Abstract
Introduction
Unstable angina (UA), referred to as acute coronary syndrome (ACS), causes unexpected chest pain. Xueshuantong injection (lyophilised) (XST) is a traditional Chinese herbal injection having the potential to treat ACS. However, no clinical trial has been performed in this field. This clinical trial aims to examine the efficacy and safety of XST.
Methods and analysis
This is a randomised, parallel-arm, controlled, double-blind and multicentre clinical trial. A total of 1200 participants with UA will be enrolled in a 1:1 ratio, with 600 patients included in the XST treatment group and 600 with 1/20th dose in the control group. The efficacy assessment and major adverse cardiovascular events will be observed, and the frequency of angina attack, angina pectoris will be examined at the start and end of the run-in period. All adverse events will be recorded, regardless of the severity, to assess the safety of XST. The baseline characteristics of patients will be summarised and compared using the t test or non-parametric statistical test. Qualitative data will be analysed using the χ2 or Fisher exact tests, Cochran–Mantel–Hasenszel test and Wilcoxon test.
Ethics and dissemination
This trial has been approved by the Research Ethics Committee of The First Affiliated Hospital of Guangzhou University of Chinese Medicine, China (approval number: ZYYEC [2017] 0021). Written informed consent will be obtained from all participants. The results of this trial will be disseminated to the public through academic conferences and peer-reviewed journals.
Trial registration
This study was registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn/) with the ID ChiCTR1800015911.
Keywords: cardiology, adult cardiology, coronary heart disease
Strengths and limitations of this study.
This is a randomised, parallel-arm, controlled, double-blind and multicentre clinical trial.
The trial will be conducted in 17 medical centres with 1200 participants.
In our study, to better implement the blind method, the extremely low dose 25 mg is used as the control group.
The efficacy assessment and major adverse cardiovascular events will be observed, and the frequency of angina attack, angina pectorals will be examined at the start and end of the run-in period.
Our experiments will be conducted in different regions of China, and whether similar effects are available to other ethnic groups and regions remains uncertain.
Introduction
Unstable angina (UA), referred to as acute coronary syndrome (ACS), causes unexpected chest pain. Reduced blood flow to the heart muscle is the most common cause of UA because the coronary arteries are narrowed by atherosclerosis, leading to the rupture of coronary blood vessels and hence blood clotting which blocks the flow of blood to the heart muscle. The risk factors for UA include diabetes, obesity, family history of heart disease, high blood pressure, high low-density lipoprotein cholesterol, low high-density lipoprotein cholesterol, male sex and use of any form of tobacco.1 2 With the wide application of percutaneous coronary intervention (PCI) in patients with ACS, current guidelines recommend potent platelet inhibition with prasugrel or ticagrelor for 12 months after ACS management with PCI. However, the greatest anti-ischaemic benefit of potent antiplatelet drugs over the less-potent clopidogrel occurs early, while most excess bleeding events occur during chronic treatment. It is related to the efforts of physicians to reduce the adverse incidence of cardiovascular events, promote the patient’s early recovery and improve their health-related quality of life.
