Abstract
Objectives
We aimed to calculate cumulative hepatitis C virus (HCV) treatment coverage among individuals enrolled in opioid agonist therapy (OAT) in Norway between 2013 and 2017 and to document the treatment transition to direct-acting antiviral (DAA) agents. Moreover, we aimed to describe adherence to DAAs in the same cohort.
Design
Prospective cohort, registry data.
Setting
Specialist healthcare service (secondary)
Participants and outcomes
This observational study was based on data from The Norwegian Prescription Database. We studied dispensed OAT and HCV treatment annually to calculate the cumulative frequency, and employed secondary sources to calculate prevalence, incidence and HCV treatment coverage from 2013 to 2017, among the OAT population. Factors associated with adherence to DAAs were identified a priori and subject to logistic regression.
Results
10 371 individuals were identified with dispensed OAT, 1475 individuals of these were identified with dispensed HCV treatment. Annual HCV treatment coverage increased from 3.5% (95% CI: 3.2 to 4.4) in 2013 to 17% (95% CI: 17 to 20) in 2017, giving a cumulative HCV coverage among OAT patients in Norway of 38.5%. A complete shift to interferon-free treatment regimens occurred, where DAAs accounting for 32% of HCV treatments in 2013 and 99% in 2017. About two-thirds of OAT patients were considered adherent to their DAA regimens across all genotypes. High level of OAT continuity was associated with improved adherence to DAAs (adjusted OR 1.4, 95% CI: 1 to 2, p=0.035).
Conclusions
A large increase in HCV treatment coverage attributed by a complete shift to interferon-free regimens among the Norwegian OAT population has been demonstrated. However, treatment coverage is inadmissibly too low and a further substantial scale-up in HCV treatment is required to reach the universal targets of controlling and eliminating the HCV endemic.
Keywords: infectious diseases, epidemiology, organisation of health services, health policy, substance misuse, infection control
Strengths and limitations of this study.
All dispensed drugs from pharmacies in Norway are registered in the database.
The completeness, precision and validity of data are high among a hard-to-reach population.
Data were not linked on an individual level to diagnosis codes of chronic hepatitis C virus (HCV).
HCV prevalence and incidence data are imprecise.
Treatment with direct-acting antivirals was limited during the study period from 2013 to 2017 based on stage of liver fibrosis.
Background
The large burden of chronic hepatitis C virus (HCV) among people who inject drugs (PWIDs) and recent developments in HCV medications creates an opportunity to eliminate HCV epidemics. Worldwide, about 71 million people are chronically infected with the virus and 399 000 died annually from HCV-related complications such as liver cirrhosis or hepatocellular carcinoma.1 2 Despite the low aggregated HCV prevalence in many countries (1.5%–3.5% in Western Europe and <1.5% in North America), prevalence is much higher among PWID (50% or more).3–5 WHO’s Global Health Sector Strategy aims to eliminate viral hepatitis as a public health threat by 2030.2 The even bolder Norwegian HCV strategy aims to reduce national incidence by 90% by 2023.6 Eliminating chronic HCV requires a significant effort in terms of increasing uptake of testing, diagnosing and linking to care. In addition, other strategies have been proposed alongside increasing antiviral treatment, such as opioid agonist therapy (OAT) scale-up, safe injection sites and sterile injection equipment to reach these objectives.2 7
Injecting drug use and needle sharing is the major driver of HCV incidence;8 however, the coverage of preventive interventions, such as needle and syringe programmes, remains poor among PWIDs.9 Number of people who actively inject drugs in Norway have been stable at around 9000 since 2012 till 2017 (2.6 per 1000 inhabitants aged 15–64 years),10 11 and opioids and amphetamines are the main injected drugs.11 12 Modelling studies suggest that around 7000 former and current PWIDs are living with chronic HCV with an estimated 400 new cases annually in the same time period.13 14 Both HCV-related liver morbidity and mortality are increasing among PWIDs and are likely to continue to increase until 2022.14
OAT has been put forward to play a vital role in the management of chronic HCV among people with opioid dependence and has been shown to reduce the risk of HCV acquisition.15 For these reasons, OAT may be crucial intervention for achieving large reductions in HCV transmissions by reducing risk behaviours such as injecting use and sharing of injecting equipment.16 HCV testing rates have been low in the national OAT programme in Norway with great annual and regional variations.5 17–20 Only in parts of Western Norway, as part of the multicenter INTRO-HCV study, all patients receiving OAT have been systematically tested and examined with elastography as part of an annual health assessment since 2017.21 Even if access to HCV treatment is improving, HCV treatment coverage remains low.8 22–25 Globally, the coverage of HCV curative treatment was 13% by 2016.26 In Norway, annual HCV treatment coverage among OAT patients was between 1.3% and 2.6% in the period from 2004 to 2013, giving a cumulative HCV coverage for the period of 14%.27
The introduction of direct-acting antiviral (DAA) medications, with a curation rate of approximately 95%, safer and better tolerated than interferon-based therapy, has dramatically changed the treatment of chronic HCV infections.28 29 Even if currently expensive, they are considered cost-effective from a societal perspective as universal coverage with antiretroviral treatment could prevent large expenses related to future complications.30–35 Combining DAAs with the OAT delivery platform may, thus, prove critical for achieving reductions in HCV prevalence and incidence.22 A number of treatment barriers exist, which should in turn be carefully addressed; nevertheless, treatment barriers should not exclude PWIDs from HCV treatment.8 36 37 Both WHO and Norwegian guidelines support DAA treatment among PWIDs and have also shown good outcomes in systematic reviews.24 25 38
The pathway to universal HCV treatment coverage has not been well documented at country levels; hence, the primary aim of the study was to:
Document HCV treatment annually and cumulatively after the introduction of DAAs among patients receiving OAT in Norway from 2013 to 2017 and to calculate HCV treatment coverage, both annually and cumulatively.
