Abstract
Background
Several endocrine therapy (ET)-based treatments are available for patients with advanced breast cancer. We assessed the efficacy of different ET-based treatments in patients with hormone receptor-positive/HER2-negative advanced breast cancer with endocrine-sensitive or endocrine-resistant disease.
Methods
We searched Medline and Cochrane Central Register of Controlled Trials up to 15 October 2019 and abstracts from major conferences from 2016 to October 2019. We included phase II/III randomised trials, comparing ≥2 ET-based treatments. Progression-free survival (PFS) and overall survival (OS) were analysed by network meta-analyses using MTC Bayesian models based on both fixed-effect and random-effect models; relative treatment effects were measured as HRs and 95% credibility intervals (CrI). All statistical tests were two-sided. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed and this systematic review is registered in the PROSPERO database.
Results
55 publications reporting on 32 trials (n=12 293 patients) were included. Regarding PFS in the endocrine sensitive setting (n=5200; 12 trials), the combination of cyclin-dependent kinases (CDK)4/6-inhibitors (CDK4/6i)+fulvestrant 500 mg (F500) was likely the most effective treatment (surface under the cumulative ranking curve (SUCRA)=97.3%), followed by CDK4/6i+aromatase inhibitor ±goserelin; there was no significant difference between them (HR 0.82; 95% CrI 0.54–1.25). Regarding OS (n=2157; five trials), the most effective treatment was probably CDK4/6i+F500 (SUCRA=97.3%); comparing CDK4/6i+F500 versus F500 held a HR of 0.77 (95% CrI 0.63–0.95). Regarding PFS in the endocrine-resistant setting (n=6635; 20 trials), CDK4/6i+F500 was likely the most effective treatment (SUCRA=95.7%), followed by capivasertib+F500, without significant difference between them (HR 0.91; 95% CrI 0.60–1.36). For OS (n=4377; 11 trials), the most effective treatments were capivasertib+F500 (SUCRA=84.7%) and CDK4/6i+F500 (SUCRA=69.9%). Comparing CDK4/6i+F500 versus F500 held a HR of 0.77 (95% CrI 0.67–0.89).
Conclusions
CDK4/6i+F500 is likely the best treatment option in both endocrine-sensitive and endocrine-resistant diseases for PFS, and in endocrine-sensitive patients for OS. Concerning OS in endocrine-resistant patients, capivasertib+F500 and CDK4/6i+F500 are likely the best treatments.
PROSPERO registration number
CRD42018104628.
Keywords: breast neoplasms, molecular targeted therapy, systematic review, network meta-analysis, endocrine therapy
Key questions.
What is already known about this subject?
A considerable research effort has been made in the previous years to develop more targeted and effective treatments for patients with hormone receptor-positive/HER2-negative advanced breast cancer. It is now recognised that endocrine therapy (ET) represents the cornerstone of systemic treatment for these patients. The addition of new targeted agents to ET have further improved prognosis in these patients by delaying and/or reversing resistance to endocrine treatment. Nevertheless, most of these agents have never been directly compared in clinical trials.
What does this study add?
Our data show that, in terms of progression-free survival, the combination of a cyclin-dependent kinases 4/6 inhibitor with fulvestrant 500 mg appears to be the best treatment option for both the endocrine-sensitive and endocrine-resistant populations. In addition, we have shown that this combination significantly increases overall survival in both settings, as compared with fulvestrant 500 mg. We also analysed specific subgroup of patients, such as patients with de novo metastatic, recurrent, visceral and bone-only disease.
How might this impact on clinical practice?
In the absence of direct head-to-head comparisons for all regimens, our results may help guiding physicians and patients in the decision regarding the choice of regimen of ET with/without targeted agents for their advanced breast cancer.
Introduction
Advanced breast cancer includes both unresectable locally advanced and metastatic breast tumours and is usually considered an incurable disease.1 More than 70% of breast cancers are hormone receptor-positive (HR+)/HER2-negative (HER2−).2 In this setting, endocrine therapy (ET) is the cornerstone of systemic treatment, being the preferred frontline therapy for these patients even in the presence of visceral metastasis, unless visceral crisis is present.1 3 Yet, despite the significant clinical benefit of ET, almost all patients eventually acquire endocrine resistance over the course of treatment.1
In recent years, a considerable amount of research has allowed to better understand, define and target the mechanisms of resistance to ET.4 Many new targeted agents have emerged, in combination with ET, and are now available in daily clinical practice or are in the late stages of drug development.1 Some of them target the cyclin-dependent kinases (CDK)4/6, which are involved in cell-cycle regulation; or the PI3K and mTOR enzymes, which are responsible for proliferation and survival, among other functions.4
Nonetheless, these new combinations have never been directly compared in clinical trials. Having pair-wise comparative data for all of these regimens would not be feasible considering the large number of possible head-to-head comparisons, and the substantial expenses associated with running such clinical trials. Furthermore, thanks to the growing sequential use of ET-based regimens in order to avoid chemotherapy, and the numerous available treatment options, clinicians face the problem of deciding which are the best ET-based regimens to be sequentially prescribed to these patients. In such scenario of multiple available options, a network meta-analysis offers a unique opportunity to summarise and rank the relative efficacy of the different ET-based treatments, which could improve the treatment decision-making process in daily clinical practice. With this network meta-analysis, we assessed the efficacy of different ET-based treatments in patients with HR+/HER2− advanced breast cancer, both with endocrine-sensitive and endocrine-resistant disease.
Methods
Search strategy and selection criteria
Randomised phase II/III controlled trials including patients with HR+/HER2− advanced breast cancer, comparing at least one single-agent ET to any ET-based treatments were included. Definitions of endocrine-sensitivity and endocrine-resistance were as per the 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC4).1 Endocrine-sensitive patients were defined as patients who never received ET in early breast cancer stage, or relapsing ≥12 months after completing adjuvant ET, or diagnosed with de novo stage IV breast cancer. Endocrine-resistant patients were defined as patients relapsing during adjuvant ET, or <12 months after its completion, or with progressive disease under ET for advanced breast cancer.
Primary endpoint was progression-free survival (PFS), defined as time from randomisation until progressive disease or death.5 PFS was reported separately for patients with endocrine-sensitive and endocrine-resistant disease, without further molecular selection beyond HR+/HER2− status. Secondary endpoint was overall survival (OS), defined as time from randomisation until death from any cause. Pre-defined subgroup analyses for PFS benefit were: patients with de novo metastatic disease; recurrent disease; visceral disease; bone-only disease; and with specific somatic mutations (e.g., PIK3CA-mutated tumours).
We excluded studies without information on HER2 status; with patients with HER2+ tumours with no separate analysis for the cohort of patients with HR+/HER2− disease; with mixed endocrine-sensitive and endocrine-resistant populations for which a clear assignment was not possible; without data on PFS; or using chemotherapy as a comparator. Trial reports focusing solely on endpoints (eg, quality of life) or subgroup analyses that were not included in our protocol were also deemed not eligible.
A systematic search of the literature without language restrictions was performed up to 15 October 2019. Keywords like breast cancer and endocrine and targeted therapy drugs were combined in the search strategy (online supplementary methods), which was applied to Medline and adapted for use in the Cochrane Central Register of Controlled Trials. We also searched abstracts from major conferences (American Society of Clinical Oncology (ASCO) Annual Meeting, European Society for Medical Oncology Congress and San Antonio Breast Cancer Symposium) from 2016 to October 2019, in order to include unpublished trials.
esmoopen-2020-000842supp001.pdf (2.4MB, pdf)
This systematic review and network meta-analysis was based on the Cochrane Collaboration Handbook6 and registered in the PROSPERO database. The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for network meta-analysis guidelines was followed to report the present study.7
Data collection and assessment of risk of bias
Two reviewers (MB, CM) independently evaluated the screened titles and abstracts; in case of disagreement, a third author (ML) resolved it. Full papers were reviewed by five authors (MB, ML, CM, NP, MD). Multiple reports from the same trials were collated and data extraction was performed by one reviewer (MB) and verified by a second reviewer (AF). Disagreements were resolved by a third reviewer (ML).
We extracted trial name/identifier; first author; year of publication; study phase; endocrine-sensitive and/or endocrine-resistant disease population; median number of previous lines of ET and allowance of previous chemotherapy for advanced breast cancer; treatment arms; number of patients; estimates of HRs and their CIs for PFS and OS in the intention-to-treat population; and PFS parameters in the specified subgroups. For multiple reports of the same trial, we used the first publication of the primary endpoint to extract PFS data in the intention-to-treat population; for OS, we used the report with the longest follow-up data for analysis; for subgroup analysis, we used publications specifically reporting subgroup analysis (when available) or, alternatively, the first published report. Two reviewers (NP, MD) assessed the risk of bias using the Cochrane tool (version 5.1.0).6
Definition of treatment arms
Treatments were grouped according to their pharmacological class, with the use of clinical judgement whenever required. As examples, palbociclib, ribociclib and abemaciclib were included in the ‘CDK4/6 inhibitors’ (CDK4/6i) group; aromatase inhibitors (AI; anastrozole, letrozole and exemestane) were pooled together as a group, irrespective of being administered as monotherapy or combined with goserelin (the ‘AI±goserelin’ group), as the latter can be used to induce menopause in premenopausal patients.1 In cases in which there was only one drug representative of a class (eg, bortezomib), the specific name of the drug was used. If different doses of the same drug were tested (eg, fulvestrant 250 mg and fulvestrant 500 mg) or if different compounds of the same class were compared head-to-head (eg, mTOR inhibitors vistusertib and everolimus), each drug was considered separately. All other treatment arms were then defined as single-agent ET (eg, tamoxifen), and the combinations of different ET agents between them (eg, AI+fulvestrant 250 mg) and/or with targeted therapies (eg, everolimus+AI).
