Abstract
Chimeric antigenreceptor (CAR) T cell therapy has demonstrated efficacy in B cell malignancies, particularly for acute lymphoblastic leukaemia (ALL) and non‑Hodgkin lymphomas. However, this regimen is not harmless and, in some patients, can lead to a multi organ failure. For this reason, the knowledge and the early recognition and management of the side effects related to CAR-T cell therapy for the staff is mandatory. In this review, we have summarised the current recommendations for the identification, gradation and management of the cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, as well as infections, and related to CAR-T cell therapy.
Keywords: CAR-T cells, cellular therapy, chimeric antigen receptor, cytokine release syndrome, immune effector cell–associated neurotoxicity syndrome
Cytokine release syndrome
Cytokine release syndrome (CRS) is a systemic reaction, generally related to the tumour burden,1 which usually occurs between the first day and second week after chimeric antigen receptor (CAR)-T-cell infusion.2–4 CRS rate differs among the pivotal studies for tisagenlecleucel (tisa-cel; Kymriah, Novartis, Switzerland) in paediatric and young patients with refractory B-cell acute lymphoblastic leukaemia2 and tisa-cel and axicabtagene-ciloleucel (axi-cel; Yescarta, Kite/Gilead, USA) in adult patients with refractory B-cell non-Hodgkin’s lymphoma.3 4 However, these studies used different grading systems scores and, as a result, the incidence of CRS and treatment guidelines cannot be compared for the two approved CAR-T cells products.5
CRS gradation scale
Recently, the American Society for Transplantation and Cellular Therapy (ASTCT) has proposed a new grade scale for CRS based exclusively on the presence of fever ≥38°C, hypotension (defined as any circumstance that requires intravenous fluid boluses or vasopressors to maintain normal blood pressure), hypoxia (requirement of supplemental oxygen) and end organ dysfunction.6 This scale is also recommended for the European Society for Blood and Marrow Transplantation (EBMT) for the management of adult and children undergoing CAR-T-cell therapy.7
CRS management
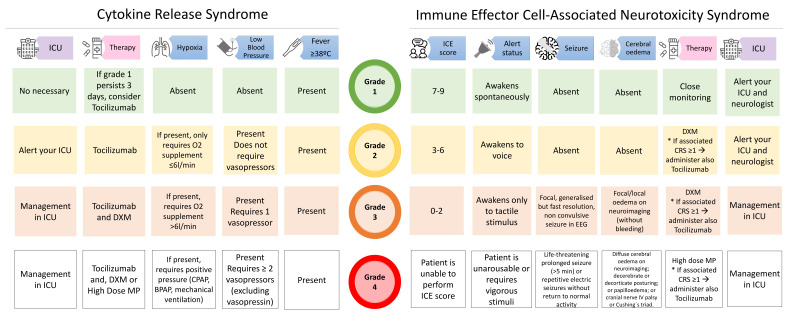
Currently, tocilizumab (Actemra, Roche, Switzerland), a monoclonal antibody against interleukin (IL)-6 receptor, is the only approved treatment for CRS grade ≥2 or persistent CRS grade 1. Siltuximab, a monoclonal antibody against IL-6, and anakinra, an anti-IL-1 receptor antagonist are under investigation.8 9 Steroids are recommended in severe CRS cases and when CRS is associated with neurotoxicity,7 however recent studies suggest they can be used earlier without deleterious effect on the CAR-T.10 11 In addition, fractionated dose of the CAR-T cells may be also an option to diminish the CRS incidence and severity without compromising efficacy.1 Figure 1 shows the current CRS grade scale and its management.
Figure 1.
The American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading and recommended management for cytokine release syndrome (CRS) and neurological toxicity associated with immune effector cells (ICANS). DXM, Dexamethasone; ICE, immune effector cell-associated encephalopathy; ICU, intensive care unit; MP, Methylprednisolone.
