as for the behavioural data, we used the Fishers LSD procedure (consistent with the procedure in Quoilin et al., 2018) for post-hoc tests when appropriate. Statistical significance was set at p < 0.05 for all the analyses except for the multiple one-side t-tests against 100 (i.e., t-tests assessing the presence of preparatory suppression at TMSDelay) where we applied Bonferroni corrections to alpha p-levels by dividing the significance level by a factor of 4 (i.e., corresponding to the number of conditions; significance level at p < 0.0125). MEP data are always expressed as mean ± SE.
3. Results and discussion
3.1. Demographic and psychopathological measures
As illustrated in Table 1, BDs and HCs were fully matched for age (t38 = −1.20, p = 0.24) and education level (t38 = −0.17, p = 0.87). In addition, the independent-sample two-sided t-tests showed that BDs did not significantly differ from HCs for depression (BDI; t38 = −0.99, p = 0.33), state of anxiety (STAI-A; t38= −0.30, p = 0.76), trait of anxiety (STAI-B; t38 = −1.88, p = 0.07), impulsivity (UPPS; t38 = 0.95, p = 0.35), reward discounting (MCQ; t38 = −1.20, p = 0.24), years of regular alcohol consumption (t38 = −0.92, p = 0.36) as well as for the rMT of the left hemisphere (t37 = 0.13, p = 0.90), the rMT of the right hemisphere (t37 = 0.47, p = 0.64) and the age at first alcohol consumption (t38 = −1.87, p = 0.07). By contrast, the two groups significantly differed for the AUDIT score (t38 = 6.81, p < 0.001), the binge drinking score (t38 = 5.75, p < 0.001), the consumption speed (t38 = 6.34, p < 0.001) and the number of alcohol drinks taken within two hours (t38 = −6.34, p < 0.001).
Taken together, these results imply that BDs and HCs only differed with respect to the pattern of alcohol consumption exhibited in drinking occasions. At first sight, this might seem odd as scores of impulsivity and sensation seeking are often higher in BDs than HCs (Adan et al., 2017). Note though that there is a high heterogeneity in the profile of BDs that could explain such seemingly contradictory results found in the literature (Gierski et al., 2017, Lannoy et al., 2017a). For instance, Lannoy and colleagues showed with cluster analysis that the impulsivity traits, drinking motives, and alcohol consumption pattern strongly vary among BDs (Lannoy et al., 2017a); whilst BDs belonging to the cluster “emotional BDs” strongly differ from HCs for many subscales of impulsivity, BDs belonging to the “Hazardous BDs” cluster show similar impulsivity traits as HCs. To decrease heterogeneity, future studies could select subjects beforehand to investigate a specific subgroup of BDs or they could enlarge their sample size to perform cluster analyses later.
3.2. Behaviour
Even though the TMS experiment was carried out on 20 HCs, only 19 of them were included in the behavioural analyses because of a technical problem that occurred during the task for one subject (the computer did not save any data). The full data set of BDs was considered (n = 20) for the behavioural analyses.
3.2.1. Reaction times
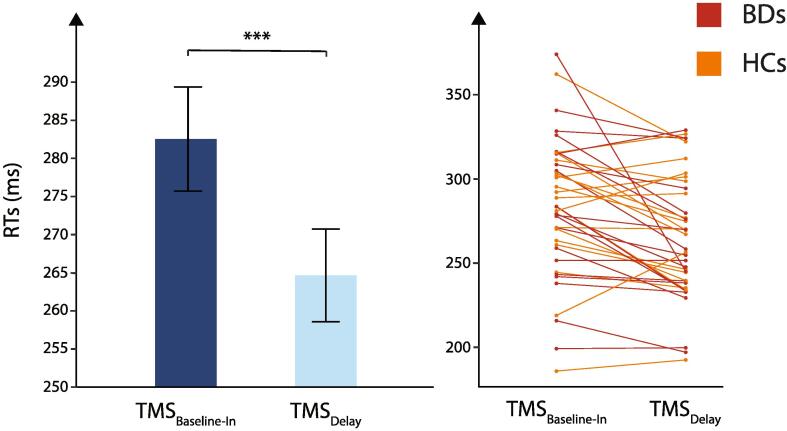
The mixed-design ANOVA showed no significant effect of the factor GROUP (F(1,37) = 0.34, p = 0.56) but a significant main effect of the factor TMS TIMING (F(1,37) = 15.86, p < 0.001): RTs at TMSDelay were significantly shorter than RTs at TMSBaseline−In (264.65 ± 6.08 ms vs 282.54 ± 6.82 ms, respectively, see Fig. 2), both in HCs and BDs (no GROUP × TMS TIMING interaction; F(1,37) = 2.46, p = 0.13). This effect is consistent with many previous reports showing that a TMS pulse applied close to the imperative signal can speed up the release of a motor response (Greenhouse et al., 2015b, Ibáñez et al., 2018, Vassiliadis et al., 2018); similar to the effect of a startling acoustic stimulus when triggered close to an imperative signal (Carlsen et al., 2011, Grandjean et al., 2019, Valls-Solé et al., 1999). Besides, none of the interactions were significant (all F < 2.92 and all p > 0.10). Hence, BDs and HCs responded equally fast in the instructed-delay RT task.
Fig. 2.
Reaction times. Mean reaction times (RTs, in ms) recorded in trials with TMSBaseline−In (dark blue bar) or TMSDelay (light blue bar). Note that RTs were significantly shortened at TMSDelay when compared to TMSBaseline−In. Data from the two GROUPS (binge drinkers and healthy controls) were comparable and thus pooled together on the figure. *** = p < 0.001. Individual data for binge drinkers and healthy controls are also displayed. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Percentage of errors
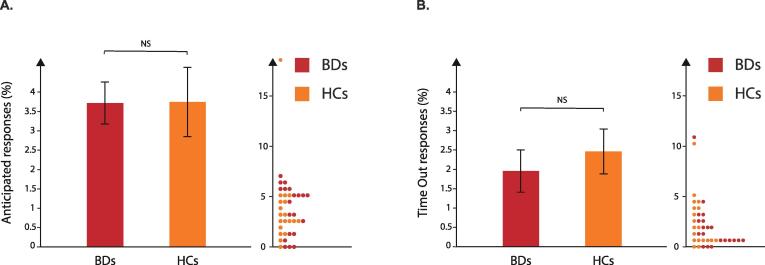
On average, the percentage of anticipated responses corresponded to 3.72 ± 0.54% and 3.75 ± 0.89% for BDs and HCs respectively (Fig. 3A) whilst the percentage of time-out responses equalled 1.96 ± 0.55% and 2.46 ± 0.58% for BDs and HCs respectively (Fig. 3B). The two-sided t-tests performed on the data did not reveal any difference between groups for either variable (t37 = − 0.03, p = 0.98 and t37 = − 0.64, p = 0.53 for anticipated and time-out responses, respectively). These results suggest that BDs and HCs committed a similar amount of errors, as expected in such a simple task (Quoilin et al., 2020).