A second objective is to evaluate adherence to DAAs among OAT patients in Norway.
Methods
Study design and data sources
This is an observational study among OAT patients from 2013 to 2017 in Norway. Data were extracted from The Norwegian Prescription Database (NorPD) from 1 January 2013 to 31 March 2018. The database covers the entire Norwegian population and records all drugs dispensed from pharmacies in Norway. All drugs are classified according to The Anatomical Therapeutic Chemical (ATC) classification system.39 Defined daily doses (DDDs) according to 201840 were employed to quantify the dispensed OAT and HCV medications, respectively. The DDDs are the assumed average maintenance dose per day for a drug used for its main indication.41
Data from The Norwegian Centre for Addiction Research were used for estimating the prevalence of chronic HCV among OAT patients, whereas incidence data among Norwegian PWIDs were gathered from The Norwegian Institute of Public Health and Meijerink et al.14
Study population and definitions
The study population included all individuals with at least one dispensed prescription of buprenorphine (ATC code N07BC01), methadone (N07BC02), buprenorphine–naloxone (N07BC51) and levomethadone (N07BC05). Patients<18 years and with other indications than OAT were excluded from the study on the basis of formulation and route of administration (online supplementary figure S1).
bmjopen-2019-036355supp001.pdf (378.5KB, pdf)
Exposure to HCV treatment was defined as being dispensed either pegylated interferon alpha (L03AB05 and L03AB11) and ribavirin (J05AP01) or any of the DAAs (in group J05AP, see online supplementary table S1 for complete list of DAAs by ATC code) during the study period. The first dispensed DAA according to ATC code was noted, and to prevent over-counting, patients were only counted once at initiation. Thus, definition of treatment was any individual on OAT who has been dispensed HCV treatment. Any individual who died was censored in the calendar year they passed away. Rates were calculated by dividing number of individuals with dispensed HCV treatment by individuals on OAT, stratified by each calendar year. The cumulative frequency, which is the addition of successive years of treatment, was then calculated. HCV treatment was stratified as overall treatment with any chronic HCV medication and treatment with solely DAAs.
HCV treatment coverage was defined as individuals on OAT identified in NorPD annually, adjusted for death, HCV prevalence and new cases of chronic HCV each year, which had received treatment for chronic HCV during the study period. Mean prevalence during the study period among patients enrolled in OAT ranged from 51% in 2013 to 43% in 20175 17–20 and proportional prevalence among OAT individuals were calculated per calendar year. Incidence was around 400 per year for PWIDs during the study period.14 It proved methodologically challenging to estimate number of new cases of chronic HCV among OAT patients. As the OAT coverage among people with opioid dependence is between 50% and 60% in Norway,5 OAT patients account for only a proportion of overall PWIDs, and thus needed to be adjusted for in our calculation. For this reason, expert opinion were obtained from clinicians in addiction medicine and set to a 0.70 (70%) proportion, giving between 277 and 256 new cases annually during the study period. We developed the following basic model for our coverage calculation:
where HCVcov is HCV coverage, tHCV=number of OAT patients with dispensed HCV treatment, pHCV=number of OAT patients with chronic HCV and iHCV=number of new cases of chronic HCV among OAT patients. Coverage was calculated annually for Norway and by Health County, and as cumulative frequencies.
We defined adherence to DAA as having collected prescriptions equivalent to 3 months of treatment. DAAs for adults, which is only prescribed by specialists, are collected for 1 month at-a-time basis where a typical DAA treatment course is 12 weeks, that is, 3 dispensed prescriptions and ≥84 DDDs. The exception is the drug combination ledipasvir/sofosbuvir, which may be prescribed for 8 weeks (two collections and ≥56 DDD) for cases of previously untreated genotype 1 infections. This allowed us to examine adherence based on number of dispensed prescriptions and DDDs. Impending factors associated with treatment adherence to DAAs were identified a priori and included gender, age and OAT continuity, and subject to multivariate analyses in a step-by-step model.
Finally, OAT continuity was defined according to dispensed DDDs and stratified into three categories, ranging from a high level of OAT continuity in category I (≥2 DDD), medium in category II (1–2 DDD) and to a low level of OAT continuity in category III (<1 DDD). One DDD for methadone and buprenorphine is 25 and 8 mg, respectively.
Statistical analyses and strategy
Descriptive data are presented as frequencies, percentages, means and with corresponding 95% confidence intervals where appropriate. Logistic regression on factors associated with adherence is presented as adjusted OR when adjusted for age, gender and OAT continuity.
The data were analysed with SPSS V.24 and Stata SE V.15 (StataCorp, Texas, USA). Map figures were made in R.
