Abstract
Context
Gestational diabetes (GDM) has profound effects on the intrauterine metabolic milieu and is linked to obesity and diabetes in offspring, but the mechanisms driving these effects remain largely unknown. Alterations in DNA methylation and gene expression in amniocytes exposed to GDM in utero represent a potential mechanism leading to metabolic dysfunction later in life.
Objective
To profile changes in genome-wide DNA methylation and expression in human amniocytes exposed to GDM.
Design
A nested case-control study (n = 14 pairs) was performed in amniocytes matched for offspring sex, maternal race/ethnicity, maternal age, gestational age at amniocentesis, and gestational age at birth. Sex-specific genome-wide DNA methylation analysis and RNA-sequencing were completed and differentially methylated regions (DMRs) and gene expression changes were identified. Ingenuity pathway analysis identified biologically relevant pathways enriched after GDM exposure. In silico high-throughput chromosome conformation capture (Hi-C) analysis identified potential chromatin interactions with DMRs.
Results
Expression of interferon-stimulated genes was increased in GDM amniocytes, accounting for 6 of the top 10 altered genes (q < 0.05). Enriched biological pathways in GDM amniocytes included pathways involving inflammation, the interferon response, fatty liver disease, monogenic diabetes, and atherosclerosis. Forty-two DMRs were identified in male GDM-exposed amniocytes and 20 in female amniocyte analysis (q < 0.05). Hi-C analysis identified interactions between DMRs and 11 genes with significant expression changes in male amniocytes and 9 in female amniocytes (P < .05).
Conclusion
In a unique repository of human amniocytes exposed to GDM in utero, transcriptome analysis identified enrichment of inflammation and interferon-related pathways and novel DMRs with potential distal regulatory functions.
Keywords: gestational diabetes, epigenetics, DNA methylation, interferon, immune activation, fetal programming
Gestational diabetes (GDM) has profound effects on the intrauterine metabolic milieu and is linked to diabetes and obesity in the offspring (1-5). GDM induces changes in the intrauterine environmental by inducing abnormalities in glucose homeostasis, insulin secretion, and fatty acid metabolism in the fetus (6-11). The Hyperglycemia and Adverse Pregnancy Outcomes study definitively demonstrated that maternal and neonatal outcomes were directly related to maternal glucose levels, and screening for maternal hyperglycemia is now recommended in high-risk women before 24 weeks’ gestation (12, 13). However, outcomes for pregnancies of women identified with GDM early in pregnancy are similar to those seen in preexisting diabetes, suggesting that early exposure to the metabolic abnormalities induced in GDM have significant programming effects very early in gestation (13). Epidemiologic and animal studies support the concept of a critical window of developmental programming during which in utero exposures are associated with an increased susceptibility to obesity and diabetes (14, 15) but the molecular mechanisms underlying this phenomenon are largely unknown. Alterations in epigenetic modifications, including DNA methylation and histone modifications, are potential mechanisms by which in utero exposure can lead to permanent changes in cellular function and ultimately metabolic disease later in life (16). Although a number of studies have linked GDM exposure to changes both in site-specific and genome-wide DNA methylation in placenta and cord blood in offspring, few have linked changes in DNA methylation to changes in gene expression on a genome-wide basis (17-20).
Recently, we reported that second-trimester amniotic fluid (AF) obtained from women who developed GDM later in pregnancy had profound changes in the AF metabolome, specifically in pathways involving glucose, amino acids, and fatty acid metabolism (6) preceding the GDM diagnosis in the third trimester. In the present study, we sought to determine whether GDM exposure in utero is associated with changes in the transcriptome and methylome in a unique repository of human amniocytes, given that amniocytes are pluripotent progenitor cells primarily derived from the fetus (21). We hypothesized specific alterations in gene expression and DNA methylation may reveal underlying mechanisms by which in utero exposure to GDM leads to the development of diabetes and obesity in the offspring. Therefore, we performed genome-wide DNA methylation analysis and RNA sequencing (RNA-Seq) in second-trimester human amniocytes exposed to GDM in utero, as well as conducted in silico high-throughput chromosome conformation capture (Hi-C) to determine how differentially methylated regions (DMRs) might be potential regulators of distal genes that interact in the 3-dimensional (3D) space.
Methods
Study population and sample collection
We used an established biospecimen repository containing AF and amniocytes collected from women who underwent amniocentesis between 2002 and 2006, when it was a standard procedure for pregnant women older than 35 years. Amniocytes were obtained from cytogenetic cell cultures collected for clinical cytogenetic testing at gestational ages 16 to 18 weeks from women with healthy singleton pregnancies who delivered at term (37-42 weeks’ gestation), without maternal health conditions, pregnancy complications, fetal anomalies, or exposures to maternal smoking or illicit drugs (22). Maternal and offspring demographics were obtained from reproductive genetics charts and a postbirth outcome survey. The study was approved by the Children’s Hospital of Philadelphia and the University of Pennsylvania Institutional Review Boards (IRB-13-010626). Written informed consent was obtained from individuals prior to participating in the study.
A nested case-control design was used to decrease variance between experimental groups. A participant was classified as GDM exposed if she received a diagnosis of GDM during the pregnancy as indicated on the postdelivery outcome questionnaire. Women were tested for GDM between 24 and 28 weeks’ gestation, based on standard clinical procedures. GDM status was confirmed by measurement of AF C-peptide concentration (representing fetal insulin production) via enzyme-linked immunosorbent assay (ELISA) as previously described (6, 23). Matching criteria included offspring sex, maternal race/ethnicity, maternal age, gestational age at amniocentesis, and gestational age at birth. Exclusion criteria included preterm delivery, congenital birth defects or chromosomal abnormalities, birth asphyxia, congenital infection, offspring metabolic disease, or history of prepregnancy maternal health problems (eg, pregestational diabetes, hypertension). Samples selected for RNA-Seq and genome-wide DNA methylation profiling were required to meet quality control criteria outlined below.
Statistical analysis
Two-sample t tests or Mann-Whitney tests were used to compare means of clinical variables between GDM and control groups. For quantitative polymerase chain reaction (qPCR) and ELISA studies, P < .05 was considered significant. GraphPad Prism 7.0 was used to perform statistical analyses.
RNA sequencing
Total RNA was extracted from amniocytes (Arcturus Picopure, Thermo Fisher) (total RNA A260/280 >2 and RNA integrity number >7 [Agilent Bioanalyzer]) and used to generate libraries with the Illumina TruSeq stranded total RNA kit (Illumina). RNA-Seq was performed on a HiSeq 2000 system using 50 base, single-end read sequencing to generate 50 million reads per sample. FASTQ data were processed with HiSeq-count v0.6.1 and aligned to Hg19 using STARv2.4.0 software. EdgeRv3.12.1 was used to find differentially expressed genes via log2 fold change (FC), P values, and q values after Bonferroni correction. Differentially expressed transcripts were identified in the analysis of all samples followed by sex-specific analyses. Differentially expressed gene lists were used for Ingenuity Pathway Analysis (IPA) (Ingenuity Systems) to identify pathways of biological significance. Venn diagrams were created with BioVenn (24).