Fig. 3.
A. Anticipated responses. Percentage of anticipated responses (in %) obtained for binge drinkers (BDs; red bars) and healthy controls (HCs; orange bars). B. Time-out responses. Percentage of time-out responses (in %) obtained for BDs (red bars) and HCs (orange bars). Individual data for BDs and HCs are also displayed. NS = not-significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. MEP Amplitude
MEP analyses were carried out on the full data set of BDs (n = 20) but on only 17 out of the 20 HCs who participated in the study. As such, 3 HCs were excluded due to several technical problems that occurred during the experiment (i.e., the cap moved for one subject, the hotspot turned out not being appropriate in another one and a last subject exhibited a history of concussion, precluding us from obtaining MEP measures in this subject).
3.3.1. Meps elicited at TMSBaseline-Out and TMSBaseline-In
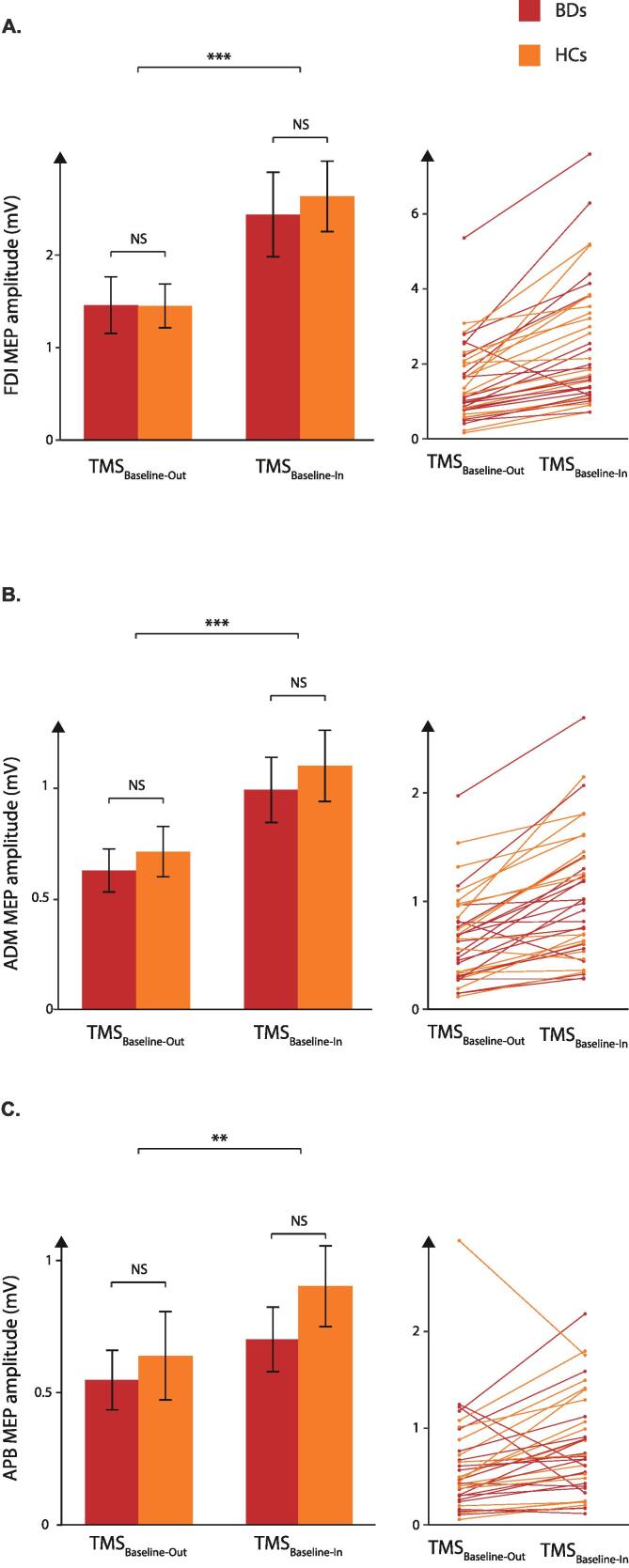
With regard to the FDI, prime mover in the task, the ANOVA showed a main effect of the factor TMS TIMING (F(1;35) = 41.58, p < 0.001) but no main effect of the factor GROUP (F(1;35) = 0.05, p = 0.83) or TMS TIMING × GROUP interaction (F(1,35) = 0.38, p = 0.54). As such, both in HCs and BDs, the amplitude of FDI MEPs elicited at TMSBaseline-In (2.52 ± 0.30 mV) was greater than the amplitude of MEPs at TMSBaseline-Out (1.45 ± 0.20 mV, Fig. 4), consistent with several past reports on HCs (Grandjean et al., 2019, Quoilin et al., 2019, Vassiliadis et al.,2018b). Besides, there was no effect of the factor MEP-SIDE (F(1;35) = 0.03, p = 0.85) and none of the interactions were significant (all F(1;35) < 2.95, all p > 0.09).
Fig. 4.
MEPs at baseline. Amplitude of motor-evoked potentials (MEPs) recorded at baseline (in mV) in the first dorsal interosseous (FDI, A), the abductor digiti minimi (ADM, B) and the abductor pollicis brevis (APB, C). MEPs were always greater when elicited at TMSBaseline-In that when elicited at TMSBaseline-Out both for binge drinkers (BDs; red bars) and healthy controls (HCs; orange bars), regardless of muscle within which MEPs were recorded. Individual data for BDs and HCs are also displayed. NS = not significant; ** = p < . 01*** = p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
MEPs elicited in the task-irrelevant muscles (ADM and APB) showed a similar pattern as those reported above for the FDI. Indeed, the ANOVAs revealed a main effect of the factor TMS-TIMING for the ADM (F(1,35) = 45.20, p < 0.001) and APB (F(1,35) = 8.56, p < 0.01): MEPs were significantly greater when elicited at TMSBaseline-In (1.04 ± 0.11 mV and 0.79 ± 0.1 mV, respectively) than at TMSBaseline-Out (0.67 ± 0.07 mV and 0.59 ± 0.1 mV, respectively) in these task-irrelevant muscles. No other main effect or interaction was significant (ADM: all F(1,35) < 0.14, all p > 0.71; APB: all F(1,35) < 0.58, all p > 0.45). Hence, resting CS excitability was higher during the blocks compared to when subjects were not involved in the task and this effect was present in both groups of subjects and in all hand muscles, regardless of their contribution to the task. This is likely due to differences in the subjects’ state of arousal between the two conditions. Indeed, a previous study (Labruna et al., 2011) reported that MEPs are larger when elicited in the context of a task requiring subjects to passively view hand or landscape images than when MEPs are elicited outside the context of the task, suggesting that the level of vigilance has a significant influence on CS excitability.