Data synthesis and statistical analysis
Network plots describing the geometry of all comparisons were generated. Relative treatment effects were measured as HR to compare the different treatment regimens regarding PFS and OS. Results from the included trials were pooled using both pairwise frequentist meta-analysis and network meta-analysis. Network meta-analysis is a generalisation of pairwise meta-analysis that allows all evidence to be taken into account in a single model (both direct and indirect). Direct evidence comes from head-to-head trials and indirect evidence comes from trials that has a common comparator arm. In a network meta-analysis, the final evidence for each pair of treatments will come from direct evidence only, from indirect evidence only or from a combination between direct and indirect evidence, all depending on the geometry of the network. Our network meta-analysis was performed using MTC Bayesian models based on both fixed-effect and random-effect models to yield comparative frameworks among all included arms, based on direct and indirect evidence.8 Decision between fixed-effect and random-effect were made using deviance information criterion.9 In random-effects network meta-analysis models, the same variance among studies was assumed for all pairwise comparisons. As none of our networks had pairwise comparisons with both direct and indirect evidence, consistency was not checked. Closed loops appearing in the plots come from three and four-arms individual studies.
The Meta and Gemtc packages from R (V.3.4.2) were used for the analysis. HR point estimates and their 95% CIs or 95% credibility intervals (CrI) were used to report the results. Additionally, in the network analysis, regimens were ordered based on posterior rank probabilities (which indicate the probability of each regimen being the best, the second best, and so on) as well the surface under the cumulative ranking curve (SUCRA) values. SUCRA is a value between 0% and 100% calculated based on the posterior rank probabilities (larger value indicates a more effective treatment).10 Difference between two regimens were considered significant if their 95% CrI did not cross the value of 1.
Results
Characteristics of included studies
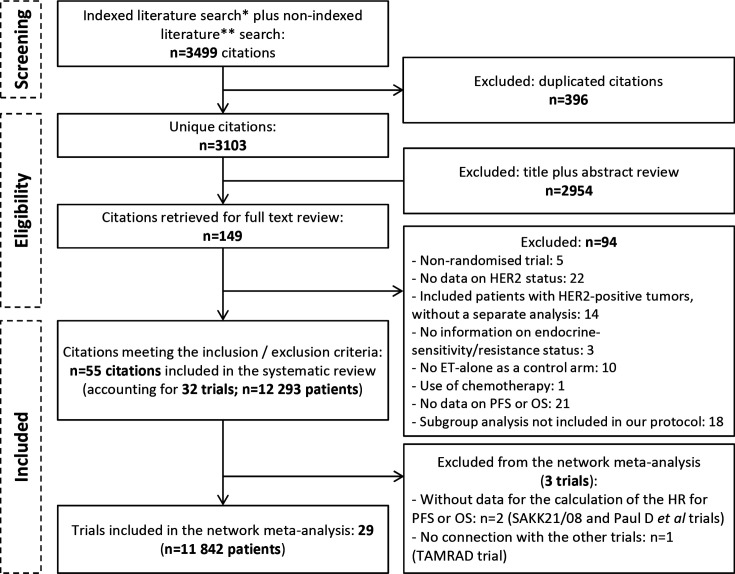
The systematic search of the literature yielded 3499 records, of which 149 were reviewed as full text (figure 1). Among them, 55 publications reporting on 32 trials (n=12 293 patients) were included: 10 trials reported on endocrine-sensitive patients only,10–31 18 trials on endocrine-resistant patients only19 29 32–56 and 3 trials on both but with distinct analyses for each group57–60 (table 1). Publication dates ranged from 2009 to 2019, reflecting the fact that widespread standardised testing of HER2 status was only implemented in 2007,61 rendering most trials conducted before that ineligible for this study.
Figure 1.
Adapted Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram. *Indexed literature search (until 15 October 2019): Medline and Cochrane Central Register of Controlled Trials. **Non-indexed literature search: American Society of Clinical Oncology, San Antonio Breast Cancer Symposium, European Society for Medical Oncology annual conferences from 2016 to October 2019. ET, endocrine therapy; OS, overall survival; PFS, progression-free survival.
Table 1.
Characteristics of the randomised controlled trials included in the systematic review
| Trial name/author | Phase | Meno-pausal status | Patients (N)* | Comparisons | Median of ET (N)† | Previous CT | ITT* PFS HR (95% CI) |
ITT* OS HR (95% CI) |
De novo* PFS HR (95% CI) | Recurrent* PFS HR (95% CI) |
Visceral* PFS HR (95% CI) |
Bone-only* PFS HR (95% CI) |
| Endocrine-sensitive patients only | ||||||||||||
| Ibrahim et al11 | II | Post-meno | 110 | AS1402+AI (letrozole) versus AI (letrozole) | 0 | No | 0.95 (0.50 to 1.81) |
– | – | – | – | – |
| SWOG S022612 | III | Post-meno | 536 | Fulvestrant 250 mg+AI (anastrozole) versus AI (anastrozole) | 0 | No | 0.81 (0.67 to 0.98) |
–‡ | – | – | – | – |
| Paul et al‡§13 | II | Post-meno | 120 | MultiTKI (dasatinib)+AI (letrozole) versus AI (letrozole) | 0 | ≤1 line | No HR | – | – | – | – | – |
| PALOMA-114–16 | II | Post-meno | 165 | CDK4/6i (palbociclib)+AI (letrozole) versus AI (letrozole) | 0 | No | 0.49 (0.32 to 0.75) |
0.90 (0.62 to 1.29) |
0.34 (0.19 to 0.60) |
0.54 (0.30 to 0.96) |
0.55 (0.32 to 0.94) |
0.29 (0.09 to 0.95) |
| PALOMA-217–20 | III | Post-meno | 666 | CDK4/6i (palbociclib)+AI (letrozole) versus AI (letrozole) | 0 | No | 0.58 (0.46 to 0.72) |
– | 0.61 (0.44 to 0.85) |
0.58 (0.41 to 0.82) |
0.62 (0.47 to 0.81) |
0.41 (0.26 to 0.63) |
| MONALEESA-221–24 | III | Post-meno | 668 | CDK4/6i (ribociclib)+AI (letrozole) versus AI (letrozole) | 0 | No | 0.56 (0.43 to 0.72) |
0.75 (0.52 1.08) |
0.45 (0.27 to 0.75) |
0.60 (0.45 to 0.81) |
0.54 (0.39 to 0.74) |
0.69 (0.38 to 1.25) |
| FALCON25–27 | III | Post-meno | 462 | Fulvestrant 500 mg versus AI (anastrozole) | 0 | Allowed | 0.80 (0.64 to 0.999) |
0.88 (0.63 to 1.22) |
– | – | 0.99 (0.74 to 1.33) |
– |
| MINT28 | II | Post-meno | 359 | Sapatinib 20 mg+AI (anastrozole) versus Ai | 0 | ≤1 line | 1.37 (0.91 to 2.06) |
– | – | – | – | – |
| Sapatinib 40 mg+AI (anastrozole) versus AI (anastrozole) | 1.16 (0.77 to 1.75) |
|||||||||||
| MONARCH 329 30 | III | Post-meno | 493 | CDK4/6i (abemaciclib)+AI (anastrozole or letrozole) versus AI (anastrozole or letrozole) | 0 | No | 0.54 (0.41 to 0.72) |
– | 0.49 (0.31 to 0.76) |
0.58 (0.42 to 0.81) |
0.61 (0.42 to 0.87) |
0.58 (0.27 to 1.25) |
| MONALEESA-731 32 | III | Pre-meno | 672 | CDK4/6i (ribociclib)+tamoxifen+goserelin§ versus tamoxifen +goserelin§ | 0 | ≤1 line | 0.59 (0.39 to 0.88) |
0.79 (0.45 to 1.38) |
– | – | – | – |
| CDK4/6i (ribociclib)+AI (anastrozole or letrozole)+goserelin versus AI (anastrozole or letrozole)+goserelin | 0.57 (0.44 to 0.74) |
0.70 (0.50 to 0.98) |
||||||||||
| Endocrine-resistant patients only | ||||||||||||
| TAMRAD§33 | II | Post-meno | 111 | Everolimus+tamoxifen versus tamoxifen | NR | ≤1 line | 0.54 (0.36 to 0.81) |
0.45 (0.24 to 0.81) |
– | – | – | – |
| BOLERO-234–38 | III | Post-meno | 724 | Everolimus+AI (exemestane) versus AI (exemestane) | NR | ≤1 line | 0.45 (0.38 to 0.54) |
0.89 (0.73 to 1.10) |
– | – | 0.47 (0.37 to 0.60) |
0.33 (0.21 to 0.53) |
| SoFEA39 | III | Post-meno | 405 | Fulvestrant 250 mg+AI (anastrozole) versus fulvestrant 250 mg | 1 | ≤1 line | 0.95 (0.75 to 1.22) |
0.85 (0.64 to 1.14) |
– | – | – | – |
| Fulvestrant 250 mg versus AI (exemestane) | 1.06 (0.83 to 1.34) |
1.26 (0.95 to 1.66) |
||||||||||
| CALGB 40 30240 | III | Post-meno | 235 | Lapatinib+fulvestrant 250 mg versus fulvestrant 250 mg | NR | ≤1 line | 1.00 (0.76 to 1.30) |
– | – | – | – | – |
| SAKK21/08§41 | II | Post-meno | 43 | Selumetinib+fulvestrant 500 mg versus fulvestrant 500 mg | 1 | ≤1 line | No HR | – | – | – | – | – |
| Adelson et al42 | II | Post-meno | 116 | Bortezomib+fulvestrant 500 mg versus fulvestrant 500 mg | 1 | ≤1 line | 0.73 (0.49 to 1.09) |
