Abstract
Introduction
The aim of this study is to investigate the effects of time-restricted eating (TRE) on change in body weight and describe changes in behaviour and metabolism in individuals at high risk of type 2 diabetes.
Methods and analysis
The REStricted Eating Time (RESET) study is a randomised controlled parallel-group open-label trial. 100 women and men with (1) overweight (body mass index (BMI)≥25 kg/m2) and prediabetes (glycated haemoglobin 39–47 mmol/mol); or (2) obesity (BMI≥30 kg/m2) will be randomised to a control group (habitual living) or TRE (self-selected 10-hours eating window within the period from 06:00 to 20:00 in a 1:1 ratio. Testing is scheduled at baseline and after 6 weeks (mid-intervention), 3 months (post-intervention) and 6 months (follow-up). The primary outcome is change in body weight after 3 months of intervention. Secondary outcomes include changes in body composition; measures of glucose metabolism including glycaemic variability, hormones and metabolites; subjective and metabolic markers of appetite, food preferences and reward; dietary intake; physical activity, sleep, chronotype; gastric emptying, gastrointestinal transit time and motility; respiratory and glycolytic capacities; the plasma proteome and metabolome; blood pressure, resting heart rate and heart rate variability; and resting energy expenditure and substrate oxidation. Motivation and feasibility will be examined based on interviews at baseline and after 3 months. After the 3-month intervention, a 3-month follow-up period and subsequent testing are scheduled to assess maintenance and longer-term effects.
Ethics and dissemination
The study has been approved by the Ethics Committee of the Capital Region of Denmark (H-18059188) and the Danish Data Protection Agency. The study will be conducted in accordance with the Declaration of Helsinki. Results from the study will address whether TRE is effective and feasible in improving health outcomes in individuals at risk of lifestyle-related diseases and can potentially inform the design of feasible health recommendations.
Trial registration number
Keywords: diabetes & endocrinology, diabetes & endocrinology, nutrition & dietetics
Strengths and limitations of this study.
The study includes state-of-the-art and novel technologies to assess the effects of the intervention on food preferences and reward, the gastrointestinal tract, respiratory and glycolytic capacities, as well as proteomics and metabolomics.
The interdisciplinary nature of the study and assessment of feasibility and sustainability using qualitative methods allow an understanding of the participants’ experiences and potential barriers and strategies for integration and maintenance of time-restricted eating in everyday life.
The duration of the trial does not allow for the investigation of long-term effects and hard endpoints, but the follow-up visit allows for the evaluation of maintenance 3 months after the end of the intervention.
Except for reminders regarding reporting of daily eating windows, no support to comply with the prescribed intervention is provided to the participants during the trial; adherence is therefore entirely dependent on the motivation and self-determination of the participants.
While the broadness of the inclusion criteria allows for the recruitment of a study population more alike to the general population at risk for type 2 diabetes and cardiovascular disease, it at the same time increases the risk for heterogeneity in the effects of some of the secondary outcomes.
Introduction
Overweight and pre-diabetes increase the risk of developing type 2 diabetes and cardiovascular disease.1–3 Weight loss is associated with improved glycaemic control and cardiometabolic health among individuals with pre-diabetes and type 2 diabetes4 5; therefore, the development of effective, feasible and sustainable weight loss strategies is essential. Current prevention and treatment of obesity and type 2 diabetes include energy-restricted diets and increased levels of physical activity.6 However, adherence and maintenance to such strategies are difficult,7 8 underscoring an unmet need for more acceptable and feasible regimens.
Circadian rhythms are ~24-hour rhythms of behaviour and metabolism that are closely related to the daily light/dark cycle and sleep–wake patterns.9 10 The timing of food intake may affect the circadian rhythms of metabolic organs.9 Factors including the 24-hour availability of energy-dense foods and different eating and sleep patterns during weekdays and weekends (ie, ‘social jetlag’) may lead to an irregular feeding–fasting rhythm.11 12 Observational studies suggest that irregular eating patterns and late-night food consumption are associated with increased cardiometabolic risk.13 Experimental studies in rodents and humans have shown that circadian misalignment of food intake and sleep may have adverse effects on energy balance, glucose metabolism and appetite regulation,14–17 suggesting a great therapeutic potential of aligning food intake to circadian rhythms of metabolism. Studies in rodents and flies suggest that time-restricted feeding is associated with improvements in metabolic health including improved glucose and lipid metabolism and reductions in adiposity and systemic inflammation.9 18 However, there is a lack of randomised controlled trials (RCTs) investigating the effects of timing of food intake on human behaviour and metabolism.
Recent cross-over intervention studies in humans have investigated the short-term (4 days–5 weeks) effects of time-restricted eating (TRE) under well-controlled conditions. Among men at high risk of type 2 diabetes, ‘early TRE’ (eating window: 6–9 hours/day, between 08:00 and 17:00) improved glucose metabolism19–21 and reduced appetite.19 22 A few small pilot intervention studies (n=8–23) have investigated the effects of 10–16 weeks of TRE (eating window: ~8–12 hours/day) in individuals with overweight and obesity and reported reductions in energy intake and body weight11 23 24 and adiposity.24 25 Furthermore, in one of the studies, in which a clinically relevant weight loss (3.9%) was observed, the participants felt more energetic and reported less hunger and improved sleep quality; however, no control group was included.11 In the same study, maintenance was assessed at a 1-year follow-up. Importantly, on completion of the 16-week intervention, all eight participants in the same study were interested in continuing the regimen, and they maintained weight loss at follow-up (3.4%),11 suggesting that TRE may be feasible, acceptable and sustainable. Additionally, the long fasting period during ‘early TRE’ seems to be well tolerated19; however, challenges associated with social events including drinking and eating may exist.25 Nevertheless, an in-depth investigation of the feasibility and sustainability of TRE is needed to understand motivation and potential barriers for integration and maintenance in everyday life. In this study, the effects and feasibility of TRE in individuals at high risk of type 2 diabetes will be assessed using an interdisciplinary approach including state-of-the-art and novel quantitative and qualitative methods.