3.3.2. Meps elicited at TMSDelay
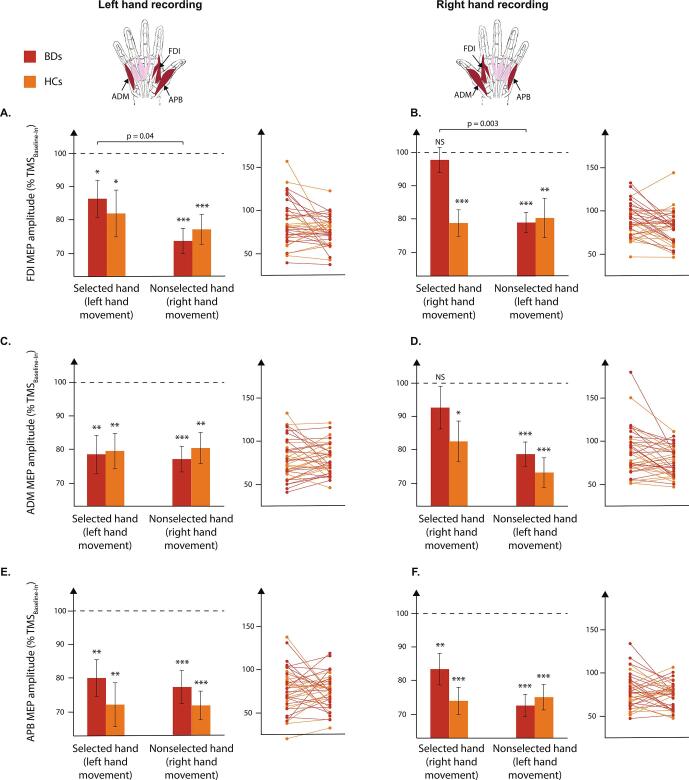
In the FDI of HCs, the t-tests against 100 (i.e., standing for MEPs elicited at TMSBaseline-In) revealed that percentage MEPs were consistently suppressed at TMSDelay. This effect occurred regardless of the hand within which MEPs were elicited and the side of the response (all t > 2.57 and all p < 0.0125), consistent with previous studies (Bestmann and Duque, 2016, Duque et al., 2017, Grandjean et al., 2019, Greenhouse et al., 2015b, Vassiliadis et al., 2018) (Fig. 5). BDs also displayed some preparatory suppression in the FDI, yet contrary to HCs, they did not as consistently: MEPs were suppressed in most of the conditions but not when they fell in a right (i.e., dominant) hand that was selected to respond shortly (t = 0.59 and p = 0.28). The mixed-design ANOVA did not reveal any main effect of the factors GROUP, MEP-SIDE or RESPONDING HAND but a significant GROUP × RESPONDING HAND × MEP-SIDE interaction (F(1,35) = 5.18, p < 0.05). For BDs, FDI MEPs were significantly less suppressed in a responding hand, either left or right, than in a nonresponding hand (LSD all p < 0.05), contrasting with MEPs in HCs, which were evenly suppressed across all conditions in this task-relevant muscle.
Fig. 5.
MEPs at delay. A/B. Amplitude of motor-evoked potentials (MEPs) elicited at TMSDelay (expressed in percentage of MEPs elicited at TMSBaseline-In) in the first dorsal interosseous (FDI) muscles of the left (left panel) or right (right panel) hand for binge drinkers (BDs, red bars) and healthy controls (HCs, orange bars). Note the absence of preparatory suppression for BDs in the right FDI in a condition in which this muscle was selected for the forthcoming response. Of importance also is the fact that FDI MEPs were significantly less suppressed for BDs when they were elicited in a hand that was selected for the forthcoming response than when recorded in a hand that was nonselected. C/D. Amplitude of MEPs elicited at TMSDelay (expressed in percentage of MEPs elicited at TMSBaseline-In) in the abductor digiti minimi (ADM) muscles of the left (left panel) or right (right panel) hand for BDs and HCs. Note the absence of preparatory suppression for BDs in the right ADM in a condition in which the right (i.e., dominant) hand was selected for the forthcoming response. E/F. Amplitude of MEPs elicited at TMSDelay (expressed in percentage of MEPs elicited at TMSBaseline-In) in the abductor pollicis brevis (APB) muscles of the left (left panel) or right (right panel) hand for BDs and HCs. Note that APB MEPs were significantly suppressed at TMSDelay in all conditions for both groups. Individual data for BDs and HCs are also displayed. NS = not-significant; * = p < 0.0125; ** = p < 0.0025; *** = p < 0.00025. Horizontal bars represent the GROUP × RESPONDING HAND × MEP-SIDE interaction. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Surprisingly, preparatory suppression turned out to be quite normal in the task-irrelevant muscles of BDs. In the ADM, MEPs did not show the usual suppression when they fell in the right responding hand of BDs (t = 1.16, p = 0.13) but despite that, the ANOVA did not reveal any GROUP × RESPONDING HAND × MEP-SIDE interaction (F(1,35) = 0.39 and p = 0.54). For the APB, the MEPs were suppressed in all conditions (all values smaller than 100%) in BDs as in HCs (all t > 3.52 and all p < 0.01) and no main effect or interaction were found (all F < 1.16 and all p > 0.29).