Messenger RNA and protein expression analysis
RNA was extracted from amniocytes (Arcturus Picopure) and complementary DNA was generated, using Superscript IV Vilo Mastermix kit (Invitrogen). Real-time PCR was performed using TaqMan Gene expression assays in the Applied Biosystems 7900HT PCR System (Supplemental Table 1) (25). Target gene expression data were normalized to ITCH based on high abundance and minimal expression change in RNA-Seq data. Expression of interferon α (IFNα) and IFNγ were quantified in AF by ELISA (Thermo Fisher) and normalized to total AF protein concentration by bicinchoninic acid assay (Pierce).
Enhanced reduced representation bisulfite sequencing
Total DNA was extracted from amniocytes (DNeasy Mini kit, Qiagen). RNA-free DNA of molecular weight greater than 40 kb was used to prepare sequencing libraries. Enhanced reduced representation bisulfite sequencing (ERRBS) was conducted at the Epigenomics Core, Cornell Medical College, as previously described (26). ERRBS provides 2 times higher CpG coverage yielding more than 3 million CpG sites, including CpG islands, promoters, exons, introns, and intergenic regions (26). Only libraries that passed quality control thresholds were analyzed. Table 1 includes maternal and offspring characteristics of samples included in the ERRBS analysis.
Table 1.
Maternal and offspring characteristics
| All control | All GDM | Male control | Male GDM | Female control | Female GDM | |
|---|---|---|---|---|---|---|
| No. | 14 | 14 | 7 | 7 | 7 | 7 |
| Maternal age, y | 36.9 (3.0) | 36.8 (4.1) | 36.1 (2.2) | 36.3 (2.1) | 38.1 (3.6) | 37.3 (5.6) |
| Gestational age amniocentesis, wk | 15.9 (0.3) | 16 (0.6) | 16 (0) | 16.1 (0.4) | 15.9 (0.4) | 15.9 (0.7) |
| AF C-peptide, pM | 30.2 (29.9) | 237.6a (100.9) | 30.3 (29.9) | 281.3a (118.1) | 36.1 (33.2) | 187.1a (63.4) |
| Gestational age birth, wk | 39.2 (1.2) | 39.3 (0.7) | 39.3 (1.2) | 39.7 (0.5) | 39.1 (0.9) | 38.9 (0.7) |
| Birth weight, g | 3528 (358) | 3531 (461) | 3527 (358) | 3845b (323) | 3580 (361) | 3165 (295) |
Data presented as mean value (SD).
Abbreviations: AF, amniotic fluid; GDM, gestational diabetes.
a P less than .05 GDM vs control.
b P less than .05 GDM male vs GDM female.
Bioinformatics for methylation analysis
Sequence data were demultiplexed and converted to FASTQ files using the Illumina bcl2fastq software. Raw data were analyzed by a custom ERRBS pipeline (26), reporting the locations and methylation levels of all CpG sites with 10 times or more coverage. Analyses used to determine DMRs and changes in DNA methylation at individual CpGs are described as follows.
Differentially methylated regions
DMRs were identified based on Weighted-Welch Expansion using DEFIANT (DMRs: Easy, Fast, Identification and ANnoTation) (27) and characterized by 2 or more CpGs with significant methylation changes defined by an absolute change in percentage methylation of 5% or more at each CpG, a q value < 0.05, and a total read count > 10 over the entire DMR (27)
Single CpG methylation
RnBeads was used for downstream differential methylation analysis (28) to identify single CpG sites with significant changes in DNA methylation. Thresholds used to determine a significant change in DNA methylation for the single CpG analysis included absolute change of methylation of at least 7.5% or more and a q value < 0.05 (29).
Chromosomal conformation assessment via in silico high-throughput chromosome conformation capture
To determine whether DMRs represented regulatory regions for nonproximal genes, we performed in silico analysis of 3D chromosomal interactions between identified DMRs and regions of the genome with the 3D Genome browser (http://promoter.bx.psu.edu/hi-c/index.html) (30). Data from virtual 4C (circular chromosomal conformation capture) were harnessed to generate 3D interactions (± 500 kbp). Virtual 4C is a chromatin ligation-based method that measures the interaction frequencies between a bait locus of interest and any other genomic loci. The DMRs identified from ERRBS were used as bait to locate interactions within the published 4C data derived from human embryonic stem cells (H1 ESC line). The gene names of the loci interacting with the DMR were integrated with amniocyte RNA-Seq expression data.
Data and resource availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.” The full data sets generated and analyzed are available at (Supplemental Tables 1-5: https://upenn.box.com/s/k1yv7ji1rszu4gj372pgp2pdlxxr992p [25], Supplemental Tables 6-11: https://upenn.box.com/s/y0xedjsnewll4fvxnkeym392i6cxzrig [31]; RNA-Seq sequence data are available in GEO [GSE150621]).
Results
Maternal characteristics and offspring birth weight
The 28 samples selected for this study (14 GDM-exposed samples and 14 controls) were a subset of a previously published cohort (6, 23). Owing to the nested case-control study design, there were no significant differences in maternal age, gestational age at amniocentesis, gestational age at birth, or distribution of offspring sex between the samples (Table 1). The mean maternal age was greater than 35 years reflective of the participants from the biospecimen repository, because the most common indication for amniocentesis was advanced maternal age. The mean AF C-peptide concentration in samples from women who were diagnosed with GDM in the third trimester was 237.6 pM (187.1 pM in female GDM samples and 281.3 pM in male GDM samples), and this was 7.9 times greater than AF from women without GDM (P < .05), indicating that abnormal maternal glucose homeostasis was present at the time of amniocentesis. The mean birth weight for GDM-exposed offspring was 3531 g, which was not significantly different from control offspring (3528 g), likely reflecting that the women had well-controlled diabetes after diagnosis. In the sex-specific analysis, the GDM-exposed males had a higher average birth weight than GDM-exposed females (P < .05) but neither were significantly different from sex-matched controls.
In utero GDM exposure results in an enrichment of immune-related pathways in human amniocytes at the second trimester
Prior studies have linked exposure to GDM and maternal obesity in utero to the development of diabetes, obesity, and fatty liver disease in offspring, but the molecular mechanisms responsible for these programming effects have not been elucidated (1-5). To determine whether in utero exposure to GDM is associated with an altered expression profile in amniocytes, we conducted whole-transcriptome analysis via RNA-Seq to identify transcripts with significant changes in expression. Amniocytes are fetal-derived cells exhibiting stem-like properties including expression of CD117 (CKIT), POU domain transcription factor OCT4 (OCT4), and nanog homeobox (NANOG) (21). Using a nested case-control study design, we analyzed all amniocyte samples together followed by separate analyses for male and female amniocytes (Supplemental Table 2) (25), based on prior studies that identified sex-specific manifestations in fetal programming studies (20, 32-34). After Bonferroni correction and employing a significance threshold of q less than 0.10 and filtering for read counts per million (log CPM) greater than 1, we identified 20 transcripts with significant differences in expression in GDM-exposed amniocytes compared to controls (19 increased, 1 decreased) (Fig. 1A). When analyzing male and female samples separately, we identified only 4 transcripts with significant expression changes in GDM-exposed male amniocytes (3 increased and 1 decreased) and only 2 transcripts with significantly increased expression in GDM-exposed female amniocytes, q less than 0.10 (Fig. 1, Supplemental Table 3) (25). These results indicate that the sex of the offspring did not have a profound independent effect on gene expression changes in amniocytes induced by in utero GDM exposure. However, a review of the enriched gene list from all 3 analyses (all, male, female) identified profound enrichment of IFN-stimulated genes (ISGs), a class of genes induced by an IFN response (Table 2). ISGs exert antiproliferative effects, stimulate the adaptive immune response, and promote an antiviral defense (35-37). Specifically, ISGs comprised 6 of the top 10 genes with significant changes in expression in the analysis of all amniocytes, 4 in the male analysis, and 1 in the female analysis (Table 2). RNA-Seq data sets are available in Supplemental Tables 6 to 8 (31).
