Abstract
Background
COVID-19 appeared in late 2019, causing a pandemic spread. This led to a reorganisation of oncology care in order to reduce the risk of spreading infection between patients and healthcare staff. Here we analysed measures taken in major oncological units in Europe and the USA.
Methods
A 46-item survey was sent by email to representatives of 30 oncological centres in 12 of the most affected countries. The survey inquired about preventive measures established to reduce virus spread, patient education and processes employed for risk reduction in each oncological unit.
Results
Investigators from 21 centres in 10 countries answered the survey between 10 April and 6 May 2020. A triage for patients with cancer before hospital or clinic visits was conducted by 90.5% of centres before consultations, 95.2% before day care admissions and in 100% of the cases before overnight hospitalisation by means of phone calls, interactive online platforms, swab test and/or chest CT scan. Permission for caregivers to attend clinic visits was limited in many centres, with some exceptions (ie, for non-autonomous patients, in the case of a new diagnosis, when bad news was expected and for terminally ill patients). With a variable delay period, the use of personal protective equipment was unanimously mandatory, and in many centres, only targeted clinical and instrumental examinations were performed. Telemedicine was implemented in 76.2% of the centres. Separated pathways for COVID-19-positive and COVID-19-negative patients were organised, with separate inpatient units and day care areas. Self-isolation was required for COVID-19-positive or symptomatic staff, while return to work policies required a negative swab test in 76.2% of the centres.
Conclusion
Many pragmatic measures have been quickly implemented to deal with the health emergency linked to COVID-19, although the relative efficacy of each intervention should be further analysed in large observational studies.
Keywords: COVID-19, oncological care
Key questions.
What is already known about this subject?
The COVID-19 pandemic led to a reorganisation of oncological care, based on expert advice, in order to reduce the spread of infection between patients and healthcare staff.
What does this study add?
The practical aspects of managing patients with cancer in multiple cancer centres during the COVID-19 health crisis have been analysed in this report. Many pragmatic measures have been quickly implemented, although the relative efficacy of each intervention should be further analysed in large observational studies.
How might this impact on clinical practice?
This analysis could be extremely interesting from an organisational point of view for possible future epidemics, for countries actually at the peak of the pandemic and in case a second COVID-19 peak occurs in the next months.
Introduction
A novel coronavirus disease, named COVID-19, responsible for SARS, appeared in late 2019, originating most likely from a seafood market in Wuhan, in the Hubei province of China.1 The causative agent was identified as a new coronavirus, called SARS-CoV-2, phylogenetically similar to SARS-CoV-1, responsible for the SARS outbreak that occurred in 2002.2 SARS-CoV-2 derives from natural selection likely in bats or pangolins with zoonotic transfer, leading to high affinity binding to the human ACE2 receptor.3
COVID-19 infection has rapidly spread and was declared a pandemic by the WHO on 11 March 2020. More than five million cases and more than 300 000 deaths have been reported in May worldwide.4 Several countries have adopted measures to limit virus spread, including lockdown, social distancing and reinforcement of hygiene requirements. Hospital activities have also been reorganised in order to reduce the risk of contagion among patients and caregivers.
Patients with cancer belong to a high-risk category for complications from infection due to organ damage from both tumour growth and treatment toxicity, immunosuppression resulting from tumour and anticancer therapies, and the generally advanced age group and presence of frequent comorbidities. Moreover, patients with cancer require frequent access to the hospital for assessment of disease status and toxicity, and in order to receive appropriate care, with increased risk of exposure to germs.
An analysis of 1524 patients admitted to a radiation and medical oncology unit in Wuhan from December 2019 to February 2020 found a twofold increase of the risk of COVID-19 infection rate in patients with cancer compared with the general population (0.79% vs 0.37%) over the same period of time.5 Data derived from a nationwide analysis in China reported that COVID-19-positive patients with cancer (N=18) had a higher risk of severe events compared with patients without cancer (39% vs 8%, p=0.0003), especially patients in active treatment compared with cancer survivors.6 In an Italian cohort published last March, 72 deaths out of 355 (20.3%) occurred in patients with cancer, while only 0.8% of the deaths occurred in patients without comorbidities, including cancer.7
Facing this exceptional and rapidly developing situation, no standard operating procedures for care organisations existed in oncologiocal institutions, although general recommendations for healthcare in hospital units, for COVID-19 and suspected case management were rapidly drafted.8 Several recent expert statements have been published addressing how to manage care in specialist oncology departments, but few reports guide effective organisation for daily clinical practice.9–20 We aimed to assess how oncology centres and departments reacted to the health crisis related to the COVID-19 pandemic, in order to improve oncological care and to implement preventive measures.