Data handling and ethical considerations
Since all registry data were received pseudo-anonymously from the registry administrator and subsequently analysed anonymously no written consent was obtained from any of the individuals in the study.42
Patient and public involvement
No patients were directly involved in this study; however, as part of the bigger Integrated treatment of hepatitis C (INTRO-HCV) project patients through user organisations, such as Pro-LAR, were involved in the planning process, workshops that included design and recruitment, protocol writing and assessment of the burden of the intervention in the randomised controlled trial.
Results
Basic characteristics of study population
A total of 10 371 individuals were identified in NorPD having received ≥1 OAT prescriptions during the study period from 2013 to 2017 (table 1). Almost 70% were men, mean age of 43 and 45 years in 2013 and 2017, respectively. The majority of the OAT patients were treated with buprenorphine-based OAT medication (55% in 2013 and 61% in 2017). Over 50% of individuals on OAT had a high level of continuity. Altogether, 692 individuals died during the study period.
Table 1.
Basic characteristics of patients receiving OAT from 2013 to 2017 in Norway
| Basic characteristics | Total | 2013 | 2014 | 2015 | 2016 | 2017 |
| Individuals>1 OAT | 10 371 | 7709 | 7914 | 7958 | 7804 | 7709 |
| Deaths | 692 | 165 | 151 | 138 | 114 | 124 |
| Gender, n (%) | ||||||
| Male | 7135 (69) | 5221 (69) | 5390 (69) | 5430 (69) | 5354 (70) | 5254 (69) |
| Female | 3236 (31) | 2323 (31) | 2373 (31) | 2390 (31) | 2336 (30) | 2340 (31) |
| Age, n (%) | ||||||
| <25 | 211 (3) | 185 (2) | 171 (3) | 135 (2) | 120 (2) | |
| 26–40 | 2813 (37) | 2797 (36) | 2718 (40) | 2574 (33) | 2432 (32) | |
| 41–60 | 4289 (57) | 4537 (58) | 3644 (53) | 4627 (60) | 4613 (61) | |
| >60 | 231 (3) | 244 (3) | 287 (4) | 354 (5) | 420 (6) | |
| OAT medication, n (%) | ||||||
| Methadone/levomethadone | 3406 (45) | 3264 (42) | 3216 (41) | 3066 (40) | 2981 (39) | |
| Buprenorphine based* | 4138 (55) | 4499 (58) | 4604 (59) | 4624 (60) | 4604 (61) | |
| Dispensions of HCV drugs† | 1475 | 146 | 167 | 243 | 322 | 597 |
| OAT continuity category, n (%) | ||||||
| I: ≥2 DDD | 5310 (51) | |||||
| II: 1–2 DDD | 3078 (30) | |||||
| III: <1 DDD | 1983 (19) | |||||
*Buprenorphine and buprenorphine/naloxone.
†HCV drugs: interferon-based and DAAs.
DDDs, Daily defined Doses; HCV, hepatitis C virus; NorPD, Norwegian prescription database; OAT, opioid agonist therapy.
HCV treatment and coverage
HCV and DAA treatment
All individuals were stratified according to the year in which they received OAT and HCV treatment. Excluding deaths, this gave a fairly stable OAT population just in excess of 7500 annually. In 2013, 146 OAT patients received HCV treatment. Treatment increased over time with 597 patients receiving HCV treatment in 2017. Overall, 1475 patients on OAT received HCV treatment during the study period, with an annual HCV treatment increasing from 1.9% (95% CI: 1.6 to 2.3) in 2013 to 7.9% (95% CI: 7.3 to 8.5) in 2017 (online supplementary table S2). By 2017, the cumulative frequency of HCV treatment reached 19% among patients on OAT.
Of the 1475 individuals who received HCV treatment during the study period, 1235 were treated with DAAs. The annual DAA treatment ranged from 0.6% (95% CI: 0.4 to 0.8) in 2013 to 7.8 (95% CI: 7.2 to 8.4) in 2017. The proportion of treated individuals receiving DAAs increased over time from 32% of HCV-treated OAT patients in 2013 to 99% in 2017.
HCV treatment: coverage
We calculated annual HCV coverage among the estimated number of OAT patients who are HCV infected, which ranged from 3.5% (95% CI: 3.2 to 4.4) in 2013 to 17% (95% CI: 16.9 to 19.6) in 2017. This gave a cumulative frequency that reached 38.5% in 2017 (table 2). Figure 1 shows cumulative HCV coverage from 2013 to 2017 by the four health counties in Norway (HCVcov and data from online supplementary table S3 were used for these calculations). There were little variation in treatment coverage across the four health counties.
Table 2.
