Abstract
Objective:
The aim of this study was to determine whether downstream [peroxisome proliferator-activated-receptor alpha (PPARα) and the G-protein coupled receptor, GPR119] and upstream (a fatty acid translocase, CD36) signaling targets of N-oleoylethanolamide (OEA) were necessary for weight loss, metabolic improvements, and diet preference following vertical sleeve gastrectomy (VSG).
Summary Background Data:
OEA is an anorectic N-acylethanolamine produced from dietary fats within the intestinal lumen that can modulate lipid metabolism, insulin secretion, and energy expenditure by activating targets such as PPARα and GPR119.
Methods:
Diet-induced obese mice, including wild-type or whole body knockout (KO) of PPARα, GPR119, and CD36, were stratified to either VSG or sham surgery before body weight, body composition, diet preference, and glucose and lipid metabolic endpoints were assessed.
Results:
We found increased duodenal production of OEA and expression of both GPR119 and CD36 were upregulated in wild-type mice after VSG. However, weight loss and glucose tolerance were improved in response to VSG in PPARαKO, GPR119KO, and CD36KO mice. In fact, VSG corrected hepatic triglyceride dysregulation in CD36KO mice, and circulating triglyceride and cholesterol levels in PPARαKO mice. Lastly, we found PPARα-mediated signaling contributes to macronutrient preference independent of VSG, while removal of CD36 signaling blunts the VSG-induced shift toward carbohydrate preference.
Conclusions:
In the search for more effective and less invasive therapies to help reverse the global acceleration of obesity and obesity-related disease OEA is a promising candidate; however, our data indicate that it is not an underlying mechanism of the effectiveness of VSG.
Keywords: bariatric surgery, metabolism, N-oleoylethanolamide (OEA)
Despite advances in treatment options and increased risk awareness, obesity rates over the past 18 years have steadily increased in adults (+9%) and children (+4.5%) within the United States.1 Bariatric surgery is currently the most effective and sustainable treatment for obesity resulting in long-term average weight loss of 30% excess body weight, improvements in glucose and insulin profiles, and the ability to rapidly suspend use of type 2 diabetes mellitus medication in many patients.2 Vertical sleeve gastrectomy (VSG), which excises ~80% of the stomach along the greater curvature without intestinal perturbation, is favored by both patients and surgeons as it is less invasive and results in similar metabolic improvements compared with other bariatric procedures. VSG has been shown to alter food preference,3,4 normalize lipid handing,5–7 and improve glucose homeostasis in both humans2,8,9 and rodents.4 However, the mechanisms that underlie weight loss and associated metabolic improvements after VSG remain unresolved.
N-oleoylethanolamide (OEA) is an endogenous lipid analogue synthesized in the proximal small intestine from the precursor molecule, oleic acid.10–12 Oleic acid is generated in the gut lumen after postprandial digestive breakdown of dietary lipids and subsequently detected and transported intracellularly by the cell-surface protein, cluster of differentiation 36 (CD36).13,14 Diet influences OEA levels, as intraduodenal infusion of intralipid, but not glucose or protein, stimulates OEA production.13 Pharmacological administration of OEA delays meal initiation and prolongs the interval between successive meals, resulting in decreased food intake, reductions in body weight, and improved glucose and lipid metabolism.11,15–17 However, chronic high-fat diet (HFD) reduces intestinal OEA,18–20 suggestive of physiological regulation of OEA in response to increased body mass and impaired metabolism.
Interestingly, OEA has been shown to be increased within the small intestine of rats after Roux-en Y gastric bypass (RYGB),21 suggesting this molecule may play an important role in metabolic improvements after bariatric surgery. Anorexic effects of OEA have been primarily attributed to activation of the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα) and subsequent stimulation of the vagal afferent neurons that innervates the gut.21–24 OEA also activates the G-protein coupled receptor 119 (GPR119), located in intestinal enteroendocrine cells,25,26 leading to the release of the satiety factor and incretin glucagon-like peptide-1 (GLP-1).27
Taken together, there is evidence that OEA signaling may be an important mechanism though which VSG leads to metabolic improvements. Here, we examine if OEA signaling through 2 downstream targets, GPR119 and PPARα, is necessary for VSG-induced improvements in glucose homeostasis, lipid handling, or diet preference. Furthermore, we examine if the upstream fatty acid translocase CD36 is necessary for the metabolic changes induced after VSG.
METHODS
Animals
Male rodents were ordered from the Jackson Laboratory, Bar Harbor, ME, at 6 to 8 weeks of age and included 1) peroxisome proliferator-activated receptor alpha knockout (PPARαKO) mice (Stock #008154), 2) C57BL/6J wild-type (WT) mice (Stock #000664), and 3) cluster of differentiation 36 receptor knockout (CD36KO) mice (Stock #019006). Long Evans rats were ordered from Envigo (New Jersey). Whole body G-protein coupled receptor 119 knockout (GPR119KO) breeding mice were gifted from Arena Pharmaceuticals28 and bred in-house. All mice were backcrossed with a C57BL/6J background, and C57BL/6J mice were used as controls unless wild-type littermates were available. Rodents were singly housed and fed a 40% high-fat butter diet (40% fat, 4.54 kcal/g, D03082706 Research Diets, New Brunswick, NJ) in the PPARαKO and rat cohorts, or a more obesogenic 60% high-fat lard-based diet (60% kcal fat, 5.24k/cal, D12492 Research Diets, New Brunswick, NJ) in the CD36KO and GPR119KO cohorts. The more obesogenic diet was used due to concerns of HFD resistance and not generating substantial fat mass before surgery. Surgical groups were age- and weight-matched within each genotype prior to surgical intervention. Rodents were housed under controlled temperature (22°C) and light (12:12-h light– dark cycle) conditions. Tissue mass was measured using nuclear magnetic resonance (Echo MRI: Echo Medical Systems, Houston, TX). All studies were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Michigan or the University of Cincinnati.
Surgical Procedure
All rodents received either sham surgery or VSG after 9 to 12 weeks of HFD feeding. VSG was performed by a midline skin incision in the ventral abdomen and underlying muscle. The stomach was exposed and transected along the greater curvature to form a sleeve. The stomach sleeve was closed and the greater curvature portion was removed using an Endopath ETS-FLEX 35 mm Stapler (Ethicon endo-surgery, LLC, Cincinnati, OH). The stomach sleeve was returned to the abdominal cavity and the body wall and skin were sutured. The sham procedure involved exposure of the stomach before applying pressure on the stomach with blunt forceps along a vertical line between the esophageal sphincter and the pylorus. Postoperatively, animals were maintained on liquid Osmolite for 4 days and received subcutaneous analgesic meloxicam (0.5 mg/kg) daily for 3 days and warm saline (1 mL) on first postoperative day for fluid replacement.
OEA Measures
Ad lib fed rodent intestinal tissue and mucosal scraps were harvested 10 to 12 weeks postsurgery and were stored at −80°C until lipid extraction was performed as described previously for brain specimens.29 Intestinal OEA was determined using isotope-dilution, liquid chromatography-mass spectrometry.30
Glucose Tolerance Test
During oral glucose tolerance tests (OGTT), all mice were fasted 4 to 5 hours prior to an oral gavage of 20% dextrose at a dose of 2 g/kg body weight. Blood was sampled via the lateral tail vein and assessed for glucose concentration using a handheld AccuChek glucometer prior to gavage (time 0), and 15, 30, 45, 60, and 120 minutes after gavage.
Lipid Measurements
Hepatic and plasma triglyceride and cholesterol levels were either analyzed at the Mouse Metabolic Phenotyping Center at the University of Cincinnati or by colorimetric assays using Triglycerides and Cholesterol Reagent Set (Pointe Scientific, Canton, MI). Blood collection for plasma lipid analysis was performed via tail nick 2 hours after feeding and again after 20 hours of fasting within the home cage at standard room temperature in the GPR119KO and PPARαKO mouse cohorts or thermoneutrality (30°C) for the CD36KO mouse cohort, as prolonged fasting may lead to hypothermia in CD36KO mice.31
qRT-PCR Gene Expression
Intestinal tissue was homogenized in Trizol reagent, RNA was extracted using the TaqMan Reverse Transcription kit (Thermo Fisher Scientific, Inc, Waltham, MA), cDNA was isolated (iScript cDNA synthesis kit, BioRad, Hercules, CA), and real-time quantitative PCR was performed using a TaqMan 7900 Sequence Detection System with TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assays (all from Applied Biosystems, Foster City, CA) mRNA expression was evaluated by the SYBR Green (PPARα, GPR119, and CD36) real-time kinetic PCR and normalized to the ribosomal RPL32 gene (Thermo Fisher, #4331182).