Figure 1.
Genes with significant changes in expression identified by RNA sequencing (RNA-Seq). A, Genes with q < 0.05. B) Genes with P < .05, absolute value (AV) log fold change (FC) > 1, log counts per million (CPM)+. X-axis represents the comparison pairs: All gestational diabetes (GDM) vs control, male GDM vs male control, female GDM vs female control. Y-axis represents the number of genes with increased (positive values, blue bars) or decreased (negative values, red bars) expression compared to control for each bar. RNA-Seq expression changes were confirmed via quantitative polymerase chain reaction. C, Relative FC of IFI44 in GDM vs control amniocytes. D, Relative FC of SAMD9L in male GDM vs male control amniocytes. E, Relative FC of ULBP1 in female GDM vs female control amniocytes. Relative FC was determined by 2ΔΔCt with ITCH as housekeeping gene. Data are statistically analyzed by unpaired t test; P < .05. F, IFNγ concentration measured in second-trimester amniotic fluid (AF) by enzyme-linked immunosorbent assay. Values normalized to total protein content of AF. Data are statistically analyzed by unpaired t test; P < .05.
Table 2.
Top 10 differentially expressed genes identified via RNA sequencinga
| All | ||||||
|---|---|---|---|---|---|---|
| Rank | Gene symbol | Log FC | Log CPM | P | q | Complete gene name |
| 1 | IFI6 | 2.72 | 4.44 | 3.56e-08 | 4.30e-04 | Interferon alpha inducible protein 6 |
| 2 | IFI44 | 2.12 | 3.71 | 4.71e-08 | 4.30e-04 | Interferon-induced protein 44 |
| 3 | IFIT1 | 2.13 | 3.88 | 1.83e-06 | 3.82e-03 | Interferon-induced protein with tetratricopeptide repeats 1 |
| 4 | IFIT3 | 1.48 | 4.81 | 3.53e-06 | 6.34e-03 | Interferon-induced protein with tetratricopeptide repeats 3 |
| 5 | CMPK2 | 1.70 | 1.52 | 3.74e-06 | 7.62e-03 | Cytidine/uridine monophosphate kinase 2 |
| 6 | TFEC | 2.52 | 0.08 | 9.16e-06 | 1.13e-02 | Transcription factor EC |
| 7 | IFI44L | 2.44 | 4.06 | 1.23e-05 | 1.41e-02 | Interferon-induced protein 44 like |
| 8 | OAS1 | 2.45 | 2.92 | 1.47e-05 | 1.54e-02 | 2’-5’-Oligoadenylate synthetase 1 |
| 9 | IFI27 | 2.23 | 0.78 | 2.37e-05 | 2.30e-02 | Interferon alpha inducible protein 27 |
| 10 | SERPINA9 | 6.43 | 0.44 | 3.12e-05 | 2.82e-02 | Serpin family A member 9 |
| Male | ||||||
| Rank | Gene name | Log FC | Log CPM | P | q | Complete gene name |
| 1 | IFI44 | 2.52 | 3.71 | 6.44e-06 | 1.75e-02 | Interferon-induced protein 44 |
| 2 | SAMD9L | 2.74 | 3.73 | 1.19e-05 | 2.95e-02 | Sterile alpha motif domain containing 9 like |
| 3 | MGP | –4.59 | 0.81 | 3.14e-05 | 6.85e-02 | Matrix Gla protein |
| 4 | IFIT1 | 2.62 | 3.88 | 5.37e-05 | 8.09e-02 | Interferon-induced protein with tetratricopeptide repeats 1 |
| 5 | PDGFRA | 5.01 | 2.01 | 7.59e-05 | 1.07e-01 | Platelet-derived growth factor receptor alpha |
| 6 | IFI6 | 2.83 | 4.44 | 1.03e-04 | 1.21e-01 | Interferon alpha inducible protein 6 |
| 7 | CMPK2 | 2.00 | 1.52 | 1.48e-04 | 1.54e-01 | Cytidine/uridine monophosphate kinase 2 |
| 8 | C3AR1 | 2.32 | 1.28 | 2.42e-04 | 1.88e-01 | Complement C3a receptor 1 |
| 9 | OAS1 | 2.96 | 2.68 | 3.02e-04 | 2.02e-01 | 2’-5’-oligoadenylate synthetase 1 |
| 10 | IFI44L | 2.68 | 4.06 | 4.09e-04 | 2.23e-01 | Interferon-induced protein 44 like |
| Female | ||||||
| Rank | Gene name | Log FC | Log CPM | P | q | Complete gene name |
| 1 | ULBP1 | 2.72 | 2.45 | 2.16e-05 | 8.38e-02 | UL16 binding protein 1 |
| 2 | TFEC | 3.31 | 0.09 | 2.61e-05 | 8.84e-02 | Transcription factor EC |
| 3 | EPGN | 2.86 | 2.76 | 3.54e-05 | 1.07e-01 | Epithelial mitogen |
| 4 | TMEM47 | 2.34 | 3.98 | 7.62e-05 | 1.38e-01 | Transmembrane protein 47 |
| 5 | ZNF257 | 3.21 | 1.45 | 2.01e-04 | 2.10e-01 | Zinc finger protein 257 |
| 6 | MX1 | 3.31 | 5.31 | 2.10e-04 | 2.10e-01 | MX dynamin like GTPas2 1 |
| 7 | IFI6 | 2.63 | 4.44 | 2.81e-04 | 2.26e-01 | Interferon alpha inducible protein 6 |
| 8 | ZNF208 | 4.13 | 1.21 | 3.91e-04 | 2.26e-01 | Zinc finger protein 208 |
| 9 | BCAM | –2.06 | 1.69 | 4.72e-04 | 2.62e-01 | Basal cell adhesion molecule (Lutheran blood group) |
| 10 | PARM1 | 2.59 | 1.95 | 4.94e-04 | 2.62e-01 | Prostate androgen- regulated mucin-like protein 1 |
Abbreviations: CPM, counts per million; FC, fold change; RNA-Seq, RNA sequencing.
a RNA-Seq criteria used for ranking: P less than .01, absolute value log FC greater than 1.