– | – | – | – | – |
| PALOMA-320 43–45 | III | Both | 521 | CDK4/6i (palbociclib)+fulvestrant 500 mg versus fulvestrant 500 mg | 1 | ≤1 line | 0.46 (0.36 to 0.59) |
0.81 (0.64 1.03) |
– | – | 0.47 (0.34 to 0.63) |
0.63 (0.38 to 1.06) |
| O'Shaughnessy et al46 | II | Post-meno | 297 | Anti-androgen (abiraterone acetate) versus AI (exemestane) | 1 | ≤1 line | 1.1 (0.82 to 1.60) |
– | – | – | – | – |
| Anti-androgen (abiraterone acetate)+AI (exemestane) versus AI (exemestane) | 0.96 (0.70 to 1.32) |
0.51 (0.32 to 0.80) |
2.09 (1.04 to 4.19) |
|||||||||
| FERGI64 | II | Post-meno | 168 | Pan-PI3Ki (pictilisib)+fulvestrant 500 mg versus fulvestrant 500 mg | 1 | ≤1 line | 0.74 (0.52 to 1.06) |
– | – | – | 0.74 (0.46 to 1.18) |
– |
| BELLE-247 48 | III | Post-meno | 1147 | Pan-PI3Ki (buparlisib)+fulvestrant 500 mg versus fulvestrant 500 mg | 1 | ≤1 line | 0.78 (0.67 to 0.89) |
0.87 (0.74 to 1.02) |
– | – | 0.76 (0.63 to 0.90) |
0.66 (0.46 to 0.95) |
| MONARCH 230 49 | III | Both | 669 | CDK4/6i (abemaciclib)+fulvestrant 500 mg versus fulvestrant 500 mg | 0 | No | 0.55 (0.45 to 0.68) |
0.76 (0.61 to 0.95) |
– | – | 0.48 (0.37 to 0.63) |
0.54 (0.36 to 0.83) |
| Musolino et al50 | II | Post-meno | 97 | MultiTKI (dovitinib)+fulvestrant 500 mg versus fulvestrant 500 mg | NR | No | 0.68 (0.41 to 1.14) |
0.81 (0.39 to 1.65) |
– | – | – | – |
| Zhao et al51 | II | Post-meno | 60 | Meftormin+AI (letrozole or exemestane) versus AI (letrozole or exemestane) | 1 | Allowed | 1.20 (0.7 to 2.1) |
1.10 (0.50 to 2.40) |
– | – | – | – |
| MANTA55 | II | Post-meno | 326 | Vistusertib continuous+fulvestrant 500 mg versus fulvestrant 500 mg | 1 | ≤1 line | 0.88 (0.63 to 1.24) |
– | – | – | – | – |
| Vistusertib intermittent+fulvestrant 500 mg versus fulvestrant 500 mg | 0.79(0.55 to 1.12) | |||||||||||
| Everolimus+fulvestrant 500 mg versus fulvestrant 500 mg | 0.63 (0.42 to 0.92) |
|||||||||||
| Everolimus+fulvestrant 500 mg versus vistusertib continuous+fulvestrant 500 mg | 0.63 (0.45 to 0.90) |
|||||||||||
| Vistusertib continuous+fulvestrant 500 mg versus vistusertib intermittent+fulvestrant 500 mg | 1.11 (0.81 to 1.52) |
|||||||||||
| PrE010252 | II | Post-meno | 130 | Everolimus+fulvestrant 500 mg versus fulvestrant 500 mg | NR | ≤1 line | 0.61 (0.40 to 0.92) |
1.31 (0.72 to 2.38) |
– | – | – | – |
| BELLE-353 | III | Post-meno | 432 | Pan-PI3Ki (buparlisib)+fulvestrant 500 mg versus fulvestrant 500 mg | 2 | Allowed | 0.67 (0.53 to 0.84) |
– | – | – | 0.56 (0.43 to 0.74) |
– |
| KCSG BR10−04/FLAG54 | II | Both | 138 | Fulvestrant 500 mg+goserelin versus goserelin | 0 | Allowed | 0.61 (0.37 to 0.998) |
0.60 (0.28 to 1.32) |
0.73 (0.26 to 2.01) |
– | 0.67 (0.34 to 1.34) |
– |
| AI (anastrozole)+goserelin versus goserelin | 0.98 (0.62 to 1.55) |
0.52 (0.23 to 1.19) |
0.69 (0.24 to 1.96) |
1.04 (0.54 to 1.97) |
||||||||
| ACE56 | III | Post-meno | 365 | Tucidinostat+AI (exemestane) versus AI (exemestane) | NR | ≤1 line | 0.75 (0.58 to 0.98) |
– | – | – | 0.69 (0.50 to 0.96) |
– |
| FAKTION**62 | Capivasertib+fulvestrant 500 mg versus fulvestrant 500 mg | 0.58 (0.39 to 0.84) |
0.59 (0.34 to 1.05) |
|||||||||
| Both endocrine-sensitive and endocrine-resistant patients | ||||||||||||
| EGF3000857 | III | Post-meno | 752 ES 200 ER |
Lapatinib+AI (letrozole) versus AI (letrozole) | NR | No | ES: 0.94 (0.79 to 1.13) ER: 0.78 (0.57 to 1.07) |
– | – | – | – | – |
| Krop et al**58 | II | Post-meno | 127 ES 120 ER |
Anti-androgen (enzalutamide)+AI (exemestane) versus AI (exemestane) | NR | ≤1 line | ES: 0.82 (0.54 to 1.26) ER: 1.02 (0.66 to 1.59) |
– | – | – | – | – |
| MONALEESA-359 60 | III | Post-meno | 367 ES 345 ER |
CDK4/6i (ribociclib)+fulvestrant 500 mg versus fulvestrant 500 mg | 0 | No | ES: 0.58 (0.42 to 0.80) |
ES: 0.70 (0.48 to 1.02) |
– | – | – | – |
| 1 | No | ER: 0.57 (0.43 to 0.74) |
ER: 0.73 (0.53 to 1.004) |
|||||||||
*Only for hormone receptor-positive/HER2-negative patients, without other molecular selection.
†Median number of ET lines for advanced breast cancer.
‡Result was not provided separately for hormone-receptor positive/HER2-negative patients, therefore it was not included in the analysis.
§Arm/trial not included in the network meta-analysis.
¶Results published only in meeting abstracts (without a full publication) at the time of literature search.
AI, aromatase inhibitor; CDK, cyclin-dependent kinase; CDK4/6i, CDK4/6 inhibitor; CT, chemotherapy; ER, endocrine-resistant; ES, endocrine-sensitive; ET, endocrine therapy; ITT, intention-to-treat population; multiTKI, multi-tyrosine kinase inhibitor; NR, not reported; OS, overall survival; pan-PI3Ki, pan-PI3K inhibitor; PFS, progression-free survival; post-meno, post-menopausal patients only; pre-meno, pre-menopausal patients only.
Quality of the evidence
Of the 32 trials included, 11 (34%) presented a low risk in at least 6 of the 7 assessed areas of potential bias (online supplementary table 1). Regarding the ‘double-blinded’ and ‘outcome-blind’ areas, a high risk of bias was present in 14 (44%) trials. The risk of bias was frequently high/unclear in trials with results published only in the form of meeting abstracts.13 58 62
For both PFS and OS, most of the direct treatment comparisons had only one trial providing direct evidence, thus heterogeneity was not assessable for those comparisons. There was minimal heterogeneity (I2=0%) in all direct comparisons with two or more trials in the PFS and OS networks, except for the comparison between fulvestrant 500 mg (F500) versus pan-PI3K inhibitors (pan-PI3Ki)+F500 in the PFS network of endocrine-resistant patients with visceral disease (I2=42%).
Network meta-analyses results
From the 32 trials included in the systematic review, 3 were excluded from the network meta-analysis: 2 trials did not provide HR for PFS or OS (Paul et al13 and SAKK21/0841 and one trial had treatment arms that could not be connected to the rest of the network (TAMRAD33). In addition, the tamoxifen-containing arms of the MONALEESA-7 trial31 32 could not be connected to the rest of the network either. Thus, 29 trials (n=11 842 patients) were included in network meta-analysis models.
Primary endpoint: PFS
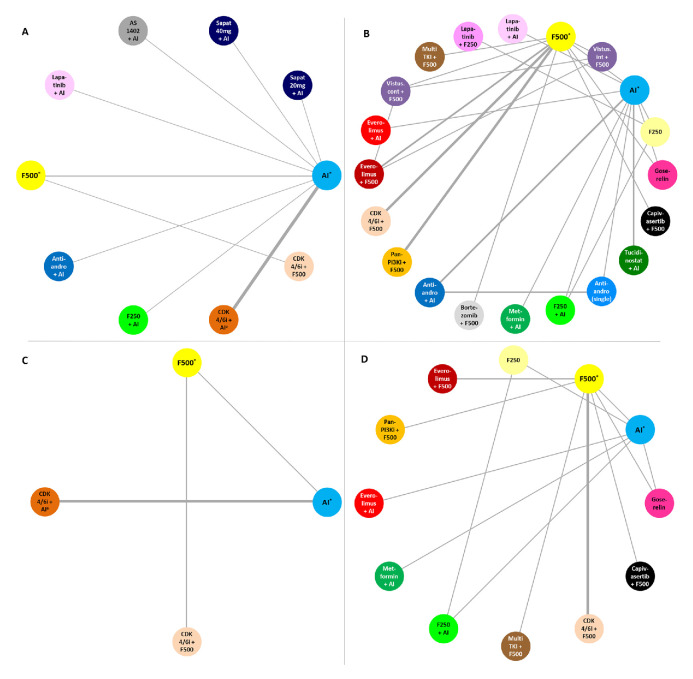
The endocrine-sensitive network included 12 randomised controlled trials (n=5200 patients), testing 10 ET-based regimens (figure 2A). Using a fixed-effects model, the combination of CDK4/6i with F500 was likely the most effective treatment (SUCRA=97.3%), followed by CDK4/6i+AI±goserelin (SUCRA=89.8%)—online supplementary table 2. When comparing the efficacy of CDK4/6i+F500 versus CDK4/6i+AI±goserelin, the HR was 0.82 (95% CrI 0.54–1.25)—table 2. The endocrine-resistant network included 20 trials (n=6635 patients), comparing 20 ET-based regimens (figure 2B). Using a fixed-effects model, CDK4/6i+F500 was probably the most effective treatment (SUCRA=95.7%), followed by capivasertib+F500 (SUCRA=88.7%)—online supplementary table 3. The comparison of CDK4/6i+F500 versus capivasertib+F500 held a HR of 0.91 (95% CrI 0.60–1.36)—table 3.