Materials and methods
A survey (online supplementary material) was sent by email to 30 representatives of oncological departments in 12 of the most affected countries by COVID-19 in Europe and the USA in order to provide quality assessment.
esmoopen-2020-000853supp001.pdf (103.7KB, pdf)
The topics investigated in the survey included preventive measures taken before and after admission to the hospital, instructions given to patients and professionals, general measures for risk reduction of virus spread, specific measures in the hospitalisation unit, general organisation of the centre, organisation of multidisciplinary meetings and activities of other healthcare professionals, staff management and antibody testing. The survey was composed of 46 items, for which response could be yes, no, not applicable or unknown. Additional information was provided by comments, such as describing procedures used in particular situations.
The survey was completed by all the centres over a short time period in order to avoid bias from changing practices, modification of diagnostic technologies and from national regulations on the availability and applicability of tests and isolation procedures.
The data obtained are presented as a percentage of responses out of the total number of participating centres, and bar chart was used to summarise data on triage, screening and general measures taken for risk reduction and specific measures in inpatient units.
The burden on the health system for each country at the moment of the survey completion was reported as the number of affected cases per 100 000 inhabitants, with data extracted from the Johns Hopkins University COVID-19 Data Repository.4
Results
Between 10 April and 6 May 2020, a total of 21 institutions from 10 different countries answered the survey by email: from Belgium (5), Italy (4), Spain (2), Germany (2), Switzerland (2), USA (2), Austria (1), the Netherlands (1), France (1) and Luxembourg (1). Overall, 15 centres were university general hospitals, 5 were comprehensive cancer centres and 1 was a private clinic. All the centres included experienced high-capacity oncological units. Among these, 21 centres had consultations and day care facilities, and 20 had an overnight facility.
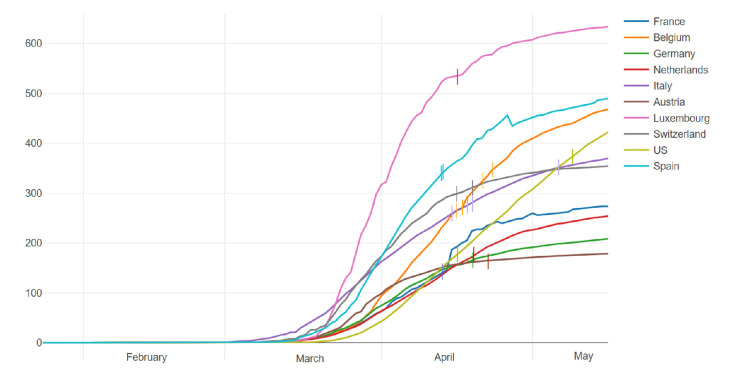
The burden of COVID-19 cases on the health system is calculated as the number of total infections per 100 000 inhabitants in each country (figure 1). However, considering the different applicability of the test in the acute phase of the outbreak in different countries, the number of cases does not fully reflect the real load of infection. At the moment of survey completion, a lower burden, with less than 200 cases per 100 000 inhabitants, was observed for France, Germany, Austria, the Netherlands and one centre from USA. For Italy, Belgium, Switzerland, Spain and the other centre from USA, 200–400 patients were affected by COVID-19 per 100 000 inhabitants. The most affected country was Luxembourg, with more than 500 cases per 100 000 inhabitants.
Figure 1.
COVID-19 infection per 100 000 inhabitants. The curves represent the number of patients per 100 000 inhabitants with positive results for COVID-19 over time in the 10 countries included in the survey from 22 January to 13 May 2020. Each bar on the curves corresponds to one investigator. On the x-axis, the timing from February to May is shown; on the y-axis, the N of patients per 100 000 inhabitants is shown.