Macronutrient Preference Test
Three pure macronutrient diets (TD.02521 [carbohydrate], TD. 02522 [fat], and TD02523 [protein], Harlan Teklad, Indianapolis, IN) were simultaneously presented in separate containers to allow singly-housed mice to self-select the macronutrient content of their diet for 5 testing days after 3 acclimation days. Each macronutrient container was weighed at the same time daily to determine 24-hour intake. Mice were removed from analysis if nutrients were unable to be accurately measured due to excessive nutrient spillage. Data are expressed as % macronutrient of total kilocalories (kcal) intake (mean ± SEM) over the 5-day period of testing or as percentage average daily intake in kcals.
Plasma Assays
Blood obtained via tail nick was collected 10 minutes after a mixed meal gavage of Ensure Plus with 25% dextrose (200 uL) in heparinized microvette tubes with a mixture of heparin, EDTA, aprotinin, and dipeptidyl peptidase IV inhibitor (Millipore, DPP4–010) and subsequently assayed for total GLP-1 (Sandwich ELISA; Mesoscale Discovery, Rockville, MD).
Statistical Analysis
The data were analyzed using mixed-model ANOVAs with a Tukey post-hoc analysis where appropriate, unless otherwise stated. Statistical significance was set at P < 0.05. Data are presented as mean ± SEM. Statistical main effect outcomes are indicated with letters and statistical interactions are represented by significance indicators within figures. Throughout the manuscript the letter “a” will signify a main effect of surgery and the letter “b” will signify a main effect of genotype.
RESULTS
VSG Decreases Fat Mass in PPARαKO and GPR119KO Mice
To determine the effect of VSG on OEA levels, WT mice and rats were fed a HFD and underwent either sham surgery or VSG prior to quantification of intestinal OEA content. VSG-treated mice and rats showed significantly higher OEA levels within the duodenum, but not in the distal jejunum or ileum, compared with respective sham controls (Fig. 1 A, B). VSG-treated WT mice also showed significantly higher expression of duodenal GPR119 and CD36, but similar expression of duodenal PPARα mRNA compared with sham controls (Fig. 1C).
FIGURE 1.
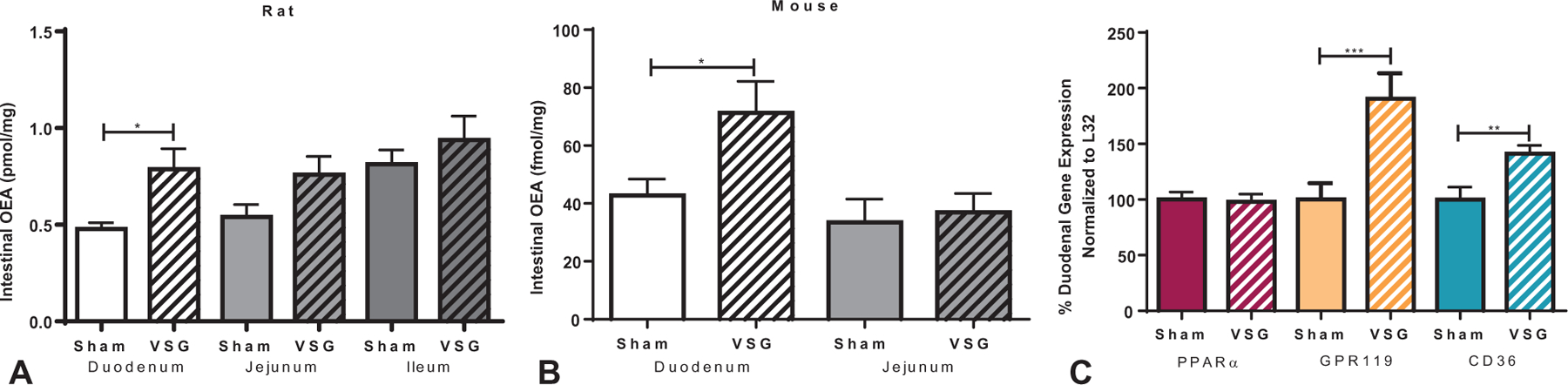
OEA levels and receptor expression in rodent intestine after VSG. Duodenal OEA was increased in WT high-fat diet fed A, rats and B, mice after VSG compared with sham-surgery control mice after ad lib feeding. No such increase was seen within the jejunum of mice and rats or the ileum of rats. C, WT mouse duodenal mRNA expression of OEA receptor targets showed an upregulation of GPR119 and CD36 after VSG compared with sham surgery counterparts. No change in WT PPARα mRNA expression was seen after VSG. (Student t test; *P < 0.05; **P < 0.01; ***P < 0.001).
We then performed VSG or sham surgery on PPARαKO and GPR119KO mice, to determine the role of these receptors in postoperative metabolic improvements. As previously described,32 PPARαKO mice had significantly more body mass compared with WT controls after 12 weeks on a 40% HFD (Supplemental Figure 1A, http://links.lww.com/SLA/B540), due to increased fat mass (Supplemental Figure 1B, http://links.lww.com/SLA/B540). In contrast, GPR119KO mice had similar body mass and composition profiles as WT control mice 9 weeks after 60% HFD exposure (Supplemental Figure 1C–D, http://links.lww.com/SLA/B540).
VSG-treated PPARαKO, GPR119KO, and WT mice had significantly lower body mass compared with genotype-matched sham controls (Fig. 2A, C; Supplemental Figure 2A, C, http://links.lww.com/SLA/B540). When calculated as a percentage of baseline, postoperative weight loss was greater in PPARαKO mice compared with WT mice (Fig. 2A), due to increased preoperative body mass in PPARαKO mice (Supplemental Figure 1A, http://links.lww.com/SLA/B540). The pre- to postoperative change in fat mass was significantly less in the VSG-treated groups compared with genotype-matched sham controls in both PPARαKO and GPR119KO cohorts (Fig. 2B, D). However, a slight decrease in lean mass was noted after VSG regardless of genotype in the GPR119KO cohort (Supplemental Figure 2D, http://links.lww.com/SLA/B540). Food intake after surgery was decreased in all VSG-treated groups 1 to 3 week after surgery, but then matched sham-operated levels for the remainder of the study (data not shown) in line with our previous work.33,34
FIGURE 2.