Xueshuantong injection (lyophilised) (XST) is a traditional Chinese herbal injection comprising a series of saponins extracted from Panax notoginseng. It has been approved by the China Food and Drug Administration (China drug approval number: Z 20025652) and collected according to the ‘2012 national essential drugs list’ and People’s Republic of China Pharmacopoeia, respectively. It has been reported to have anti-inflammatory effects that correct endothelial dysfunction in vivo3 and in vitro.4 Clinically, as a common medicine in China’s Grade-A Tertiary Hospital, XST has been reported to be beneficial in treating ACS.5 6 In preliminary studies, including small samples, XST has been found to platelet aggregation inhibition, antimyocardial ischaemia, anti-inflammation and antioxidation, protecting endothelial cells (ECs), which could reduce the incidence of major adverse cardiovascular events (MACE).7 A recent study found that XST inhibits platelet activation and suppresses leucocytes adhesion to injured ECs under controlled shear stress in vitro which not only dose-dependent and showed stronger anti-platelet activation and adhesion effect under low shear stress.7 XST also played an effect in fighting against thrombosis induced by k-carrageenan in rats. High dose could significantly increase the microcirculatory blood flow perfusion of the tail and significantly inhibit platelet aggregation rate.8 Besides, XST could significantly inhibit platelet piezo1 protein expression which may improve blood flow and antithrombotic. The meta-analysis reported that XST combined with routine basic treatment (RBT) can alleviate UA pectorals, especially for frequency relief of angina, frequency reduction of nitroglycerin and the effective rate is more than 80%. It can significantly reduce blood high-sensitivity C-reactive protein, fibrinogen concentration.9 As the primary component,10 Panax notoginseng has been extensively verified that it can ameliorate ischaemia-reperfusion-induced injury in cardiovascular and neuronal systems mainly by upregulating the activity of oestrogen receptorα-dependent phosphoinositide 3-kinase/protein kinase B and nuclear factor erythroid-2-related factor 2 pathways and downregulating nuclear factor-κB and mitogen-activated protein kinase pathways. Therefore, high-quality trials and evidence are needed to prove the efficacy of XST. This randomised, parallel, controlled, double-blind and multicentre clinical trial aims to examine the efficacy and safety of XST in patients with UA. The results of this trial may provide clinical evidence for treating patients with UA.
Method and design
Study design
This randomised, parallel-arm, controlled, double-blind and multicentre clinical trial will be conducted in 17 medical centres in China: The First Affiliated Hospital of Guangzhou University of Chinese Medicine, The First Affiliated Hospital of Henan University of Chinese Medicine, Luoyang No. 1 Hospital of Traditional Chinese Medicine, Shanxi Fenyang Hospital, The First Affiliated Hospital of Henan University of Science and Technology, Zhengzhou People’s Hospital, Zhengzhou Central Hospital, Ruikang Hospital Affiliated to Guangxi University of Traditional Chinese Medicine, Nanjing Hospital of Chinese Medicine, Shuguang Hospital Affiliated to Shanghai University of Chinese Medicine, Changsha Fourth Hospital, The First Affiliated Hospital of Hunan University of Traditional Chinese Medicine, The Second Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, The Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Affiliated Hospital of Tianjin Academy of Chinese Medicine, Tianjin Beichen District Hospital of Traditional Chinese Medicine and Wenzhou Hospital of Traditional Chinese Medicine. A total of 1200 patients with UA who met the selection will be enrolled in a 1:1 ratio, with 600 patients included in the XST treatment group (high-dose, 500 mg) and 600 in the control group (extremely low dose, 25 mg).
All the visits will be recorded in electronic case report forms through the Electronic Data Capture system, which is accessed online via the Internet for data collection and management. The protocol for this study was developed in accordance with the standard protocol project: Interventional Trial Recommendations guidelines.11
Patient and public involvement
This clinical trial was designed to evaluate the efficacy and safety of high-dose XST in patients with UA. Clinically, XST has been found to be beneficial in treating ACS, which had been widely used in China’s Grade-A Tertiary Hospital. More high-quality trials and evidence are needed to prove the efficacy of XST. The outcome measures used in this trial were considered as important endpoints in clinical practice. The participants in this trial will be recruited from 17 medical centres in China. However, patients were not directly involved in the design, recruitment or conduct of the study. After the trial completes, the results of this study will be disseminated to the public through academic conferences and peer-reviewed journals. Once the manuscript is published, the results will be briefly summarised in a simple language and inform all trial participants through the telephone. The burden of intervention will not be assessed by the trial participants.
Study population
Patients will be included after written informed consent and enrolled in the study when the inclusion and exclusion criteria are met (table 1).
Table 1.
Inclusion and exclusion criteria
| Exclusion criteria | |
|
|
PCI, percutaneous coronary intervention.
Withdrawal criteria
Patients with some comorbidities, complications or special physiological changes during the trial.
Patients with poor compliance in the trial; the use of the drug not reaching 80% (except for those recovered in advance) or exceeding 120% of the prescribed amount.
Patients with blindness or emergency unblinding during the trial.
Patients with serious adverse events (AEs) and those not appropriate to continue the test.
Patients failing to use the test drug.