Triage, screening procedures and patients’ education
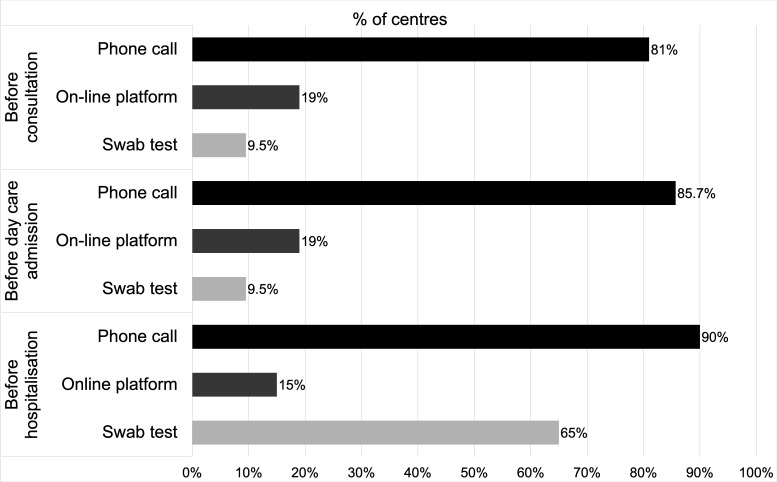
Patients were subjected to a triage for signs of infection prior to presenting to the oncology units in the majority of the centres, notably in 19/21 institutions (90.5%) before consultations, in 20/21 (95.2%) before day care outpatient infusion room admissions and in 20/20 (100%) before overnight hospitalisation (figure 2). More specifically, the triage/screening tools used were phone calls, interactive online platforms, nasopharyngeal or oropharyngeal swab tests, and/or chest CT scans. Phone call was the most common sorting method, used in 17/21 (81%) institutions before outpatient consultations, in 18/21 (85.7%) before day care admissions and in 18/20 (90%) before hospitalisation. Less extensive use of the interactive online platform was noted: platforms were in implementation in two centres, routinely used in 4/21 (19%) before consultations and day care unit admissions and in 3/20 (15%) before hospitalisations. Swab test was not routinely performed for outpatients as it was used in only 2/21 centres (9.5%). Its use was more common for sorting patients in overnight wards, with 13/20 centres (65%) using it routinely on unselected patients prior to admission, with a result available within 12 hours in all the institutions. Chest CT scan was used in only one institution (4.8%) as a screening method before surgery and invasive procedures.
Figure 2.
Triage/screening measures for COVID-19 in oncological units. Triage (phone call and on-line platform) and screening (swab test) measures taken before consultation, day care admission and hospitalisation in 21 oncological units.
A clinical check of symptoms was performed in the hospital/clinic before entering the consultation room in 17/21 centres (81%), before entering the day care unit in 20/21 (95.2%) and before hospitalisation in 19/20 (95%) units.
Patients were educated in the majority of centres to take precautions in order to avoid virus transmission. In particular, 20/21 (95.2%) centres educated patients to contact the medical oncology/haematology department if they developed symptoms potentially related to COVID-19, and in 19/21 (90.5%) centres, patients were advised to avoid visits to the unit in the presence of suspected symptoms.
Allowance of visitors with patients
Permission for family members and caregivers to accompany patients was limited at this stage of pandemic spread, with only 8/21 (38.1%) centres allowing attendance to consultations, 4/21 (19%) to day care admissions and 8/20 (40%) to visit patients in overnight wards. Some exceptions were allowed: in 17/21 (81%) centres when a patient was unable to enter without assistance, in case of a new diagnosis of cancer in 3/21 (14.3%) centres, when bad news has to be announced in 4/21 (19%) centres and for terminally ill patients in 18/20 (90%) hospitalisation units. Some centres implemented virtual meetings with the family concomitantly to physical meetings with the patients.