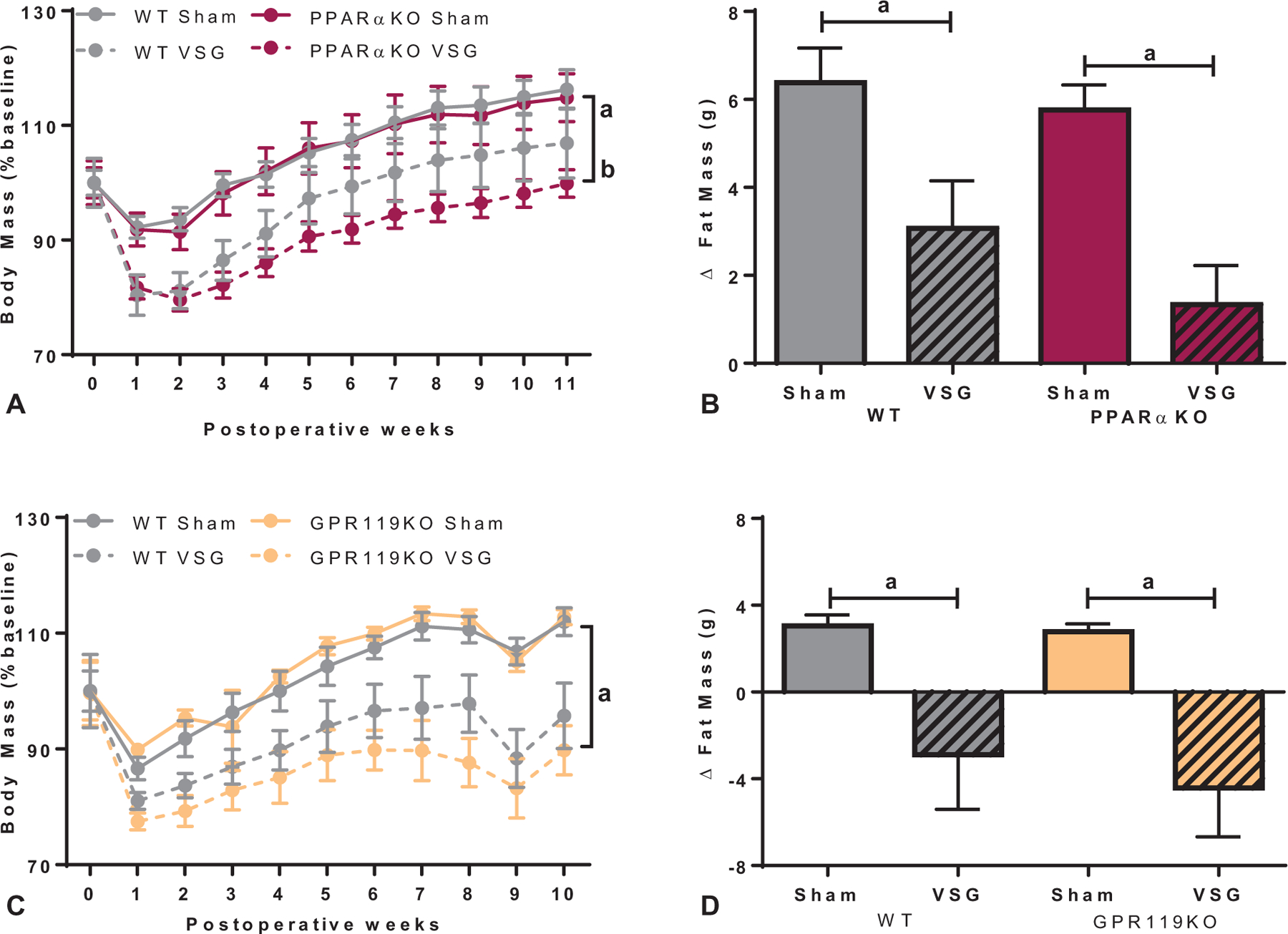
Ablation of PPARα and GPR119 receptors did not impact body weight loss after VSG. A, Body mass, expressed as percent of baseline, after VSG decreased throughout the 11 postoperative weeks in WT and to a greater extent in PPARαKO mice compared with respective sham controls (aP < 0.05, main effect of surgery; bP < 0.05, main effect of genotype). B, The change in fat mass from presurgical to 11 weeks after surgery was reduced in VSG groups of both WT and PPARαKO mice compared with respective sham controls (aP < 0.001, main effect of surgery). C, VSG led to decreased body mass, expressed as percent of baseline, in GPR119KO and WT mice compared with respective sham controls throughout 10 weeks postoperatively (aP < 0.0001, time × surgery interaction). D, A similar decrease in fat mass after VSG was seen in GPR119KO and WT mice compared with respective sham controls from presurgical to 9 weeks after surgery (aP < 0.001, main effect of surgery).
PPARα and GPR119 Signaling Is Not Required for Improved Glucose Homeostasis After VSG
PPARαKO mice had lower ad lib fed glucose levels compared with sham-operated WT mice, which was not further improved after VSG (Fig. 3A). During an OGTT, PPARαKO mice had overall lower basal glucose levels that more rapidly returned to baseline compared with WT mice (Fig. 3B). VSG-treated WT and PPARαKO mice showed improved glucose excursion at the 30- and 45-minute time points compared with respective sham control mice (Fig. 3B). VSG also lowered ad lib fed glucose levels in WT and GPR119KO mice (Fig. 3C), and improved the glucose excursion at the15- and 30-minute time points during an OGTT (Fig. 3D) compared with sham controls.
FIGURE 3.
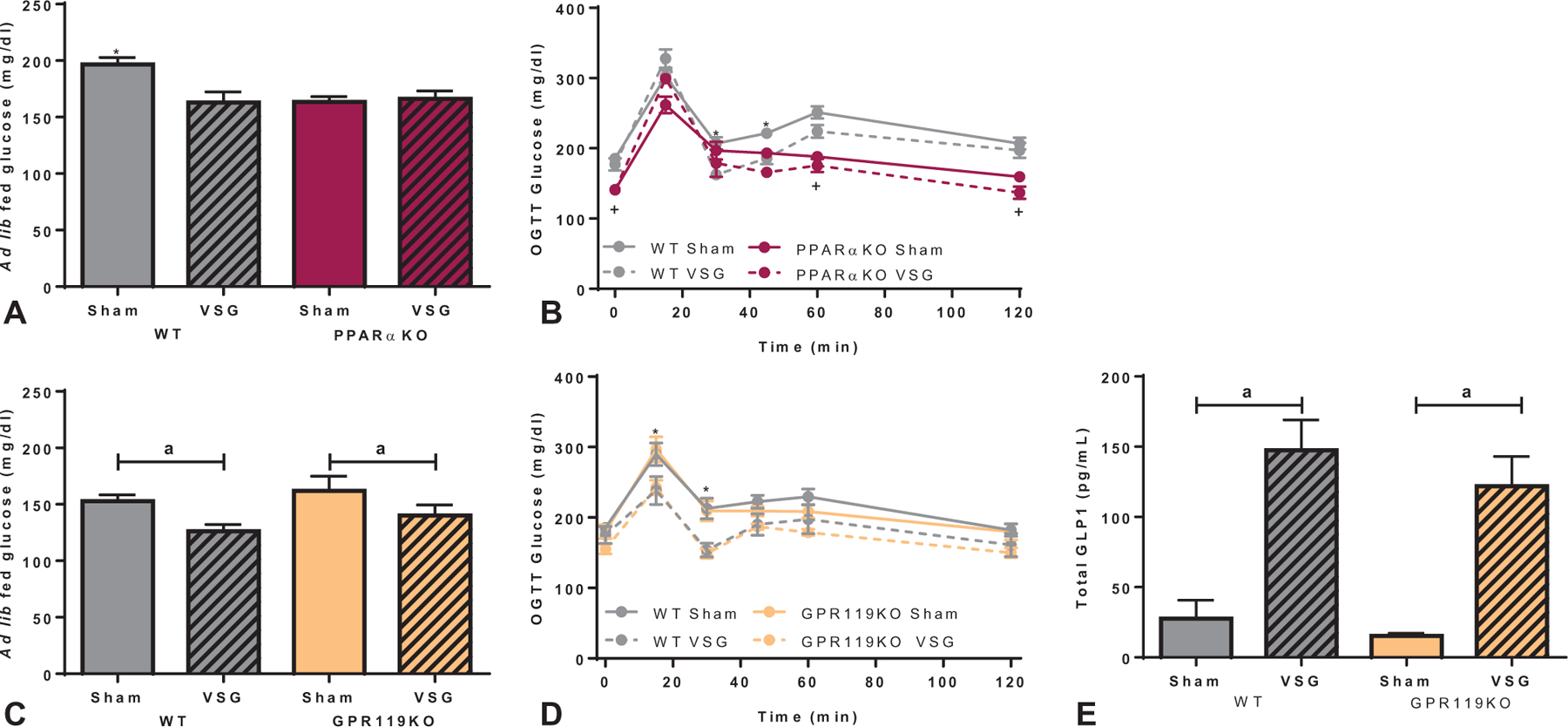
Glucose regulation through PPARα and GPR119 signaling after VSG. A, Ad lib fed glucose levels were decreased after VSG in the WT group but no change was seen after VSG in PPARαKO mice. PPARαKO mice had overall lowered ad lib fed glucose levels compared with WT sham mice (*P < 0.05 compared with all other groups). B, Regardless of surgical intervention, PPARαKO mice had lower fasted glucose levels at baseline, 60-, and 120-min time points compared with WT mice after an oral glucose (2 g/kg) challenge (+P < 0.05, time × genotype interaction). VSG improved glucose tolerance in both genotypes at the 30- and 45-minutes time points (*P < 0.05, time × surgery interaction). C, Both WT and GPR119KO mice decreased ad lib fed glucose levels after VSG compared with sham-operated controls (aP < 0.05, main effect of surgery). D, After an oral glucose load, VSG improved glucose tolerance at the 15- and 30-minute time points in both WT and GPR119KO mice (*P < 0.05, time × surgery interaction). E, After a mixed meal gavage (EnsurePlus with 25% glucose; 200 µL), total plasma GLP-1 increased in both WT and GPR119KO groups after VSG compared with respective sham controls (aP < 0.001, main effect of surgery).