Immune effector cell-associated neurotoxicity syndrome
Immune effector cell-associated neurotoxicity syndrome (ICANS) is the second most common adverse event related to CAR-T cell therapies and can occur concurrently with or without CRS.2–4 ICANS incidence seems to be closely related to high disease burden, patient’s age as well as the specific CAR-T cell product.6 8
ICANS gradation scale
Similar to CRS, the ASTCT consensus-based grading system also includes ICANS for a uniform assessment for clinical trials and daily use.6 The ASTCT consensus system combines the immune effector cell-associated encephalopathy (ICE) score, based on the patient’s orientation and their ability to name three objects (nomination), follow simple commands, write a standard sentence and count backwards from 100 to 10, with the level of consciousness, presence and severity of seizures, motor impairment and clinical and/or imaging signs of cerebral oedema or elevated intracranial pressure. The ICE score is substituted by the Cornell Assessment of Paediatric Delirium for children aged <12 years. Table 1 shows ICANS grading for children and adults.
Table 1.
ICANS gradation scale for children and adults
| CAPD score (children <12 years) | Never (4 points) | Rarely (3 points) | Sometimes (2 points) | Often (1 point) | Always (0 point) |
| Eye contact with the caregiver | |||||
| Actions deliberated | |||||
| Aware of their surroundings | |||||
| Communicate their needs and wants | |||||
| Never (0 points) | Rarely (1 point) | Sometimes (2 points) | Often (3 points) | Always (4 points) | |
| Is the child restless? | |||||
| Is the child inconsolable? | |||||
| Is the child underactive? | |||||
| Does it take the child a long time to respond to interactions? | |||||
| ICE score (adults and children ≥12 years) | Orientation to year, month, city, hospital: 4 points | Naming three objects: 3 points | Following simple commands: 1 point | Writing a standard sentence: 1 point | Counting backwards from 100 to 10: 1 point |
| ASTCT ICANS consensus grading* | |||||
| Age (years) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| <12 | CAPD score | 1–8 | 1–8 | ≥9 | Unable to perform CAPD |
| ≥12 | ICE score | 7–9 | 3–6 | 0–2 | Unable to perform ICE |
| All ages | Depressed level of consciousness | Awakens spontaneously | Awakens to voice | Awakens only to tactile stimuli | Requires vigorous or repetitive tactile stimuli |
| All ages | Seizure | N/A | N/A | Any clinical seizure that resolves quickly or non-convulsive seizures on EEG that resolve with intervention | Life-threatening prolonged seizure (>5 min); or repetitive clinical or electrical seizures without return to baseline in between |
| All ages | Motor weakness | N/A | N/A | N/A | Hemiparesis, paraparesis |
| All ages | Elevated ICP/cerebral oedema | N/A | N/A | Focal oedema on neuroimaging | Decerebrate or decorticate posturing, cranial nerve VI palsy, papilloedema, Cushing’s triad or signs of diffuse cerebral oedema or neuroimaging |
*Original version in Lee et al.6
ASTCT, American Society for Transplantation and Cellular Therapy; CAPD, Cornell Assessment of Pediatric Delirium; ICANS, immune effector cell-associated encephalopathy score; ICE, immune effector cell-associated encephalopathy; ICP, intracranial pressure; N/A, not applicable.