Annual and cumulative chronic HCV treatment coverage among OAT patients in Norway between 2013 and 2017
| Source | 2013 | 2014 | 2015 | 2016 | 2017 | Total | |
| Chronic HCV treatment, n (overall) | NorPD | 146 | 167 | 243 | 322 | 597 | 1475 |
| DAAs, n | NorPD | 46 | 95 | 212 | 290 | 592 | 1235 |
| DAAs, % of HCV | 32 | 57 | 87 | 90 | 99 | 84 | |
| Study population, n, yearly including deaths | NorPD | 7709 | 7914 | 7958 | 7804 | 7709 | 10 371 |
| Deaths | NorPD | 165 | 151 | 138 | 114 | 124 | 692 |
| Study population, n, yearly, excluding deaths | NorPD | 7544 | 7763 | 7820 | 7690 | 7585 | 9679 |
| Prevalence chronic HCV, mean % | SERAF | 51 | 52 | 52 | 46 | 43 | |
| Prevalence chronic HCV, n | SERAF | 3847 | 4037 | 4066 | 3537 | 3262 | |
| Incidence chronic HCV among PWIDs, n | NIPH, Mejierick et al14 | 396 | 388 | 381 | 374 | 366 | |
| Incidence chronic HCV OAT from PWIDs, n | Expert opinion | 277 | 272 | 267 | 262 | 256 | |
| Treatment coverage chronic HCV, % | 3.5 | 3.9 | 5.6 | 8.5 | 17 | ||
| Cumulative frequency chronic HCV | 3.5 | 7.4 | 13 | 21.5 | 38.5 | ||
| 95% CI treatment coverage chronic HCV | 3.2 to 4.4 | 3.5 to 4.8 | 5.3 to 6.7 | 8.2 to 10.1 | 16.9 to 19.6 |
DAA, direct-acting antivirals; HCV, hepatitis C virus; NIPH, Norwegian Institute for Public Health; NorPD, Norwegian Prescription Database; OAT, opioid agonist therapy;PWID, people who inject drugs; SERAF, The Norwegian Centre for Addiction Research.
Figure 1.
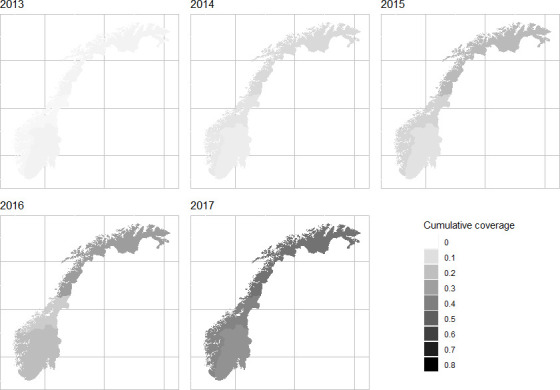
Cumulative chronic HCV treatment coverage among OAT patients, from 0 (white) to 100% (black) in Norway between 2013 and 2017 by health counties*. Meijerink et al 14 (2017) calculations in online supplementary table S2 *Cumulative coverage in %, the four health counties: Helse Vest, Helse Midt, Helse Nord and Helse Sør-Øst. OAT, opioid agonist therapy; HCV hepatitis C virus. NorPD, Norwegian Prescription Database; SERAF, The Norwegian Centre for Addiction Research; NIPH, Norwegian Institute for Public Health.
Adherence to DAAs
Overall, almost 70% of the OAT patients were adherent to their DAA regimen and considered to have finished their DAA treatment course (table 3). There were no major differences by gender or OAT drug. However, for age, patients in the age group 18–35 were less adherent (42%) compared with older age groups. The drug combination of elbasvir/grazoprevir, commonly used for treatment of genotype 1 infections, had by far the utmost adherence (93%) compared with treatment combinations of sofosbuvir/velpatasvir and ledipasvir/sofosbuvir, which both were around 70%. However, sometimes ledipasvir/sofosbuvir is prescribed for 8 weeks, in which case yields an overall adherence of 78%.
Table 3.
Adherence* to DAAs among OAT patients in Norway between 2013 and 2017
| Adherent | Non-adherent | Total | |
| Adherence by gender, n (%) | |||
| Male | 551 (67) | 277 (33) | 828 |
| Female | 191 (67) | 92 (33) | 283 |
| Total | 742 (67) | 369 (33) | 1111 |
| Adherence by age, n (%) | |||
| 18–35 | 119 (58) | 85 (42) | 204 |
| 36–45 | 259 (68) | 122 (32) | 381 |
| 46–55 | 302 (70) | 128 (30) | 430 |
| >56 | 62 (65) | 34 (35) | 95 |
| Total | 742 (67) | 369 (33) | 1111 |
| Adherence by OAT medication, n (%) | |||
| Methadone/levomethadone | 298 (65) | 157 (35) | 455 |
| Buprenorphine based | 444 (68) | 212 (32) | 656 |
| Total | 742 (67) | 369 (33) | 1111 |
| Logistic regression on factors associated with adherence* | |||
| aOR (CI 95%) | P value | ||
| Age | 0.98 (0.97 to 1.00) | 0.17 | |
| Gender | |||
| Male | 1.00 | ||
| Female | 0.92 (0.69 to 1.23) | 0.57 | |
| OAT continuity | |||
| Category I: ≥2 DDD | 1.00 | ||
| Category II: 1–2 DDD | 1.36 (1.02 to 1.82) | 0.035 | |
| Category III: <1 DDD | 1.36 (0.93–1.99) | 0.11 | |
*Adherence defined as collected ≥3 prescriptions and >84 DDDs (unless ledipasvir and sofosbuvir which also calculated as ≥2 prescriptions and >56 DDDs). Analyses included 1111 patients as inclusion were ceased 01.10.17 to avoid counting treatment initiation after this date non-adherent.
DAA, direct-acting antivirals; DDD, daily defined doses; NorPD, Norwegian Prescription Database; OAT, opioid agonist therapy.
In multivariate analyses, only OAT continuity was associated with adherence to DAAs (adjusted OR 1.4, 95% CI: 1.0 to 1.8, p=0.035).