Figure 2.
Network plots. (A) Progression-free survival (PFS) in endocrine-sensitive (ES) patients. (B) PFS in endocrine-resistant (ER) patients. (C) Overall survival (OS) in ES patients. (D) OS in ER patients. The width of the connecting lines is proportional to the number of trials comparing each pair of treatments, with bolder lines indicating comparisons with a higher number of trials. *±goserelin. AI, aromatase inhibitor; anti-andro, anti-androgen agent; CDK, cyclin-dependent kinases; CDK4/6i, CDK4/6 inhibitor; cont, continuous; F250, fulvestrant 250 mg; F500, fulvestrant 500 mg; int, intermittent; multiTKI, multi-tyrosine kinase inhibitor; pan-PI3Ki, pan-PI3K inhibitor; sapat, sapatinib; vistus., vistusertib.
Table 2.
Comparisons between treatments (HR, 95% credibility interval (CrI)) in endocrine-sensitive patients, both for progression-free survival (column vs row) and overall survival (row vs column)
| Progression-free survival, HR (95% CrI) | |||||||||||
| Treatments | Sapat 20 mg+AI | Sapat 40 mg+AI | AI* | Lapatinib+AI | AS1402+AI | Anti-andro+AI | F250+AI | F500* | CDK4/6i+AI* | CDK4/6i+F500 | |
| Overall survival, HR (95% CrI) | Sapat 20 mg+AI | 0.85 (0.56–1.28) |
0.73 (0.49–1.10) |
0.69 (0.44–1.07) |
0.69 (0.32–1.48) |
0.60 (0.33–1.08) |
0.59 (0.38–0.93) |
0.58 (0.37–0.93) |
0.41 (0.27–0.62) |
0.34 (0.19–0.60) |
|
| Sapat 40 mg+AI | – | 0.86 (0.57–1.30) |
0.81 (0.52–1.27) |
0.82 (0.38–1.74) |
0.71 (0.39–1.28) |
0.69 (0.44–1.10) |
0.69 (0.43–1.10) |
0.48 (0.31–0.74) |
0.40 (0.22–0.70) |
||
| AI* | – | – | 0.94 (0.79–1.12) |
0.95 (0.51–1.80) |
0.82 (0.54–1.26) |
0.81 (0.67–0.98) |
0.80 (0.64–1.00) |
0.56 (0.49–0.63) |
0.46 (0.31–0.69) |
||
| Lapatinib+AI | – | – | – | 1.01 (0.52–1.96) |
0.87 (0.55–1.39) |
0.86 (0.67–1.12) |
0.85 (0.64–1.13) |
0.59 (0.48–0.74) |
0.49 (0.32–0.76) |
||
| AS1402+AI | – | – | – | – | 0.86 (0.40–1.85) |
0.85 (0.44–1.65) |
0.84 (0.42–1.64) |
0.59 (0.31–1.12) |
0.49 (0.23–1.02) |
||
| Anti-andro+AI | – | – | – | – | – | 0.99 (0.62–1.56) |
0.97 (0.60–1.56) |
0.68 (0.44–1.06) |
0.56 (0.31–1.00) |
||
| F250+AI | – | – | – | – | – | – | 0.98 (0.73–1.32) |
0.69 (0.55–0.86) |
0.57 (0.36–0.88) |
||
| F500* | – | – | 0.80 (0.47–1.38) |
– | – | – | – |
0.70 (0.54–0.91) |
0.58 (0.41–0.80) |
||
| CDK4/6i+AI* | – | – | 0.70 (0.48–1.02) |
– | – | – | – | 0.87 (0.59–1.29) |
0.82 (0.54–1.25) |
||
| CDK4/6i+F500 | – | – | 0.62 (0.37–1.02) |
– | – | – | – |
0.77 (0.63–0.95) |
0.88 (0.63–1.22) |
||
Treatments in the cells closer to the right-upper corner of the table are usually better than treatments in the cells closer to the upper-left corner. Cells in bold: statistically significant difference.
*±goserelin.
AI, aromatase inhibitor; anti-andro, anti-androgen agent; CDK, cyclin-dependent kinase; CDK4/6i, CDK4/6 inhibitor; F250, fulvestrant 250 mg; F500, fulvestrant 500 mg; sapat, sapatinib.
Table 3.
Comparisons between treatments (HR, 95% credibility interval) in endocrine-resistant patients, both for progression-free survival (column vs row) and overall survival (row vs column)
| Progression-free survival, HR (95% CrI) | |||||||||||||||||||||
| Treatment | Metformin +AI |
Anti-andro (single) | F250 | Lapatinib +F250 |
Goserelin | AI | F250 +AI |
Anti-andro +AI |
Lapatinib +AI |
Tucidinos-tat+AI | F500 | Vistus. cont +F500 |
Vistus. int +F500 |
Bortezo-mib +F500 |
Pan-PI3Ki +F500 |
Everolim-us+AI | MultiTKI +F500 |
Everolim-us+F500 | Capivaser -tib +F500 |
CDK4/6i +F500 |
|
| Overall survival, HR (95% CrI) | Metformin +AI |
0.92 (0.49–1.75) |
0.88 (0.48–1.61) |
0.88 (0.46–1.31) |
0.85 (0.42–1.71) |
0.83 (0.48–1.44) |
0.84 (0.46–1.53) |
0.81 (0.45–1.50) |
0.65 (0.35–1.22) |
0.63 (0.34–1.15) |
0.52 (0.25–1.07) |
0.45 (0.21–0.98) |
0.40 (0.18–0.88) |
0.38 (0.16–0.86) |
0.38 (0.19–0.81) |
0.37 (0.21–0.67) |
0.35 (0.14–0.86) |
0.32 (0.15–0.69) |
0.30 (0.13–0.68) |
0.27 (0.13–0.57) |
|
| Anti-andro (single) | – | 0.96 (0.64–1.43) |
0.96 (0.59–1.54) |
0.91 (0.53–1.57) |
0.90 (0.66–1.24) |
0.90 (0.60–1.36) |
0.88 (0.64–1.21) |
0.70 (0.45–1.10) |
0.67 (0.45–1.02) |
0.56 (0.31–0.98) |
0.49 (0.25–0.93) |
0.43 (0.22–0.83) |
0.41 (0.20–0.82) |
0.42 (0.23–0.75) |
0.41 (0.28–0.58) |
0.38 (0.18–0.81) |
0.35 (0.18–0.66) |
0.32 (0.16–0.64) |
0.29 (0.16–0.53) |
||
| F250 | 1.15 (0.50–2.62) |
– | 1.00 (0.76–1.31) |
0.96 (0.58–1.58) |
0.94 (0.74–1.20) |
0.95 (0.74–1.21) |
0.92 (0.65–1.31) |
0.74 (0.50–1.09) |
0.71 (0.50–1.01) |
0.59 (0.34–1.00) |
0.51 (0.28–0.94) |
0.45 (0.24–0.84) |
0.43 (0.22–0.83) |
0.44 (0.25–0.75) |
0.42 (0.32–0.57) |
0.40 (0.19–0.83) |
0.36 (0.20–0.66) |
0.34 (0.18–0.65) |
0.31 (0.18–0.53) |
||
| Lapatinib +F250 |
– | – | – | 0.96 (0.54–1.69) |
0.94 (0.66–1.35) |
0.95 (0.66–1.35) |
0.92 (0.59–1.44) |
0.74 (0.46–1.19) |
0.71 (0.45–1.10) |
0.58 (0.32–1.06) |
0.51 (0.26–0.99) |
0.45 (0.23–0.89) |
0.43 (0.21–0.88) |
0.44 (0.24–0.80) |
0.42 (0.28–0.63) |
0.40 (0.18–0.87) |
0.36 (0.19–0.70) |
0.34 (0.17–0.69) |
0.31 (0.17–0.56) |
||
| Goserelin | 1.76 (0.56–5.55) |
– | 1.53 (0.64–3.67) |
– | 0.98 (0.63–1.53) |
0.99 (0.60–1.64) |
0.96 (0.58–1.60) |
0.77 (0.45–1.32) |
0.74 (0.44–1.23) |
0.61 (0.37–1.00) |
0.53 (0.30–0.95) |
0.47 (0.26–0.85) |
0.45 (0.24–0.85) |
0.46 (0.27–0.76) |
0.44 (0.28–0.71) |
0.42 (0.20–0.85) |
0.38 (0.21–0.67) |
0.35 (0.19–0.66) |
0.32 (0.19–0.53) |
||
| AI | 0.91 (0.42–1.99) |
– | 0.79 (0.60–1.05) |
– | 0.52 (0.22–1.18) |
1.01 (0.78–1.29) |
0.98 (0.76–1.27) |
0.78 (0.57–1.07) |
0.75 (0.58–0.97) |
0.62 (0.39–0.99) |
0.54 (0.31–0.95) |
0.48 (0.27–0.85) |
0.45 (0.24–0.84) |
0.46 (0.28–0.75) |
0.45 (0.38–0.54) |
0.42 (0.21–0.85) |
0.38 (0.22–0.67) |
0.36 (0.20–0.66) |
0.33 (0.20–0.53) |
||
| F250 +AI |
0.97 (0.42–2.23) |
– | 0.85 (0.64–1.13) |
– | 0.56 (0.23–1.33) |
1.07 (0.80–1.42) |
0.97 (0.68–1.40) |
0.78 (0.52–1.16) |
0.75 (0.52–1.07) |
0.62 (0.36–1.05) |
0.54 (0.29–0.99) |
0.48 (0.26–0.89) |
0.45 (0.23–0.88) |
0.46 (0.27–0.80) |
0.45 (0.33–0.61) |
0.42 (0.20–0.88) |
0.38 (0.21–0.70) |
0.36 (0.19–0.69) |
0.32 (0.19–0.56) |
||
| Anti-andro +AI |
– | – | – | – | – | – | – | 0.80 (0.53–1.19) |
0.77 (0.53–1.11) |
0.63 (0.37–1.08) |
0.55 (0.30–1.02) |
0.49 (0.26–0.91) |
0.46 (0.24–0.90) |
0.47 (0.27–0.82) |
0.46 (0.34–0.63) |
0.43 (0.21–0.91) |
0.39 (0.21–0.72) |
0.37 (0.19–0.71) |
0.33 (0.19–0.58) |
||
| Lapatinib +AI |
– | – | – | – | – | – | – | – | 0.96 (0.64–1.44) |
0.79 (0.45–1.41) |
0.69 (0.36–1.32) |
0.61 (0.32–1.18) |
0.58 (0.29–1.17) |