Patients misdiagnosed or not matching the inclusion criteria and accidentally included.
Patients with no follow-up records.
Patients failing to comply with the treatment during the trial, changing medicines or adding non specified therapeutic medications by themselves, especially those medications that may affect the evaluation of the test drug, affecting the validity and safety.
Study setting and recruitment
Between December 2018 and December 2020, 1200 outpatients or inpatient will be recruited at 17 centres mentioned earlier through the official website of the hospitals, posters and networks. Physicians will diagnose the participants, and the research assistants will manage the recruitment.
Randomisation
On the day of enrolment, statistical analysis system software will be used to generate the random arrangement of 1200 people in two groups (XST and placebo groups) with the method of central stratified regional group randomisation. The randomisation numbers will be kept in opaque sealed envelopes. Physicians and patients will not be aware of the grouping and intervention.
Blinding
This study has a double-blind design. Because XST was lyophilised powder form in this study, which was a kind of white or light yellow amorphous powder or loose solid, and was dissolved with an appropriate amount of injection sodium chloride injection before use, there were colour changes and a small amount of powder precipitation. Therefore, in order to better implement the blind method, an extremely low dose (25 mg) was used as the control group, which is invalid for UA from our previous pharmacokinetic experiment, did not increase the efficacy of the experimental group.
The number of cases in the study and control group will be in the ratio of 1:1. The blinding work will be completed by statisticians. To ensure the blinding of investigators and participants to study treatment, the study drug or placebo will be provided in identical packaging and labelling. Due to some natural variability in the colour of the study drug, which is batch dependent, the colour of the placebo will match the same as the average colour of the study drug. Study drug and placebo will label with a unique label letter that will be used to assign treatment to the patient but will not indicate treatment allocation to investigators or participants. No member of the study team and their extended staff, except for pharmacists and biostatisticians, will have access to the randomisation scheme during the conduct of the study. In the event of a medical emergency, where breaking the blind is required to provide medical care to the participant, the investigator will obtain the treatment assignment from the trial pharmacists.
Sample size calculation
MACE will be used as the effective index according to the statistical requirements of the optimal validity test design.
Based on the expert advice combined with clinical practical considerations and according to the loss rate of less than 20% estimated beforehand, the study sample was 1200 cases (600 cases in each group), assuming that the incidence of MACE was 12% after dual antiplatelet therapy for 6 months which was reduced by 6% after lyophilisation (alpha=0.05; power=0.9).
Interventions
All participants will receive dual antiplatelet therapy (aspirin 100 mg/d+clopidogrel 75 mg/d) and anticoagulation therapy (unfractionated heparin) according to the 2014 American College of Cardiology/American Heart Association Guidelines for the Diagnosis and Treatment of Non-ST-Segment Acute Coronary Syndrome, and in accordance with the guidelines to accept statins, angiotensin-converting enzyme inhibitors, beta-blockers and nitrates. Patients with mild UA will undergo a standardised baseline assessment before the treatment, including detailed medical history, physical examination and laboratory testing. Meanwhile, the following treatments will be given to different groups:
XST treatment group: The patients will be treated via an intravenous drip with 500 mg XST (lyophilised) diluted with 250–500 mL of 5% glucose injection or sodium chloride injection, once per day for 7–14 days.
Control group: The patients will be treated via an intravenous drip with 25 mg XST (lyophilised) diluted with 250–500 mL of 5% glucose injection or sodium chloride injection, once per day for 7–14 days.
The experimental drugs will be distributed by a drug administrator and injected by trained nurses.
Patients will be admitted to the hospital on the day of registration for the first intervention, and patients who discharge will move directly to the follow-up period. The efficacy and safety of XST will be assessed after treatment for 7–14 days and follow-up for 1, 3 and 6 months (figure 1 and table 2).
Figure 1.