In summary, our results are consistent with the view that withholding responses during action preparation involves a global suppression of motor activity (Derosiere et al., 2020, Derosiere and Duque, 2020, Duque et al., 2017). This phenomenon was clear in HCs who systematically displayed smaller MEP amplitudes at TMSDelay in both relevant (FDI) and task-irrelevant (ADM, APB) effectors compared to MEPs probed at TMSBaseline-In. Contrary to our predictions, the MEP suppression was also quite strong in BDs, although CS excitability were not quite normal at TMSDelay either. In fact, preparatory suppression was absent in a very specific condition, when MEPs were elicited in the right FDI (and to some extent in the right ADM too) in right hand trials (i.e. when MEPs were elicited in the right selected hand). Consistently, right FDI MEPs were systematically larger when the right hand was selected for the forthcoming movement compared to when it was nonselected (i.e. in left hand trials) and a comparable effect was also found for left FDI MEPs : they were larger preceding left hand responses (selected condition) than right hand responses (nonselected condition). Importantly, preparatory suppression was perfectly normal in the APB, a muscle innervated separately from the FDI, on top of being irrelevant. Altogether, these findings suggest that generic inhibition operated quite normally in the group of BDs recruited for the current study. If anything, it seems that motor activity associated with the prime mover was larger in BDs than in HCs. This is quite clear from the fact that MEPs were larger (they did not show as much - or any - suppression) in the responding muscle.
It is well accepted that preparatory suppression reflects an initial neural state made of the balance of excitatory and inhibitory influences that foster the preparation of the movement but eventually cancel it out until a go-signal appears (Elsayed et al., 2016, Hannah et al., 2018, Ibáñez et al., 2018, Kaufman et al., 2014). Hence, in BDs, the weaker preparatory suppression observed in the FDI when this muscle was selected for the forthcoming action could reflect a stronger activity of facilitatory inputs to this particular muscle rather than a real lack of inhibition, which again seems normal when considering the other muscles (irrelevant in the task). Interestingly, the strongest effect was observed when movements were performed with the right hand. Even if this was not the case in HCs here, some past studies have reported less preparatory suppression in the responding hand, especially on the dominant side (Quoilin et al.,2016, Wilhelm et al., 2016). Direct comparisons between studies remain however tricky as these experiments substantially differ with regards to the device used to record the response (i.e., standard keyboard vs. response device vs. response provided « in the air ») (Quoilin et al., 2016) and/or with regards to the task parameters (i.e., TMS timings, number of catch trials, individualized score, etc.) (Labruna et al., 2011, Lebon et al., 2016, Wilhelm et al.,2017). Noteworthy, it has been proposed that the larger right hand MEPs preceding responses on that side reflect a greater amount of excitatory influences supporting a strong prepotency or readiness for moving with that hand (Bestmann and Duque, 2016, Wilhelm et al., 2016). Hence, action readiness (and related excitatory influences) may be particularly strong in BDs, resulting in an apparent lack of preparatory suppression, even in a task where inhibitory influences usually take over the changes due to facilitatory inputs at the time of the TMS pulse.
In our prior work on ADIs (Quoilin et al., 2018), we proposed a deficit of preparatory suppression based on observations that were limited to the prime mover (i.e. FDI), as this was the standard in most past studies where task-irrelevant muscles were only rarely considered (Bestmann and Duque, 2016, Lebon et al., 2016, Vassiliadis et al., 2018). Our findings in BDs clearly question this first reading of our data and emphasize the importance of considering task-irrelevant effectors to assess preparatory suppression. As such, the suppression effect is known to expand to various muscles within the moving limb, even if they are not involved in the task (Labruna et al., 2019), as also supported by the current study. Moreover, irrelevant effectors are not (or little) influenced by facilitatory inputs that are mainly centered on the prime mover. Hence, these muscles are likely to provide even cleaner probes of preparatory suppression than the prime movers, where excitatory and inhibitory changes mix up. Given this, at this stage, we do not know whether preparatory suppression is really lacking in ADIs (Nardone et al., 2019) as the weak preparatory suppression may also be due to an abnormally strong facilitation of the prime mover in ADIs. Chronic alcohol exposure has indeed been shown to trigger changes in the functions of both inhibitory and excitatory systems leading to decreased GABAergic and increased glutamatergic functions (Littleton, 2001, Nardone et al., 2010, Olsen and Liang, 2017, Rao et al., 2015). As a result, the motor cortex of ADIs is often reported to be hyper excitable at rest (Naim-Feil et al., 2016, Nardone et al., 2019), though not systematically (Quoilin et al., 2018).
Importantly, it is unclear whether the altered preparatory activity evidenced in BDs arose from heavy alcohol consumption or whether it was present beforehand and rather caused heavy drinking. Indeed, the question of what comes first and what follows (Wise and Koob, 2014) is prominent in the addiction literature and, depending on the proposal, the reading of the data strongly differs. According to the first view, drinking is what altered preparatory activity, while with the second view, this abnormality may have led BDs to drink, thus representing a vulnerability marker of heavy alcohol consumption (Lannoy et al., 2019a). Importantly, both hypotheses are not exclusive and whilst it is possible that altered preparatory activity represents a biomarker for excessive drinking, it is now clearly recognised that heavy alcohol consumption has deleterious consequence on the human brain (López-Caneda et al., 2014). Ideally, longitudinal studies should be performed to shed light on this important question.
In conclusion, future studies are required to identify the cause of abnormal preparatory activity, both in BDs and in ADIs. A clear understanding of preparatory suppression in ADIs should involve measures in task-irrelevant muscles. Only then, will we be able to conclude whether BDs and ADIs share similar alterations at the motor level as implied by the continuum hypothesis (ENOCH, 2006, Lannoy et al., 2019a). Moreover, the use of paired-pulse or directional TMS approaches (Derosiere and Duque, 2020, Hallett, 2000, Reis et al., 2008, Rossini et al., 2015, Derosiere et al., 2020, Di Lazzaro et al., 2001, Di Lazzaro et al., 2017, Hannah et al., 2018) or the combination of TMS with functional/diffusion magnetic resonance imaging techniques represent interesting lines of future investigation to evaluate the involvement of specific cortical circuits to preparatory suppression (including facilitatory and inhibitory inputs).
4. Conclusions
This study provides first evidence that BDs present abnormal preparatory activity at the level of the motor output system, alike ADIs. It remains however unclear whether the underlying causes of these alterations are shared amongst BDs and ADIs. Ultimately, for both populations, the question remains wide open as to whether peculiar preparatory activity accounts for a deficit of inhibition, from an excess of facilitation, or from both.