To perform pathway analyses we broadened the significance criteria to include transcripts with change in expression of log fold change greater than 1 (log FC > 1) and P < .05 and identified 1449 genes (936 upregulated, 513 downregulated, P < .05) with significant changes in expression (Fig. 1) when we analyzed data from all amniocytes exposed to GDM in utero. Similar numbers of differentially expressed genes were identified in the analysis of male amniocytes (1258 genes, 860 upregulated, 398 downregulated, P < .05) and female amniocytes (1418 genes; 883 upregulated, 535 downregulated, P < .05) separately (Fig. 1B), again suggesting that the sex of the offspring did not have a profound effect on gene expression changes in midgestation amniocytes exposed to GDM. Venn diagrams were generated from the list of significantly upregulated (log FC > 1) and downregulated (log FC < 1) genes (P < .05) from all 3 analyses (all, male, female) and identified 132 specific genes that were upregulated and 34 genes that were downregulated in all 3 separate analyses (Fig. 2; Supplemental Table 4) (24). In all 3 analyses (all, male, female) there were more differentially expressed genes reported with a gain in expression after GDM exposure.
Figure 2.
Overlap in genes with significant changes in gene expression in gestational diabetes exposed amniocytes identified with RNA-sequencing. Venn diagrams depict the number of genes with significant changes in gene expression in 3 independent analyses: all (green), male (purple), and female (pink), including the overlap of identified genes. A, Genes with significant increased expression (log fold change > 1.0). B, Genes with significant decreased expression (log fold change < 1.0).
To gain further insight into how in utero exposure to GDM may lead to increased risk of developing metabolic disease in offspring, we determined biological pathways that were enriched after GDM exposure by performing IPA analysis with lists of genes with significant changes in expression (P < .05, log CPM > 1). Top enriched canonical pathways included inflammation-related pathways involving cytokines and chemokines, communication with immune cells, IFN signaling, and farnesoid X receptor (FXR)/retinoid X receptor (RXR) signaling in the all-sample analysis, inflammation-related pathways involving cytokines and chemokines, communication with immune cells and mature onset of diabetes in young signaling in the male sample analysis, and multiple sclerosis pathways, cytokines and chemokines, and communication with immune cells, neuroinflammation, and glucocorticoid receptor signaling pathways in the female sample analysis (Table 3). The strong enrichment of inflammation and immune response pathways was present in all 3 analyses of GDM-exposed amniocytes.
Table 3.
Top 15 canonical pathways identified by RNA-sequencing
| All n = 1575 genes | Male n = 1279 genes | Female n = 1459 genes | |||
|---|---|---|---|---|---|
| Pathway | log, P | Pathway | log, P | Pathway | log, P |
| Role of cytokines and chemokines in inflammation | 9.97 | Role of cytokines in mediating communication between immune cells | 5.53 | Pathogenesis of multiple sclerosis | 6.41 |
| Role of cytokines in mediating communication between immune cells | 6.56 | Interferon signaling | 5.40 | Role of hypercytokinemia/ hyperchemokinemia in inflammation | 5.08 |
| Role of pattern recognition receptors in recognition of bacteria and viruses | 6.13 | Role of pattern recognition receptors in recognition of bacteria and viruses | 4.91 | Agranulocyte adhesion and diapedesis | 4.75 |
| Granulocyte adhesion and diapedesis | 5.16 | Role of hypercytokinemia/ hyperchemokinemia in inflammation | 4.72 | Granulocyte adhesion and diapedesis | 4.72 |
| Neuroinflammation signaling pathway | 4.99 | MODY signaling | 4.13 | Role of pattern recognition receptors in recognition of bacteria and viruses | 4.07 |
| Interferon signaling | 4.86 | Cellular effects of nitrous oxide signaling | 3.55 | Neuroinflammation signaling pathway | 3.88 |
| FXR/RXR activation | 4.65 | Altered T-cell and B-cell signaling in rheumatoid arthritis | 3.4 | Glucocorticoid receptor signaling | 3.79 |
| Atherosclerosis signaling | 4.56 | Neuroinflammation signaling pathway | 3.33 | Axonal guidance signaling | 3.23 |
| IL-10 signaling | 4.55 | Retinol biosynthesis | 3.15 | Role of macrophages, fibroblasts, and endothelial cells in rheumatoid arthritis | 3.21 |
| Hepatic cholestasis | 4.46 | Th2 pathway | 2.9 | Interferon signaling | 3.21 |
| LXR/RXR activation | 4.30 | Complement system | 2.71 | FXR/RXR activation | 3.11 |
| Superpathway of melatonin degradation | 3.8 | FXR/RXR activation | 2.68 | MSP-RON signaling pathway | 3.02 |
| Agranulocyte adhesion and diapedesis | 3.73 | Granulocyte adhesion and diapedesis | 2.65 | TREM1 signaling | 2.97 |
| Pathogenesis of multiple sclerosis | 3.48 | Communication between innate and adaptive immune cells | 2.61 | Cardiomyocyte differentiation via BMP receptors | 2.94 |
| LPS/IL-1 mediated inhibition of RXR function | 3.26 | Inhibition of matrix metalloproteases | 2.59 | Glutamate-dependent acid resistance | 2.76 |
Abbreviations: CPM, counts per million; FC, fold change; FXR, farnesoid X receptor; IL-1, interleukin 1; IL-10, interleukin 10; LPS, lipopolysaccharide; LXR, liver X receptor; MODY, maturity onset diabetes of young; RXR, retinoid X receptor.
We confirmed our RNA-Seq findings with qPCR by measuring gene expression of IFN-induced protein 44 (IFI44), sterile alpha motif domain containing 9 like (SAMD9L), and UL16 binding protein 1 (ULBP1) (Fig. 1). IFI44 (all amniocytes), SAMD9L (male amniocytes), and ULBP1 (female amniocytes) were chosen based on RNA-Seq data showing robust changes in expression and high abundance across all samples, and they represent novel changes with respect to fetal programming by GDM. IFI44 is a member of the ISG class, which aggregates to form microtubular structures within the cytoskeleton, facilitating intracellular transport of secretory vesicles, and regulates cell division through mitosis and meiosis. IFI44 is expressed in the trophoblast and may be a part of the innate immune response of pregnancy (35) and exert antiproliferative effects (38) but its role in fetal development is unknown. SAMD9L encodes for a cytoplasmic protein that regulates cell proliferation and is involved in the innate immune response and IFN signaling (39). ULBP1 is a ligand of natural killer group 2, member D, which is an immune system–activator receptor described in natural killer and T cells (40). We observed consistent changes between gene expression levels of IFI44, SAMD9L, and ULBP1 from qPCR and RNA-Seq (Fig. 1C-1E, Table 2).
We sought to determine whether the enrichment of ISGs was due to increased exposure to IFNα or IFNγ in pregnancies complicated by GDM. Therefore, we quantified IFNα and IFNγ in second-trimester AF from corresponding amniocytes exposed to GDM in utero with high-sensitivity ELISA. Although we measured no change in IFNα concentration, we measured a 5-fold increase in IFNγ concentration in GDM-exposed AF (P < .001), suggesting that IFNγ leads to the induction of ISG expression (Fig. 1F).
In utero GDM exposure alters the genome-wide DNA methylation profile in human amniocytes in a sex-specific manner
Next we aimed to determine whether exposure to GDM in utero alters DNA methylation in human amniocytes as a potential mechanism linking GDM exposure to adverse health outcomes in offspring. Therefore, we performed ERRBS to identify both DMRs and single CpG sites with significant changes in DNA methylation after GDM exposure.