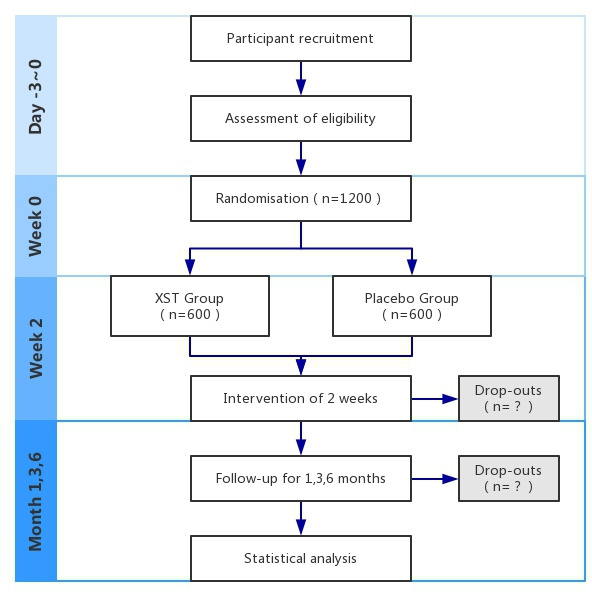
Flow chart of the study procedure. XST, Xueshuantong injection.
Table 2.
Study schedule of assessments
| Study period | ||||
| Enrolment | Allocation | Intervention | Follow-up | |
| Time point | −3~0 days | 0 | 7~14 days | 1, 3 and 6 months |
| ENROLMENT: | X | |||
| Eligibility screen | X | |||
| Informed consent | X | |||
| Allocation | X | |||
| INTERVENTIONS: | ||||
| XST treatment | X | |||
| Placebo treatment | X | |||
| ASSESSMENTS: | ||||
| MACE | X | X | ||
| CK-MB | X | X | ||
| cTnT | X | X | ||
| cTnI | X | X | ||
| hsTnI | X | X | ||
| Adverse events | X | X | ||
CK-MB, creatine kinase MB; cTnI, cardiac troponin I; cTnT, cardiac troponin T; hsTnI, high-sensitivity troponin I; MACE, major adverse cardiac events; UA, unstable angina; XST, Xueshuantong injection.
Outcome measurements
Primary outcomes
The primary endpoints will include the incidence of the composite endpoints for MACE which is a commonly used indicator to evaluate the prognosis of patients with coronary heart disease or UA,12 including cardiovascular death, non-fatal myocardial infarction and revascularisation.
The primary endpoint is the time of enrolment to the end of the study (including the medication observation period and follow-up period) when any of the MACE events occur for the first time. The researchers will record through telephone interviews, in-patient and outpatient medical records of patients, and information provided by their family members. Patients without MACE during the study will be defined as censored at the end of the study. For patients who quit the trial early due to reasons other than MACE, the time of occurrence is defined as censoring at the time of early termination. Death not due to cardiovascular diseases or that occurs after the MACE will not be evaluated.
Secondary outcomes
The efficacy of angina pectorals will be observed at the time of enrolment and by the end of treatment, including the frequency of angina attack, the clinical manifestations, ECG and laboratory examination.
Myocardial injury markers (serum creatine kinase MB; cardiac troponin T/cardiac troponin I (cTnI)/high-sensitivity troponin I) will be monitored, to observe changes in myocardial injury during treatment and to assess the efficacy and safety during treatment.
Safety assessment
An AE is any adverse medical event that occurs during the trial. The researchers will record the observation of vital signs, testing of blood and urine samples, renal and liver function at the start and end of the run-in period, and recorded abnormal changes and AEs at any time.
In view of the particularity of the disease, the condition of the participants may change significantly during the observation, including the need for hospitalisation for the deterioration of the disease, or even life-threatening. Since cardiovascular death, non-fatal myocardial infarction and revascularisation were the endpoints of this study, the above events would not be reported as serious AEs (and described in the study history). Known adverse reaction of the XST: systemic injury: fever, chills, anaphylactic reaction, anaphylactic shock and so on; respiratory system damage: chest tightness, breathing difficulties, shortness of breath, asthma, laryngeal oedema and so on; skin and its appendages damage: rash, pruritus and exfoliative dermatitis; heart rate and arrhythmia: palpitations, tachycardia and so on; central and peripheral nervous system damage: dizziness, headache, convulsions, tremor and so on; gastrointestinal system damage: nausea, vomiting and so on; cardiovascular system damage: cyanosis, flushing, decreased blood pressure, elevated blood pressure and so on; other damage: blood in the urine, abnormal liver function and so on.