ICANS management
Today, there are no approved therapies for the prevention/treatment of neurotoxicity; thus, it is primarily managed with supportive care. The use of levetiracetam as antiepileptic prophylaxis is controversial, but it is recommended, for at least 1 month after CAR-T cell infusion, in patients with a history of seizures or central nervous disease.7 9
Manifestations of ICANS can range from mild headache to coma and the continuous observation of patients who develop neurological symptoms after CAR-T cell infusion is mandatory.7 12 The EBMT recommendations also suggest to alert the intensive care unit (ICU) and a neurologist at onset of neurological findings regardless of the ICANS grade.7
In general, at the first sign of neurological symptoms, the bed’s head should be elevated by ≥30° to minimise aspiration risks and to improve cerebral venous flow. A neurological evaluation should be requested, independently of ICANS grade. Neuroimaging or lumbar puncture should be considered to exclude increased intracranial pressure and cerebral oedema, as well as ruling out other aetiologies. Repeated neuroimaging is recommended to detect early signs of cerebral oedema in patients with ICANS grade ≥3 or with rapid changes in grade. Brain MRI is preferred, but if not feasible, CT is an alternative option.7
Steroids are typically used as first-line therapy of ICANS grade ≥2,7 9 and dexamethasone and high dose of methylprednisolone are the most frequently used.2–4 7 9 Doses and length of therapy are variable and depend on the ICANS grade.7 9 Whereas dexamethasone is generally used in ICANS with low scores,7 repeated high pulse dose methylprednisolone is mostly used in grade 4 ICANS.7–9 Steroids are typically tapered over 2–3 weeks but patients should be monitored closely for recurrence of ICANS.8
Tocilizumab combined with corticosteroids is recommended for grade ≥1 ICANS and concurrent CRS,7 9 however it should not be administered for isolated ICANS because it can cause worsening of symptoms.8 Although currently not approved for this indication, siltuximab and anakinra have been used in severe cases of neurotoxicity.7–9 12
ICU monitoring is mandatory for all patients with grade 4 ICANS and recommended for patients with grade 2–3 ICANS.7 9
Non-convulsive/convulsive seizures or status epilepticus can be managed with benzodiazepines and additional antiepileptics (preferably levetiracetam), as needed. Patients with raised intracranial pressure or cerebral oedema should be managed promptly with anti-oedema measures as per standard guidelines.7–9
Infections, antibiotic prophylaxis and vaccinations
Infections can be observed for a long period after CAR-T cell infusion6 13 14 and severe CRS is a major risk factor.13
Bacterial infections, especially bacteraemia, and viral infections are the most common events within the first months after CAR-T cell therapy,13 whereas fungal infections are a rare complication.13 Beyond day 90 postinfusion, the most common cause of infections is upper (48%) and lower (23%) respiratory tract infections.14 15 Of them, the majority receive treatment in an outpatient setting (80%) and only 5% of patients need therapy in the ICU.14
In contrast to allogeneic stem cell transplantation, reactivation of herpes viruses such as cytomegalovirus, Epstein-Barr virus or human herpesvirus 6 is infrequent.13 Scarce information about the risk of CAR-T cell therapy in patients with hepatitis B or C is available because of the exclusion of these patients from CAR-T cell trials.12
Infection prophylaxis may follow institutional guidelines, covering bacteria, fungi and herpes simplex virus and varicella zoster virus. Prophylaxis for bacteria and candida species may be stopped when neutropenia resolves; in contrast Pneumocystis jirovecii prophylaxis and acyclovir prophylaxis may last at least 6 and 12 months after CAR-T cell infusion, respectively.12 Finally, mould fungi prevention may be individualised depending on patient risk of infection and antibacterial prophylaxis will be according to local bacterial resistance patterns.7 12
Because long-lived plasma cells are not a direct target of CD19+ CAR-T cells, the humoral immunity may be preserved.16 In the absence of data, we recommend complete vaccinations according to the patient’s age and seroprotection status.
Management of cytopoenias post-CAR-T cell therapy
Cytopoenia after CAR-T cell are usually observed up to day +28 (early cytopoenia) but some patients can experience them beyond day +90 (late cytopoenia).
Early cytopoenia
Within the first month after CAR-T cell infusion, grade ≥3 neutropenia, anaemia and thrombocytopoenia have been reported.2–4 17 In this period, cytopoenias are related to the lymphodepletion, and to a prior stem cell transplant, the severity of CRS and macrophage syndrome activation.17 For patients with neutropenia, granulocyte cell stem factor may be considered after CRS period risk, in general after the second week.12 Some patients can experience prolonged cytopoenia.
Delayed cytopoenia
Cytopoenia beyond the third month have been described in 16% of patients with ongoing complete remission14 and they are more frequent in patients with grade ≥3 CRS. The mechanism related to late cytopoenia is not well known but inflammation may have a role. In addition, it is important to keep in mind that the majority of patients have received many prior lines of therapy and MDS diagnosis needs to be ruled out.