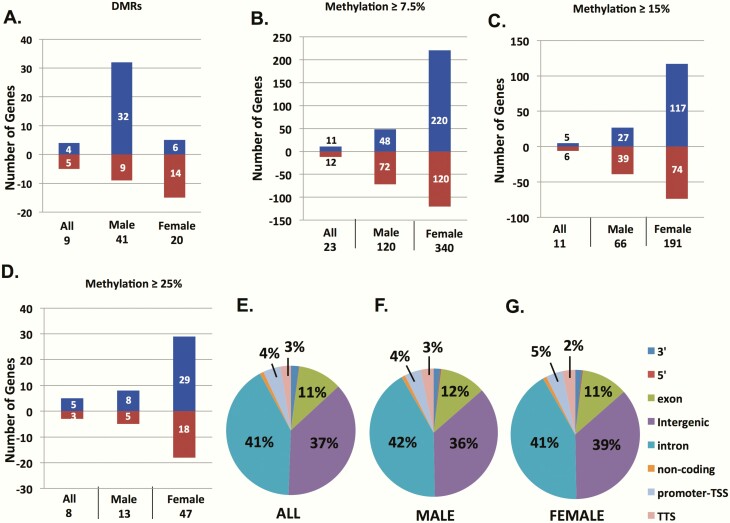
In the DNA methylation analysis, we characterized DMRs as having 2 or more sequential CpGs with an absolute value (AV) percentage DNA methylation change of 5% or more and q < 0.05 across the entire DMR. Initially we examined DNA methylation data derived from all samples and identified 9 DMRs (4 increased methylation, 5 decreased methylation; q < 0.05). We subsequently performed sex-specific analyses and identified 41 DMRs in male amniocytes exposed to GDM (32 increased methylation and 9 decreased methylation; q < 0.05) and 20 DMRs in female amniocyte analysis (6 increased methylation and 14 decreased methylation, q < 0.05) (Fig. 3A; Supplemental Table 5) (25).
Figure 3.
DNA methylation changes associated with gestational diabetes (GDM) exposure. A, Differentially methylated regions (DMRs) identified via enhanced reduced representation bisulfite sequencing with 2 or more sequential CpGs with absolute value (AV) change in percentage methylation of > 5% at each CpG, a q value across the entire DMR of < 0.05, and a total read count of > 10 over the entire DMR. B, CpGs with AV change in percentage methylation ≥ 7.5% compared to controls, P < .001. C, CpGs with AV change in percentage methylation ≥ 15% compared to controls, P < .001. D, CpGs with AV change in percentage methylation ≥ 25% compared to controls, P < .001. X-axis represents each comparison pair: all GDM vs control, male GDM vs male control, and female GDM vs female control, including total number of differentially methylated CpGs for each pair. Y-axis represents number of significant differentially methylated CpGs with increased (positive, blue bars) or decreased (negative, red bars) percentage methylation levels. E to G, Distribution of location of single CpGs with significant changes in DNA methylation AV ≥ 7.5% compared to control. Values represent percentage of single CpGs within a specific genomic regulatory region.
We further explored our data set to identify single CpG sites with significant changes in DNA methylation after in utero GDM exposure. In the all-sample analysis, we identified only 23 CpGs with a 7.5% or greater change in the AV of DNA methylation, 11 CpGs with a 15% or greater change in the AV of DNA methylation, and 8 CpGs with a 25% or greater change in the AV of DNA methylation (q < 0.05). However, the number of single CpGs with significant changes in DNA methylation was increased when we performed separate analyses of male and female amniocytes exposed to GDM in utero. In the male analysis, we identified 120 (≥ 7.5%), 66 (≥ 15%), and 13 (≥ 25%) single CpGs with significant changes in DNA methylation (q < 0.05), and in the female analysis we identified 340 (≥ 7.5%), 191 (≥ 15%), and 47 (≥ 25%) single CpGs with significant changes in DNA methylation (q < 0.05) (Fig. 3B-3D). Although the absolute number of differentially methylated single CpGs varied between the all, male, and female analyses, the distribution of the location of the differentially methylated single CpGs across gene regulatory regions was consistent (Fig. 3E-3G), with the largest number of changes in the intronic (41%-42%) and intergenic (36%-39%) regions. In contrast to the DMR analysis, there were more single CpG sites identified in the female amniocytes analysis compared to male amniocytes regardless of the threshold for percentage change in DNA methylation, and there were a greater number of significant CpG sites identified with a gain in DNA methylation. Overall these findings suggest that GDM exposure in utero is associated with an overall increase in DNA methylation and that the sex of the offspring has a profound effect on DNA methylation changes induced by GDM exposure. A full list of all identified single CpGs is available in Supplemental Tables 9-11 (31).
Differentially methylated regions identified in human amniocytes after in utero exposure to GDM interact with multiple distal genes that are involved in epigenetic and immune-related pathways
After identifying changes in DNA methylation associated with in utero GDM exposure in amniocytes, we sought to determine whether DMRs were associated with transcriptional changes at the gene proximal to the DMR. However, we did not observe a consistent functional relationship between the identified DMRs and expression of the nearest gene because only 4 of the 70 genes proximal to the DMRs had significant changes in expression as measured by RNA-Seq (Supplemental Table 5) (25). Therefore, we investigated whether DMRs interact with distal genes by conducting in silico Hi-C analysis to assess 3D genomic interactions between each DMR and distal regions of the genome. Interestingly, using in silico Hi-C analysis, we identified a large number of genes with potential interactions with each DMR in the 3D space (Fig. 4). For the 9 DMRs identified in the analysis of all amniocytes, we identified 154 potential interacting genes with in silico Hi-C analysis. Additionally, we found that the 41 DMRs identified in the male GDM-exposed amniocytes potentially interact with 662 genes and in female GDM-exposed amniocytes, the 20 DMRs potentially interact with 365 genes based on the in silico Hi-C analysis (Fig. 4). To determine whether the identified DMRs could regulate gene expression of distal interacting genes identified by Hi-C, we merged the DMR data with the RNA-Seq data set. In amniocytes exposed to GDM in utero, DMRs were associated with 5 potential interacting genes with significant change in expression (4 upregulated, 1 downregulated) (P < .05) (Fig. 4, Table 4). In male GDM-exposed amniocytes, DMRs were associated with 11 potential interacting genes with significant changes in expression (7 genes upregulated, 4 genes downregulated) and in female GDM-exposed amniocytes, DMRs were associated with 9 potential interacting genes with significant changes in expression (1 gene upregulated, 8 genes downregulated) (P < .05) (Fig. 4, Table 4). The list of distal interacting genes with significant expression changes identified 4 microRNAs, a noncoding RNA, 4 immune-related genes (killer cell immunoglobulin-like receptor, 3 Ig domains and long cytoplasmic tail (KIR3DL1), Fc fragment of IgA receptor (FCAR), natural cytotoxicity triggering receptor 1 (NCR1), and leukocyte immunoglobulin like receptor A2 (LILRA2) and 2 genes involved in embryogenesis (POU class 3 homeobox 1 [POU3F1] and SRY-box transcription factor 10 [SOX10]).
Figure 4.