Objectives
The primary objective of the REStricted Eating Time (RESET) study is to investigate the effects of 3 months of TRE (10 hours/day) on change in body weight in individuals at high risk of type 2 diabetes. Secondary objectives are to describe changes in body weight and composition, metabolism and behaviour and to assess aspects related to motivation, feasibility and maintenance during the 3-month intervention and after additional 3 months of follow-up.
Hypotheses
We hypothesise that 3 months of TRE will induce a clinically relevant weight loss in individuals with overweight and obesity at high risk of type 2 diabetes (ie, TRE superior to control). Furthermore, we expect that weight loss is maintained in the TRE group at the 3-month follow-up visit (ie, TRE superior to control).
Methods and analysis
Study design
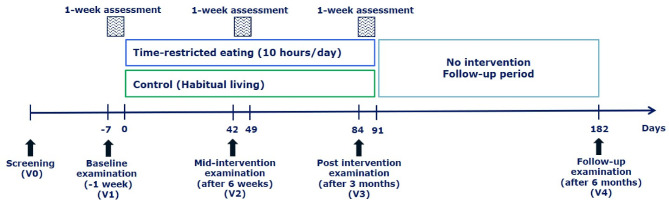
The study is a single-centre, parallel-group, randomised, controlled, superiority, open-label trial (figure 1). A total of 100 individuals will be randomised to 3 months of TRE or habitual living (control) in a 1:1 ratio. Habitual living was chosen as the comparator to evaluate the effects of TRE when included in everyday routines. Randomisation is performed after completion of screening and baseline testing (visit 1 (V1)). The primary outcome is assessed after 3 months of intervention (V3). At baseline, mid-intervention (after 6 weeks; V2) and after the intervention (3 months; V3), outcomes are assessed during test days and free-living measurements during the week following the test days. After the 3-month intervention, a 3-month follow-up period and subsequent testing (V4) are scheduled to assess maintenance and longer-term effects. The trial will be performed at Steno Diabetes Center Copenhagen and will be reported according to the Consolidated Standards of Reporting Trials (CONSORT).26 The study protocol follows the Standard Protocol Items: Recommendations for Interventional Trials statement.27 A copy of the WHO Trial Registration Data Set is supplied in the online supplementary table 1.
Figure 1.
Study design.
bmjopen-2020-037166supp001.pdf (108.8KB, pdf)
Participants
Women and men, 30–70 years of age, with (a) overweight (body mass index (BMI)≥25 kg/m2) with concomitant pre-diabetes as defined by glycated haemoglobin (HbA1c) 39–47 mmol/mol6 or (b) obesity (BMI≥30 kg/m2), who are eligible according to the inclusion and exclusion criteria (box 1) will be included.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Age: ≥30 to ≤70 years.
Body mass index ≥30 kg/m2 or body mass index ≥25 kg/m2 in combination with pre-diabetes (HbA1c ≥39 to <48 mmol/mol).
Habitual eating/drinking window ≥12 hours (including foods/snacks and energy-containing beverages, eg, soft drinks (except water)) and an eating/drinking window of ≥14 hours minimum 1 day/week.
Exclusion criteria
Daily smoking.
For women: pregnancy, planned pregnancy (within the study period) or lactating.
Frequent travels over time zones (more than one return trip/travel over times zones (˃1 hour time difference) during the 13-week intervention).
Shift work or partner engaged in shift work (if it affects the person’s sleep and eating pattern).
Unable to understand the informed consent and the study procedures.
Self-reported history of an eating disorder during the past 3 years.
Self-reported weight change (>5 kg) within 3 months prior to inclusion.
Known diabetes or diabetes detected at screening (HbA1c ≥48 mmol/mol).
Uncontrolled medical issues including but not limited to cardiovascular, pulmonary, rheumatological, haematological, oncological, infectious, gastrointestinal or psychiatric disease; endocrine disease; immunosuppression.
Current treatment with medication or medical devices that significantly affect glucose metabolism, appetite or energy balance.
Current treatment with antidepressants.
Bariatric surgery.
Implanted or portable electromechanical medical device such as a cardiac pacemaker, defibrillator or infusion pump.
Coeliac disease, Crohn’s disease, ulcerative colitis or proctitis.
Alcohol/drug abuse or in treatment with disulfiram at time of inclusion.
Concomitant participation in other intervention studies.
Not able to eat ≥85% of the test meal because of, eg, allergy.
Specific exclusion criteria for participants receiving SmartPill
Gastrointestinal symptoms or diseases such as regular (weekly) abdominal pain, dysphagia, gastric bezoars, strictures, fistulas, bowel obstructions or diverticulitis.
Current treatment with medication or medical devices that significantly affect gastrointestinal motility or transit time (prokinetics, antidiarrhoeals, laxatives or opioids).
Gastrointestinal surgery within 3 months before inclusion.
Other criteria for withdrawal and exclusion after inclusion
Participant’s withdrawal of the informed consent.
Pregnancy or other safety concerns—judged by the investigator.
HbA1c, glycated haemoglobin.
Eligibility criteria
Inclusion and exclusion criteria are listed in box 1. We include high-risk individuals aged 30–70 years because our focus is on preventing diabetes at an early stage. Individuals with overweight and pre-diabetes and obesity with/without pre-diabetes are included to target individuals at high risk of type 2 diabetes. The rationale of choosing 70 years as the upper limit is that the potential for prevention is limited in older individuals. To prevent sources of circadian irregularity during the intervention, shift workers and individuals with a partner engaged in shift work affecting the circadian rhythm of the participant are not eligible for participation.