CRediT authorship contribution statement
Julien Grandjean: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. Julie Duque: Conceptualization, Methodology, Formal analysis, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Auriane Fierens, Chloé Warnauts, Émilie Lancelon and Mathilde Verdure for their greatly appreciated help in the data collection and analyses. This work was supported by grants from the ‘‘Fonds Spéciaux de Recherche’’ (FSR) of the Université catholique de Louvain, the Belgian National Funds for Scientific Research (FRS—FNRS: MISF.4512.14) and the ‘‘Fondation Médicale Reine Elisabeth’’ (FMRE). J.G. was a graduate student supported by a Fund for Research Training in Industry and Agriculture (FRIA).
References
- Adan A., Forero D.A., Navarro J.F. Personality traits related to binge drinking: a systematic review. Front. Psychiatry. 2017;8:1–11. doi: 10.3389/fpsyt.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and statistical manual of mental disorders. Arlington. 2013 doi: 10.1176/appi.books.9780890425596.744053. [DOI] [Google Scholar]
- Baler R.D., Volkow N.D. Drug addiction: the neurobiology of disrupted self-control. Trends Mol. Med. 2006;12(12):559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bestmann S., Duque J. Transcranial magnetic stimulation: decomposing the processes underlying action preparation. Neuroscientist. 2016;22(4):392–405. doi: 10.1177/1073858415592594. [DOI] [PubMed] [Google Scholar]
- Bestmann S., Krakauer J.W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015;233(3):679–689. doi: 10.1007/s00221-014-4183-7. [DOI] [PubMed] [Google Scholar]
- Billieux J., Gay P., Rochat L., Khazaal Y., Zullino D., Van der Linden M. Lack of inhibitory control predicts cigarette smoking dependence: Evidence from a non-deprived sample of light to moderate smokers. Drug Alcohol. Depend. 2010;112(1-2):164–167. doi: 10.1016/j.drugalcdep.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Bø R., Aker M., Billieux J., Landrø N.I. Binge drinkers are fast, able to stop – but they fail to adjust. J. Int. Neuropsychol. Soc. 2016;22(1):38–46. doi: 10.1017/S1355617715001204. [DOI] [PubMed] [Google Scholar]
- Brainard D.H. The psychophysics toolbox. Spatial Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Brion M., D’Hondt F., Pitel A.-L., Lecomte B., Ferauge M., de Timary P., Maurage P. Executive functions in alcohol-dependence: a theoretically grounded and integrative exploration. Drug Alcohol. Depend. 2017;177:39–47. doi: 10.1016/j.drugalcdep.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Brust, J., 2010. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int. J. Environ. Res. Public Health 7, 1540–1557. https://doi.org/10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed]
- Camchong, J., Endres, M., Fein, G., 2014. Decision making, risky behavior, and alcoholism. pp. 227–236. https://doi.org/10.1016/B978-0-444-62619-6.00014-8. [DOI] [PubMed]
- Carlsen A.N., Maslovat D., Lam M.Y., Chua R., Franks I.M. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci. Biobehav. Rev. 2011;35(3):366–376. doi: 10.1016/j.neubiorev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Collet L., Cottraux J. Inventaire abrégé de la dépression de Beck (13 items): Étude de la validité concurrente avec les échelles de Hamilton et de ralentissement de Widlöcher. Encéphale. 1986;12:77. [PubMed] [Google Scholar]
- Cos I., Duque J., Cisek P. Rapid prediction of biomechanical costs during action decisions. J. Neurophysiol. 2014;112(6):1256–1266. doi: 10.1152/jn.00147.2014. [DOI] [PubMed] [Google Scholar]
- Courtney, K.E., Polich, J., 2009. Binge drinking in young adults: data, definitions, and determinants. Psychol. Bull. 135, 142–156. https://doi.org/10.1037/a0014414. [DOI] [PMC free article] [PubMed]
- Crabbe, J.C., Harris, R.A., Koob, G.F., 2011. Preclinical studies of alcohol binge drinking. Ann. N. Y. Acad. Sci. 1216, 24–40. https://doi.org/10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed]
- Cservenka A., Brumback T. The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L., Charlson F., Ferrari A., Santomauro D., Erskine H., Mantilla-Herrara A., Whiteford H., Leung J., Naghavi M., Griswold M., Rehm J., Hall W., Sartorius B., Scott J., Vollset S.E., Knudsen A.K., Haro J.M., Patton G., Kopec J., Carvalho Malta D., Topor-Madry R., McGrath J., Haagsma J., Allebeck P., Phillips M., Salomon J., Hay S., Foreman K., Lim S., Mokdad A., Smith M., Gakidou E., Murray C., Vos T. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L., O'Loughlin C., Swift W., Romaniuk H., Carlin J., Coffey C., Hall W., Patton G. The persistence of adolescent binge drinking into adulthood: findings from a 15-year prospective cohort study. BMJ Open. 2013;3(8):e003015. doi: 10.1136/bmjopen-2013-003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosiere G. A dynamical system framework for theorizing preparatory inhibition. J. Neurosci. 2018;38(14):3391–3393. doi: 10.1523/JNEUROSCI.0069-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosiere G., Duque J. Tuning the corticospinal system: how distributed brain circuits shape human actions. Neuroscientist. 2020 doi: 10.1177/1073858419896751. [DOI] [PubMed] [Google Scholar]
- Derosiere G., Thura D., Cisek P., Duque J. Motor cortex disruption delays motor processes but not deliberation about action choices. J. Neurophysiol. 2019;122(4):1566–1577. doi: 10.1152/jn.00163.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosiere G., Vassiliadis P., Duque J. Advanced TMS approaches to probe corticospinal excitability during action preparation. NeuroImage. 2020;213:116746. doi: 10.1016/j.neuroimage.2020.116746. [DOI] [PubMed] [Google Scholar]