Patients will be required to report all AEs at each visit. All AEs will be recorded, regardless of the severity, to assess the safety of XST.
If an AE occurs, the researchers will have to determine whether to stop the observation and proceed with the diagnosis and corresponding treatment. If any severe AE occurs, the researchers must take immediate action to ensure the safety of the participants. They must also report to the ethics committee within 24 hours. The responsible staff from The First Affiliated Hospital of Guangzhou University of Chinese Medicine must promptly notify other participating centres and initiate any necessary legal procedures.
Follow-Up
All included participants will be evaluated for the occurrence of MACE after 1, 3 and 6 months through phone calls after the end of the medication. The trial will be ended if the following endpoints occur: death, non-fatal myocardial infarction or revascularisation (including PCI and coronary artery bypass grafting). The academic research on haemorrhage in Europe and the USA proposed a unified definition of haemorrhage type 3–5.
The occurrence of the MACE must be recorded in an original supporting document, including but not limited to a copy of the discharge summary, a copy of the medical record or other documents that can be used to verify the occurrence of the MACE and the date of its occurrence.
Statistical analysis
Enrolment and case completion
The completion of the trial at each centre must be recorded and described. All cases of shedding must be listed.
Baseline comparability analysis
The baseline characteristics of patients will be summarised and compared using the t test or non-parametric statistical test. Qualitative data will be analysed using the χ2 or Fisher exact tests, Cochran–Mantel–Hasenszel (CMH) test and Wilcoxon test.
Analysis of efficacy
Baseline comparability analysis: This includes the description of demographic data, symptoms, and general conditions. The t test or non-parametric statistical method will be used for quantitative data. Qualitative data will be determined using the χ2 test, Fisher's exact probability method, CMH test and Wilcoxon rank-sum test.
Primary and secondary outcomes: The incidence of the composite endpoints for MACE and the efficacy of angina pectorals will be compared between the two groups and analysed using the χ2 or Fisher exact tests and two-sample t tests or Wilcoxon rank-sum test. The laboratory data on myocardial injury markers will be analysed for the changes before and after the intervention. The average value of each laboratory data after the treatment will be compared.
For cases of rejection and shedding, statistical descriptions will be performed one by one. Adverse reactions will be statistically described. The incidence of adverse reactions will be compared using the χ2 or Fisher exact tests.
Discussion
XST is a traditional Chinese herbal injection consisting of a series of saponins extracted from Panax notoginseng. It has been approved by the China Food and Drug Administration (China drug approval number: Z 20025652) and collected according to the ‘2012 national essential drugs list’ and the People’s Republic of China Pharmacopoeia, respectively. Total saponins, isolated from the root and rhizome of P. notoginseng, are the main components of XST.
P. notoginseng is known for promoting blood circulation, preventing thrombosis and dilating blood vessels. It is widely applied to treat acute cerebral infarction, stroke and coronary heart disease in clinical practice.13 14 Wang et al15 found that in a rat model of middle cerebral arteryocclusion-reperfusion, administration of XST combined with salvianolate lyophilised injection not only significantly decreased neurological deficit scores and infarct volumes, and increased regional cerebral blood flow. Gan et al16 evaluated the efficacy and safety of the P. notoginseng extract via intracoronary injection for treating the post-PCI slow-reflow phenomenon in patients with ST-segment elevation myocardial infarction and its impact on patients' prognosis. They found that coronary injection with tirofiban and XST was more effective in improving coronary blood flow and showed no increase in the incidence of haemorrhagic complications compared with the injection with tirofiban only. P. notoginseng has been found to be beneficial in patients with UA. The use of the extract has been recommended for patients with UA in clinical practice as a complementary and alternative therapy.17 However, more randomised controlled trials with reliable designs, large samples and long-term observations are needed for further evaluations.