Genes predicted to interact with differentially methylated regions (DMRs) through 3-dimensional (3D) chromatin looping. A, Number of genes with potential interactions with DMRs through 3D chromatin looping identified by in silico high-throughput chromosome conformation capture (Hi-C) analysis. Blue bars indicate number of interacting genes with gain in expression, teal bars indicate no change in expression, and red bars indicates number of interacting genes with decreased expression via RNA-sequencing (RNA-Seq). B, Number of genes with potential interactions with DMRs through 3D chromatin looping identified by in silico Hi-C that also had significant changes in gene expression as measured by RNA-Seq (P < .05). Blue bars indicate number of interacting genes with gain in expression, and red bars indicates number of interacting genes with decreased expression via RNA-Seq.
Table 4.
Differentially methylated regions and expression data from potential distally regulated genes based on in silico high-throughput chromosome conformation capture analysis
| All (GDM vs control) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proximal gene: RNA-Seq | Hi-C gene interactions: RNA-Seq | |||||||||||
| Chr | DMR start-end | No. CpGs | UCSC regulatory region | Methyl % | ERRBS q | Name | Log FC | P | Name | Log FC | P | Full form |
| 2 | 143626118- 143626133 | 2 | Strong enhancer/ exon 1 | –9.8 | 0.01 | KYNU | 0.91 | .24 | ARHGAP15 (RHG15)* | 2.01 | .02 | Rho GTPase activating protein 15 |
| 9 | 137537491- 137537663 | 2 | Strong or weak enhancer/ exon 1 | –25.6 | 0 | COL5A1 | –0.19 | .77 | MIR3689A | –3.18 | .002 | MicroRNA 3689a |
| 19 | 55477808- 55478162 | 3 | Active promoter/ exon 1 | –21.9 | 0.01 | NLRP2 | –0.77 | .34 | KIR3DL1 | 2.64 | .01 | Killer cell immunoglobulin- like receptor, 3 Ig domains and long cytoplasmic tail 1 |
| FCAR | 2.33 | .03 | Fc fragment of IgA receptor | |||||||||
| NCR1 | 3.12 | .003 | Natural cytotoxicity triggering receptor 1 | |||||||||
| Female (GDM vs control) | ||||||||||||
| Proximal gene: RNA-Seq | Hi-C gene interactions: RNA-Seq | |||||||||||
| Chr | DMR start-end | No. CpGs | UCSC regulatory region | Methyl % | ERRBS q | Name | Log FC | P | Name | Log FC | P | Full form |
| 1 | 38899434- 38899466 | 2 | Exon 1 | 31.6 | 0.001 | RHBDL2 | 0.12 | .83 | POU3F1 | –3.21 | .04 | POU class 3 homeobox 1 |
| 7 | 95025855- 95025906 | 2 | CpG island/active promoter/ intergenic | 15.6 | 0.03 | PON3/PON1 | 0.49/– 0.20 | 0.62/0.78 | DYNC1I1 | 1.49 | .03 | Dynein cytoplasmic 1 intermediate chain 1 |
| 8 | 143879721- 143879783 | 2 | CpG island/exon 1 | -23 | 0.02 | RP11-706C16.8 | 0.01 | 0.99 | SLURP1 | –2.39 | .02 | Secreted LY6/PLAUR domain containing 1 |
| CYP11B2 | 3.52 | .02 | Cytochrome P450 family 11 subfamily B member 2 | |||||||||
| 9 | 137625891- 137626053 | 2 | Strong enhancer/ exon 1 | 33.9 | 0.01 | COL5A1 | –0.5 | 0.5 | MIR3689A | –4.96 | .002 | MicroRNA 3689A |
| MIR3689F | –2.46 | .02 | MicroRNA 3689F | |||||||||
| 19 | 55477808- 55478162 | 3 | Active/weak promoter exon 1 | -35.2 | 0.01 | NLRP2 | –1.66 | 0.14 | LILRA2 | 3.84 | .008 | Leukocyte immunoglobulin- like receptor 2 |
| KIR3DL1 | 3.53 | .02 | Killer cell immunoglobulin- like receptor, 3 Ig domains, and long cytoplasmic tail 1 | |||||||||
| NCR1 | 4.04 | .005 | Natural cytotoxicity triggeringreceptor 1 | |||||||||
| Male (GDM vs control) | ||||||||||||
| Proximal gene: RNA-Seq | Hi-C interacting genes: RNA-Seq | |||||||||||
| Chr | DMR start-end | No. CpGs | UCSC regulatory region | Methyl % | ERRBS q | Name | Log FC | P | Name | Log FC | P | Full form |
| 3 | 49471923- 49472056 | 2 | Intergenic | 11.6 | 0.004 | NICN1 | 0.45 | .31 | MIR425 | –2.81 | .005 | MicroRNA 425 |
| 11 | 130416252- 130416291 | 2 | Intergenic | 16.9 | 0.015 | ADAMTS15 | –1.11 | .24 | ADAMTS8 | –3.15 | .04 | ADAM metallopeptidase with thrombospondin type 1 motif 8 |
| 12 | 53069066- 53069130 | 2 | Exon 9 | 7.7 | 0.01 | KRT1 | 0 | 1 | KRT83 | 4.42 | .001 | Keratin 83 |
| KRT78 | 2.99 | .01 | Keratin 6B | |||||||||
| KRT6C | 2.62 | .05 | Keratin 6C | |||||||||
| KRT5 | 3.68 | .04 | Keratin 5 | |||||||||
| KRT4 | 2.59 | .03 | Keratin 4 | |||||||||
| KRT78 | 2.84 | .006 | Keratin 78* all type 2 keratins in a cluster on chromosome 12q1 | |||||||||
| 17 | 5925022- 5925042 | 2 | Intergenic | 19.1 | 0.008 | WSCD1 | –0.01 | .99 | LOC339166 | 2.13 | .02 | Noncoding RNA LOC339166 |
| 22 | 38522378- 38522389 | 2 | Exon 10/weak enhancer | 7.4 | 0.01 | PLA2G6 | 0.28 | .62 | SOX10 | –2.08 | .04 | SRY-box 10 |
| LOC400927 (TPTEP2) | –3.47 | .003 | Transmembrane phosphoinositide 3-phosphatase and tensin homolog 2 | |||||||||
Abbreviations: Chr, chromosome; DMR, differentially methylated regions; ERRBS, enhanced reduced representation bisulfite sequencing; FC, fold change; GDM, gestational diabetes; Hi-C, high-throughput chromosome conformation capture; Ig, immunoglobulin; RNA-Seq, RNA sequencing; SOX10, SRY-box transcription factor 10; UCSC, University of California Santa Cruz.