- Derosiere G., Zénon A., Alamia A., Duque J. Primary motor cortex contributes to the implementation of implicit value-based rules during motor decisions. NeuroImage. 2017;146:1115–1127. doi: 10.1016/j.neuroimage.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Devos G., Clark L., Maurage P., Kazimierczuk M., Billieux J. Reduced inhibitory control predicts persistence in laboratory slot machine gambling. Int. Gambl. Stud. 2015;15(3):408–421. [Google Scholar]
- Di Lazzaro V., Oliviero A., Saturno E., Pilato F., Insola A., Mazzone P., Profice P., Tonali P., Rothwell J.C. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp. Brain Res. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Rothwell J., Capogna M. Noninvasive stimulation of the human brain: activation of multiple cortical circuits. Neuroscientist. 2018;24(3):246–260. doi: 10.1177/1073858417717660. [DOI] [PubMed] [Google Scholar]
- Dum R., Strick P. Motor areas in the frontal lobe of the primate. Physiol. Behav. 2002;77(4-5):677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Duque J., Greenhouse I., Labruna L., Ivry R.B. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 2017;40(4):219–236. doi: 10.1016/j.tins.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J., Ivry R.B. Role of corticospinal suppression during motor preparation. Cereb. Cortex. 2009;19(9):2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J., Labruna L., Verset S., Olivier E., Ivry R.B. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J. Neurosci. 2012;32(3):806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J., Lew D., Mazzocchio R., Olivier E., Ivry R.B. Evidence for two concurrent inhibitory mechanisms during response preparation. J. Neurosci. 2010;30(10):3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J., Petitjean C., Swinnen S.P. Effect of aging on motor inhibition during action preparation under sensory conflict. Front. Aging Neurosci. 2016;8 doi: 10.3389/fnagi.2016.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed G.F., Lara A.H., Kaufman M.T., Churchland M.M., Cunningham J.P. Reorganization between preparatory and movement population responses in motor cortex. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENOCH M.-A. Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann. N. Y. Acad. Sci. 2006;1094(1):193–201. doi: 10.1196/annals.1376.019. [DOI] [PubMed] [Google Scholar]
- Gierski F., Benzerouk F., De Wever E., Duka T., Kaladjian A., Quaglino V., Naassila M. Cloninger's temperament and character dimensions of personality and binge drinking among college students. Alcohol. Clin. Exp. Res. 2017;41(11):1970–1979. doi: 10.1111/acer.13497. [DOI] [PubMed] [Google Scholar]
- Grandjean J., Derosiere G., Vassiliadis P., Quemener L., de Wilde Y., Duque J. Validation of a double-coil TMS method to assess corticospinal excitability. Brain Stimulation. 2017;10(2):507. doi: 10.1016/j.brs.2017.01.480. [DOI] [Google Scholar]
- Grandjean, J., Derosiere, G., Vassiliadis, P., Quemener, L., de Wilde, Y., Duque, J., 2017b. Investigating the reliability of a double-coil TMS method to assess corticospinal excitability. In: 27th Neural Control Mov. Meet. Dublin - Irel. https://doi.org/10.3389/conf.fnins.2017.94.00118.
- Grandjean J., Derosiere G., Vassiliadis P., Quemener L., Wilde Y.d., Duque J. Towards assessing corticospinal excitability bilaterally: validation of a double-coil TMS method. J. Neurosci. Methods. 2018;293:162–168. doi: 10.1016/j.jneumeth.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Grandjean J., Quoilin C., Duque J. Investigating the effect of anticipating a startling acoustic stimulus on preparatory inhibition. Neurophysiol. Clin. 2019;49(2):137–147. doi: 10.1016/j.neucli.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Greenhouse I., Saks D., Hoang T., Ivry R.B. Inhibition during response preparation is sensitive to response complexity. J. Neurophysiol. 2015;113(7):2792–2800. doi: 10.1152/jn.00999.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I., Sias A., Labruna L., Ivry R.B. Nonspecific inhibition of the motor system during response preparation. J. Neurosci. 2015;35(30):10675–10684. doi: 10.1523/JNEUROSCI.1436-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406(6792):147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hanajima, R., Ugawa, Y., Machii, K., Mochizuki, H., Terao, Y., Enomoto, H., Furubayashi, T., Shiio, Y., Uesugi, H., Kanazawa, I., 2001. Interhemispheric facilitation of the hand motor area in humans. J. Physiol. 531, 849–859. https://doi.org/10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed]
- Hannah R., Cavanagh S.E., Tremblay S., Simeoni S., Rothwell J.C. Selective suppression of local interneuron circuits in human motor cortex contributes to movement preparation. J. Neurosci. 2018;38(5):1264–1276. doi: 10.1523/JNEUROSCI.2869-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D.E., Elsabagh S., File S.E. Binge drinking and sex: effects on mood and cognitive function in healthy young volunteers. Pharmacol. Biochem. Behav. 2004;78(3):611–619. doi: 10.1016/j.pbb.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T., Kaneko H., Akamatsu M., Possamaï C.-A. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Cogn. Brain Res. 1997;5(3):185–192. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Heikkinen N., Niskanen E., Könönen M., Tolmunen T., Kekkonen V., Kivimäki P., Tanila H., Laukkanen E., Vanninen R. Alcohol consumption during adolescence is associated with reduced grey matter volumes: adolescent alcohol use and grey matter. Addiction. 2017;112(4):604–613. doi: 10.1111/add.13697. [DOI] [PubMed] [Google Scholar]
- Henderson K.E., Vaidya J.G., Kramer J.R., Kuperman S., Langbehn D.R., O'Leary D.S. Cortical thickness in adolescents with a family history of alcohol use disorder. Alcohol. Clin. Exp. Res. 2018;42(1):89–99. doi: 10.1111/acer.13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R.W., Heeren T., Winter M.R. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch. Pediatr. Adolesc. Med. 2006;160(7):739. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Ibáñez, J., Hannah, R., Rocchi, L., Rothwell, J.C., 2018. Premovement suppression of corticospinal excitability may be a necessary part of movement preparation. bioRxiv 1–35. https://doi.org/http://dx.doi.org/10.1101/470153. [DOI] [PubMed]