0.59 (0.33–1.06) |
0.58 (0.40–0.83) |
0.54 (0.25–1.17) |
0.49 (0.26–0.93) |
0.46 (0.23–0.91) |
0.42 (0.23–0.75) |
||
| Tucidinos-tat +AI |
– | – | – | – | – | – | – | – | – | 0.83 (0.48–1.42) |
0.72 (0.39–1.35) |
0.64 (0.34–1.20) |
0.60 (0.31–1.19) |
0.62 (0.35–1.08) |
0.60 (0.44–0.83) |
0.56 (0.27–1.19) |
0.51 (0.28–0.95) |
0.48 (0.25–0.93) |
0.43 (0.25–0.76) |
||
| F500 | 1.06 (0.34–3.27) |
– | 0.92 (0.39–2.18) |
– | 0.60 (0.28–1.33) |
1.16 (0.52–2.61) |
1.09 (0.46–2.60) |
– | – | – | 0.87 (0.65–1.18) |
0.77 (0.56–1.06) |
0.73 (0.49–1.09) |
0.75 (0.67–0.84) |
0.73 (0.44–1.20) |
0.68 (0.41–1.13) |
0.62 (0.47–0.82) |
0.58 (0.40–0.85) |
0.52 (0.46–0.60) |
||
| Vistus. cont+F500 | – | – | – | – | – | – | – | – | – | – | – | 0.89 (0.69–1.14) |
0.84 (0.51–1.38) |
0.86 (0.62–1.18) |
0.83 (0.46–1.50) |
0.78 (0.43–1.41) |
0.71 (0.54–0.94) |
0.66 (0.41–1.08) |
0.60 (0.43–0.84) |
||
| Vistus. int +F500 |
– | – | – | – | – | – | – | – | – | – | – | – | 0.95 (0.57–1.58) |
0.97 (0.69–1.36) |
0.94 (0.52–1.71) |
0.88 (0.48–1.61) |
0.80 (0.60–1.08) |
0.75 (0.46–1.24) |
0.68 (0.48–0.96) |
||
| Bortezomib +F500 |
– | – | – | – | – | – | – | – | – | – | – | – | – | 1.02 (0.67–1.55) |
0.99 (0.52–1.90) |
0.93 (0.49–1.78) |
0.85 (0.52–1.38) |
0.79 (0.45–1.38) |
0.72 (0.47–1.10) |
||
| Pan-PI3Ki+F500 | 0.92 (0.29–2.86) |
– | 0.80 (0.33–1.92) |
– | 0.52 (0.24–1.17) |
1.01 (0.44–2.32) |
0.95 (0.39–2.28) |
– | – | – | 0.87 (0.74–1.02) |
– | – | – | 0.97 (0.58–1.63) |
0.91 (0.54–1.53) |
0.83 (0.61–1.13) |
0.78 (0.52–1.16) |
0.70 (0.59–0.84) |
||
| Everolimus +AI |
0.81 (0.36–1.83) |
– | 0.71 (0.50–1.00) |
– | 0.46 (0.20–1.08) |
0.89 (0.73–1.09) |
0.83 (0.58–1.18) |
– | – | – | 0.76 (0.33–1.77) |
– | – | – | 0.88 (0.38–2.08) |
0.94 (0.46–1.92) |
0.85 (0.48–1.52) |
0.80 (0.42–1.50) |
0.72 (0.43–1.22) |
||
| MultiTKI+F500 | 0.86 (0.22–3.27) |
– | 0.75 (0.24–2.28) |
– | 0.49 (0.17–1.40) |
0.94 (0.32–2.78) |
0.88 (0.28–2.71) |
– | – | – | 0.81 (0.39–1.67) |
– | – | – | 0.93 (0.44–1.94) |
1.06 (0.35–3.20) |
0.91 (0.51–1.64) |
0.85 (0.45–1.61) |
0.77 (0.46–1.31) |
||
| Everolimus +F500 |
1.38 (0.38–5.00) |
– | 1.21 (0.42–3.45) |
– | 0.79 (0.29–2.13) |
1.52 (0.56–4.20) |
1.43 (0.50–4.09) |
– | – | – | 1.31 (0.72–2.39) |
– | – | – | 1.51 (0.81–2.82) |
1.71 (0.61–4.83) |
1.61 (0.64–4.12) |
0.93 (0.58–1.50) |
0.85 (0.62–1.16) |
||
| Capivaser-tib+F500 | 0.63 (0.18–2.20) |
– | 0.54 (0.20–1.53) |
– |
0.35 (0.13–0.93) |
0.69 (0.26–1.85) |
0.64 (0.23–1.81) |
– | – | – | 0.59 (0.34–1.04) |
– | – | – | 0.68 (0.38–1.22) |
0.77 (0.28–2.13) |
0.73 (0.29–1.83) |
0.45 (0.20–1.02) |
0.91 (0.60–1.36) |
||
| CDK4/6i +F500 |
0.82 (0.26–2.54) |
– | 0.71 (0.30–1.71) |
– | 0.46 (0.21–1.03) |
0.90 (0.39–2.05) |
0.84 (0.35–2.02) |
– | – | – |
0.77 (0.67–0.89) |
– | – | – | 0.89 (0.71–1.10) |
1.01 (0.43–2.35) |
0.95 (0.46–1.99) |
0.59 (0.32–1.08) |
1.31 (0.73–2.33) |
||
Treatments in the cells closer to the right-upper corner of the table are usually better than treatments in the cells closer to the upper-left corner. Cells in bold: statistically significant difference.
*±goserelin.
AI, aromatase inhibitor; anti-andro, anti-androgen agent; CDK, cyclin-dependent kinase; CDK4/6i, CDK4/6 inhibitor; cont, continuous; F250, fulvestrant 250 mg; F500, fulvestrant 500 mg; int, intermittent; multiTKI, multi-tyrosine kinase inhibitor; pan-PI3Ki, pan-PI3K inhibitor; vistus., vistusertib.
Secondary endpoint: OS
The number of trials reporting on OS was smaller as compared with those reporting on PFS. In the endocrine-sensitive population, five trials were included (n=2157 patients), comparing four treatments (figure 2C). Using a fixed-effects model, CDK4/6i+F500 was likely the most effective treatment (SUCRA=97.3%), followed by CDK4/6i+AI±goserelin (SUCRA=89.8%) and F500 (SUCRA=61.8%)—online supplementary table 2. Comparing CDK4/6i+F500 to F500 held a HR of 0.77 (95% CrI 0.63–0.95) and when comparing CDK4/6i+AI±goserelin to AI±goserelin the HR was 0.70 (95% CrI 0.48–1.02)—table 2.
In the endocrine-resistant population, 11 trials (n=4377 patients), testing 12 treatments, reported data on OS (figure 2D). Using a fixed-effects model, treatments with the highest chance of improving OS were capivasertib+F500 (SUCRA=84.7%) and CDK4/6i+F500 (SUCRA=69.9%)—table 3 and online supplementary table 3. The comparison of capivasertib+F500 to F500 held a HR of 0.59 (95% CrI 0.34–1.04), and when comparing CDK4/6i+F500 to F500 the HR was 0.77 (95% CrI 0.67–0.89).
Subgroup analysis of PFS
Four trials reported subgroup analysis for PFS separately for endocrine-sensitive patients with de novo metastatic or recurrent disease.15 23 29 63 All trials tested CDK4/6i+AI±goserelin versus AI±goserelin and the pairwise meta-analysis showed a HR of 0.50 (95% CI 0.39 to 0.63) among patients with de novo metastatic disease, favouring the combination with CDK4/6i; similarly, the HR was 0.58 (95% CI 0.49 to 0.70) among patients with recurrent metastatic disease (online supplementary figure 1).
Five trials reported subgroup analysis for PFS on patients with visceral disease in the endocrine-sensitive setting,15 19 24 25 29 and nine in the endocrine-resistant setting (online supplementary figure 2).33 42 44–46 48 52 53 55 On endocrine-sensitive patients, CDK4/6i+AI±goserelin was likely the most effective treatment (SUCRA=98.4%), with a HR of 0.59 (95% CrI 0.34–1.04) when compared with F500. On endocrine-resistant patients, CDK4/6i+F500 was probably the most effective treatment (SUCRA=94.7%), with a HR of 0.68 (95% CrI 0.53–0.88) when compared with pan-PI3Ki+F500 (online supplementary tables 4 and 5).
Patients with bone-only disease were analysed separately for PFS in nine trials, of which four were on endocrine-sensitive,15 19 24 29 and five on endocrine-resistant patients (online supplementary figures 3 and 4).20 35 46 47 49 In endocrine-sensitive patients, all trials compared CDK4/6i+AI±goserelin versus AI±goserelin: the pairwise meta-analysis showed a HR of 0.49 (95% CI 0.36 to 0.67) favouring the combination with CDK4/6i. In endocrine-resistant patients, treatments had to be split between two networks, due to the absence of direct or indirect connection between all tested regimens. In network 1, everolimus+AI was probably the best treatment (SUCRA=100%) and, in network 2, it was CDK4/6i+F500 which was most likely to be the best treatment (SUCRA=80.3%)—online supplementary tables 6 and 7.