As GPR119 activation increases GLP-1 levels,26 we assessed GLP-1 levels after a mixed meal oral gavage. Circulating GLP-1 levels were similarly increased in VSG-treated WT and GPR119KO mice compared with respective sham controls (Fig. 3E).
VSG Normalizes PPARαKO Mouse Plasma Lipid Levels
Postprandial and fasted plasma lipid levels were assessed approximately 7-weeks after surgery. Postprandial plasma triglyceride levels were lower in PPARαKO mice after VSG compared with sham-operated controls, but fasting levels were unchanged between surgery groups (Fig. 4A). Additionally, PPARαKO mice had overall higher fasting triglyceride levels compared with WT mice, regardless of surgical intervention. Postprandial plasma cholesterol levels were elevated in PPARαKO mice compared with WT mice (Fig. 4B), while VSG-treated mice of both genotypes had lower plasma cholesterol levels compared to respective sham controls in both dietary states (Fig. 4B). VSG decreased hepatic triglyceride and cholesterol levels equally in WT and PPARαKO mice compared with respective sham controls (Fig. 4C, D). No difference in fed or fasted plasma triglyceride levels was detected between surgical groups or genotypes within the GIPR119KO cohort (Fig. 4E). However, postprandial circulating cholesterol levels were decreased in VSG-treated groups compared with sham controls (Fig. 4F). GPR119KO mice, like their WT counterparts, showed a significant decrease in hepatic triglycerides after VSG (Fig. 4G). There were no significant changes in hepatic cholesterol levels in WT or GPR119KO mice after VSG (Fig. 4H).
FIGURE 4.
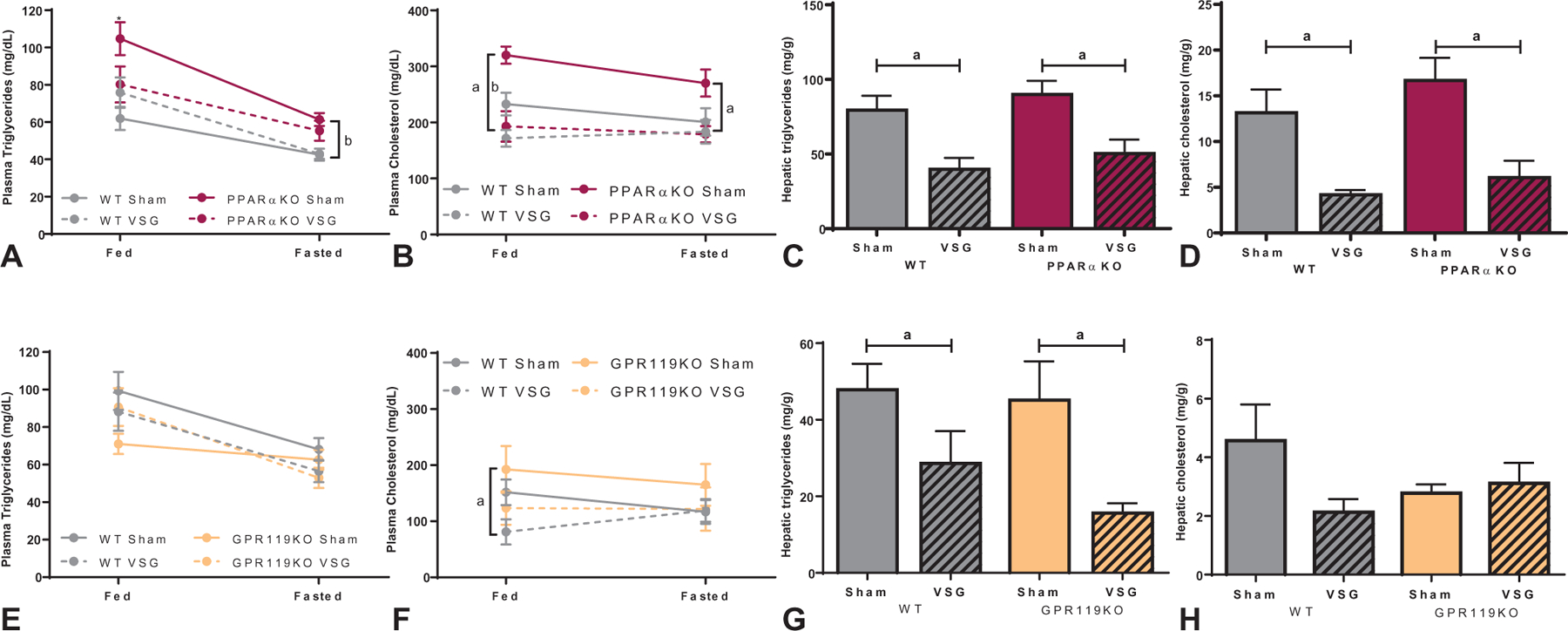
Lipid responses in PPARαKO and GPR119KO mice after VSG. A, Plasma triglyceride levels in sham-operated PPARαKO mice were significantly higher than all other groups in the fed state (fed: *P < 0.05), while under fasting conditions PPARαKO mice showed increased triglyceride levels compared with WT mice regardless of surgery (fasted: bP < 0.01, main effect of genotype). B, Postprandial plasma cholesterol levels were elevated in PPARαKO mice compared to WT mice but decreased after VSG in both genotypes (fed: bP < 0.01, main effect of genotype; aP < 0.0001, main effect of surgery). VSG decreased fasted plasma cholesterol levels in both genotypes (fasted: aP < 0.05, main effect of surgery). C, Hepatic triglyceride (aP < 0.001, main effect of surgery) and D, hepatic cholesterol (aP < 0.0001, main effect of surgery) levels were significantly decreased in both WT and PPARαKO mice after VSG compared with respective sham controls. E, No statistical change in plasma triglyceride levels was identified between genotypes or surgical groups in either dietary condition within the GPR119KO cohort. F, Neither genotype nor surgery impacted plasma cholesterol levels after fasting, however, VSG decreased fed levels of plasma cholesterol in WT and GPR119KO mice (fed: aP < 0.05, main effect of surgery). G, Hepatic triglyceride levels in GPR119KO and WT mice decreased after VSG compared with respective sham controls (aP < 0.001, main effect of surgery). H, Hepatic cholesterol levels were statistically unchanged after VSG and between genotypes within the GPR119KO cohort.
VSG-induced Changes in Food Preference Are Not Replicated in PPARαKO Mice
Next, macronutrient preference for fat, carbohydrate, and protein was assessed over a 5-day period. As we and others have previously shown, VSG in WT mice shifts macronutrient preference away from fat and towards carbohydrate consumption.4,33 However, VSG induced no significant change in macronutrient preference in PPARαKO mice. Interestingly, PPARαKO mice had overall higher preference for carbohydrates and less preference for fat compared with WT mice, while PPARαKO mice showed similar macronutrient preferences between surgical groups (Fig. 5A, B). Although total food intake is typically constant during macronutrient preference tests,4,33,35 sham-operated WT mice had increased total nutrient intake compared with sham-operated PPARαKO mice (Fig. 5C). Conversely, VSG-treated WT and GPR119KO mice had lower fat intake (Fig. 5D) and higher carbohydrate intake (Fig. 5E) compared with respective sham-controls. The GPR119KO mouse cohort exhibited no differences in total food intake (Fig. 5F). Additionally, protein intake was comparable between surgical groups and genotypes in both mouse cohorts (data not shown).