The meta-analysis of XST injection combined with RBT was superior to RBT alone in alleviating clinical symptoms, with statistically significant differences between the groups. XST injection combined with RBT could alleviate UA pectorals. However, due to the low quality of the included studies, further well-designed, multicentre and large-scale RCTs are still needed to evaluate the efficacy of XST injection.18 Moreover, the total revenue from XST in the Chinese market in 2013 was over $700 million.15 Therefore, enormous consumption requires stricter and accurate evidence on its safety. However, many reports of XST were case reports, and hence large-sample and high-level evidence for the efficacy and safety of XST is still lacking. Therefore, this study will be conducted to investigate the efficacy and safety of XST in reducing the incidence of MACE in patients with UA. The findings of this study will provide clinical evidence for the use of XST in reducing the incidence of MACE in patients with UA.
Supplementary Material
Acknowledgments
The authors are grateful to Professor Licheng Zhao from Guangzhou University of Chinese Medicine for his guidance during this study. We also thank Miss Jingyi Xu, who carefully checked the references. We also thank MedSci for the language editing of the manuscript.
Footnotes
WL and HL contributed equally.
Contributors: ZY and WL wrote the study protocol. SX, HL, TY and YT developed the original study design. XH, JL, YH, JD, YL and WL were all involved in the revision of the study design and contributed in the review process of the protocol manuscript. YT and QL are jointly responsible for the collection of data and administration of study participants. LL provides methodological guidance on research statistics. ZY is the principal investigator and responsible for the funding and overall management of the trial.
Funding: This study has been supported by he National Key Research and Development Plan (2018YFC1707401) and The second batch of national Traditional Chinese Medicine(TCM) clinical research base project (2018131).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study has been approved by the ethics committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (approval number: ZYYEC[2017]002), the Ethics Committee of Drug Clinical Trials of Zhengzhou People's Hospital(approval number: YW201800501), the Ethics Committee of Shanxi Fenyang Hospital(approval number: 2018002), the Ethics Committee of Ruikang Hospital Affiliated to Guangxi University of Traditional Chinese Medicine(approval number: RK201709), the Ethics Committee of Changsha Fourth Hospital (approval number: CSSDSYY-LL-SC-2017-03-03), the Ethics Committee of The First Affiliated Hospital of the Henan University of Chinese Medicine(approval number: 2018HL-046-01), the Ethics Committee of the Second Affiliated Hospital of Heilongjiang University of Chinese Medicine (approval number: 2015R000774), the Ethics Committee of The First Affiliated Hospital of Hunan University of Traditional Chinese Medicine (approval number: HN-LL-2017-018-01), the Medical Ethics Committee of Luoyang NO.1 Hospital of TCM (approval number: 2018-01), the Ethics Committee of Zhengzhou Central Hospital (approval number: 2018-006-02), the Ethics Committee of Tianjin Beichen District Hospital of Traditional Chinese Medicine(approval number: BCZYK201901), the Ethics Committee of Nanjing Hospital of Chinese Medicine(approval number: 2017NJL033), the Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of Chinese Medicine(approval number: 2017-563-46-01), the Ethics Committee of Affiliated Hospital of Tianjin Academy of Chinese Medicine(approval number: LLSY207-04), the Ethics Committee of The Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine (approval number: EC.AT/03.19-02/08.0), the Ethics Committee of The First Affiliated Hospital of Henan University of Science and Technology (approval number: 2018-0020)and the Ethics Committee of Wenzhou Hospital of Traditional Chinese Medicine (approval number: WTCM-H-2017024-2018-002) and registered on the Chinese Clinical Trial Registry with the ID ChiCTR1800015911.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ueda P, Gulayin P, Danaei G. Long-Term moderately elevated LDL-cholesterol and blood pressure and risk of coronary heart disease. PLoS One 2018;13:e0200017. 10.1371/journal.pone.0200017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB, Feinleib M. Natural history of angina pectoris in the Framingham study. prognosis and survival. Am J Cardiol 1972;29:154–63. 10.1016/0002-9149(72)90624-8 [DOI] [PubMed] [Google Scholar]