- Kaufman M.T., Churchland M.M., Ryu S.I., Shenoy K.V. Cortical activity in the null space: permitting preparation without movement. Nat. Neurosci. 2014;17(3):440–448. doi: 10.1038/nn.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby, K.N., Petry, N.M., Bickel, W.K., 1999. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 128, 78–87. https://doi.org/10.1037/0096-3445.128.1.78. [DOI] [PubMed]
- Klein P.-A., Duque J., Labruna L., Ivry R.B. Comparison of the two cerebral hemispheres in inhibitory processes operative during movement preparation. NeuroImage. 2016;125:220–232. doi: 10.1016/j.neuroimage.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E., Kuntsche S., Thrul J., Gmel G. Binge drinking: health impact, prevalence, correlates and interventions. Psychol. Health. 2017;32(8):976–1017. doi: 10.1080/08870446.2017.1325889. [DOI] [PubMed] [Google Scholar]
- Labruna L., Fernández-del-Olmo M., Ivry R.B. Comparison of different baseline conditions in evaluating factors that influence motor cortex excitability. Brain Stimulation. 2011;4(3):152–155. doi: 10.1016/j.brs.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Labruna L., Tischler C., Cazares C., Greenhouse I., Duque J., Lebon F., Ivry R.B. Planning face, hand, and leg movements: anatomical constraints on preparatory inhibition. J. Neurophysiol. 2019;121(5):1609–1620. doi: 10.1152/jn.00711.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy, S., Billieux, J., Dormal, V., Maurage, P., 2019a. Behavioral and cerebral impairments associated with binge drinking in youth: a critical review. Psychol. Belg. 59, 116–155. https://doi.org/10.5334/pb.476. [DOI] [PMC free article] [PubMed]
- Lannoy S., Billieux J., Poncin M., Maurage P. Binging at the campus: motivations and impulsivity influence binge drinking profiles in university students. Psychiatry Res. 2017;250:146–154. doi: 10.1016/j.psychres.2017.01.068. [DOI] [PubMed] [Google Scholar]
- Lannoy S., Dormal V., Billieux J., Maurage P. A joint exploration of executive subcomponents in binge drinking. Addict. Res. Theor. 2019;27(6):498–506. [Google Scholar]
- Lannoy S., Dormal V., Brion M., Gaudelus B., Billieux J., Maurage P. Affective impairments in binge drinking: investigation through emotional facial expression decoding. Compr. Psychiatry. 2018;83:59–63. doi: 10.1016/j.comppsych.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Lannoy S., Heeren A., Moyaerts N., Bruneau N., Evrard S., Billieux J., Maurage P. Differential impairments across attentional networks in binge drinking. Psychopharmacology. 2017;234(7):1059–1068. doi: 10.1007/s00213-017-4538-4. [DOI] [PubMed] [Google Scholar]
- Lebon F., Greenhouse I., Labruna L., Vanderschelden B., Papaxanthis C., Ivry R.B. Influence of delay period duration on inhibitory processes for response preparation. Cereb. Cortex. 2016;26(6):2461–2470. doi: 10.1093/cercor/bhv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Thayer R., Squeglia L.M., McQueeny T.M., Tapert S.F. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. Neuroimag. 2013;211(1):17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton, J., 2001. Receptor regulation as a unitary mechanism for drug tolerance and physical dependence - not quite as simple as it seemed! Addiction 96, 87–101. https://doi.org/10.1046/j.1360-0443.2001.961877.x. [DOI] [PubMed]
- López-Caneda, E., Rodríguez Holguín, S., Cadaveira, F., Corral, M., Doallo, S., 2014. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol. 49, 173–181. https://doi.org/10.1093/alcalc/agt168. [DOI] [PubMed]
- Luciana M., Collins P.F., Muetzel R.L., Lim K.O. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am. J. Drug Alcohol Abuse. 2013;39(6):345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P., Bestelmeyer P.E.G., Rouger J., Charest I., Belin P. Binge drinking influences the cerebral processing of vocal affective bursts in young adults. NeuroImage: Clin. 2013;3:218–225. doi: 10.1016/j.nicl.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P., Joassin F., Speth A., Modave J., Philippot P., Campanella S. Cerebral effects of binge drinking: respective influences of global alcohol intake and consumption pattern. Clin. Neurophysiol. 2012;123(5):892–901. doi: 10.1016/j.clinph.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Michelet T., Duncan G.H., Cisek P. Response competition in the primary motor cortex: corticospinal excitability reflects response replacement during simple decisions. J. Neurophysiol. 2010;104(1):119–127. doi: 10.1152/jn.00819.2009. [DOI] [PubMed] [Google Scholar]
- Naim-Feil J., Bradshaw J.L., Rogasch N.C., Daskalakis Z.J., Sheppard D.M., Lubman D.I., Fitzgerald P.B. Cortical inhibition within motor and frontal regions in alcohol dependence post-detoxification: a pilot TMS-EEG study. World J. Biol. Psychiatry. 2016;17(7):547–556. doi: 10.3109/15622975.2015.1066512. [DOI] [PubMed] [Google Scholar]
- Nardone, R., Bergmann, J., Kronbichler, M., Caleri, F., Lochner, P., Tezzon, F., Ladurner, G., Golaszewski, S., 2010. Altered motor cortex excitability to magnetic stimulation in alcohol withdrawal syndrome. Alcohol. Clin. Exp. Res. 34, 628–632. https://doi.org/10.1111/j.1530-0277.2009.01131.x. [DOI] [PubMed]
- Nardone R., Trinka E., Sebastianelli L., Versace V., Saltuari L. Commentary: deficient inhibition in alcohol-dependence: let’s consider the role of the motor system! Front. Neurosci. 2019;13:147–150. doi: 10.3389/fnins.2019.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism, [NIAAA], 2004. NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsl. No. 33. Bethesda, MD NIAAA.