Discussion
The HCV treatment coverage has increased substantially, yet it seems too low if the ambitious targets of ending the endemic are to be met. Annual treatment rate increased from 1.9% of all OAT patients in Norway in 2013 to 7.9% in 2017, which gives a cumulative frequency of around 19% over the study period. However, cumulative HCV treatment coverage among OAT patients with assumed chronic HCV in Norway was just above 38%, with annual treatment coverage that ranged from 3.5% in 2013 to 17% in 2017. Second, we observed a complete shift in the HCV treatment among OAT patients in Norway during the study period, from two-thirds treated with DAAs in 2013 to nearly all in 2017. Finally, about two-thirds of all OAT patients with chronic HCV were considered adherent to their DAAs regimen, which improved with level of OAT continuity.
It can be useful to compare our results at country levels. Immense advances have been made in chronic HCV treatment since the introduction of DAAs in recent years; however, multiple studies have demonstrated continued low treatment uptake among PWIDs and OAT patients,23 27 43 partly explained by varying and restricted treatment access policies that prevented a widespread scale-up of DAA treatment during the study period.44 For instance, England saw one of the most restricted access policies to DAA treatment compared with for example France and Germany, which had the least restrictions.45 Consequently HCV treatment rates varied dramatically across European countries ranging from 0.6% to 10.2% in 2015.46 In the same year, we found HCV treatment rate of 5.6% in Norway, which is similar to Sweden; however, higher than Denmark that saw treatment rate more in line with the overall 3.7% that year among European countries.46 Prior to the introduction of DAAs, Midgard et al showed an annual treatment coverage of 1.3%–2.6% between 2004 and 2013 among Norwegian OAT patients, giving a cumulative treatment coverage of 14% during the entire study period. Considering there is not in place a national and systematic programme for testing and linking to HCV care among PWIDs, nor has the full effectiveness of integrated treatment combining OAT and HCV treatment been fully demonstrated,47 HCV coverage would probably be substantially higher with a comprehensive model of integrative care where both testing and treatment were provided in OAT outpatient clinics.
The Norwegian hepatitis C policy identifies improved access to treatment, prevention and surveillance of the endemic as crucial to succeed with HCV elimination strategies.48 Treatment with DAAs in Norway was until 1 February 2018, limited by eligibility criteria based on stage of liver fibrosis. Since then, DAA treatment has been offered to all regardless of genotype and level of liver fibrosis. As a result, treatment demand increased and coverage of curative HCV treatment has amplified. From 2014 to June 2018, around 5000 patients were treated for chronic HCV in Norway; however, these patients are mostly former PWIDs and immigrants being infected prior to the arrival in Norway.13 It is unclear how many of these patients were on OAT and overlapped with our results. Nonetheless, despite continued falling prices of DAAs, which have made unrestricted treatment possible for all, HCV treatment and coverage remains low among active PWIDs,13 which is in line with our results demonstrating the need for a significant scale-up to improve HCV coverage and being able to plan elimination strategies. It may, therefore, be crucial to identify other barriers to treatment for this vulnerable patient group. Arguably, even with DAA treatment for all, low threshold OAT, needle and syringe programmes in place, it is hard to see how this can be achieved unless testing and linkage to care is provided where PWIDs and OAT patients actually are. This opts for decentralised testing and treatment and probably a change in how the specialist healthcare delivers treatment for current PWIDs. A substantial scale-up in DAA treatment requires Norway’s capacity and health system infrastructure at large, in addition to take place among this group of patients, which have the highest transmission risk in order for treatment-as-prevention strategies to succeed. In terms of surveillance, chronic HCV prevalence and incidence data are not readily available for Norway. The infection is regarded as a Group A infectious disease and it has been mandatory to notify The Norwegian Surveillance System for Communicable Diseases (MSIS) since 1990. However, only cases of acute HCV were notifiable initially. Since 1 January 2016 it was changed to merely include HCV RNA and HCV core antigen.13 Thus, it is impossible to tell whether cases before 2016 were acute or chronic, or whether patients achieved sustained virological response (SVR) on their own, or how many cases were actually notified.27
About two-thirds of all patients were considered adherent to the DAA regimens. At first, this may seem low; however, this may be related to patients being categorised as adherent (100%) and non-adherent (<100%) according to recommendations from the prescribing specialist. For instance, the SIMPLIFY study, while demonstrating that 97% of PWIDs completed DAA treatment, overall 32% were considered non-adherent (<90% adherence) with median adherence at 94%.49 Another study among PWIDs and OAT patients, were 97% completed DAA treatment, found a non-adherence of 40% (<90%) and median adherence at 92%.50 Other studies have shown that high adherence to DAAs is achievable with appropriate supportive strategies.51 52 As such, adherence can be a key predictor for response to DAAs.51 Perhaps the most compelling evidence among PWIDs and OAT patients is a recent systematic review that showed DAA completion rate of above 97% among almost 4500 participants.53 Our intention was to evaluate to what extent patients initiated and complied to treatment, rather than drawing a comparison between individual DAAs. The main reason for this is varying adherence to drug protocol and guidelines for DAAs during the study period from a prescriber’s perspective, which was only moderate after introduction of DAAs, although it increased markedly after 2015.54 In addition, since included patients were only counted once on DAA initiation, there is some uncertainty whether patients in the non-adherent group had lengthier treatment courses due to for instance awaiting liver transplantation or becoming reinfected with HCV. Rate of reinfection is controversial and less understood; however, it seems to be low between 1% and 5% in the interferon era.55 After the introduction of DAAs, a study found six cases of reinfection among 301 patients (4.6 reinfections per 100 person-years), with three of those experiencing spontaneous clearance of their reinfection.56
Adherence to DAAs was associated with OAT continuity, and as such, predicted a higher adherence compared with lower level of OAT continuity in our model. Studies have shown that patients receiving higher doses of OAT, for example methadone, above 60 mg/day, have better treatment outcomes compared with lower doses48 57 and for this reason we set high level of continuity above two DDD. This is in line with previous studies demonstrating that OAT continuity is a factor for HCV treatment.27 Age was not considered statistical significant; however, less adherence was noted in the younger age groups. Dissimilarities in methodology and study settings, however, prevent for precise comparisons of adherence, including the above. Linking these data, on an individual level, to biomarkers of SVR12 was, however, beyond the scope of this paper. In addition, we had no system in place to control whether these patients actually swallowed and metabolised these drugs and as such cannot comment to the extent the medications were actually taken.