Recruitment and screening
Participants are recruited through advertisements on different publicly available platforms (newspapers, webpages, pharmacies and so on). A pre-screening is performed as a telephone interview focusing on the participant’s age, BMI and habitual eating window to reduce the number of screen failures. Participants who are eligible based on the pre-screening receive written information about the study and are scheduled for a screening visit (V0). At the screening visit, participants provide oral and written informed consent to medical staff, and a health examination including medical history and assessment of inclusion and exclusion criteria is performed (box 1). After the screening, eligible participants will be scheduled for four visits (V1–V4, figure 1). Baseline testing (V1) takes place as soon as possible and within 6 weeks from the screening visit (V0). The first participant signed consent on 25 February 2019, and participants are recruited continuously.
Outcomes
Primary outcome
The primary outcome is mean change in body weight (kg) from baseline (V1) to the end of intervention (after 3 months, V3). Change in body weight was chosen as the primary outcome for several reasons. First, weight loss is associated with a reduction in all-cause mortality in individuals with obesity28 and with improvement in glycaemic control in individuals with overweight and obesity.4 Second, according to the American Diabetes Association, weight loss is recommended for all individuals with pre-diabetes.6 Third, body weight is easy to measure with high precision and available in most clinical studies that enable determination of sample size and comparison across studies.
Secondary exploratory outcomes
The secondary exploratory outcomes include a variety of metabolic and behavioural outcomes potentially associated with the intervention. These include changes in body composition, hormones involved in glucose metabolism and appetite regulation (eg, pancreatic and gastrointestinal hormones), metabolites and glycaemic variability; subjective appetite, food preferences and reward, and eating behaviour; gastric emptying, gastrointestinal motility and transit time; gut microbiome; physical activity, dietary intake and sleep; inflammatory markers; respiratory and glycolytic capacities; the plasma proteome and metabolome; blood pressure, resting heart rate and heart rate variability; and resting energy expenditure and substrate oxidation (table 1). We describe changes from baseline to mid-intervention (after 6 weeks; V2), post-intervention (after 3 months; V3) and follow-up testing (after 6 months; V4).
Table 1.
Overview of study visits
| Visit | V0 | V1 | V2 | V3 | V4 |
| Time, weeks from start of intervention | −7* | −1 | 6 | 12 | 26 |
| Participant information | |||||
| Informed consent | X | ||||
| Medical history (individual and family) | X | ||||
| Inclusion and exclusion criteria | X | ||||
| Pregnancy test (fertile women only) | X | X | X | X | |
| Efficacy outcomes | |||||
| HbA1c | X | X | X | X | X |
| Body weight | X | X | X | X | X |
| Waist and hip circumference | X | X | X | X | |
| Body composition (DXA scanning) | X | X | X | X | |
| Blood pressure and resting heart rate | X | X | X | X | X |
| Stool sample | X | X | |||
| Fasting blood samples | X | X | X | X | |
| Postprandial blood samples | X | X | |||
| Indirect calorimetry | X | X | |||
| Heart rate variability (Vagus) | X | X | |||
| Mixed meal test with SmartPill | X | X | |||
| Event registration related to SmartPill | X | X | |||
| Physical activity and sleep measurement | X | X | X | ||
| Food records | X | X | X | ||
| Continuous glucose monitoring | X | X | X | ||
| Fasting food reward and biometric measurements | X | X | X | X | |
| Postprandial food reward and biometric measurements | X | X | |||
| Questionnaires | |||||
| Sociodemographic characteristics | X | ||||
| Health and well-being | X | X | X | X | |
| Physical activity | X | X | X | X | |
| Fasting appetite sensations | X | X | X | X | |
| Postprandial appetite sensations | X | X | |||
| Gastrointestinal symptoms | X | X | X | X | |
| Autonomic symptoms | X | ||||
| Pain | X | ||||
| Sleep quality and sleepiness | X | X | X | X | |
| Chronotype | X | X | X | X | |
| Night eating | X | X | X | X | |
| Eating behaviour and control over eating | X | X | X | X | |
| Interviews | |||||
| Interview† | X | ||||
| Interview (participants in the TRE group)‡ | X | X |
*Max 6 weeks before baseline testing (V1).
†Interview regarding motivation for participation.
‡Interview regarding feasibility and maintenance.
DXA, dual-energy X-ray absorptiometry; HbA1c, glycated haemoglobin; TRE, time-restricted eating; V0, screening; V1, baseline testing; V2, mid-intervention testing (after 6 weeks); V3, post-intervention testing (after 3 months); V4, follow-up testing (after 6 months).
Study visits and free-living assessment periods
The study includes identical test days and free-living assessment periods at baseline (before randomisation, V1) and postintervention (V3). The test day at V3 is scheduled after 12 weeks intervention; however, participants are instructed to follow their group allocation during the subsequent 1-week free-living assessment period (ie, 13th week of the intervention). Mid-intervention testing after 6 weeks (V2) includes a short test day and a subsequent free-living assessment period during which participants follow their group allocation. After the 13-week follow-up period (26 weeks from baseline), a short test day (V4) is scheduled to assess the maintenance of potential intervention effects. If possible, all 4 test days are scheduled on similar weekdays and at the same time in the morning. An overview of the study visits is presented in table 1.
Test days
All clinical examinations are conducted at Steno Diabetes Center Copenhagen. Participants arrive in the morning at ∼08:00 am after a ∼12 hours overnight fast. All participants are instructed to have a last meal between 19:00 and 20:00 the day prior to the test days to minimise the potential acute effects of varying fasting duration on the outcomes of interest.29 Furthermore, no alcohol consumption or strenuous physical activity is allowed 48 hours prior to testing. The participants are instructed to avoid physically demanding transportation to the research facility.
Anthropometry
Height is measured using a stadiometer (SECA, Vogel&Halke, Hamburg, Germany) and body weight is measured using a digital scale (Tanita BWB-620A, Amsterdam, The Netherlands) while participants are wearing only light clothes/underwear. Waist circumference is measured at the midpoint between the lowest point of the lowest rib and the highest point of the iliac crest. Hip circumference is measured at the point of the greater femoral trochanter. An average of two repeated measurements of hip and waist circumference is used. In the case of ˃3 cm difference between the two measurements, a third measurement is conducted, and the average of the two closest measurements is used. Body composition (fat mass and fat-free mass) is measured using whole-body dual-energy X-ray absorptiometry (Discovery, Hologic, Bedford, Massachusetts, USA). A urine sample is collected, and a pregnancy test is performed for all fertile women <60 years before the scan.