Protective equipment and disinfection
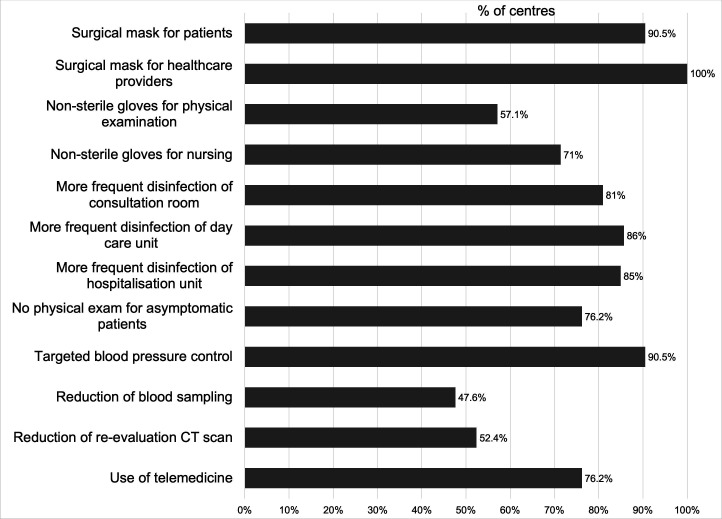
In addition to social distancing and frequent hand washing/disinfection, some general measures were implemented to reduce risk of contagion (figure 3), such as the use of surgical masks for all patients in 19/21 (90.5%) institutions and for healthcare providers in all of the 21 centres (100%), the use of non-sterile gloves for physical examinations in 12/21 (57.1%) and for nursing in 15/21 (71.4%) centres. Filtering facepiece 2 and 3 masks were used in 1/21 (4.8%) institutions for non-aerosolising procedures, if no previous swab test had been done.
Figure 3.
General measure for risk reduction. Measures taken to reduce the risk of COVID-19 transmission in 21 oncological units.
Increased rates of disinfection process were also noted: of consultation rooms in 17/21 (81%) centres, of the day care units in 18/21 (85.7%) and of the hospitalisation units in 17/20 (85%) centres.
Reduction in physical contact
To reduce contact (figure 3), physical examination was abolished for asymptomatic patients in 16/21 (76.2%) centres, and a targeted examination was preferred when necessary, for example, of the known sites of metastasis or directed by symptoms. Blood pressure control was performed only when clinically indicated and not routinely in 19/21 (90.5%) institutions. Blood sampling was reduced by at least 25% in 10/21 (47.6%) centres, and the number of CT scans for evaluation of tumour response was reduced by at least 25% in 11/21 (52.4%) institutions. The use of telemedicine was implemented in 16/21 (76.2%) centres.
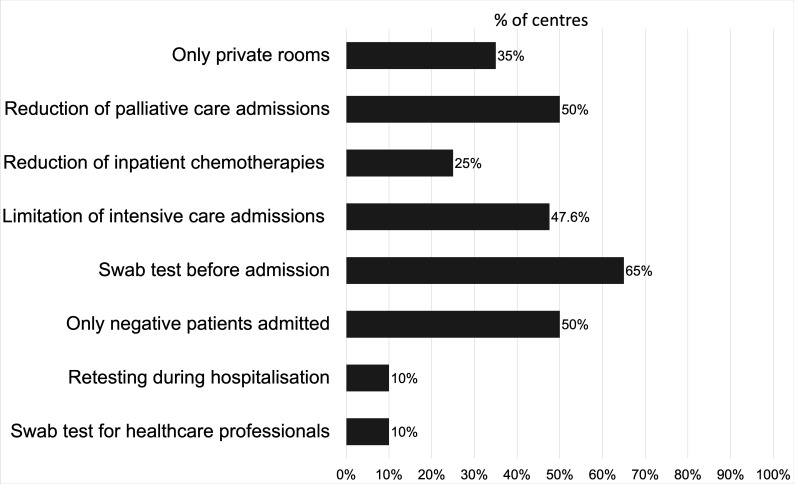
Several specific measures have been taken in inpatient units to reduce patients’ stay and physical contacts (figure 4). Only private rooms were used in 7/20 (35%) centres; palliative care admissions were reduced in 10/20 (50%) centres; chemotherapy was transferred from overnight hospitalisation to day care unit in 5/20 (25%) centres. In 10/21 (47.6%) centres, physicians discussed the option to avoid intensive care in case of worsening of clinical conditions in those with terminal illness, regardless of COVID-19 infection, accepting the risk of early death. In 13/20 (65%) centres, swab tests were performed before hospitalisation, and in 10/20 (50%) centres, admissions were limited to COVID-19-negative patients. Two centres (10%) allow emergency admissions without a negative test result. Retesting during hospitalisation was done in only two centres (10%), weekly (one centre) or biweekly (one centre). Nasopharyngeal or oropharyngeal swab test for healthcare staff in the hospitalisation unit was done weekly by only one centre and biweekly by another one, while 18/20 (90%) centres did not do swab testing for healthcare professionals in the absence of symptoms.