- Norman Andria, L, Pulido Carmen, Squeglia Lindsay, M, Spadoni Andrea, D, Paulus Martin, P, Tapert Susan., F Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119(3):216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olsen R.W., Liang J. Role of GABAA receptors in alcohol use disorders suggested by chronic intermittent ethanol (CIE) rodent model Tim Bliss. Mol. Brain. 2017;10:1–20. doi: 10.1186/s13041-017-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A., Leung J., Larney S., Colledge S., Hickman M., Rehm J., Giovino G.A., West R., Hall W., Griffiths P., Ali R., Gowing L., Marsden J., Ferrari A.J., Grebely J., Farrell M., Degenhardt L. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018;113(10):1905–1926. doi: 10.1111/add.14234. [DOI] [PubMed] [Google Scholar]
- Pelli D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Petit, G., Maurage, P., Kornreich, C., Verbanck, P., Campanella, S., 2014. Binge drinking in adolescents: a review of neurophysiological and neuroimaging research. Alcohol Alcohol. 49, 198–206. https://doi.org/10.1093/alcalc/agt172. [DOI] [PubMed]
- Pfefferbaum A., Rohlfing T., Pohl K.M., Lane B., Chu W., Kwon D., Nolan Nichols B., Brown S.A., Tapert S.F., Cummins K., Thompson W.K., Brumback T.y., Meloy M.J., Jernigan T.L., Dale A., Colrain I.M., Baker F.C., Prouty D., De Bellis M.D., Voyvodic J.T., Clark D.B., Luna B., Chung T., Nagel B.J., Sullivan E.V. Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cereb. Cortex. 2016;26(10):4101–4121. doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C., Fievez F., Duque J. Preparatory inhibition: impact of choice in reaction time tasks. Neuropsychologia. 2019;129:212–222. doi: 10.1016/j.neuropsychologia.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Quoilin, C., Grandjean, J., Duque, J., 2020. Considering motor excitability during action preparation in gambling disorder: a transcranial magnetic stimulation study 11, 1–13. https://doi.org/10.3389/fpsyt.2020.00639. [DOI] [PMC free article] [PubMed]
- Quoilin, C., Lambert, J., Jacob, B., Klein, P.-A., Duque, J., 2016. Comparison of motor inhibition in variants of the instructed-delay choice reaction time task. PLoS One 11, e0161964. https://doi.org/10.1371/journal.pone.0161964. [DOI] [PMC free article] [PubMed]
- Quoilin C., Wilhelm E., Maurage P., de Timary P., Duque J. Deficient inhibition in alcohol-dependence: let’s consider the role of the motor system! Neuropsychopharmacology. 2018;43(9):1851–1858. doi: 10.1038/s41386-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P.S.S., Bell R.L., Engleman E.A., Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Front. Neurosci. 2015;9 doi: 10.3389/fnins.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J., Mathers C., Popova S., Thavorncharoensap M., Teerawattananon Y., Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Reis, J., Swayne, O.B., Vandermeeren, Y., Camus, M., Dimyan, M.A., Harris-Love, M., Perez, M.A., Ragert, P., Rothwell, J.C., Cohen, L.G., 2008. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 586, 325–351. https://doi.org/10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R., Di Lazzaro V., Ferreri F., Fitzgerald P.B., George M.S., Hallett M., Lefaucheur J.P., Langguth B., Matsumoto H., Miniussi C., Nitsche M.A., Pascual-Leone A., Paulus W., Rossi S., Rothwell J.C., Siebner H.R., Ugawa Y., Walsh V., Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, J.B., Aasland, O.G., Babor, T.F., De La Fuente, J.R., Grant, M., 1993. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88, 791–804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed]
- Siqueira L., Smith V.C. Binge drinking. Pediatrics. 2015;136(3):e718–e726. doi: 10.1542/peds.2015-2337. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Mattick R.P., Jamadar S.D., Iredale J.M. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol. Depend. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Psychol. Press; Palo Alto, CA Consult: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Stavro, K., Pelletier, J., Potvin, S., 2013. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict. Biol. 18, 203–213. https://doi.org/10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed]
- Sullivan E.V., Harris A. Alcohol’s effects on brain and behavior. Alcohol Res. Heal. 2010;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- Townshend J.M., Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol. Clin. Exp. Res. 2005;29(3):317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- Valls-Solé, J., Rothwell, J.C., Goulart, F., Cossu, G., Muñoz, E., 1999. Patterned ballistic movements triggered by a startle in healthy humans. J. Physiol. 516, 931–938. https://doi.org/10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed]
- Van der Linden M., d'Acremont M., Zermatten A., Jermann F., Larøi F., Willems S., Juillerat A.-C., Bechara A. A French adaptation of the UPPS impulsive behavior scale. Eur. J. Psychol. Assessment. 2006;22(1):38–42. [Google Scholar]
- van Velzen L.S., Vriend C., de Wit S.J., van den Heuvel O.A. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front. Hum. Neurosci. 2014;8:1–22. doi: 10.3389/fnhum.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermeeren Y., De Volder A., Bastings E., Thonnard J.-L., Duqué J., Grandin C., Sébire G., Olivier E. Functional relevance of abnormal fMRI activation pattern after unilateral schizencephaly. NeuroReport. 2002;13(14):1821–1824. doi: 10.1097/00001756-200210070-00027. [DOI] [PubMed] [Google Scholar]
- Vassiliadis, P., Derosiere, G., Grandjean, J., Duque, J., 2020. Motor training strengthens corticospinal suppression during movement preparation. bioRxiv 2020.02.14.948877. https://doi.org/10.1101/2020.02.14.948877. [DOI] [PubMed]
- Vassiliadis P., Grandjean J., Derosiere G., de Wilde Y., Quemener L., Duque J. Using a double-coil TMS protocol to assess preparatory inhibition bilaterally. Front. Neurosci. 2018;12:1–14. doi: 10.3389/fnins.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliadis, P., Grandjean, J., Derosiere, G., de Wilde, Y., Quemener, L., Duque, J., 2018b. Probing preparatory inhibition bilaterally with double-coil TMS. Front. Neurosci. Conf. Abstr. Belgian Brain Congr. 2018 — Belgian Brain Counc. https://doi.org/10.3389/conf.fnins.2018.95.00029.
- Vassiliadis, P., Grandjean, J., Derosiere, G., Duque, J., 2019. Training-related modulation of preparatory activity in the motor system. Progr. No. 312.09. 2019 Neurosci. Meet. Planner. Chicago, Soc. Neurosci. 2019. Online.
- Verdejo-García A., Lawrence A.J., Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wagner F. From first drug use to drug dependence developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wessel J.R., Aron A.R. On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron. 2017;93(2):259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J.R., Reynoso H.S., Aron A.R. Saccade suppression exerts global effects on the motor system. J. Neurophysiol. 2013;110(4):883–890. doi: 10.1152/jn.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox, C.E., Dekonenko, C.J., Mayer, A.R., Bogenschutz, M.P., Turner, J.A., 2014. Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev. Neurosci. 25, 1–24. https://doi.org/10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed]
- Wilhelm, E., Duque, J., Grandjean, J., 2017. Testing the influence of various parameters on preparatory motor inhibition: a possible explanation for discrepancies between previous studies? Front. Neurosci. Conf. Abstr. 12th Natl. Congr. Belgian Soc. Neurosci. https://doi.org/10.3389/conf.fnins.2017.94.00080.
- Wetherill Reagan, R, Squeglia Lindsay, M, Yang Tony, T, Tapert Susan., F A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230(4):663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm E., Quoilin C., Petitjean C., Duque J. A double-coil TMS method to assess corticospinal excitability changes at a near-simultaneous time in the two hands during movement preparation. Front. Hum. Neurosci. 2016;10:1–11. doi: 10.3389/fnhum.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A., Koob G.F. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao PeiRong, Dai ZhenYu, Zhong JianGuo, Zhu YingLing, Shi HaiCun, Pan PingLei. Regional gray matter deficits in alcohol dependence: a meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend. 2015;153:22–28. doi: 10.1016/j.drugalcdep.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Zahr N.M., Pfefferbaum A. Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol Res. 2017;38:183–206. [PMC free article] [PubMed] [Google Scholar]