Blood pressure and resting heart rate
Blood pressure (mmHg) and resting heart rate (beats per minute) are measured three times with 2 min intervals using a digital blood pressure monitor (UA-852, A&D Instruments, Abingdon, UK) after a minimum of 10 min rest, and the average of the two lowest values of three consecutive measurements are used to avoid falsely high blood pressure caused by an unfamiliar and potentially stressful environment.
Heart rate variability
Heart rate variability and cardiovascular reflex are measured by electrocardiography using a handheld device (Vagus, Medicus Engineering, Aarhus, Denmark) during four consecutive tests: (1) resting heart rate is measured while the participant is in the supine position holding the device; (2) heart rate response to standing up from the supine position; (3) heart rate response to inhalation and exhalation is measured in the seated position; (4) heart rate response to increased intrathoracic pressure (Valsalva manoeuvre).
Resting energy expenditure and substrate oxidation
Resting energy expenditure and substrate oxidation are measured for 30 min using indirect calorimetry and a ventilated hood (Vyntus CPX, CareFusion, Hoechberg, Germany) with the participant resting in the supine position in a quiet room. Energy expenditure30 and substrate oxidation31 are calculated based on respiratory gas exchange, that is, carbon dioxide production and oxygen consumption.
Blood samples
Venous blood samples are collected in the fasting state at all 4 test days (V1–V4) and postprandially during a mixed meal test at V1 and V3 at time points 15, 30, 45, 60, 90, 120, 180 and 240 min via a catheter in an antecubital vein. Analyses include assessment of HbA1c, circulating levels of glucose and lipids, inflammatory markers and hormones involved in the regulation of appetite and metabolism (eg, insulin, glucagon, glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, peptide YY and acylated ghrelin). Furthermore, metabolomics and proteomics will be applied, and assessment of circulating proteins and metabolites that correlate with low-grade inflammation and markers of lipid metabolism will be captured using mass-spectrometry-driven analyses of the plasma proteome and metabolome.32 33 Gene expression of proinflammatory and anti-inflammatory proteins including cytokines and chemokines and genes involved in the energy metabolism of isolated peripheral blood mononuclear cells will be measured by real-time PCR. Cellular bioenergetic activity (mitochondrial respiration and glycolysis) of isolated peripheral blood mononuclear cells will be determined using a Seahorse XFe24 Analyzer.34 The seahorse technology measures real-time oxygen consumption rate as an indicator of mitochondrial activity and extracellular acidification rate as an indicator of glycolytic activity. Thus, these measurements will provide mechanistic knowledge of TRE-induced metabolic changes at the cellular level. Serum and plasma will be stored in a biobank for future analyses.
Meal test
At V1 and V3, after assessments in the fasting state, participants are asked to consume a standard breakfast meal (300 g, 498 kcal, 49% of total energy (%E) carbohydrate, 34 E% fat and 17 E% protein) consisting of bread roll, rye bread, cheese, yoghurt, muesli, butter, marmalade and 150 mL water. For the following 4 hours after initiation of the meal, blood samples are collected and subjective appetite is assessed using visual analogue scales35 at time points 15, 30, 45, 60, 90, 120, 180 and 240 min.
Subjective appetite
Hunger, satiety, fullness, thirst, estimated prospective food consumption and desire for sweet, salt and fat, and potential nausea are rated by the participants using electronic visual analogue scales35 after the blood samples in the basal state at all 4 test days (V1–V4) and postprandially at V1 and V3.
Food preferences and food reward
At V1 and V3, components of food reward and biometric responses to standardised photographic images of foods will be assessed in the fasting state and 60 min after ingestion of the standardised breakfast meal to examine meal-induced changes in food reward and responses to food stimuli. At V2 and V4, only fasting measurements of food reward and biometric responses will be performed. In a computerised questionnaire, different food reward outcomes (food choice, implicit wanting, explicit liking and explicit wanting) are measured using the Leeds Food Preference Questionnaire36–38 in combination with measures of autonomic nervous system activity including arousal estimated from galvanic skin response (Biopac MP160, Biopac Systems, Goleta, California, USA), emotional response using facial expression analyses (AFFDEX algorithm, Affectiva, Massachusetts, USA), and motivated visual attention using eye tracking (Tobii X2-60, Tobiipro, Stockholm, Sweden). The Leeds Food Preference Questionnaire is integrated into a biometric software platform (iMotions A/S, Frederiksberg, Denmark) to enable the simultaneous collection of data on eye tracking, galvanic skin response and facial expressions.
Questionnaires
At V1–V4, participants fill in questionnaires regarding health and well-being, gastrointestinal and autonomic symptoms, eating behaviour, chronotype, sleep and physical activity (table 1). At V1, participants fill in a questionnaire regarding sociodemographic characteristics including age, sex, ethnicity, education, occupation, civil status, children and personal and household income.
Interviews
Interviews will be conducted at V1, V3 and V4 to obtain insights into the participants’ experiences and perceptions of the intervention. This will provide an understanding of the feasibility and integration of the intervention into the everyday life of the participants as well as maintenance of the regimen. At V1, participants will be interviewed for ~25 min to examine their reasons and motivation for participation, their expectations towards the intervention and their everyday life activities and eating practices.
At V3 and V4, participants in the TRE group will be invited to individual semistructured interviews of ~45 min to explore the feasibility and maintenance of TRE in everyday life. If participants withdraw from the study, then they will be invited to individual interviews about their reasons for doing so and their experiences with the intervention. The interviews will draw on social practice theory39 and will be recorded and transcribed verbatim. Malterud’s systematic text condensation approach40 will be used to analyse the interview data.