Discussion
Although exposure to GDM in utero is associated with metabolic abnormalities such as development of diabetes and obesity as well as chronic inflammation and cognitive abnormalities in the offspring (41-45), the molecular mechanisms responsible for these findings remain largely unknown. Here we are the first to report genome-wide changes both in expression and DNA methylation in second-trimester human amniocytes collected from women who were subsequently diagnosed with GDM during the third trimester. Multiple studies suggest that DNA methylation may be a critical intermediate in GDM-related adverse health outcomes in offspring (17-19, 46-48). However, there is a paucity of in vivo data linking GDM exposure to changes in DNA methylation and gene expression in human stem cells. Using expansive genome-wide techniques, we were able to identify novel changes both in the transcriptome and the methylome of human amniocytes associated with gestational GDM exposure. We recently reported that second-trimester AF samples from women who subsequently developed GDM have a distinct metabolic signature including profound sex-specific changes in glucose and amino acid metabolism and medium- and long-chain fatty acid species (6). In addition, we reported sex-specific changes in microRNA expression in AF and offspring liver exposed to GDM and maternal obesity (23). Here we expand on these findings by using an unbiased whole-transcriptomic and epigenomic (methylation) approach and provide evidence of novel genome-wide changes associated with midgestation in utero GDM exposure that may lead to later onset metabolic disease. Although we did not observe a profound sex-specific effect on amniocyte gene expression after GDM exposure, we did measure sex-specific changes in DNA methylation indicating that GDM-exposed female amniocytes had a greater number of significant changes at single CpG sites, whereas male GDM-exposed amniocytes had a greater number of DMRs. In addition, we report that the majority of the changes in DNA methylation were found in intergenic and intronic regions, similar to other genome-wide DNA methylation studies on fetal programming (49, 50).
Although DNA methylation changes did not consistently correlate with the expression level of the nearest gene, using in silico Hi-C analysis, we identified previously unknown correlations between DMRs and expression of distal genes that interact through chromatin looping. Enrichment of immune-regulated pathways and genes involved in the development of metabolic disease as well as sex-specific outcome effects are commonly reported in studies of offspring exposed to GDM in utero (8, 10, 20, 32-34, 45). Our findings on the effects of GDM exposure on the transcriptome and methylome of human amniocytes support results from these previous studies and provide evidence for possible mechanisms responsible for these observations, including the identification of DMRs that may function to regulate expression of distal genes. Interestingly, we observed that most differentially expressed genes in amniocytes after GDM exposure were enriched in immune- and inflammation-related pathways, which are recognized as critical factors in the development of obesity and diabetes. Findings from our RNA-Seq analysis of human amniocytes exposed to GDM highlight the potential role that the ISGs may play in the programming of offspring development. Recently the role of ISGs in placental growth and function has been described (35), but the role of ISGs in the pathophysiology of GDM and the effects on placental and fetal development have not been previously recognized. Although increased levels of IFNγ have been observed in cord blood of women with GDM (51), additional studies need to be performed to understand the role that the IFN response may play in the development of obesity and diabetes in the offspring. Despite the fact that we do not have access to additional health outcome data from the offspring in this study, our results suggest that such data should be collected from future cohorts.
Although our data represent the largest known cohort of human amniocytes in which GDM exposure is correlated with genome-wide transcriptomic and methylome changes, because of the high cost of genome-wide techniques and limited sample availability we analyzed relatively few amniocyte samples. In addition, because amniocentesis is now a rare clinical procedure with the introduction of cell-free fetal DNA testing, we were unable to replicate these findings in a separate cohort of GDM-exposed human amniocytes. Amniocytes are a unique cell type derived from the fetus and have a distinct phenotype at specific gestational ages (52, 53). The nested case-control study design and strict protocols employed for sample collection and preparation allowed us to profile amniocyte samples that were matched for gestational age and offspring sex, and thereby control for variables that have been associated with changes in amniocyte gene expression in prior studies (53). Given the unique characteristics of the amniocyte cell type, it is possible but unlikely that the samples were clinically mischaracterized and the changes described are related to inherent sample differences and not GDM exposure. The mean maternal age in this cohort was greater than 35 years, reflective of the participants in the biospecimen repository, for which the most common indication for amniocentesis was advanced maternal age. Although the results may not be reflective of GDM in younger women, advanced maternal age is a strong risk factor for developing GDM and our findings are relevant to that population. Despite these limitations, we have confidence in our findings based on the robust changes described in the GDM-exposed samples and the validation studies that were performed reflecting that the changes reported are persistent.
In conclusion, using a large, unique repository of human amniocytes, we identify novel sex-specific, genome-wide transcriptomic and methylome changes associated with in utero GDM exposure, and these changes provide insight into mechanisms responsible for the development of diabetes, obesity, and fatty liver disease in the offspring. Importantly, by integrating the data from the transcriptome and DNA methylome, this study identifies potential novel regulatory regions of the DNA induced by GDM exposure that may be future pharmacological targets. Finally, the results from studies of human amniocytes exposed to GDM identified inflammation-related and immune-mediated changes that may be critical to the development of diabetes and obesity in the offspring.
Acknowledgments
We would like to thank Jeanne Manson, PhD, and Deborah Driscoll, MD, for their work in establishing the amniotic fluid and amniocyte biospecimen repository. We would also like to acknowledge the Epigenomics Core Facility of Weill Cornell Medical College for conducting RNA-Seq and enhanced reduced representation bisulfite sequencing.
Financial Support: This work was supported by the National Institute of Environmental Health Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (award numbers: K08 DK090302, P30 ES013508, UL1TR001878 to S.E.P.), the McCabe Foundation, and the creation of the biospecimen repository: 5R21-ES11675. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: S.E.P. researched the data, wrote the manuscript, reviewed and edited the manuscript, and is the guarantor for the research described in this manuscript. A.J., V.Y., S.W.M., C.R., D.E.C., and P.Z.W. researched the data and reviewed and edited the manuscript.