Strengths and limitations
All dispensed drugs from pharmacies in Norway are registered in NorPD. This provide researchers and other stakeholders alike with sound, precise and a near complete database. The main strength of the study is, thus, it provides a large sample of hard-to-reach patients being treated for chronic HCV.
However, this study has some limitations, which should be considered when interpreting both results and conclusions. Treatment with OAT in Norway is not uniform. It is estimated that NorPD captures around 90% of the patients with dispensed OAT from pharmacies.5 The 10%, which is not included in our study, could represent OAT patients with more need for follow-up in the OAT outpatient clinics, and as such, can represent patients with higher disease burden and in need of HCV treatment. This could skew our results toward underestimating the HCV treatment coverage as these patients would not be included in our study. On the other hand, our estimates can also be overestimates. OAT patients have successfully entered the healthcare system, and therefore more likely to accept other medical care, including HCV treatment, and thus bias our results toward improved HCV treatment coverage.
OAT and HCV treatment administered to hospitalised and institutionalised patients are not recorded on an individual level in NorPD. Nonetheless, it should be stated that almost all HCV treatment is initiated in outpatient clinics in Norway, and hence included in NorPD.27 58 In addition, some dispensed prescriptions may lack reimbursement codes and medical indication for use, and DDDs does not necessarily reflect the prescribed daily dose.
Furthermore, data were not linked on an individual level to diagnosis codes of chronic HCV. This is due to the quality of MSIS prior to 2016 is poor and the authors had to employ other data sources when estimating HCV prevalence and incidence rates from a number of different sources, including modelling and expert opinion. This could lead to either overestimating or underestimating the HCV coverage. We believe, however, that the 0.7 (70%) proportion represents a liberal estimate and the biggest risk is that we overestimated the HCV incidence. When calculating the HCV prevalence, mean population data for Norway were used, rather than more accurate regional data as the latter were not readily available. In addition, treatment with DAAs was limited by stage of liver fibrosis during the study period. Only from 1 February 2018, it was offered universally regardless of level of liver fibrosis. Thus, it is likely that younger patients and patients with Metavir F0–F1 Score were excluded from DAA treatment during the study period.
When measuring adherence among different age groups, we should be careful when interpreting results. Older patients are more likely to have cirrhosis and longer HCV treatment courses compared with younger patients. This could bias our results toward higher adherence among the latter. Finally, PWIDs are a heterogenic group of individuals, and one should be careful not to generalise OAT patients to include all PWIDs.
Conclusion
This is the first population-based study documenting the transition to DAA treatment regimens among Norwegian OAT patients. A marked scale-up in HCV treatment coverage attributed by a complete shift to interferon-free regimens among Norwegian OAT patients has been demonstrated. Adherence to DAAs across all genotypes remained sound, especially for high level of OAT continuity. Annual HCV treatment coverage ranged from 3.5% in 2013 to 17% in 2017, giving a cumulative HCV coverage among OAT patients for the study period just above 38%. Despite a large increase in treatment, overall HCV coverage is inadmissibly too low in order to meet the ambitious national and WHO targets of controlling and eliminating chronic HCV. There is a need to establish more accurate monitoring system and more precision in prevalence and incidence rates of chronic HCV among PWID to get more precise coverage data. Efficacy of health system strategies is needed in order to further scale-up of the most effective HCV policies to this group and for countries to be able to control and eliminate HCV.
Supplementary Material
Footnotes
Contributors: This observational study was led by CFA in terms of study design, analyses, drafting and writing the article. SS and JHV was particularly involved with acquisition of data, analyses and interpretation. Maps were made by JMØ and KAJ. SS, JHV, IO, FC, JMØ, RAML, PV, KAJ and LTF contributed to the conception, writing and revising the draft(s) critically. All authors have read and approved the version to be published.