Free-living assessment period
A 1-week free-living assessment period is scheduled after each of the test days at V1–V3 and includes the procedures described further. Participants are instructed to follow group allocation during the assessment period at V2 and V3. After the 1-week free-living assessment period at V3, the participants will be instructed to live as they wish during the subsequent 3-month follow-up period.
Gastric emptying, gastrointestinal transit times and motility
At V1 and V3, participants will be instructed to ingest a wireless motility capsule (SmartPill, Medtronic, Minnesota, USA) immediately after ingesting the standardised breakfast meal with 150 mL water. The capsule measures pH, temperature and pressure through the gastrointestinal tract until expulsion. From these data, regional transit time, pH profile and motility in different parts of the gastrointestinal tract can be estimated.41–43 Participants will be instructed to wear a SmartPill receiver unit within 0.25 m of their body for the following week or until the expulsion of the capsule. As this is an expensive measurement, only the first ~60 participants will be offered the capsule.
Events related to the gastrointestinal tract
At V1 and V3, participants register all events related to the gastrointestinal tract (passing stool, eating, sleeping, and gastrointestinal symptoms such as nausea, vomiting, pain in the abdominal region, bloating and so on), until the expulsion of the SmartPill, using an ‘event button’ on the SmartPill receiver. Additionally, the participants register the time and type of each event in a diary.
Physical activity and sleep
Physical activity and sleep will be measured using accelerometry. Participants will be equipped with one accelerometer on the thigh and one on the lower back for 1 week (Axivity AX3, Newcastle upon Tyne, UK). Concomitant with wearing the accelerometers, participants will be asked to fill out a physical activity and sleep diary.
Continuous glucose monitoring
A 1-week continuous glucose monitoring (CGM) system (Ipro, Medtronic Denmark A/S, Copenhagen, Denmark) will be initiated at the test days at V1–V3. The CGM will be attached to the lower part of the abdomen in the morning at the test days. Participants will be instructed to measure blood glucose levels using a glucometer (Contour XT, Ascensia Diabetes Care Denmark ApS, Copenhagen, Denmark) four times a day during the measurement period for the calibration of the CGM (before breakfast, lunch, main evening meal and bedtime).
Dietary intake
During the week following V1, V2 and V3, participants will be asked to fill in a food record diary (pen and paper) for 3 days (day 1, 3 and 5 after the test day; 2 weekdays and 1 weekend day). Participants will be instructed to register weight, time and content of all meals and beverages (except water). During the same week, participants will be asked to register their eating window (see the Assessment of adherence section).
Participants will be instructed to send the CGM, SmartPill receiver, accelerometers and diaries to the researchers after completion of each assessment period.
Gut microbiome
Participants will be provided with a kit for stool samples including storage equipment at V0 and V2. They will be instructed to collect and immediately freeze (−20°C) three samples from the same stool sample ≤72 hours before test days at V1 and V3. The participants will transport the samples to the laboratory in provided cooling bags and the samples will be stored at −80°C until analysis. Bacterial DNA and RNA will be purified from the stool samples. The microbial content, composition and function will be estimated based on the sequencing of the microbiome.
Randomisation and intervention
After completing baseline testing, participants are randomly allocated to either the control group or the TRE group. Randomisation is performed in blocks varying in size, unknown to the researchers, to ensure an equal distribution of participants in the two groups in case the study, for unexpected reasons, must be terminated before the inclusion of all participants. The randomisation list was generated by an external statistician and uploaded to the electronic data management system REDCap (V.8.10.18, Vanderbilt University, Tennessee, USA). When participants leave the research facilities on the test day at V1, they receive a sleeve with a combination lock that contains information about group allocation. On day 7, when all baseline assessments are completed, the participants are provided with the code for the lock by an investigator. This approach ensures that participants are blinded to the group allocation during the 7 day free-living assessment period. Over the phone, the investigator provides a detailed description and introduction to the specific group allocation. For practical reasons, randomisation is open for participants and research staff. However, the outcome assessors (data analysts) will be blinded during the statistical analyses of all experimental outcomes.
Time-restricted eating
Participants allocated to the TRE group are instructed to consume all foods and beverages (except water) within a self-selected time window of 10 hours/day between 06:00 and 20:00 for the 13-week intervention. Furthermore, the participants are instructed to keep the eating window stable during the entire week and advised to select a window which starts at least 2 hours after habitual wake-up time and stops 3 hours before habitual bedtime if possible. Participants are advised to follow the Danish dietary recommendations.44 No other dietary restrictions are prescribed.
Control
Participants allocated to habitual living are advised to follow the Danish dietary recommendations44 but are otherwise instructed to continue their habitual lifestyle during the 13-week intervention.
Assessment of adherence
All participants are asked to register time for initiating first and terminating the last eating/drinking episode (except water) every day from the test day at V1–V4. Every week during the 26-week period (intervention and follow-up), a link to an online form will be sent by email to the participants for them to register the time for eating/drinking episodes for the previous week. In case participants in the control group restrict their eating window to less than their habitual ≥12 hours/day or if the eating window of participants in the TRE group deviates from their self-selected 10 hours eating window ≥4 days during the first week, then the participant will be contacted per telephone to ensure that the participant has understood the concept of their designated group allocation. During the first week of the intervention, participants in the TRE group can change their eating window once, in case they are not satisfied with the originally selected window. After the first week no changes are allowed. To ensure similar contact with participants in the control group and the TRE group, the participants will not be contacted in case of non-adherence after the first week. However, if the participants fail to register their eating window, they will be contacted only to remind them to register. No other feedback is provided during the intervention. To account for variations in daily eating windows around 10 hours, participants in the TRE group are considered adherent if their eating window is less than 11 hours/day. Adherence to the intervention is calculated as the number/percentage of days during the intervention the participants’ eating window is <11 hours/day. Per protocol is defined as ≥80% compliance. The eating window will be calculated for both groups, but no compliance criterion is applied in the control group. Regardless of the degree of adherence all participants allocated to both groups will be invited for test days with an emphasis on participating in V3 and if participants are not willing to attend a full test day, then they will be asked to come in for a measurement of the primary outcome.