Hypogammaglobulinaemia and immunoglobulin replacement
Secondary moderate (IgG >400 mg/dL) to severe (IgG ≤400 mg/dL) hypogammaglobulinaemia due to B-cell aplasia is commonly ‘the price to pay’ for the success of the CD19 antigen targeting malignant B cells in acute lymphoplastic leukaemia,2 however at least three of four patients with ongoing responses treated with axi-cel in the ZUMA-1 trial showed evidence of B-cell recovery by 2 years.15
Recently, Hill et al18 proposed a practical algorithm for hypogammaglobulinaemia management:
Screening for serum IgG prior to and in the first 3 months post-CAR-T cell therapy.
Consider prophylactic IgG replacement in patients with IgG ≤400 mg/dL.
Beyond the third month post-CAR, the only recommend IgG replacement if IgG is ≤400 mg/dL and the patient is experiencing infections.
In conclusion, CAR-T cell therapy is emerging as a curative option for some haematological malignancies. The recent ASTCT grading consensus provides a common approach for the grading of CRS and ICANS and may help guide common treatment guidelines. Furthermore, an improved understanding of the pathophysiology of cytopoenia may uncover new strategies to improve supportive care.
Acknowledgments
AT is supported by a grant from the Alfonso Martin Escudero Foundation.
Footnotes
Twitter: @DrLucreciaYSS
Contributors: LYSS, AAT and MS-E wrote and reviewed the manuscript. M-AP reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: LYSS reports honoraria from AbbVie, Gilead-Kite, Janssen, Celgene, Roche-La Hoffman, Merck, Novartis, Pfizer and Sandoz. M-AP reports honoraria from AbbVie, Bellicum, Celgene, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, Omeros and Takeda. He serves on DSMBs for Cidara Therapeutics, Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte, Kite/Gilead and Miltenyi Biotec. He serves in a volunteer capacity as a member of the Board of Directors of American Society for Transplantation and Cellular Therapy (ASTCT) and Be The Match (National Marrow Donor Program (NMDP)), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Committee.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol 2020;38:415–22. 10.1200/JCO.19.01892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–54. 10.1056/NEJMoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv 2020;4:676–86. 10.1182/bloodadvances.2019000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yakoub-Agha I, Chabannon C, Bader P, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for blood and marrow transplantation (EBMT) and the joint accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2020;105:297–316. 10.3324/haematol.2019.229781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santomasso B, Bachier C, Westin J, et al. The other side of car T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book 2019;39:433–44. 10.1200/EDBK_238691 [DOI] [PubMed] [Google Scholar]
- 9.Brudno JN, Kochenderfer JN. Recent advances in car T-cell toxicity: mechanisms, manifestations and management. Blood Rev 2019;34:45–55. 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topp MS, van Meerten T, Wermke M, et al. Preliminary results of earlier steroid use with axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory large B-cell lymphoma (R/R LBCL). JCO 2019;37:7558 10.1200/JCO.2019.37.15_suppl.7558 [DOI] [Google Scholar]
- 11.Liu S, Deng B, Yin Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J 2020;10 10.1038/s41408-020-0280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain T, Bar M, Kansagra AJ, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for transplantation and cellular therapy. Biol Blood Marrow Transplant 2019;25:2305–21. 10.1016/j.bbmt.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 13.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018;131:121–30. 10.1182/blood-2017-07-793760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-Targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant 2020;26:26–33. 10.1016/j.bbmt.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31–42. 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JA, Krantz EM, Hay KA, et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Adv 2019;3:3590–601. 10.1182/bloodadvances.2019000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant 2019;54:1643–50. 10.1038/s41409-019-0487-3 [DOI] [PubMed] [Google Scholar]
- 18.Hill JA, Giralt S, Torgerson TR, et al. CAR-T - and a side order of IgG, to go? - Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev 2019;38:100596. 10.1016/j.blre.2019.100596 [DOI] [PMC free article] [PubMed] [Google Scholar]