FIGURE 5.
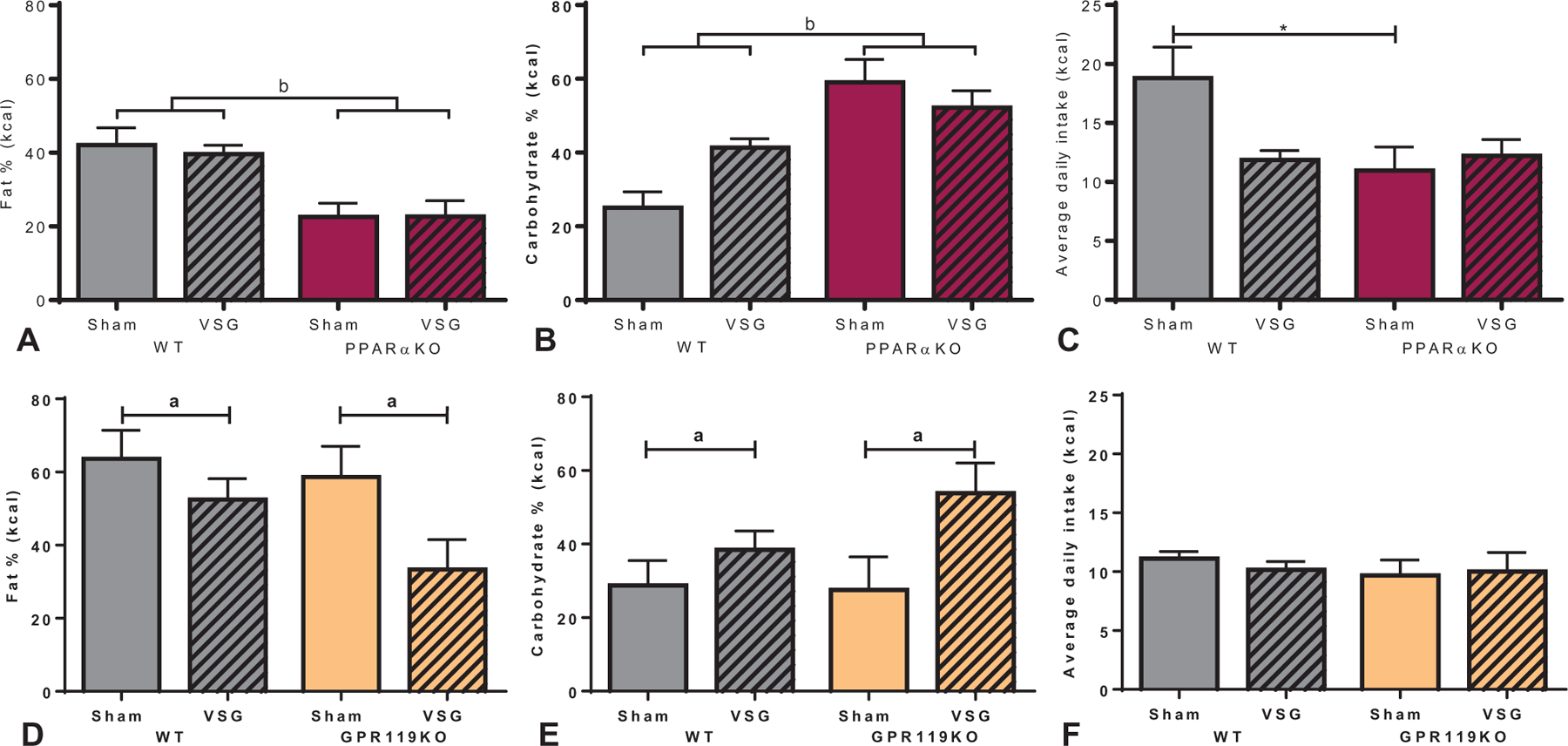
Macronutrient preference test in PPARaKO and GPR119KO mice after VSG. A, Although macronutrient preference for fat was unchanged after VSG in WT and PPARαKO mice, an overall decrease in fat preference was observed in PPARαKO mice (bP < 0.01, main effect of genotype). B, Similarly, carbohydrate preference was unchanged after VSG in WT and PPARαKO mice, but an overall increase in carbohydrate preference was observed in PPARαKO mice (bP < 0.001, main effect of genotype). C, Average daily kilocalorie (kcal) intake was increased in sham-operated WT mice compared with sham-operated PPARαKO mice (*P < 0.05). D, Fat intake was decreased (aP < 0.05, main effect of surgery) and E, carbohydrate intake was increased (aP < 0.05, main effect of surgery) in both WT and GPR119KO mice after VSG compared with respective sham surgery counterparts. F, No differences in overall daily kilocalorie intake was detected between surgery or genotype in the GPR119KO mouse cohort. Data are expressed as % macronutrient of total kcal intake (mean ± SEM) over a 5-day period of testing.
VSG Decreased Body Mass and Improved Glucose Regulation in CD36KO Mice
As PPARαKO and GPR119KO mice showed similar metabolic changes as WT mice after VSG, these receptors may not be necessary for postoperative metabolic improvements. Therefore, postoperative increases in OEA are either a noncontributing byproduct of surgical intervention or operate through alternative signaling pathways. To test the latter possibility, we targeted CD36, an upstream regulator of OEA. After 9 weeks of 60% HFD feeding, CD36KO mice gained less body weight, lean mass, and fat mass than WT mice (Supplemental Figure 1E–F, http://links.lww.com/SLA/B540). Both CD36KO mice and WT mice had overall reduced absolute body mass and expressed as percent of baseline after VSG (Supplemental Figure 2E, http://links.lww.com/SLA/B540; Figure 6A). CD36KO mice showed an initial decrease in body mass within the first 3 weeks, before surgical groups showed similar body weight throughout the remainder of the study. Although CD36KO mice had less overall fat and lean mass (Supplemental Figure 2F, http://links.lww.com/SLA/B540), VSG decreased fat mass from preoperative to 8 weeks postsurgery in WT and CD36KO mice compared with respective sham controls (Fig. 6B).
FIGURE 6.