Statistical methods
Sample size determination
There is strong evidence for the clinical relevance of a weight reduction of 3% in people with overweight or obesity, with or without pre-diabetes.45 At the time when the RESET study was designed, no RCT had investigated the effects of TRE on weight loss in individuals with overweight or obesity at high risk of type 2 diabetes. However, Gill and Panda investigated the effects of a 16-week TRE intervention on changes in body weight in eight healthy overweight individuals (BMI˃25 kg/m2) with a habitual eating window ˃14 hours/day and observed a mean reduction of body weight of 3.9% (3.3 kg, 95% CI 0.9–5.6 kg); however, no control group was included.11 Per inclusion criteria individuals with a BMI of ≥25 kg/m2 are included in the RESET study. For the participants with a BMI of 25 kg/m2 (with an expected mean height of 170 cm), a change in weight of 3% will correspond to ~2 kg. Thus, in order to detect a minimal clinically relevant difference in weight change of 3% across the allowed BMI range, the trial was dimensioned to detect a difference in change of 2 kg between the TRE group and the control group. In a recent RCT examining the effects of 13 weeks of either exercise or pharmacological therapy on cardiometabolic health in individuals with overweight or obesity and pre-diabetes, the SD for within-group changes in body weight in the control group was 2.6 kg (Færch et al, unpublished data). We expect that the SD for within-group changes will be similar in the RESET trial, but to account for uncertainties we increased the SD by 20%, resulting in an SD of 3.1 kg. In order to detect a 2 kg (SD 3.1) difference in weight change with a desired statistical power of 0.8 (two-tailed test, alpha 0.05), a total of 40 participants is required in each group. To allow for a 20% drop-out in each group, we plan to include 50 participants in each of the two groups.
Statistical analysis plan
Intention-to-treat analysis including all randomised participants will be performed after the last participant has participated in the last visit. Additionally, per protocol analysis will be performed including participants who are compliant during the intervention. Data will be presented with the use of standard descriptive statistics. Descriptive statistics will be shown as mean (SD) for normally distributed data and as median (Q1; Q3) for non-normally distributed data. Changes from baseline and differences in delta values between groups will be analysed using linear mixed-effects models with the outcome as a function of the group, time and group×time interaction and including a participant-specific random intercept. Outcomes with a known/expected bimodal distribution due to sex differences will be adjusted for sex. Adequacy of assumptions of normality and homogeneity of variances will be assessed using graphical methods and, if necessary, data will be log-transformed for analysis and back-transformed for presentation. If model assumptions are not met by logarithmically transformation, non-parametric statistical tests will be performed. P values <0.05 (two-tailed) are considered statistically significant. The potential impact of missing data on the primary outcome will be evaluated in a sensitivity analysis based on multiple imputation in which participants with missing data at follow-up will be pooled with the participants in the control group during the imputation process.
Results will be presented as estimated mean differences in changes with 95% CIs and p values when relevant. A full statistical analysis plan will be uploaded to ClinicalTrials.gov before the inclusion of participants is finalised.
Patient and public involvement statement
During the study, we enter into dialogue with participants about their experiences of the test days, examinations, participant information and so on with the aim to understand and improve participants’ experiences in current and future studies of TRE.
Ethics and dissemination
All equipment used in the studies meet the requirement for patient safety. The total amount of blood taken at each visit is maximally 300 mL, which is less than a standard blood donation of 450 mL and considered safe. Participants will be instructed not to donate blood during the trial. There may be some discomfort associated with swallowing the SmartPill. The risk of capsule retention in individuals without known stenosis is only 0.75% and in such a case, a promotility drug is often sufficient to mobilise the capsule. Alternatively, endoscopy can be performed to retrieve the capsule. Body composition is measured using dual-energy X-ray absorptiometry with a radiation dose of less than 0.01 mSv, which corresponds to less than 1 day of normal background radiation. There is no expected discomfort or risks associated with the ingestion of the meals, food reward measurements or biometric measurements (eye tracking, galvanic skin response and facial expression analyses). Participants are covered by the Patient Compensation Association according to the Danish Act on the Right to Complain and Receive Compensation within the Health Service. The intervention is considered safe in individuals with overweight and obesity.46
The study has been approved by the Ethics Committee of the Capital Region of Denmark (H-18059188) and will be conducted in accordance with the Declaration of Helsinki. Approval of data and biobank has been obtained from the Danish Data Protection Agency. Consent from the Ethics Committee of the Capital Region of Denmark to all previous and future amendments to the protocol have and will be obtained before these are instated (online supplementary table 2).
bmjopen-2020-037166supp002.pdf (116KB, pdf)
No data monitoring committee has been appointed for the trial due to the perceived very low risk of harm.
All study-related information will be recorded, handled and stored safely in a way that allows accurate reporting, interpretation and verification. Source data will be registered in the electronic data management system REDCap. For CGM measurements, source data will be registered in a web-based software (CareLink, Medtronic) using the participant’s study ID. Source data from dual-energy X-ray absorptiometry, biometric measurements, food preferences, Vagus, SmartPill, accelerometers and indirect calorimetry are registered on the device or related hardware and uploaded to a secured logged drive to which only project staff has access. Investigators at Steno Diabetes Center Copenhagen will have access to the full data set. The sponsor/investigator will provide direct access to source data/documents for regulatory inspection. Access to the full protocol and data can be obtained from the principal investigator. As we expect no harm associated with the intervention, information on harms/side-effects will not be systematically recorded.
Positive and negative as well as inconclusive study results will be presented at conferences and published in international peer-reviewed journals in accordance with the CONSORT guidelines, no publication restrictions have been imposed. All coauthors must comply with the International Committee of Medical Journal Editors guidelines and no professional writers will be engaged in the writing process.