Funding: This study is part of the main Integrated treatment of hepatitis C (INTRO-HCV) study, which was funded by The Norwegian Research Council (no. 269855) and the Western Norway Regional Health Authority (Åpen prosjektstøtte) with Department of Addiction Medicine, Haukeland University Hospital as responsible institution. The funders had no role in the study design, data collection and analyses, decision to publish, nor preparation of any content in the manuscript. Two of the authors, CFA and JHV, are funded from the above research grant, whereas the other authors are funded by their respective affiliations.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: IO is employed at the Centre for Pharmacoepidemiology, Karolinska Institutet, which receives grants from several entities (pharmaceutical companies, regulatory authorities and contract research organisations) for performance of drug safety and drug utilisation studies, unrelated to this work. None of the other authors have competing interests.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the regional committee for ethics in medical research (no. 2018/939/REK Vest). It was conducted in accordance with the Declaration of Helsinki and as an observational study in accordance with international accepted STROBE guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. No additional data are available due to data protection requirements.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333–42. 10.1002/hep.26141 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection, 2018. [PubMed] [Google Scholar]
- 3.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378:571–83. 10.1016/S0140-6736(11)61097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017;5:e1192–207. 10.1016/S2214-109X(17)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waal HBK, Clausen T, Lillevold PH, et al. Seraf report 2017: status MAR 20 years. status, evaluations and perspectives: the Norwegian centre for addiction research (Seraf, 2018. [Google Scholar]
- 6.Ministry of Health and Care Services National strategy against hepatitis 2018 - 2023: Ministry of Health and Care Services, 2018. [Google Scholar]
- 7.Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol 2018;68:402–11. 10.1016/j.jhep.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grebely J, Robaeys G, Bruggmann P, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy 2015;26:1028–38. 10.1016/j.drugpo.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health 2017;5:e1208–20. 10.1016/S2214-109X(17)30373-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amundsen EJ, Bretteville-Jensen AL, Kraus L. A method to estimate total entry to hard drug use: the case of intravenous drug use in Norway, European addiction research. 17, 2011: 129–35. [DOI] [PubMed] [Google Scholar]
- 11.European Monitoring Centre for Drugs and Drug Addiction Country drug report 2018. Norway, 2018. [Google Scholar]
- 12.The Norwegian Institute of Public Health Drugs in Norway: problematic use of drugs. The Norwegian Institute of Public Health, 2018. [Google Scholar]
- 13.The Norwegian Institute of Public Health Hepatitis C - guidelines for health professionals. The Norwegian Institue of Public Health, 2019. [Google Scholar]
- 14.Meijerink H, White RA, Løvlie A, et al. Modelling the burden of hepatitis C infection among people who inject drugs in Norway, 1973-2030. BMC Infect Dis 2017;17:541. 10.1186/s12879-017-2631-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017;9:Cd012021. 10.1002/14651858.CD012021.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gowing L, Farrell MF, Bornemann R, et al. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2011:CD004145. 10.1002/14651858.CD004145.pub4 [DOI] [PubMed] [Google Scholar]
- 17.Waal HBK, Clausen T, Håseth A, et al. Seraf report: status report 2013. The Norwegian Centre of Addiction Reserach (SERAF), 2014. [Google Scholar]
- 18.Waal HBK, Clausen T, Håseth A, et al. Seraf report: status 2014, an aging MAR-population? The Norwegian Centre for Addiction Research (SERAF), 2015. [Google Scholar]
- 19.Waal HBK, Clausen T, Håseth A, et al. Seraf report: status report 2015. The Norwegian Centre for Addiction Research, 2016. [Google Scholar]
- 20.Waal HBK, Clausen T, Håseth A, et al. Seraf report: status 2016. The Norwegian Centre for Addiction Reserach (SERAF), 2017. [Google Scholar]
- 21.Fadnes LT, Aas CF, Vold JH, et al. Integrated treatment of hepatitis C virus infection among people who inject drugs: study protocol for a randomised controlled trial (INTRO-HCV). BMC Infect Dis 2019;19:943. 10.1186/s12879-019-4598-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013;58:1598–609. 10.1002/hep.26431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner City residents. J Viral Hepat 2009;16:352–8. 10.1111/j.1365-2893.2009.01080.x [DOI] [PubMed] [Google Scholar]
- 24.Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis 2013;57 Suppl 2:S80–9. 10.1093/cid/cit306 [DOI] [PubMed] [Google Scholar]
- 25.Dimova RB, Zeremski M, Jacobson IM, et al. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis 2013;56:806–16. 10.1093/cid/cis1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization Newsroom: hepatitis C. World Health Organization (WHO), 2018. [Google Scholar]
- 27.Midgard H, Bramness JG, Skurtveit S, et al. Hepatitis C treatment uptake among patients who have received opioid substitution treatment: a population-based study. PLoS One 2016;11:e0166451. 10.1371/journal.pone.0166451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalgard O, Weiland O, Noraberg G, et al. Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-A Scandinavian real-life study. PLoS One 2017;12:e0179764. 10.1371/journal.pone.0179764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Association for Study of Liver EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015;63:199–236. 10.1016/j.jhep.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 30.Selvapatt N, Ward T, Harrison L, et al. The cost impact of outreach testing and treatment for hepatitis C in an urban drug treatment unit. Liver Int 2017;37:345-353. 10.1111/liv.13240 [DOI] [PubMed] [Google Scholar]