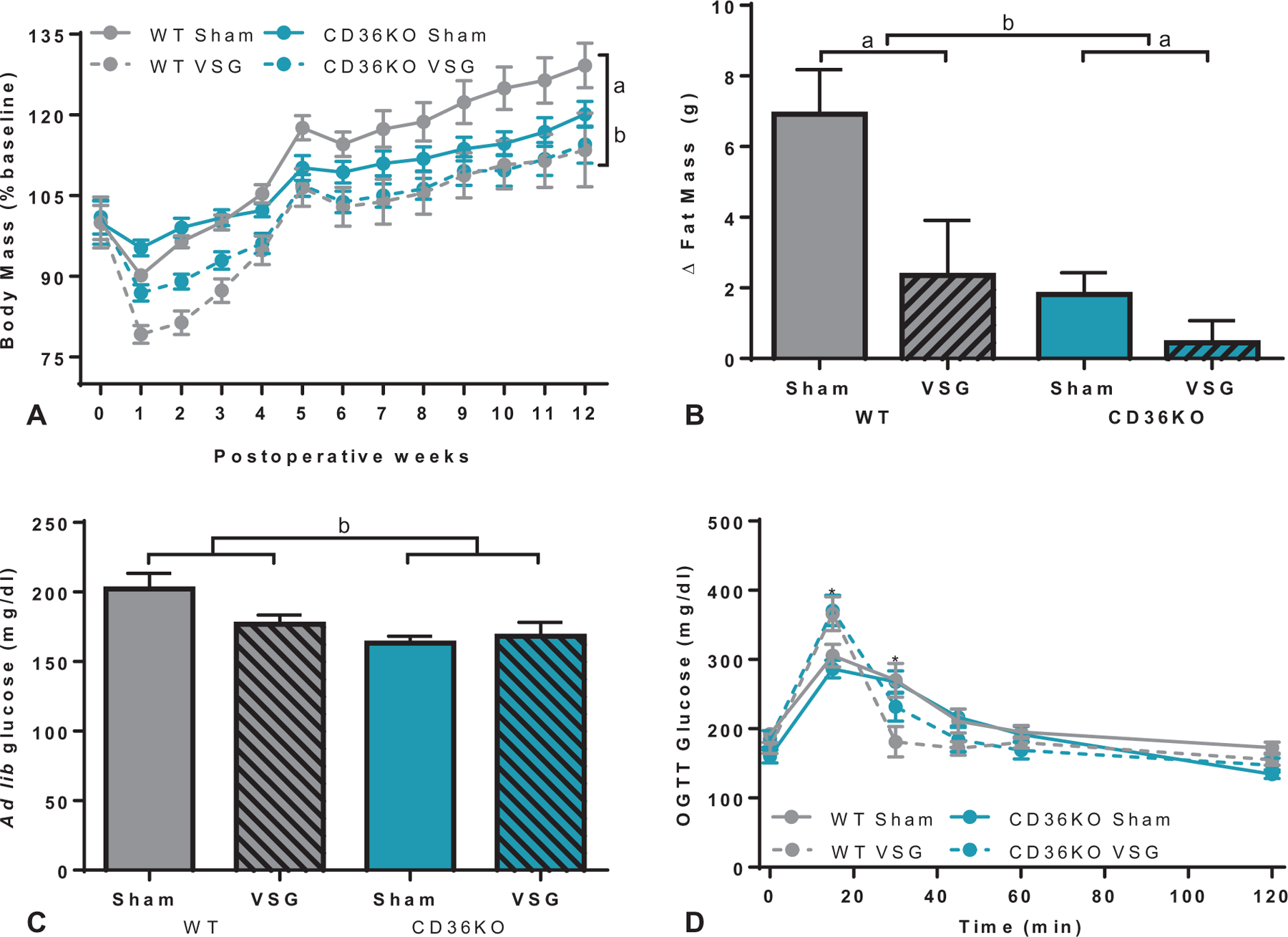
Body mass and glucose regulation after VSG in CD36KO mice. A, VSG decreased body mass (% baseline) in both WT and CD36KO up to 12 weeks postoperatively (aP < 0.01, time × surgery interaction), and CD36KO mice had overall lower body mass compared with WT mice (bP < 0.0001, time × genotype interaction). B, The change in fat mass between preoperative levels and 8 weeks after surgery revealed both WT and CD36KO lost fat-specific mass compared with respective sham controls (aP < 0.01, main effect of surgery) and that CD36KO mice had lower fat mass compared with WT mice (bP < 0.01, main effect of genotype). C, CD36KO mice had decreased ad lib fed glucose levels 2 weeks after surgery compared with WT mice (bP < 0.05, main effect of genotype). D, VSG increased the glucose response 15 minutes after an oral glucose load (2 g/kg) compared with sham-operated mice (*P < 0.001, time×surgery interaction), and improved glucose tolerance at the 30-minute time point (*P < 0.01, time×surgery interaction).
Given lower overall body mass, CD36KO mice had expectedly lower ad lib fed glucose levels compared with WT mice (Fig. 6C). During an oral glucose tolerance test, VSG-treated WT and CD36KO mice had higher peak glucose levels 15 minutes after oral gavage, but more rapid return to baseline glucose levels (within 30 min) compared with sham-operated control mice (Fig. 6D). The higher glucose values 15 minutes postoral gavage corresponds with the increased gastric emptying rate associated with VSG.36
VSG Normalizes CD36KO Hepatic Triglyceride Levels
Plasma triglyceride levels in the CD36KO mouse cohort did not significantly change with respect to surgery or genotype after either an overnight fast or 2 hours after feeding (Fig. 7A). Postprandial and fasted plasma cholesterol levels were lower in VSG-treated groups compared with shame controls in both WT and CD36KO mice (Fig. 7B). CD36KO mice had elevated hepatic triglyceride levels compared with WT controls (Fig. 7C), despite significantly decreased lean and fat mass throughout the duration of the study (Supplemental Figure 2F, http://links.lww.com/SLA/B540). However, VSG lowered hepatic triglyceride levels in CD36KO mice alongside reducing levels in WT mice (Fig. 7C). Hepatic cholesterol levels were not impacted by removal of CD36-mediated signaling or after VSG (Fig. 7D).
FIGURE 7.
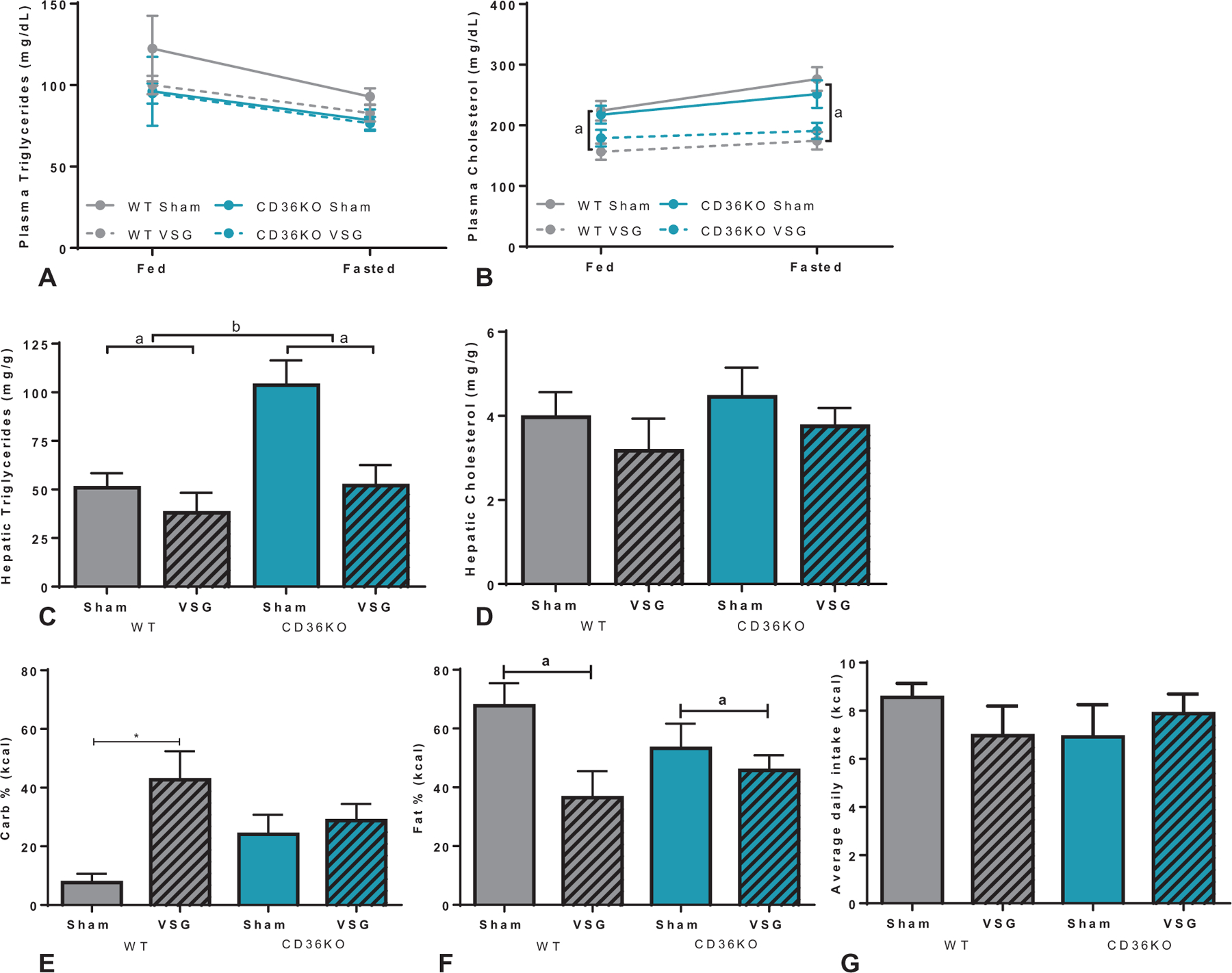
Lipid responses and macronutrient preference after VSG in CD36KO mice. A, Circulating plasma triglyceride levels were unchanged between genotype or surgery in both the fed and fasted state. B, Plasma cholesterol levels in both WTand CD36KO were improved with VSG after feeding (fed: aP < 0.001, main effect of surgery), and fasting (fasted: aP < 0.0001, main effect of surgery), without differences between genotypes. C, Hepatic triglycerides were elevated in CD36KO mice compared with WT mice but were reduced after VSG in both genotypes (aP < 0.01, main effect of surgery; bP < 0.01, main effect of genotype). D, Hepatic cholesterol levels were unchanged between genotype or surgical groups. E, During a macronutrient preference test, WT mice increased carbohydrate intake after VSG, while CD36KO mice show no effect of surgery on carbohydrate intake (*P < 0.05). F, Both WT and CD36KO mice decrease preference for fat intake after VSG (aP < 0.05, main effect of surgery). G, No change in average daily caloric intake was noted between either genotype or surgical intervention.