Discussion
Implementation and maintenance of traditional strategies for weight loss and early prevention of type 2 diabetes, that is, increased physical activity and dietary restrictions, are difficult for many,47–49 because it is time consuming and requires insights into the type and amount of foods eaten. From a public health perspective, there is a strong need for feasible lifestyle strategies to combat the current type 2 diabetes epidemic. TRE is a simple eating pattern regime that extends the daily fasting period and potentially synchronises food intake with circadian rhythms of metabolism and may, therefore, represent a feasible lifestyle modification strategy. The evidence from animal studies and well-controlled human studies suggest that TRE has the potential to improve a variety of cardiometabolic risk factors in metabolically vulnerable individuals and may be a feasible and sustainable regimen18; however, RCTs are lacking. Using an interdisciplinary approach, the present study will examine the effects of TRE on weight changes and explore effects on cardiometabolic health and behaviour, as well as on participants’ motivation and experiences with TRE.
Implicit in the trial design is that the intervention is only relevant and feasible if the participants can uphold the TRE regime without frequent contact with the research staff; otherwise we find it unlikely that this intervention will have great relevance in the public at large (who are likely less motivated than those opting to partake in an RCT). Eating is integrated into the rhythms and social relations in everyday life. Because TRE affects eating practices, it is important to get insight into participants’ experiences with TRE and to identify potential barriers and strategies for integration and maintenance of this regimen. Thus, the findings from the present study will address whether TRE is an acceptable intervention that can improve health outcomes in individuals at risk of lifestyle-related diseases. As such, it will potentially inform the design of future large-scale studies and feasible health recommendations.
Supplementary Material
Footnotes
Twitter: @Nicwin98, @@SatchinPanda
Contributors: JSQ and KF conceived the idea and initiated the study. KF is the principal investigator and JSQ, HP, MMJ, KKBC and NB are coinvestigators. JSQ, MMJ, KKBC, HP, NB, JS, MBB, NJWA, JJH, SST, DV, MEJ, SP, CB, GF and KF contributed to the design of the study. JSQ drafted the manuscript. MMJ, KKBC, HP, NB, JS, MBB, NJWA, JJH, SST, DV, MEJ, SP, CB, GF and KF critically reviewed the manuscript. JSQ, MMJ, KKBC, HP, NB, JS, MBB, NJWA, JJH, SST, DV, MEJ, SP, CB, GF and KF approved the final version.
Funding: KF is sponsor and principal investigator (e-mail: kristine.faerch@regionh.dk, Phone:+4530913061, Clinical Prevention Research, Steno Diabetes Centre Copenhagen, Niels Steensens Vej 6, DK-2820, Gentofte, Denmark). The study is funded by an unrestricted grant from the Novo Nordisk Foundation (NNF17OC0027822), a PhD scholarship from Aalborg University and an industrial PhD scholarship from the Innovation Fund Denmark.
Disclaimer: The sponsor and investigators have no economic interest in the results of the study. The funders do not take part in or have any influence on: study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication. We cover documented, reasonable travel expenses. Participants will not receive any other financial compensation for participating in the study.
Competing interests: Steno Diabetes Center Copenhagen is a hospital providing health services for the public healthcare system. Steno Diabetes Center Copenhagen is partly funded by the Novo Nordisk Foundation through unrestricted grants. The Novo Nordisk Foundation has no economic interests in the study. The Novo Nordisk Foundation will not have an influence on the study design, data collection, analysis, interpretation of data, the writing of the study report or any publication and the decision to submit the paper for publication. The investigators employed at Steno Diabetes Center Copenhagen will not benefit economically from conducting the study. HP is a coinvestigator on the project which is part of her Industrial PhD project in collaboration with iMotions A/S, where HP is employed. iMotions A/S is a collaborator on the project and gives advice for the use and analysis of biometric methods in the study design phase. SP has published a book, The Circadian Code, focusing on the concept of TRE.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lee CMY, Colagiuri S, Woodward M, et al. . Comparing different definitions of prediabetes with subsequent risk of diabetes: an individual participant data meta-analysis involving 76 513 individuals and 8208 cases of incident diabetes. BMJ Open Diabetes Res Care 2019;7:1–10. 10.1136/bmjdrc-2019-000794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt MI, Bracco PA, Yudkin JS, et al. . Intermediate hyperglycaemia to predict progression to type 2 diabetes (ELSA-Brasil): an occupational cohort study in Brazil. Lancet Diabetes Endocrinol 2019;7:267–77. 10.1016/S2213-8587(19)30058-0 [DOI] [PubMed] [Google Scholar]
- 3.Vistisen D, Witte DR, Brunner EJ, et al. . Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II study. Diabetes Care 2018;41:899–906. 10.2337/dc17-2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gummesson A, Nyman E, Knutsson M, et al. . Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab 2017;19:1295–305. 10.1111/dom.12971 [DOI] [PubMed] [Google Scholar]
- 5.le Roux CW, Astrup A, Fujioka K, et al. . 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–409. 10.1016/S0140-6736(17)30069-7 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in diabetes-2019. Diabetes Care 2019;42:S29–33. 10.2337/dc19-S003 [DOI] [PubMed] [Google Scholar]
- 7.Anderson JW, Konz EC, Frederich RC, et al. . Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001;74:579–84. 10.1093/ajcn/74.5.579 [DOI] [PubMed] [Google Scholar]