Figure 4.
Specific measures in inpatient units. Measures taken to reduce the risk of COVID-19 transmission in 20 oncological hospitalisation units.
In the majority of the hospitals, specific COVID-19 units separate from non-COVID units have been organised (20/21 centres, 95.2%), and nurses and medical staff in the oncology unit participate exclusively to COVID-19-negative patients’ care in 12/21 (57.1%) centres. In 20/21 (95.2%) centres, specific pathways for COVID-19-negative and COVID-19-positive/suspected patients have been organised. COVID-19-confirmed or suspected cases at or during hospitalisation were transferred to the COVID-19 units in 18/20 (90%) centres. Specific separate day care units for COVID-19-positive/suspected cases were established in 9/21 (42.9%) institutions, while in 1 (4.8%) case a specific room was used for these patients. At the start of the COVID-19 crisis, the oncology department, either in part or in full, moved to another location in 4/21 (19%) centres, in the same hospital in three cases and to another hospital in one case.
Multidisciplinary meetings were done by videoconferences in 17/21 (81%) centres, while in 4/21 (19%) physical meetings were still done respecting social distancing. Multidisciplinary team members other than physician and nurses could still see patients in 10/21 (47.6%) centres, while in 16/21 (76.2%) centres, they managed all (10/21) or almost all (6/21) problems by phone or videoscreen.
Management of staff suspected or confirmed as having COVID-19
Self-isolation at home was the rule for staff members presenting with fever in 20/21 (95.2%) centres and for staff members living with someone in self-isolation in 13/21 (61.9%) centres. Return to work for healthcare professionals tested COVID-19-positive was allowed after at least 7 days from diagnosis and 3 days without symptoms in 5/21 cases (23.8%). In the remaining cases (16/21, 76.2%), a negative swab test was required. In particular, in three centres, swab testing needed to be done after at least 2 weeks of self-isolation, and in three centres, two negative tests were required. In one (4.8%) centre, homework was allowed for staff members older than 60 years, with serious comorbidity or with cohabitant family members with serious morbidity.
In addition to the previously discussed testing methods, an emerging interest is the use of serology. During the survey period, this test was offered to healthcare workers in 8/21 (38.1%) centres, most frequently in the context of a research project (7/21: 33.3%), and to patients in 9/21 (42.9%) centres, in four cases as clinical study (19%) and in five (23.8%) as routine practice.
Discussion
The emergence of COVID-19 has caused a global health emergency, leading to multiple critical challenges. Not only do clinicians and healthcare workers need to manage an extremely high load of very critically ill patients, but also they need to identify ways to reduce the risk of intrahospital contagion both among patients and healthcare staff while using available resources as efficiently as possible. This, however, has often resulted in a lack of resources for routine patient care.
In the oncology field, the initial response has been focused on the protection of patients from the risk of infection. A shared approach, based on expert advice, includes limiting hospital access whenever possible, implementing of a triage prior to admission to the hospital, reducing unnecessary consultations and exams, delaying follow-up visits, giving priority to adjuvant and curative treatment over palliative ones, and avoiding advanced treatment lines with low probability of clinical benefit.9 10 In this context, the risk of jeopardising the effectiveness of cancer treatment and the risk of delayed diagnosis must be taken into high consideration and should be carefully analysed in future long-term studies.18
We conducted an observational study to analyse the activity of oncology centres located in the countries most affected by the COVID-19 outbreak. These types of studies are beneficial in order to identify best practices and to construct standardised algorithms for the prevention and management of this virus, and other emerging viruses, at long-term. It is especially crucial in light of a likely secondary recrudescence of the infection outbreak.
While for some aspects the applied procedures were almost the same in all centres, others were quite different between centres, probably due to different burden of infection, differences in the availability of viral screening and resource availability.