No network meta-analysis was performed based on molecularly defined subgroups, such as PIK3CA-mutant patients, owing to the substantial degree of heterogeneity in how to define and/or identify these subgroups among different trials.44 47 53 64
Discussion
In this network meta-analysis, all the tested ET-based treatments for patients with HR+/HER2− advanced breast cancer were compared providing a rank order for their efficacy based on clinically meaningful endpoints (PFS and OS). Results suggest that the combination of CDK4/6i+F500 is likely to be the best treatment option in terms of PFS benefit among endocrine-resistant patients and also for endocrine-sensitive patients. The second most effective treatment options were CDK4/6i+AI±goserelin in the endocrine-sensitive population, and capivasertib+F500 in the endocrine-resistant population. Notably, by directly comparing CDK4/6i+F500 to CDK4/6i+AI±goserelin or to capivasertib+AI, even if HR are below 1, the CrIs cross 1.0, meaning that these differences are non-significant. Yet, this model may still guide clinicians by indicating which treatment options are probably the most effective among all.
The robustness of OS networks was partially hampered, as half of the trials have not yet provided mature data on this endpoint and all were unpowered for it, as OS was a secondary endpoint. Even so, the analysis showed that, similarly to the PFS results, CDK4/6i+F500 is also possibly the best treatment for endocrine-sensitive patients in terms of OS, with a significantly better HR when compared with F500 (HR 0.77; 95% CrI 0.63–0.95). Interestingly, when pooling the OS data for the comparison of CDK4/6i+AI±goserelin versus AI±goserelin, the HR was non-significant (0.70; 95% CrI 0.48–1.02). In the endocrine-resistant population, the SUCRA value was higher for capivasertib+F500, but only the comparison between CDK4/6i+F500 and F500 held a significant HR (0.77; 95% CrI 0.67–0.89). These pooled results are highly relevant, as they consistently demonstrate that the addition of a CDK4/6i to F500 significantly increases the clinically important endpoint of OS, both in the endocrine-sensitive and endocrine-resistant settings. Nonetheless, we have to consider that OS gains are also influenced by post-progression therapies—in MONALEESA-3, subsequent antineoplastic therapies were received by 81.5% of patients in the ribociclib+F500 group and 84.7% of patients in the F500 group60; however, in MONALEESA-7, only 68.9% of patients in the ribociclib+tamoxifen/AI+goserelin group and 73.2% of patients in the tamoxifen/AI+goserelin group received subsequent antineoplastic therapy,32 which is lower than what would be expected. Therefore, this should also be taken into account when evaluating the OS benefits of each treatment.
For the subgroup analysis of patients with endocrine-sensitive disease, data regarding the comparison between CDK4/6i+F500 versus F500 were not available and, hence, we cannot assess the combination’s benefit in these subgroups. Taking that into account, results from subgroup analyses were not substantially different from the ones reported in the overall populations: CDK4/6i+AI±goserelin was likely the most effective treatment in terms of PFS both in endocrine-sensitive patients with bone-only, visceral, de novo and recurrent disease. In the endocrine-resistant setting, CDK4/6i+F500 was likely the best treatment for patients with visceral disease; for patients with bone-only disease, both CDK4/6i+F500 and everolimus+AI are suitable options.
The remaining preplanned subgroup analysis, namely in molecular-selected subgroups, was not carried out, as we did not have access to individual patient data and aggregated data from trial publications was scarce. In addition, trials testing selective PI3K-alpha inhibitors in endocrine-resistant patients, like SANDPIPER (fulvestrant with/without taselisib)65 and SOLAR-1 (fulvestrant with/without alpelisib),66 were not included in this systematic review, as they reported endpoints separately for patients with PI3KCA-mutated tumours and with PI3KCA-wild type tumours. Thus, our network meta-analysis only provides results regarding the use of ET-based regimens in patients with HR+/HER2− advanced breast cancer without further molecular selection. With the increasing use of multigene sequencing, clinicians will have access to the mutational landscape of the individual patient’s tumour. Nevertheless, use of somatic multigene sequencing in breast cancer is still controversial as its clinical utility has not yet been proven. Hence, ASCO and ABC4 guidelines do not support the routine use of such multigene panels in routine clinical practice when deciding treatment for patients with advanced breast cancer.1 67
Our main conclusions are based on fixed-effect model analyses, given that all of them provided better deviance information criteria compared with the respective random-effect models. These results could have been impaired by heterogeneity among trials, but we showed that heterogeneity was minimal (I2=0%) in all direct comparisons with ≥2 trials in the main PFS and OS networks, making the results robust, while still taking into account that these findings are derived from both direct and indirect evidence.
We have focused solely on efficacy parameters and did not include toxicity, due to the large number of regimens evaluated and the absence of one single parameter to measure it Furthermore, our group has previously published a meta-analysis on the risk of adverse events with the addition of targeted agents to ET in patients with HR+ advanced breast cancer.68 We concluded that the use of targeted agents significantly increased the incidence of adverse events, both of grade 1–4 and grade 3–4. Therefore, the toxicity profile of each class of agents should be taken into account when deciding which treatment to prescribe to the individual patient. The systematic use of patient-reported outcomes (PROs) in clinical trials has recently become widespread practice; however, most of the trials included in our analysis did not provide information on this endpoint. Thus, we have not extracted data regarding PROs, but this should be considered in future meta-analyses. This is especially relevant nowadays, as the Magnitude of Clinical Benefit Scale of the European Society for Medical Oncology gives limited credit to a PFS gain not associated with improved quality of life or OS benefit.69 Therefore, as this scale is used for reimbursement policies in some countries, PROs results can also influence the availability of some treatments.
We also acknowledge that there was heterogeneity in patients’ populations regarding menopausal status and prior hormonal/chemotherapy treatments received, which may have influenced the benefit from each regimen. Additionally, endocrine-sensitivity should be regarded as a continuum—hence, the time cut-off to separate endocrine-sensitive from endocrine-resistant disease does not have a specific biological basis and the benefit from treatment may have changed between trials according to the proportion of patients with a ‘more’ endocrine-sensitive or endocrine-resistant disease. Endocrine-resistant population may also be heterogeneous, as patients might have received a variable number of previous ET lines—nonetheless, in most trials including endocrine-resistant patients, the median number of previous ET lines was just one (table 1); the only exception was the BELLE-3 trial.53
Publication bias was not assessed, as there are multiple limitations to its performance on network meta-analysis, especially regarding the creation of funnel plots.70 Nonetheless, we have searched both fully published articles as well as conference abstracts, thus increasing the chances of including all potentially eligible trials.
Most of the published network meta-analyses assessing the efficacy of ET-based regimens in HR+/HER2− advanced breast cancer only included patients with endocrine-sensitive,71–73 or with endocrine-resistant disease.74–76 Of the network meta-analyses that have included both groups of patients, one specifically compared palbociclib+ET to different single-agent ET,77 another compared everolimus+exemestane to several chemotherapy regimens78 and the third evaluated palbociclib+ET versus chemotherapy,79 making them difficult to compare with our results. Another recent and very large network meta-analysis has evaluated all chemotherapy and ET-based treatments in postmenopausal women with HR+/HER2− metastatic breast cancer.80 Similarly to our results, it has showed that CDK4/6i plus ET are better than standard ET; in addition, it has demonstrated that no chemotherapy regimen was significantly better than CDK4/6i plus ET in terms of PFS. However, this network meta-analysis did not include premenopausal women, did not provide OS data or analysed efficacy according to endocrine-sensitivity status or different patients’ subgroups (visceral disease, bone-only, etc). In addition, it comprised many trials including patients with HER2+ or triple-negative disease, partially hampering the generalisation of results.
Thanks to the high number of patients included and the geometry of the networks, we could provide ranking probabilities for each treatment, which facilitates decision-making for clinicians. Yet, these probabilities should be taken together with the HR and CrI of the pairwise comparisons. For instance, when analysing OS data in endocrine-resistant patients, the first rank is occupied by capivasertib+F500, followed by CDK4/6i+F500. Nevertheless, when comparing these two regimens, we see that the CrI are wide (table 3), meaning that these rankings should be interpreted with caution, as there was no significant difference between these treatments. In addition, our findings should be tailored to the individual patient, in terms of phase of disease (ie, endocrine-sensitive vs endocrine-resistant), previous therapies and tolerance to them.
Due to the rapid pace of changes in treatment options for HR+/HER2− advanced breast cancer, this network meta-analysis provides evidence-based data to patients, clinicians and policy makers to support nowadays clinical decision-making. It clearly demonstrates that CDK4/6i combined with ET is likely the best treatment option in terms of PFS and OS among patients with endocrine-sensitive and endocrine-resistant disease and across all patient subgroups, which could help facilitating the access to CDK4/6i. Yet, data of the direct comparison between CDK4/6i+F500 versus F500 among endocrine-sensitive patients comes from a subgroup analysis of a single trial (MONALEESA-3)59 60; therefore, these subgroup analysis results should ideally be confirmed in a dedicated randomised trial for patients with endocrine-sensitive disease. Nonetheless, while such data are not available, CDK4/6i+F500 could still be considered as an option for endocrine-sensitive patients in upcoming ASCO and ABC5 guidelines.1 3 This is reinforced by the fact that, even if for many years AI have been considered the standard-of-care for these patients, the FALCON trial has demonstrated that, in terms of PFS, F500 is superior to AI.25 However, little data are available on the effectiveness of AI or tamoxifen after F500, which leads many physicians to delay its use into the second-line—a practice which the availability of alpelisib66 should reinforce. Ongoing trials are testing new, orally administered selective oestrogen receptor degraders, alone or in combination with CDK4/6i or AI, which should change the treatment landscape in coming years.81
In the future, it is expected that new genomic, proteomic, metabolomics and imaging biomarkers will be used to further tailor treatment to the individual patient. This is especially needed, not only to spare patients from the toxic effects of ineffective regimens, but also due to the potential ‘financial toxicity’ of these multiple ET-based treatments, given their potential impact on the individual patient and also on the sustainability of healthcare systems.82 Therefore, new pooled analyses will probably be conducted to compare the efficacy of different ET-based treatments in biomarker-defined populations, such as patients with PI3KCA-mutated tumours.