- 3.Wan J-B, Lee SM-Y, Wang J-D, et al. Panax notoginseng reduces atherosclerotic lesions in apoE-deficient mice and inhibits TNF-alpha-induced endothelial adhesion molecule expression and monocyte adhesion. J Agric Food Chem 2009;57:6692–7. 10.1021/jf900529w [DOI] [PubMed] [Google Scholar]
- 4.Wang N, Wan J-B, Chan S-W, et al. Comparative study on saponin fractions from Panax notoginseng inhibiting inflammation-induced endothelial adhesion molecule expression and monocyte adhesion. Chin Med 2011;6:37. 10.1186/1749-8546-6-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong X, Shen JH, GP W. Effect of Xueshuantong injection on cerebral infarction. Hainan Yixueyuan Xuebao 2009;15:603–4. [Google Scholar]
- 6.Fu X-xia, Xiao W-jian, Lu J, et al. Retrospective analysis of thrombolysis therapy for 64 cases of acute myocardial infarction with elevated ST segment. Chin J Integr Med 2009;15:462–5. 10.1007/s11655-009-0462-8 [DOI] [PubMed] [Google Scholar]
- 7.Han S, Chen Y, Wang J, et al. Anti-Thrombosis effects and mechanisms by Xueshuantong capsule under different flow conditions. Front Pharmacol 2019;10:35. 10.3389/fphar.2019.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Ding S-L, Chen Y, et al. [Effects of Xueshuantong Injection on thrombosis formation and blood flow in rats].. Zhongguo Zhong Yao Za Zhi 2020;45:2446–53. 10.19540/j.cnki.cjcmm.20191112.404 [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Lyu J, Xie Y-M, et al. [Effictiveness and safety of Xueshuantong Injection in treatment of unstable angina pectoris: a systematic review and Meta-analysis of randomized controlled trials].. Zhongguo Zhong Yao Za Zhi 2019;44:4366–78. 10.19540/j.cnki.cjcmm.20190724.501 [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Yang J, Yang W, et al. Focus on notoginsenoside R1 in metabolism and prevention against human diseases. Drug Des Devel Ther 2020;14:551–65. 10.2147/DDDT.S240511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mani P, Puri R, Schwartz GG, et al. Association of initial and serial C-reactive protein levels with adverse cardiovascular events and death after acute coronary syndrome: a secondary analysis of the VISTA-16 trial. JAMA Cardiol 2019;4:314–20. 10.1001/jamacardio.2019.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Xiong X, Wang H, et al. Protective effects of Panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med 2014;2014:1–13. 10.1155/2014/204840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gui Q, Yang Y, Ying S, et al. Xueshuantong improves cerebral blood perfusion in elderly patients with lacunar infarction. Neural Regen Res 2013;8:792–801. 10.3969/j.issn.1673-5374.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F-J, Wang S-X, Chai L-J, et al. Xueshuantong injection (lyophilized) combined with Salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress. Acta Pharmacol Sin 2018;39:998–1011. 10.1038/aps.2017.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan L-jun, Zhang C-hui, Zhang M. [Effect of intracoronary injection with xuesaitong in treating post-PCI slow-reflow phenomenon in patients with ST-segment elevation myocardial infarction]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2010;30:348–51. [PubMed] [Google Scholar]
- 17.Song H, Wang P, Liu J, et al. Panax notoginseng preparations for unstable angina pectoris: a systematic review and meta-analysis. Phytother Res 2017;31:1162–72. 10.1002/ptr.5848 [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Lyu J, Xie Y-M, et al. [Effictiveness and safety of Xueshuantong Injection in treatment of unstable angina pectoris: a systematic review and Meta-analysis of randomized controlled trials]. Zhongguo Zhong Yao Za Zhi 2019;44:4366–78. 10.19540/j.cnki.cjcmm.20190724.501 [DOI] [PubMed] [Google Scholar]
- 19.Zheng XY. Chinese medicine new drug clinical guidelines. Beijing, China: China Pharmaceutical Science and Technology Press, 2002: 392. [Google Scholar]
- 20.Mendis S, Thygesen K, Kuulasmaa K, et al. World Health organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol 2011;40:139–46. 10.1093/ije/dyq165 [DOI] [PubMed] [Google Scholar]
- 21.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–42. 10.1001/jama.284.7.835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