- 8.Lemstra M, Bird Y, Nwankwo C, et al. . Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence 2016;10:1547–59. 10.2147/PPA.S103649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melkani GC, Panda S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol 2017;595:3691–700. 10.1113/JP273094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longo VD, Panda S. Fasting, circadian rhythms, and Time-Restricted feeding in healthy lifespan. Cell Metab 2016;23:1048–59. 10.1016/j.cmet.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill S, Panda S. A smartphone APP reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 2015;22:789–98. 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wittmann M, Dinich J, Merrow M, et al. . Social jetlag: misalignment of biological and social time. Chronobiol Int 2006;23:497–509. 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- 13.St-Onge M-P, Ard J, Baskin ML, et al. . Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American heart association. Circulation 2017;135:e96–121. 10.1161/CIR.0000000000000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arble DM, Bass J, Behn CD, et al. . Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep 2015;38:1849–60. 10.5665/sleep.5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav 2014;134:44–50. 10.1016/j.physbeh.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Scheer FAJL, Hilton MF, Mantzoros CS, et al. . Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009;106:4453–8. 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonnissen HKJ, Hulshof T, Westerterp-Plantenga MS. Chronobiology, endocrinology, and energy- and food-reward homeostasis. Obes Rev 2013;14:405–16. 10.1111/obr.12019 [DOI] [PubMed] [Google Scholar]
- 18.Chaix A, Manoogian ENC, Melkani GC, et al. . Time-Restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr 2019;39:12.1–12.5. 10.1146/annurev-nutr-082018-124320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton EF, Beyl R, Early KS, et al. . Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 2018;27:1212–21. 10.1016/j.cmet.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamshed H, Beyl RA, Della Manna DL, et al. . Early Time-Restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019;11:1234–16. 10.3390/nu11061234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchison AT, Regmi P, Manoogian ENC, et al. . Time-Restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity 2019;27:724–32. 10.1002/oby.22449 [DOI] [PubMed] [Google Scholar]
- 22.Ravussin E, Beyl RA, Poggiogalle E, et al. . Early time‐restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 2019;27:1244–54. 10.1002/oby.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabel K, Hoddy KK, Haggerty N, et al. . Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 2018;4:345–53. 10.3233/NHA-170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson MJ, Manoogian ENC, Zadourian A, et al. . Ten-Hour Time-Restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 2020;31:92–104. 10.1016/j.cmet.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoni R, Robertson TM, Robertson MD, et al. . A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci 2018;7 10.1017/jns.2018.13 [DOI] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D, et al. . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. . SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma C, Avenell A, Bolland M, et al. . Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ 2017;359:j4849. 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulmán A, Færch K, Vistisen D, et al. . Effect of time of day and fasting duration on measures of glycaemia: analysis from the Whitehall II study. Diabetologia 2013;56:294–7. 10.1007/s00125-012-2770-3 [DOI] [PubMed] [Google Scholar]
- 30.Weir JBDEB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. 10.1113/jphysiol.1949.sp004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 1983;55:628–34. 10.1152/jappl.1983.55.2.628 [DOI] [PubMed] [Google Scholar]
- 32.Geyer PE, Kulak NA, Pichler G, et al. . Plasma proteome profiling to assess human health and disease. Cell Syst 2016;2:185–95. 10.1016/j.cels.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 33.Galsgaard KD, Winther-Sørensen M, Ørskov C, et al. . Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am J Physiol Endocrinol Metab 2018;314:E93–103. 10.1152/ajpendo.00198.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones N, Piasecka J, Bryant AH, et al. . Bioenergetic analysis of human peripheral blood mononuclear cells. Clin Exp Immunol 2015;182:69–80. 10.1111/cei.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flint A, Raben A, Blundell JE, et al. . Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes 2000;24:38–48. 10.1038/sj.ijo.0801083 [DOI] [PubMed] [Google Scholar]
- 36.Finlayson G, King N, Blundell JE. Is it possible to dissociate 'liking' and 'wanting' for foods in humans? A novel experimental procedure. Physiol Behav 2007;90:36–42. 10.1016/j.physbeh.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 37.Dalton M, Finlayson G. Psychobiological examination of liking and wanting for fat and sweet taste in trait binge eating females. Physiol Behav 2014;136:128–34. 10.1016/j.physbeh.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 38.Finlayson G, King N, Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite 2008;50:120–7. 10.1016/j.appet.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 39.Warde A. Consumption and theories of practice. J Consum Cult 2005;5:131–53. 10.1177/1469540505053090 [DOI] [Google Scholar]
- 40.Malterud K. Systematic text condensation: a strategy for qualitative analysis. Scand J Public Health 2012;40:795–805. 10.1177/1403494812465030 [DOI] [PubMed] [Google Scholar]
- 41.Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill Gi monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 2009;54:2167–74. 10.1007/s10620-009-0899-9 [DOI] [PubMed] [Google Scholar]
- 42.Farmer AD, Wegeberg A-ML, Brock B, et al. . Regional gastrointestinal contractility parameters using the wireless motility capsule: inter-observer reproducibility and influence of age, gender and study country. Aliment Pharmacol Ther 2018;47:391–400. 10.1111/apt.14438 [DOI] [PubMed] [Google Scholar]
- 43.Zarate N, Mohammed SD, O'Shaughnessy E, et al. . Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol 2010;299:G1276–86. 10.1152/ajpgi.00127.2010 [DOI] [PubMed] [Google Scholar]
- 44.The Danish Veterinary and Food Administration, Ministry of Environment and Food, Danish Dietary Recommendations [Internet]. Available: https://altomkost.dk/english/#c41067
- 45.Jensen MD, Ryan DH, Apovian CM, et al. . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation 2014;129(25 Suppl:S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab 2019;44:107–9. 10.1139/apnm-2018-0389 [DOI] [PubMed] [Google Scholar]
- 47.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82:222S–5. 10.1093/ajcn/82.1.222S [DOI] [PubMed] [Google Scholar]
- 48.Wadden TA, Neiberg RH, Wing RR, et al. . Four-year weight losses in the look AHEAD study: factors associated with long-term success. Obesity 2011;19:1987–98. 10.1038/oby.2011.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia Ulen C, Huizinga MM, Beech B, et al. . Weight regain prevention. Clin Diabetes 2008;26:100–13. 10.2337/diaclin.26.3.100 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037166supp001.pdf (108.8KB, pdf)
bmjopen-2020-037166supp002.pdf (116KB, pdf)