In conclusion, this network meta-analysis suggests that the combination of a CDK4/6i+F500 may be the best treatment option in terms of PFS for both endocrine-sensitive and endocrine-resistant patients with HR+/HER2− advanced breast cancer. As for OS, CDK4/6i+F500 is possibly the best choice for endocrine-sensitive patients. Concerning endocrine-resistant patients, capivasertib+F500 and CDK4/6i+F500 are likely the best treatments in this setting.
Footnotes
Twitter: @MarianaBrandao0, @ajrsferreira, @E_de_Azambuja, @matteolambe
Presented at: A preliminary result of this work was presented at the 2018 San Antonio Breast Cancer Symposium (SABCS), San Antonio, Texas, USA, on 7 December 2018.
Contributors: Conceptualisation: MB, CM, EdA, MD, ML. Data curation: MB, CM, NFP, AF, MD, ML. Formal analysis: PZ. Investigation: all authors. Methodology: MB, CM, PKZ, MD, ML. Supervision: MD, ML. Writing, original draft: MB. Writing, review and editing: all authors. MD and ML are co-last authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MB, NFP, MJP, EdA: research grants for their Institute from Radius, AstraZeneca, Lilly, MSD, GSK/Novartis, Roche/GNE, Synthon, Servier and Pfizer. MB: travel grant and speaker honoraria from Roche/GNE. CM: travel grants from Servier Deutschland GmbH, Mundipharma and Amgen. PKZ: declares no conflict of interest. NFP: speaker honoraria from AstraZeneca, Lilly, Roche/GNE and Novartis; travel grants from Lilly and Novartis; research grants from Daichii Sankyo and Novartis. AF: travel grants from Novartis and Roche. SM: consultancy for Novartis, Genomic Health and Knight Therapeutics. MJP: consultancy honoraria for AstraZeneca, Camel-IDS, Crescendo Biologics, Debiopharm, G1 Therapeutics, Genentech, Huya, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Periphagen, Pfizer, Roche, Seattle Genetics; scientific board member: Oncolytics. EdA: honoraria and advisory board for Roche/GNE, travel grants from Roche/GNE and GSK/Novartis. EA: speaker honoraria and/or advisory board for Roche/GNE, Novartis, SeaGen and Zodiac; travel grants from Roche/GNE and GSK/Novartis. MD: started working at Roche Pharmaceuticals on September/2019. ML: consultancy for Roche and Novartis; speaker honoraria from Theramex, Roche, Pfizer, Novartis, Lilly and Takeda. No other relationships or activities that could appear to have influenced the submitted work.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1.Cardoso F, Senkus E, Costa A, et al. . 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol 2018;29:1634–57. 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Altekruse SF, Li CI, et al. . US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106. 10.1093/jnci/dju055. [Epub ahead of print: 28 Apr 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugo HS, Rumble RB, Macrae E, et al. . Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of clinical oncology guideline. J Clin Oncol 2016;34:3069–103. 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- 4.Turner NC, Neven P, Loibl S, et al. . Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet 2017;389:2403–14. 10.1016/S0140-6736(16)32419-9 [DOI] [PubMed] [Google Scholar]
- 5.Gourgou-Bourgade S, Cameron D, Poortmans P, et al. . Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials)†. Ann Oncol 2015;26:873–9. 10.1093/annonc/mdv106 [DOI] [PubMed] [Google Scholar]
- 6.Cochrane Handbook for systematic reviews of interventions. Available: https://training.cochrane.org/handbook [Accessed 16 Aug 2018].
- 7.Hutton B, Salanti G, Caldwell DM, et al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 8.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24. 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 9.Spiegelhalter DJ, Best NG, Carlin BP, et al. . Bayesian measures of model complexity and fit. J R Stat Soc Ser B Stat Methodol 2002;64:583–639. 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 10.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim NK, Yariz KO, Bondarenko I, et al. . Randomized phase II trial of letrozole plus anti-MUC1 antibody AS1402 in hormone receptor-positive locally advanced or metastatic breast cancer. Clin Cancer Res 2011;17:6822–30. 10.1158/1078-0432.CCR-11-1151 [DOI] [PubMed] [Google Scholar]
- 12.Mehta RS, Barlow WE, Albain KS, et al. . Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012;367:435–44. 10.1056/NEJMoa1201622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul D, Vukelja SJ, Holmes FA, et al. . Abstract S3-07: letrozole plus dasatinib improves progression-free survival (PFS) in hormone receptor-positive, HER2-negative postmenopausal metastatic breast cancer (MBC) patients receiving first-line aromatase inhibitor (AI) therapy. Cancer Res 2013;73:S3-07-S3-07. [Google Scholar]
- 14.Finn RS, Crown JP, Lang I, et al. . The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35. 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 15.Finn RS, Crown JP, Ettl J, et al. . Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res 2016;18:67. 10.1186/s13058-016-0721-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn RS, Crown J, Lang I, et al. . Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2– advanced breast cancer (PALOMA-1; TRIO-18). J Clin Oncol 2017;35:1001 10.1200/JCO.2017.35.15_suppl.1001 [DOI] [Google Scholar]
- 17.Finn RS, Martin M, Rugo HS, et al. . Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–36. 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 18.Finn RS, Dieras V, Rugo HS, et al. . Palbociclib (PAL) + letrozole (L) as first-line (1L) therapy (tx) in estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC): Efficacy and safety across patient (pt) subgroups. J Clin Oncol 2017;35:1039 10.1200/JCO.2017.35.15_suppl.1039 [DOI] [Google Scholar]
- 19.Rugo H, Finn R, Dieras V, et al. . Abstract P5-21-03: Palbociclib (PAL) + letrozole (LET) as first-line therapy in estrogen receptor–positive (ER+)/human epidermal growth factor receptor 2–negative (HER2−) advanced breast cancer (ABC): Efficacy and safety updates with longer follow-up across patient subgroups. Cancer Res 2018;78:P5–21. [Google Scholar]
- 20.Turner NC, Finn RS, Martin M, et al. . Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol 2018;29:669–80. 10.1093/annonc/mdx797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hortobagyi GN, Stemmer SM, Burris HA, et al. . Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738–48. 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 22.Hortobagyi GN, Stemmer SM, Burris HA, et al. . Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018;29:1541–7. 10.1093/annonc/mdy155 [DOI] [PubMed] [Google Scholar]
- 23.O'Shaughnessy J, Petrakova K, Sonke GS, et al. . Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat 2018;168:127–34. 10.1007/s10549-017-4518-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burris HA, Chan A, Campone M, et al. . Abstract P4-22-16: first-line ribociclib + letrozole in patients with HR+, HER2– advanced breast cancer (ABC) presenting with visceral metastases or bone-only disease: a subgroup analysis of the MONALEESA-2 trial. Cancer Res 2017;77 :P4-22-16-P4-22-16. [Google Scholar]
- 25.Robertson JFR, Bondarenko IM, Trishkina E, et al. . Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016;388:2997–3005. 10.1016/S0140-6736(16)32389-3 [DOI] [PubMed] [Google Scholar]
- 26.Robertson JFR, Noguchi S, Shao Z, et al. . Abstract P2-08-02: progression-free survival results in patient subgroups from a phase 3 randomized trial of fulvestrant 500 mg vs anastrozole for hormone receptor-positive advanced breast cancer (FALCON). Cancer Res 2017;77. [DOI] [PubMed] [Google Scholar]
- 27.Robertson JFR, Leo AD, Fazal M, et al. . Abstract PD5-09: fulvestrant for hormone receptor-positive advanced breast cancer in patients with visceral vs non-visceral metastases: findings from falcon, first, and confirm. Cancer Res 2018;78. [Google Scholar]
- 28.Johnston S, Basik M, Hegg R, et al. . Inhibition of EGFR, HER2, and HER3 signaling with AZD8931 in combination with anastrozole as an anticancer approach: phase II randomized study in women with endocrine-therapy-naïve advanced breast cancer. Breast Cancer Res Treat 2016;160:91–9. 10.1007/s10549-016-3979-5 [DOI] [PubMed] [Google Scholar]
- 29.Goetz MP, Toi M, Campone M, et al. . MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–46. 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 30.Leo AD, Dickler M, Sledge GW, et al. . Abstract P5-21-02: efficacy and safety of abemaciclib in patients with liver metastases in the MONARCH 1, 2, and 3 studies. Cancer Res 2018;78. [Google Scholar]
- 31.Tripathy D, Im S-A, Colleoni M, et al. . Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018;19:904–15. 10.1016/S1470-2045(18)30292-4 [DOI] [PubMed] [Google Scholar]
- 32.Im S-A, Lu Y-S, Bardia A, et al. . Overall survival with Ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019;381:307–16. 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 33.Bachelot T, Bourgier C, Cropet C, et al. . Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 2012;30:2718–24. 10.1200/JCO.2011.39.0708 [DOI] [PubMed] [Google Scholar]
- 34.Baselga J, Campone M, Piccart M, et al. . Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520–9. 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campone M, Bachelot T, Gnant M, et al. . Effect of visceral metastases on the efficacy and safety of everolimus in postmenopausal women with advanced breast cancer: subgroup analysis from the BOLERO-2 study. Eur J Cancer 2013;49:2621–32. 10.1016/j.ejca.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 36.Yardley DA, Noguchi S, Pritchard KI, et al. . Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 2013;30:870–84. 10.1007/s12325-013-0060-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piccart M, Hortobagyi GN, Campone M, et al. . Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. Ann Oncol 2014;25:2357–62. 10.1093/annonc/mdu456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck JT, Hortobagyi GN, Campone M, et al. . Everolimus plus exemestane as first-line therapy in HR⁺, HER2⁻ advanced breast cancer in BOLERO-2. Breast Cancer Res Treat 2014;143:459–67. 10.1007/s10549-013-2814-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston SR, Kilburn LS, Ellis P, et al. . Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 2013;14:989–98. 10.1016/S1470-2045(13)70322-X [DOI] [PubMed] [Google Scholar]