Glossary
Abbreviations
- 3D
3-dimensional
- 4C
circular chromosomal conformation capture
- AF
amniotic fluid
- AV
absolute value
- CKIT
CD117
- DEFIANT
DMRs: easy, fast, identification and ANnoTation
- DMR
differentially methylated region
- ELISA
enzyme-linked immunosorbent assay
- ERRBS
enhanced reduced representation bisulfite sequencing
- FC
fold change
- FCAR
Fc fragment of IgA receptor
- FXR
farnesoid X receptor
- GDM
gestational diabetes
- Hi-C
high-throughput chromosome conformation capture
- IFI44
interferon-induced protein 44
- IFN
interferon
- Ig
immunoglobulin
- IPA
Ingenuity Pathway Analysis
- ISGs
interferon-stimulated genes
- KIR3DL1
killer cell immunoglobulin like receptor, 3 Ig domains and long cytoplasmic tail
- LILRA2
leukocyte immunoglobulin like receptor A2
- NANOG
nanog homeobox
- NCR1
natural cytotoxicity triggering receptor 1
- OCT4
POU domain transcription factor OCT4
- POU3F1
POU class 2 homeobox 1
- qPCR
quantitative polymerase chain reaction
- RXR
retinoid X receptor
- RNA-Seq
RNA sequencing
- SAMD9L
sterile alpha motif domain containing 9 like
- SOX10
SRY-box transcription factor 10
- ULBP1
UL16 binding protein 1
Additional Information
Disclosure Summary: The authors have nothing to declare.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340-346. [DOI] [PubMed] [Google Scholar]
- 2. Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94(7):2464-2470. [DOI] [PubMed] [Google Scholar]
- 3. Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208-2211. [DOI] [PubMed] [Google Scholar]
- 4. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54(1):87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawasaki M, Arata N, Ogawa Y. Obesity and abnormal glucose tolerance in the offspring of mothers with diabetes. Curr Opin Obstet Gynecol. 2018;30(6):361-368. [DOI] [PubMed] [Google Scholar]
- 6. O’Neill K, Alexander J, Azuma R, et al. Gestational diabetes alters the metabolomic profile in 2nd trimester amniotic fluid in a sex-specific manner. Int J Mol Sci. 2018;19(9)2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133(5Suppl 2):1674S-1683S. [DOI] [PubMed] [Google Scholar]
- 8. Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care. 2007;30(Suppl 2):S120-S126. [DOI] [PubMed] [Google Scholar]
- 9. Gauster M, Desoye G, Tötsch M, Hiden U. The placenta and gestational diabetes mellitus. Curr Diab Rep. 2012;12(1):16-23. [DOI] [PubMed] [Google Scholar]
- 10. Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52(12):2951-2958. [DOI] [PubMed] [Google Scholar]
- 11. Vambergue A, Fajardy I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J Diabetes. 2011;2(11):196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
- 13. Sweeting AN, Ross GP, Hyett J, et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care. 2016;39(1):75-81. [DOI] [PubMed] [Google Scholar]
- 14. Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62-67. [DOI] [PubMed] [Google Scholar]
- 15. Plagemann A. Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav. 2005;86(5):661-668. [DOI] [PubMed] [Google Scholar]
- 16. Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118(6):2316-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen P, Piaggi P, Traurig M, et al. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia. 2017;60(4):645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hjort L, Martino D, Groth Grunnet L, et al. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight. 2018;3(17):e122572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruchat SM, Houde AA, Voisin G, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. 2013;8(9):935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexander J, Teague AM, Chen J, et al. Offspring sex impacts DNA methylation and gene expression in placentae from women with diabetes during pregnancy. PloS One. 2018;13(2):e0190698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anchan RM, Quaas P, Gerami-Naini B, et al. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet. 2011;20(5):962-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinney SE, Mesaros CA, Snyder NW, et al. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod Toxicol. 2017;67:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joshi A, Azuma R, Akumuo R, Goetzl L, Pinney SE. Gestational diabetes and maternal obesity are associated with sex-specific changes in miRNA and target gene expression in the fetus. Int J Obes (Lond). 2020;44(7):1497-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hulsen T, de Vlieg J, Alkema W. BioVenn—a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Supplemental Tables 1 to 5. ProMED-mail website. https://upenn.box.com/s/k1yv7ji1rszu4gj372pgp2pdlxxr992p. June 29, 2020.
- 26. Garrett-Bakelman FE, Sheridan CK, Kacmarczyk TJ, et al. Enhanced reduced representation bisulfite sequencing for assessment of DNA methylation at base pair resolution. J Vis Exp. 2015;(96):e52246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Condon DE, Tran PV, Lien YC, et al. Defiant: (DMRs: easy, fast, identification and ANnoTation) identifies differentially methylated regions from iron-deficient rat hippocampus. BMC Bioinformatics. 2018;19(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Assenov Y, Müller F, Lutsik P, Walter J, Lengauer T, Bock C. Comprehensive analysis of DNA methylation data with RnBeads. Nat Methods. 2014;11(11):1138-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811-818. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Song F, Zhang B, et al. The 3D Genome Browser: a web-based browser for visualizing 3D genome organization and long-range chromatin interactions. Genome Biol. 2018;19(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Supplemental Tables 6 to 11. ProMED-mail website. https://upenn.box.com/s/y0xedjsnewll4fvxnkeym392i6cxzrig. June 29, 2020.
- 32. Lingwood BE, Henry AM, d’Emden MC, et al. Determinants of body fat in infants of women with gestational diabetes mellitus differ with fetal sex. Diabetes Care. 2011;34(12):2581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care. 2013;36(10):3045-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Retnakaran R, Kramer CK, Ye C, et al. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care. 2015;38(5):844-851. [DOI] [PubMed] [Google Scholar]
- 35. Bayer A, Lennemann NJ, Ouyang Y, et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016;19(5):705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hubel P, Urban C, Bergant V, et al. A protein-interaction network of interferon-stimulated genes extends the innate immune system landscape. Nat Immunol. 2019;20(4):493-502. [DOI] [PubMed] [Google Scholar]
- 37. Schoggins JW. Interferon-stimulated genes: what do they all do? Annu Rev Virol. 2019;6(1):567-584. [DOI] [PubMed] [Google Scholar]
- 38. Hallen LC, Burki Y, Ebeling M, et al. Antiproliferative activity of the human IFN-α-inducible protein IFI44. J Interferon Cytokine Res. 2007;27(8):675-680. [DOI] [PubMed] [Google Scholar]
- 39. de Jesus AA, Hou Y, Brooks S, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin Invest. 2020;130(4):1669-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cosman D, Müllberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123-133. [DOI] [PubMed] [Google Scholar]
- 41. Awoke Adane A, Mishra GD, Tooth LR. Diabetes in pregnancy and childhood cognitive development: a systematic review. Pediatrics. 2016;137(5):e20154234. [DOI] [PubMed] [Google Scholar]
- 42. Perna R, Loughan AR, Le J, Tyson K. Gestational diabetes: long-term central nervous system developmental and cognitive sequelae. Appl Neuropsychol Child. 2015;4(3):217-220. [DOI] [PubMed] [Google Scholar]
- 43. Vuong B, Odero G, Rozbacher S, et al. Exposure to gestational diabetes mellitus induces neuroinflammation, derangement of hippocampal neurons, and cognitive changes in rat offspring. J Neuroinflammation. 2017;14(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Money KM, Barke TL, Serezani A, et al. Gestational diabetes exacerbates maternal immune activation effects in the developing brain. Mol Psychiatry. 2018;23(9):1920-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bouchard L, Hivert MF, Guay SP, St-Pierre J, Perron P, Brisson D. Placental adiponectin gene DNA methylation levels are associated with mothers’ blood glucose concentration. Diabetes. 2012;61(5):1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Godfrey KM, Sheppard A, Gluckman PD, et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60(5):1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelstrup L, Hjort L, Houshmand-Oeregaard A, et al. Gene expression and DNA methylation of PPARGC1A in muscle and adipose tissue from adult offspring of women with diabetes in pregnancy. Diabetes. 2016;65(10):2900-2910. [DOI] [PubMed] [Google Scholar]
- 49. Thompson RF, Fazzari MJ, Niu H, Barzilai N, Simmons RA, Greally JM. Experimental intrauterine growth restriction induces alterations in DNA methylation and gene expression in pancreatic islets of rats. J Biol Chem. 2010;285(20):15111-15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bansal A, Robles-Matos N, Wang PZ, Condon DE, Joshi A, Pinney SE. In utero bisphenol A exposure is linked with sex specific changes in the transcriptome and methylome of human amniocytes. J Clin Endocrinol Metab. 2020;105(2): 453-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Atègbo JM, Grissa O, Yessoufou A, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91(10):4137-4143. [DOI] [PubMed] [Google Scholar]
- 52. Di Germanio C, Bernier M, de Cabo R, Barboni B. Amniotic epithelial cells: a new tool to combat aging and age-related diseases? Front Cell Dev Biol. 2016;4:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maguire CT, Demarest BL, Hill JT, et al. Genome-wide analysis reveals the unique stem cell identity of human amniocytes. PLoS One. 2013;8(1):e53372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”