The use of personal protective equipment was one of the cornerstones for prevention of virus spread that was almost unanimously accepted. Another interesting point is reducing the number of people accessing the hospital by limiting those accompanying or visiting patients. This second strategy is not currently used in all centres, due to the particular needs of patients with cancer. Therefore, a limitation of the number of persons could be applied to routine visits or access to the hospital during treatment, while exceptions can be made for heavily dependent patients, when bad news has to be announced and for terminally ill patients.
The use of telemedicine reduces patient exposure to the hospital and is actually used in 76.2% of the institutions. Although some legal and reimbursement issues remain, COVID-19 crisis has led to a rapid expansion of telemedicine, which could remain a good option also outside the pandemic crisis, especially when a clinical examination is not necessary and in areas of the world where reaching the hospital raises practical difficulties for patients.21
Almost all the oncological centres included in the survey performed a triage before admission to the hospital in order to recognise symptoms early in suspected cases and to avoid contamination of other patients and healthcare professionals. Triage is supplemented with diagnostic tests, usually swab, with nasal sampling resulting more efficiently than throat sampling.22 23 The main issue with this tool is the relatively low sensitivity of about 70%, the multiplicity of primers that can be used to perform the reverse-transcriptase polymerase chain reaction (RT-PCR) and the risk of cross-reactivity with other coronavirus strains.24–26 Chest CT scan seems to be more sensitive than RT-PCR for COVID-19 diagnosis and in 60%–93% of the cases becomes positive before the RT-PCR.25 27 However, the disadvantage of CT is the limited availability in terms of number of exams per day in each institution compared with the swab, which can be easily made on a large scale and is less cost-effective. On the other hand, the result of the swab test takes a longer time than CT scan. A potential solution to these concerns is the development of a rapid test, such as the serological test for IgA, IgM and IgG. Currently, its low sensitivity at the early phase of infection, the delayed and the lower rate of seroconversion in patients with cancer and the inability to detect contagiousness do not yet make it a suitable test to be used as a diagnostic standard.28–30 Overall, at the moment, it seems more appropriate to carry out a triage by telephone or interactive platform before outpatient admissions and a swab before hospitalisation. While the use of screening by means of a swab prior to hospitalisation is increasing, there was no unanimous agreement about retesting. A single baseline test does not allow early detection of intrahospital infections and could miss paucisymptomatic or presymptomatic patients.31
Another issue of concern is the implementation of a policy for return to work for healthcare professionals tested positive. While some centres require negative swab test, others do not, which may pose a risk of transmitting the infection at the workplace, considering that a duration of viral shedding up to 37 days has been described.32 33
Finally, predisposed dedicated facilities to manage cancer care during COVID-19 pandemic could be advisable.34 Although COVID-19 wards have been organised in most centres, in only 57.1% of the centres healthcare professionals caring for oncohaematological patients did not take part in the care of COVID-19-positive patients.
In conclusion, we analysed the prevention measures taken in oncological units in high-income countries in Europe and the USA, which effectiveness could be assessed in a later stage of the pandemic and in large observational studies. Although some recommendations for hospital management during the COVID-19 pandemic are actually available, such as those of the European Centre for Disease Prevention and Control, our study allows assessment of adherence to this recommendation, in addition to providing an insight into the management of oncological care during the pandemic in a real-world context.8 Many procedures adopted, in fact, require agreement at the international level. Some critical issues remain to be improved in order to increase the safety of treatment for oncohaematological patients. The same level of care should be extended to the entire healthcare system, to less well-supplied centres and to low-income countries.
Footnotes
Twitter: @Fabio Puglisi
Correction notice: This paper has been updated since first published to update affiliation for Fabio Puglisi.
Contributors: GJ conceived the work; CEO wrote the manuscript; HR, DG, MP, KZ, HW, NH, MM, MC, JC, VTH, GB, MT, MC, RB, SDP, FP, SR, VM, TR, JPM and GJ answered the survey; all the authors contributed to the survey drafting and revised and approved the manuscript.
Funding: This work was supported by Fonds de la Recherche Scientifique (grant to CEO) and Fondation Léon Fredericq (grant to GJ and CEO).