- 40.Burstein HJ, Cirrincione CT, Barry WT, et al. . Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: a randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer-CALGB 40302 (Alliance). J Clin Oncol 2014;32:3959–66. 10.1200/JCO.2014.56.7941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaman K, Winterhalder R, Mamot C, et al. . Fulvestrant with or without selumetinib, a MEK 1/2 inhibitor, in breast cancer progressing after aromatase inhibitor therapy: a multicentre randomised placebo-controlled double-blind phase II trial, SAKK 21/08. Eur J Cancer 2015;51:1212–20. 10.1016/j.ejca.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 42.Adelson K, Ramaswamy B, Sparano JA, et al. . Randomized phase II trial of fulvestrant alone or in combination with bortezomib in hormone receptor-positive metastatic breast cancer resistant to aromatase inhibitors: a New York cancer Consortium trial. NPJ Breast Cancer 2016;2:16037. 10.1038/npjbcancer.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner NC, Ro J, André F, et al. . Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015;373:209–19. 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 44.Cristofanilli M, Turner NC, Bondarenko I, et al. . Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 45.Turner NC, Slamon DJ, Ro J, et al. . Overall survival with Palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–36. 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 46.O'Shaughnessy J, Campone M, Brain E, et al. . Abiraterone acetate, exemestane or the combination in postmenopausal patients with estrogen receptor-positive metastatic breast cancer. Ann Oncol 2016;27:106–13. 10.1093/annonc/mdv487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baselga J, Im S-A, Iwata H, et al. . Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904–16. 10.1016/S1470-2045(17)30376-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campone M, Im S-A, Iwata H, et al. . Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: overall survival results from BELLE-2. Eur J Cancer 2018;103:147–54. 10.1016/j.ejca.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 49.Sledge GW, Toi M, Neven P, et al. . Monarch 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–84. 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 50.Musolino A, Campone M, Neven P, et al. . Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2- breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res 2017;19:18. 10.1186/s13058-017-0807-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y, Gong C, Wang Z, et al. . A randomized phase II study of aromatase inhibitors plus metformin in pre-treated postmenopausal patients with hormone receptor positive metastatic breast cancer. Oncotarget 2017;8:84224–36. 10.18632/oncotarget.20478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kornblum N, Zhao F, Manola J, et al. . Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-Negative metastatic breast cancer resistant to aromatase inhibitor therapy: results of PrE0102. J Clin Oncol 2018;36:1556–63. 10.1200/JCO.2017.76.9331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Leo A, Johnston S, Lee KS, et al. . Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:87–100. 10.1016/S1470-2045(17)30688-5 [DOI] [PubMed] [Google Scholar]
- 54.Kim J-Y, Im S-A, Jung KH, et al. . Fulvestrant plus goserelin versus anastrozole plus goserelin versus goserelin alone for hormone receptor-positive, HER2-negative tamoxifen-pretreated premenopausal women with recurrent or metastatic breast cancer (KCSG BR10-04): a multicentre, open-label, three-arm, randomised phase II trial (FLAG study). Eur J Cancer 2018;103:127–36. 10.1016/j.ejca.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 55.Schmid P, Zaiss M, Harper-Wynne C, et al. . Fulvestrant plus Vistusertib vs fulvestrant plus everolimus vs fulvestrant alone for women with hormone receptor-positive metastatic breast cancer: the MANTA phase 2 randomized clinical trial. JAMA Oncol 2019. 10.1001/jamaoncol.2019.2526. [Epub ahead of print: 29 Aug 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Z, Li W, Hu X, et al. . Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:806–15. 10.1016/S1470-2045(19)30164-0 [DOI] [PubMed] [Google Scholar]
- 57.Johnston S, Pippen J, Pivot X, et al. . Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27:5538–46. 10.1200/JCO.2009.23.3734 [DOI] [PubMed] [Google Scholar]
- 58.Krop I, Abramson V, Colleoni M, et al. . Abstract GS4-07: results from a randomized placebo-controlled phase 2 trial evaluating exemestane ± enzalutamide in patients with hormone receptor–positive breast cancer. Cancer Res 2018;78:GS4-07-GS4-07. [DOI] [PubMed] [Google Scholar]
- 59.Slamon DJ, Neven P, Chia S, et al. . Phase III randomized study of Ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-Negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018;36:2465–72. 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 60.Slamon DJ, Neven P, Chia S, et al. . Overall survival (OS) results of the phase III MONALEESA-3 trial of postmenopausal patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor 2-negative (HER2−) advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB). Ann Oncol 2019;30:v856–7. 10.1093/annonc/mdz394.007 [DOI] [Google Scholar]
- 61.Wolff AC, Hammond MEH, Schwartz JN, et al. . American Society of clinical Oncology/College of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–45. 10.1200/JCO.2006.09.2775 [DOI] [PubMed] [Google Scholar]
- 62.Jones RH, Carucci M, Casbard AC, et al. . Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER-positive breast cancer (FAKTION): a randomized, double-blind, placebo-controlled, phase II trial. J Clin Oncol 2019;37:1005 10.1200/JCO.2019.37.15_suppl.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rugo HS, Finn RS, Diéras V, et al. . Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 2019;174:719–29. 10.1007/s10549-018-05125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krop IE, Mayer IA, Ganju V, et al. . Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:811–21. 10.1016/S1470-2045(16)00106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baselga J, Dent SF, Cortés J, et al. . Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA -mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J Clin Oncol 2018;36:LBA1006 10.1200/JCO.2018.36.18_suppl.LBA1006 [DOI] [Google Scholar]
- 66.André F, Ciruelos EM, Rubovszky G, et al. . Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): results of the phase III SOLAR-1 trial. Ann Oncol 2018;29:viii709 10.1093/annonc/mdy424.010 [DOI] [Google Scholar]
- 67.Robson ME, Bradbury AR, Arun B, et al. . American Society of clinical oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 2015;33:3660–7. 10.1200/JCO.2015.63.0996 [DOI] [PubMed] [Google Scholar]
- 68.Martel S, Bruzzone M, Ceppi M, et al. . Risk of adverse events with the addition of targeted agents to endocrine therapy in patients with hormone receptor-positive metastatic breast cancer: a systematic review and meta-analysis. Cancer Treat Rev 2018;62:123–32. 10.1016/j.ctrv.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 69.Cherny NI, Sullivan R, Dafni U, et al. . A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for medical oncology magnitude of clinical benefit scale (ESMO-MCBS). Ann Oncol 2015;26:1547–73. 10.1093/annonc/mdv249 [DOI] [PubMed] [Google Scholar]
- 70.Chaimani A, Higgins JPT, Mavridis D, et al. . Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Huang Y, Wang C, et al. . Efficacy and safety of endocrine monotherapy as first-line treatment for hormone-sensitive advanced breast cancer: a network meta-analysis. Medicine 2017;96:e7846. 10.1097/MD.0000000000007846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang T, Feng F, Zhao W, et al. . Effect of first-line endocrine therapy in patients with hormone-sensitive advanced breast cancer: a network meta-analysis. OncoTargets Ther 2018;11:2647–56. 10.2147/OTT.S165681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ayyagari R, Tang D, Patterson-Lomba O, et al. . Progression-Free survival with first-line Endocrine-based therapies among postmenopausal women with HR+/HER2- metastatic breast cancer:: a network meta-analysis. Clin Ther 2018;40:628–39. 10.1016/j.clinthera.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 74.Telford C, Jones N, Livings C, et al. . Network meta-analysis comparing overall survival for fulvestrant 500 Mg versus alternative therapies for treatment of postmenopausal, estrogen receptor-positive advanced breast cancer following failure on prior endocrine therapy. Clin Breast Cancer 2016;16:188–95. 10.1016/j.clbc.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 75.Ayyagari R, Tang D, Patterson-Lomba O, et al. . Progression-free survival with endocrine-based therapies following progression on non-steroidal aromatase inhibitor among postmenopausal women with hormone receptor positive, human epidermal growth factor receptor-2 negative metastatic breast cancer: a network meta-analysis. Curr Med Res Opin 2018;34:1645–52. 10.1080/03007995.2018.1479246 [DOI] [PubMed] [Google Scholar]
- 76.Zhang T, Feng F, Zhao W, et al. . Comparative efficacy of different targeted therapies plus fulvestrant for advanced breast cancer following progression on prior endocrine therapy: a network meta-analysis. Cancer Manag Res 2018;10:5869–80. 10.2147/CMAR.S176172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chirila C, Mitra D, Colosia A, et al. . Comparison of palbociclib in combination with letrozole or fulvestrant with endocrine therapies for advanced/metastatic breast cancer: network meta-analysis. Curr Med Res Opin 2017;33:1457–66. 10.1080/03007995.2017.1325730 [DOI] [PubMed] [Google Scholar]
- 78.Generali D, Venturini S, Rognoni C, et al. . A network meta-analysis of everolimus plus exemestane versus chemotherapy in the first- and second-line treatment of estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat 2015;152:95–117. 10.1007/s10549-015-3453-9 [DOI] [PubMed] [Google Scholar]
- 79.Wilson FR, Varu A, Mitra D, et al. . Systematic review and network meta-analysis comparing palbociclib with chemotherapy agents for the treatment of postmenopausal women with HR-positive and HER2-negative advanced/metastatic breast cancer. Breast Cancer Res Treat 2017;166:167–77. 10.1007/s10549-017-4404-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giuliano M, Schettini F, Rognoni C, et al. . Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol 2019;20:1360–9. 10.1016/S1470-2045(19)30420-6 [DOI] [PubMed] [Google Scholar]
- 81.Dickler MN, Villanueva R, Fidalgo JP, et al. . Abstract PD5-10: a first-in-human phase I study to evaluate the oral selective estrogen receptor degrader (SERD), GDC-0927, in postmenopausal women with estrogen receptor positive (ER+) HER2-negative metastatic breast cancer (BC). Cancer Res 2018;78. [Google Scholar]
- 82.Chino F, Peppercorn JM, Rushing C, et al. . Out-of-Pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncol 2017;3:1582–4. 10.1001/jamaoncol.2017.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000842supp001.pdf (2.4MB, pdf)