VSG does not induce a food preference shift in CD36KO mice.
Macronutrient preference testing was performed to determine the role of CD36 signaling in surgery-induced shifts in nutrient preference. While VSG-operated WT mice had higher preference for carbohydrates compared with sham mice, CD36KO mice showed similar carbohydrate preferences regardless of surgical intervention (Fig. 7E). CD36KO mice after VSG showed moderately lower fat preference (−18%), while WT mice after VSG showed drastically lower fat preference (−55%) compared with respective sham controls (Fig. 7F). No differences in protein intake (data not shown) or average daily kilocalorie intake were detected between genotypes or surgical interventions (Fig. 7G).
DISCUSSION
OEA is an endocannabinoid-like lipid signaling molecule that can trigger physiological effects similar to those seen after bariatric surgery; including decreased food intake, reduced fat mass, increased GLP-1 release, and reduced serum and hepatic lipids. Due to the hypophagic actions of OEA, it has been hypothesized that decreased OEA may lead to obesity through impaired satiety signaling pathways. In fact, chronic consumption of fat-rich diets reduces OEA production.19,20,37,38 Although studies report variable relationships between OEA levels and obesity and impaired glucose homeostasis,39–42 many of these studies measured total circulating OEA. Since fats are absorbed in the proximal small intestine, duodenal, and jejunal OEA levels may better represent OEA turnover. In support of this, diet-induced obese rats have significantly lower levels of jejunal OEA than lean rats.20 Additionally, intestinal OEA is increased following RYGB in rats,21 and our data show similar findings in the duodenum of mice after VSG. To determine whether these differences in OEA were necessary for the metabolic success of surgery, we performed VSG in 3 mouse models lacking the expression of key proteins involved in OEA signaling and quantified body weight and lipid and glucose metabolic endpoints.
Here, we find that VSG induces similar changes in body mass and composition across genotypes; with lower overall body mass and fat content in VSG-treated WT, PPARαKO, and GPR119KO mice and lower fat content in VSG-treated CD36KO mice compared with sham control mice. VSG improved glucose handling in all KO mouse models compared with respective sham controls, with similar increases in postprandial GLP-1 in VSG-treated mice regardless of GPR119 expression. Plasma lipid levels were notably lower after VSG in WT and PPARαKO mice compared with sham controls, while hepatic triglyceride levels were lower in VSG-treated WT and CD36KO mice compared with sham controls. Interestingly, macronutrient preference for carbohydrates did not vary between surgical interventions in PPARαKO or CD36KO mice.
Previous data suggest that increased OEA signaling through PPARα can lead to improvements in lipid and glucose homeostasis.43 Conversely, we found that PPARαKO mice had overall improved glucose metabolism evidenced by reduced ad lib fed and fasting glucose levels and improved glucose tolerance during an oral glucose tolerance test. Unlike WT mice where glucose metabolism was greatly improved after VSG, glucose metabolism was only moderately improved by VSG in PPARαKO mice. However, VSG did normalize plasma lipids, suggesting that VSG works through PPARα-independent pathways to mediate changes in lipid metabolism. One possibility is by reduced intestinal chylomicron production, which we have previously seen after VSG.44 Although we were unable to assess chylomicron production here, samples were taken in the fed state where the predominant source of triglycerides would be intestinal chylomicron production.
OEA-induced activation of enteroendocrine GPR119 receptors contributes to GLP-1 and insulin secretion.25–27 However, our data show increased GLP-1 in VSG-treated GPR119KO mice, indicating that increased duodenal OEA production and upregulation of GPR119 expression observed after VSG in WT mice is not responsible for associated increases in postsurgical GLP-1 levels. In fact, our data generally indicate that GPR119, presumably via OEA signaling, is not necessary for VSG-induced improvements in glucose homeostasis or GLP-1 release.
Activation of an OEA-PPARα gut-brain axis has been linked to the beneficial effects of RYGB on fat intake and macronutrient preference.21 While PPARαKO mice maintained on a HFD had reduced fat preference and increased carbohydrate preference compared with WT mice, this was not further influenced by VSG. In contrast, VSG significantly decreased fat and increased carbohydrate consumption in both GPR119KO and WT mice. Regardless of changes in macronutrient preference, all groups within the PPARαKO and GPR19KO cohorts showed comparable postoperative body weight loss. Similarly, Hankir et al21 found that vagotomy blunted the RYGB-induced shift in macronutrient preference without impacting postsurgical weight loss. These data suggest that changes in food preference are not the key driver of weight loss after bariatric surgery. Although VSG and RYGB have similar postoperative outcomes, these procedures differ anatomically and physiologically with variations in weight loss and T2DM remission.45–47 Specifically, RYGB induces drastic intestinal rearrangement, which does not occur after VSG. Despite this difference, VSG and RYGB both increase OEA levels within the portion of the intestine directly connected to the stomach, either the duodenum in VSG or Roux limb in RYGB.21 A direct comparison of RYGB and VSG will be necessary to determine if OEA acts through PPARα after RYGB but not VSG.
The cell-surface enterocyte protein CD36 functions as a biosensor for dietary fats and can influence the activity of OEA catabolic enzymes among several other biological processes.48,49 Given the dual role of CD36 to translocate OEA across the membrane and as a target of OEA signaling, we utilized a CD36KO mouse as an upstream mediator of OEA synthesis.13 CD36 is also a known taste receptor for unsaturated fatty acids in the mouse oral cavity, therefore, CD36KO mice are known to be resistant to diet-induced obesity,50,51 which we also noted despite maintaining mice on a 60% lard-based HFD. However, both CD36KO and WT mice decreased body weight and fat mass after VSG, suggesting this was not a limiting factor.
A significant postsurgical reduction in hepatic triglyceride levels was noted in VSG-treated CD36KO mice despite both surgical groups having nearly identical total body weight at the time of tissue collection, which is suggestive of long-acting weight-independent effects of VSG on CD36KO mice. Similar to PPARαKO mice, a dissociation existed between circulating and hepatic triglyceride levels, but in either case VSG is able to normalize lipid levels. While the role of OEA remains unclear, this suggests that VSG is able to correct impaired plasma or hepatic lipid metabolism induced by deficient PPARα- and CD36-mediated signaling, respectively. Previous studies show that OEA-mediated suppression of food intake depends on the presence of CD36.13 Interestingly, we found no significant differences between WT and CD36KO mice with respect to fat intake during the macronutrient preference test. This is surprising as previous work has found that CD36KO mice have reduced fatty acid preference using a lick test with increasing concentrations of fatty acids.52 It is unclear whether different protocols (lick test vs. macronutrient preference) or different types of fat may have influenced our results. Interestingly, we found that VSG did not alter carbohydrate preference in CD36KO as it does in WT mice.
In conclusion, key molecular targets in OEA synthesis (CD36) or signaling (PPARα and GPR119) are not necessary for the metabolic improvements after VSG. Although 1 limitation of these mouse models is that each involves a congenital deletion, which can introduce developmental and/or compensatory physiological changes. Notably, we found that VSG was able to correct the increase in plasma and hepatic triglycerides in the PPARαKO and CD36KO mice, respectively. However, these data also dissociate the VSG-induced shift in macronutrient preference from weight loss. In the search for more effective and less invasive obesity therapies to help reverse the global acceleration of obesity and metabolic disease OEA is a promising candidate, however, our data indicate that it is not an underlying mechanism of the effectiveness of VSG.