Competing interests: HSR reported fundings to the Institution from Eisai, Genentech, Lilly, Macrogenics, Merk, Novartis, OBI Pharma, Odonate Therapeutics, Immunomedics, Daiichi-Sankyo and Pfizer; travel fees from Pfizer, Novartis, MacroGenics, Mylan, Daiichi-Sankyo and AstraZeneca; consulting roles for Samsung and Celtrion. MM reported personal fees from Roche, Novartis, Puma, AstraZeneca, Amgen, Taiho Oncology, Daiichi-Sankyo, PharmaMar, Eli-Lilly and Pfizer; grants from Roche, Novartis and Puma. MC reported a grant from Pfizer; personal fees from CytoDyn, Pfizer, Eli-Lilly, Novartis, Foundation Medicine, G1 Therapeutics, Sermionex and Genentech. JC reported funding to the institution from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer healthcare, Eisai, F. Hoffman-La Roche, Guardanth health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C and Queen Mary University of London; honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Eli-Lilly, Merck Sharp & Dohme, and Daiichi Sankyo; travel fees from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo; consulting/advisor role for Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi-Sankyo, Erytech, Athenex, Polyphor, Lilly, Servier, Merck Sharp&Dohme, GSK, Leuko, Bioasis, Clovis Oncology and Boehringer Ingelheim; stock, patents and intellectual property of MedSIR. SAH reported grants from Ambrx, Amgen, Arvinas, Bayer, Daiichi-Sankyo, Genentech/Roche, GSK, Immunomedics, Eli-Lilly, Macrogenics, Novartis, Pfizer, OBI Pharma, Pieris, Puma, Radius, Sanofi, Seattle Genetics and Dignitana; travel fees from Lilly and Novartis; stock options from NK Max; editorial support from Roche/Genetech, Eli-Lilly and Pfizer. MT declares travel, accomodations, expenses by Roche, Bristol-Myers Squibb, Astra Zeneca, Takeda; activity as medical writer supported by Novartis and Amgen, outside the present work. MC reported funding to the institution from Sanofi, Pierre-Fabre, AstraZeneca, Servier, Novartis, Abbvie, Accord and Pfizer; personal fees from GT1, Sanofi, Pierre-Fabre, AstraZeneca, Servier, Novartis, Abbvie, Accord and Pfizer. RB reported personal fees from Accord, AstraZeneca, Daiichi-Sankyo, Eisai, Eli-Lilly, MSD, Novartis, Roche, Puma, Pierre-Fabre and Sandoz; grants from Daiichi-Sankyo, Novartis and Roche; non-financial support from Eli-Lilly and MSD. SDP reported personal fees from Roche, Novartis, Pfizer, Celgene, AstraZeneca and Eli-Lilly. VM reported funding to the institution from Novartis, Roche, Seattle Genetics and Genentech; personal fees from Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva and Seattle Genetics; consultancy honoraria from Genomic Health, Hexal, Roche, Pierre-Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Eli-Lilly, Tesaro, Seattle Genetics and Nektar. J-PM reported personal fees from Roche, AstraZeneca, Bayer, Innate, Merck-Serono, Boeringher, BMS, Novartis, Janssen, Incyte, Cue Biopharma, ALX Oncology and Pfizer; grant from BMS; travel fee from Amgen; other support from MSD, Psioxus, Debio and Nanobiotix. GJ reported personal fees from Novartis, Roche, Pfizer, Eli-Lilly, Amgen, BMS, AstraZeneca, Daiichi-Sankyo and Abbvie; grants from Novartis, Roche and Pfizer; non-financial support from Medimmune and Merck KGaA.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.Zhu N, Zhang D, Wang W, et al. . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, et al. . Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen KG, Rambaut A, Lipkin WI, et al. . The proximal origin of SARS-CoV-2. Nat Med 2020;26:450–2. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 data Repository by the center for systems science and engineering (CSSE) at Johns Hopkins University. Available: https://github.com/CSSEGISandData/COVID-19
- 5.Yu J, Ouyang W, Chua MLK, et al. . SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020;6:1108 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, et al. . Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020. 10.1001/jama.2020.4683. [Epub ahead of print: 23 Mar 2020]. [DOI] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control - Covid-19 pandemic. Available: https://www.ecdc.europa.eu/en/covid-19-pandemic
- 9.Lambertini M, Toss A, Passaro A, et al. . Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists' perspective. ESMO Open 2020;5:e000759. 10.1136/esmoopen-2020-000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meattini I, Franco P, Belgioia L, et al. . Radiation therapy during the coronavirus disease 2019 (covid-19) pandemic in Italy: a view of the nation's young oncologists. ESMO Open 2020;5:e000779. 10.1136/esmoopen-2020-000779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motlagh A, Yamrali M, Azghandi S, et al. . COVID19 Prevention & Care; A Cancer Specific Guideline. Arch Iran Med 2020;23:255–64. 10.34172/aim.2020.07 [DOI] [PubMed] [Google Scholar]