- 31.Younossi Z, Henry L. The impact of the new antiviral regimens on patient reported outcomes and health economics of patients with chronic hepatitis C. Dig Liver Dis 10.1016/j.dld.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 32.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-Effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med 2015;162:407–19. 10.7326/M14-1152 [DOI] [PubMed] [Google Scholar]
- 33.Chhatwal J, Kanwal F, Roberts MS, et al. Cost-Effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med 2015;162:397–406. 10.7326/M14-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younossi ZM, Park H, Saab S, et al. Cost-Effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther 2015;41:544–63. 10.1111/apt.13081 [DOI] [PubMed] [Google Scholar]
- 35.Selvapatt N, Ward T, Harrison L, et al. The cost impact of outreach testing and treatment for hepatitis C in an urban drug treatment unit. Liver Int 2017;37:345–53. 10.1111/liv.13240 [DOI] [PubMed] [Google Scholar]
- 36.Patel K, Zickmund SL, Jones H, et al. Determinants of hepatitis C treatment adherence and treatment completion among veterans in the direct acting antiviral era. Dig Dis Sci 2019;64:3001–12. 10.1007/s10620-019-05590-x [DOI] [PubMed] [Google Scholar]
- 37.Malespin M, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol 2019;18:304–9. 10.1016/j.aohep.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization WHO Guidlines for the screening, care and treatment for persons with chronic hepatitis C infection. WHO, 2016. [PubMed] [Google Scholar]
- 39.The Norwegian Prescription Database (NorPD). About the Norwegian prescription database. The Norwegian Institute of Public Health, 2019. [Google Scholar]
- 40.WHO Who collaborating centre for drug statistics methodology. ATC classification index with DDDs 2018, 2018. [Google Scholar]
- 41.The World Health Organization Collaborating Centre for Drug Statistics Methodology Definition and general considerations. World Health Organization, 2019. [Google Scholar]
- 42.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500–24. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 43.Alavi M, Raffa JD, Deans GD, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner City residents. Liver Int 2014;34:1198–206. 10.1111/liv.12370 [DOI] [PubMed] [Google Scholar]
- 44.Lazarus JV, Safreed-Harmon K, Stumo SR, et al. Restrictions on access to direct-acting antivirals for people who inject drugs: the European Hep-CORE study and the role of patient groups in monitoring national HCV responses. Int J Drug Policy 2017;47:47–50. 10.1016/j.drugpo.2017.05.054 [DOI] [PubMed] [Google Scholar]
- 45.Alcorn K. European HCV treatment access survey shows big variations in eligibility. AIDSmap 2017. [Google Scholar]
- 46.Papatheodoridis GV, Hatzakis A, Cholongitas E, et al. Hepatitis C: the beginning of the end-key elements for successful European and national strategies to eliminate HCV in Europe. J Viral Hepat 2018;25 Suppl 1:6–17. 10.1111/jvh.12875 [DOI] [PubMed] [Google Scholar]
- 47.Vold JH, Aas C, Leiva RA, et al. Integrated care of severe infectious diseases to people with substance use disorders; a systematic review. BMC Infect Dis 2019;19:306. 10.1186/s12879-019-3918-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ministry of Health and Care Services National guidline for medicaly assisted rehabilitation (MAR) for opioid dependence. The Norwegian Ministry of health and care services, 2010. [Google Scholar]
- 49.Cunningham EB, Amin J, Feld JJ, et al. Adherence to sofosbuvir and Velpatasvir among people with chronic HCV infection and recent injection drug use: the simplify study. Int J Drug Policy 2018;62:14–23. 10.1016/j.drugpo.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 50.Cunningham EB, Hajarizadeh B, Amin J, et al. Adherence to once-daily and twice-daily direct acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clinical Infectious Diseases 2019. 10.1093/cid/ciz1089 [DOI] [PubMed] [Google Scholar]
- 51.Mason K, Dodd Z, Guyton M, et al. Understanding real-world adherence in the directly acting antiviral era: a prospective evaluation of adherence among people with a history of drug use at a community-based program in Toronto, Canada. Int J Drug Policy 2017;47:202–8. 10.1016/j.drugpo.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 52.Read P, Gilliver R, Kearley J, et al. Treatment adherence and support for people who inject drugs taking direct-acting antiviral therapy for hepatitis C infection. J Viral Hepat 2019;26:1301–10. 10.1111/jvh.13175 [DOI] [PubMed] [Google Scholar]
- 53.Hajarizadeh B, Cunningham EB, Reid H, et al. Direct-Acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018;3:754–67. 10.1016/S2468-1253(18)30304-2 [DOI] [PubMed] [Google Scholar]
- 54.Frisk P, Aggefors K, Cars T, et al. Introduction of the second-generation direct-acting antivirals (DAAs) in chronic hepatitis C: a register-based study in Sweden. Eur J Clin Pharmacol 2018;74:971–8. 10.1007/s00228-018-2456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grady BP, Schinkel J, Thomas XV, et al. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis 2013;57 Suppl 2:S105–10. 10.1093/cid/cit301 [DOI] [PubMed] [Google Scholar]
- 56.Norton BL, Akiyama MJ, Zamor PJ, et al. Treatment of chronic hepatitis C in patients receiving opioid agonist therapy: a review of best practice, infectious disease clinics of North America. 32, 2018: 347–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caplehorn JR, Bell J. Methadone dosage and retention of patients in maintenance treatment. Med J Aust 1991;154:195–9. 10.5694/j.1326-5377.1991.tb121030.x [DOI] [PubMed] [Google Scholar]
- 58.Midgard H, Weir A, Palmateer N, et al. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol 2016;65:S33–45. 10.1016/j.jhep.2016.07.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-036355supp001.pdf (378.5KB, pdf)