Supplementary Material
ACKNOWLEDGMENTS
The authors specially thank Kyle Leix and Jack Magrisso for their technical expertise on performing lipid assays and genotyping. The authors greatly thank Cecilia Hillard (Medical College of Wisconsin) for her expertise in advising and performing the OEA measurements within the mouse intestine. The Mouse Metabolic Phenotyping Center (MMPC, Cincinnati, OH) provided both tissue and serum lipid analysis.
This work is supported in part by NIH Awards, DK082480 (DAS). CRH was supported by T32 DK101357, a multidisciplinary training program in basic diabetes research. The investigators also received research support from Ethicon Endo-Surgery Inc. (DAS), Zafgen (DAS), and Novo Nordisk A/S (DAS). DAS has been a paid speaker for Novo Nordisk.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016 Key findings Data from the National Health and Nutrition Examination Survey. Available at: https://www.cdc.gov/nchs/data/databriefs/db288.pdf 2015. Accessed November 10, 2017.
- 2.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faulconbridge LF, Ruparel K, Loughead J, et al. Changes in neural responsivity to highly palatable foods following roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: an fMRI study. Obesity. 2016;24:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson-Pérez HE, Chambers AP, Sandoval DA, et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes. 2013;37:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grayson BE, Gutierrez-Aguilar R, Sorrell JE, et al. Bariatric surgery emphasizes biological sex differences in rodent hepatic lipid handling. Biol Sex Differ. 2017;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal S, Agarwal D, Kanojiya R, et al. Effect of laparoscopic sleeve gastrectomy on lipid profile of obese patients in complete nine month follow up. Int Surg J. 2016;3:42–46. [Google Scholar]
- 7.Strain GW, Saif T, Ebel F, et al. Lipid profile changes in the severely obese after laparoscopic sleeve gastrectomy (LSG), 1, 3, and 5 years after surgery. Obes Surg. 2015;25:285–289. [DOI] [PubMed] [Google Scholar]
- 8.Shah SS, Todkar JS, Shah PS, et al. Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35kg/m2. Surg Obes Relat Dis. 2010;6:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basso N, Capoccia D, Rizzello M, et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc. 2011;25:3540–3550. [DOI] [PubMed] [Google Scholar]
- 10.Fu J, Astarita G, Gaetani S, et al. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez de Fonseca F, Navarro M, Gómez R, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. [DOI] [PubMed] [Google Scholar]
- 12.Petersen G, Sørensen C, Schmid PC, et al. Intestinal levels of anandamide and oleoylethanolamide in food-deprived rats are regulated through their precursors. Biochim Biophys Acta. 2006;1761:143–150. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Fu J, Astarita G, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guijarro A, Fu J, Astarita G, et al. CD36 gene deletion decreases oleoylethanolamide levels in small intestine of free-feeding mice. Pharmacol Res. 2010;61:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaetani S, Oveisi F, Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology. 2003;28:1311–1316. [DOI] [PubMed] [Google Scholar]
- 16.Overton HA, Babbs AJ, Doel SM, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen MJ, Petersen G, Astrup A, et al. Food intake is inhibited by oral oleoylethanolamide. J Lipid Res. 2004;45:1027–1029. [DOI] [PubMed] [Google Scholar]
- 18.Hansen HS, Artmann A. Endocannabinoids and nutrition. J Neuroendocrinol. 2008;20(suppl 1):94–99. [DOI] [PubMed] [Google Scholar]
- 19.Diep TA, Madsen AN, Holst B, et al. Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J. 2011;25:765–774. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi M, DiPatrizio NV, Narayanaswami V, et al. Feeding-induced oleoylethanolamide mobilization is disrupted in the gut of diet-induced obese rodents. Biochim Biophys Acta. 2015;1851:1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankir MK, Seyfried F, Hintschich CA, et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25:335–344. [DOI] [PubMed] [Google Scholar]
- 22.Fu J, Gaetani S, Oveisi F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 2003;425:90–93. [DOI] [PubMed] [Google Scholar]
- 23.Tellez LA, Medina S, Han W, et al. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science (80-). 2013;341:800–802. [DOI] [PubMed] [Google Scholar]
- 24.Guzmán M, Lo Verme J, Fu J, et al. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α). J Biol Chem. 2004;279:27849–27854. [DOI] [PubMed] [Google Scholar]
- 25.Chu Z-L, Carroll C, Alfonso J, et al. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology. 2008;149:2038–2047. [DOI] [PubMed] [Google Scholar]
- 26.Lan H, Vassileva G, Corona A, et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol. 2009;201:219–230. [DOI] [PubMed] [Google Scholar]
- 27.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu Z-L, Jones RM, He H, et al. A role for β-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–2609. [DOI] [PubMed] [Google Scholar]
- 29.Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. [DOI] [PubMed] [Google Scholar]
- 30.Patel S, Carrier EJ, Ho W-SV, et al. The postmortal accumulation of brain N - arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. [DOI] [PubMed] [Google Scholar]
- 31.Putri M, Syamsunarno MRAA, Iso T, et al. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem Biophys Res Commun. 2015;457:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim BH, Won YS, Kim E-Y, et al. Phenotype of peroxisome proliferator-activated receptor-α(PPARa) deficient mice on mixed background fed high fat diet. J Vet Sci. 2003;4:239–244. [PubMed] [Google Scholar]
- 33.Arble DM, Sandoval DA, Turek FW, et al. Metabolic effects of bariatric surgery in mouse models of circadian disruption. Int J Obes (Lond). 2015;39:1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pressler JW, Haller A, Sorrell J, et al. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes. 2015;64: 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306:E424–E432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artmann A, Petersen G, Hellgren LI, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. [DOI] [PubMed] [Google Scholar]
- 38.Aviello G, Matias I, Capasso R, et al. Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice. J Mol Med. 2008;86:413–422. [DOI] [PubMed] [Google Scholar]
- 39.Matias I, Gatta-Cherifi B, Tabarin A, et al. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One. 2012;7:e42399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosshans M, Schwarz E, Bumb JM, et al. Oleoylethanolamide and human neural responses to food stimuli in obesity. JAMA Psychiatry. 2014;71:1254. [DOI] [PubMed] [Google Scholar]
- 41.Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes. 2007;31:692–699. [DOI] [PubMed] [Google Scholar]
- 42.Mallipedhi A, Prior SL, Dunseath G, et al. Changes in plasma levels of N-arachidonoyl ethanolamine and N-palmitoylethanolamine following bariatric surgery in morbidly obese females with impaired glucose homeostasis. J Diabetes Res. 2015;2015:680867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel S, Mace OJ, Tough IR, et al. Gastrointestinal hormonal responses on GPR119 activation in lean and diseased rodent models of type 2 diabetes. Int J Obes. 2014;38:1365–1373. [DOI] [PubMed] [Google Scholar]
- 44.Stefater MA, Sandoval DA, Chambers AP, et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141:934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Yang G, Wang W, et al. A meta-analysis: to compare the clinical results between gastric bypass and sleeve gastrectomy for the obese patients. Obes Surg. 2013;23:1001–1010. [DOI] [PubMed] [Google Scholar]
- 46.Franco JVA, Ruiz PA, Palermo M, et al. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 47.Lee W-J, Chong K, Ser K-H, et al. Gastric bypass vs sleeve gastrectomy for Type 2 diabetes mellitus. Arch Surg. 2011;146:143. [DOI] [PubMed] [Google Scholar]
- 48.Naville D, Duchampt A, Vigier M, et al. Link between intestinal CD36 ligand binding and satiety induced by a high protein diet in mice. PLoS One. 2012;7:e30686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hajri T, Hall AM, Jensen DR, et al. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2007;56:1872–1880. [DOI] [PubMed] [Google Scholar]
- 51.Cai L, Wang Z, Ji A, et al. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One. 2012;7:e36785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin C, Passilly-Degrace P, Gaillard D, et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 2011;6: e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