- 12.Shankar A, Saini D, Roy S, et al. . Cancer Care Delivery Challenges Amidst Coronavirus Disease - 19 (COVID-19) Outbreak: Specific Precautions for Cancer Patients and Cancer Care Providers to Prevent Spread. Asian Pac J Cancer Prev 2020;21:569–73. 10.31557/APJCP.2020.21.3.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson DJ, Palma D, Guckenberger M, et al. . Practice recommendations for risk-adapted head and neck cancer radiation therapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys 2020;107:618–27. 10.1016/j.ijrobp.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guckenberger M, Belka C, Bezjak A, et al. . Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. Int J Radiat Oncol Biol Phys 2020;107:631–40. 10.1016/j.ijrobp.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzone PJ, Gould MK, Arenberg DA, et al. . Management of lung nodules and lung cancer screening during the COVID-19 pandemic: chest expert panel report. Chest 2020;158:406–15. 10.1016/j.chest.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernhardt D, Wick W, Weiss SE, et al. . Neuro-Oncology management during the COVID-19 pandemic with a focus on who grade III and IV gliomas. Neuro Oncol 2020. 10.1093/neuonc/noaa113. [Epub ahead of print: 05 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. . A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international Collaborative group. Oncologist 2020;25:e936–45. 10.1634/theoncologist.2020-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortiula F, Pettke A, Bartoletti M, et al. . Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol 2020;31:553–5. 10.1016/j.annonc.2020.03.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauri D, Kamposioras K, Tolia M, et al. . Summary of international recommendations in 23 languages for patients with cancer during the COVID-19 pandemic. Lancet Oncol 2020;21:759–60. 10.1016/S1470-2045(20)30278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Wang J, He J. Active and effective measures for the care of patients with cancer during the COVID-19 spread in China. JAMA Oncol 2020. 10.1001/jamaoncol.2020.1198. [Epub ahead of print: 01 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 21.Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care 2020;26:147–8. 10.37765/ajmc.2020.42784 [DOI] [PubMed] [Google Scholar]
- 22.Xiao AT, Tong YX, Gao C, et al. . Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol 2020;127:104346. 10.1016/j.jcv.2020.104346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou L, Ruan F, Huang M, et al. . SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177–9. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JF-W, Yip CC-Y, To KK-W, et al. . Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol 2020;58. 10.1128/JCM.00310-20. [Epub ahead of print: 23 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Y, Zhang H, Xie J, et al. . Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 2020;296:E115–7. 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corman VM, Landt O, Kaiser M, et al. . Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020;25:2000045 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai T, Yang Z, Hou H, et al. . Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020;296:200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassaniti I, Novazzi F, Giardina F, et al. . Performance of VivaDiag COVID-19 IgM/IgG rapid test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J Med Virol 2020. 10.1002/jmv.25800. [Epub ahead of print: 30 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long Q-X, Liu B-Z, Deng H-J, et al. . Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020;26:845–8. 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 30.Solodky ML, Galvez C, Russias B, et al. . Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol 2020;31:1087–8. 10.1016/j.annonc.2020.04.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arons MM, Hatfield KM, Reddy SC, et al. . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020;382:2081–90. 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Yan L-M, Wan L, et al. . Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020;20:656–7. 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tagliamento M, Lambertini M, Genova C, et al. . Call for ensuring cancer care continuity during COVID-19 pandemic. ESMO Open 2020;5:e000783. 10.1136/esmoopen-2020-000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000853supp001.pdf (103.7KB, pdf)