Abstract
Purpose
Pituitary gangliocytomas (GCs) are rare neuronal tumors that present with endocrinological disorders, such as acromegaly, amenorrhea-galactorrhea syndrome, and Cushing’s disease. Most pituitary GCs coexist with pituitary adenomas pathologically and are diagnosed as mixed gangliocytoma-adenomas. Herein, we report a case of 45-year-old man who presented with the syndrome of inappropriate secretion of thyroid-stimulating hormone (SITSH) and discuss the pathogenesis of pituitary GCs.
Methods
Pituitary magnetic resonance imaging showed an 8-mm homogeneous and poorly enhanced mass inside the pituitary gland. Endoscopic transsphenoidal surgery was performed under a preoperative diagnosis of thyrotroph adenoma. However, the tumor was finally diagnosed as gangliocytoma without an adenomatous component. The tumor was further analyzed via immunohistochemistry and electron microscopy. Additionally, we searched MEDLINE and PubMed for previously published cases of isolated pituitary GCs and analyzed the reported clinicopathological findings.
Results
The patient showed complete clinical and endocrinological recovery after an operation. The tumor was positive for thyrotropin (TSH), TSH-releasing hormone (TRH), Pit-1, GATA-2, and most neuronal markers. Electron microscopy demonstrated the presence of intracytoplasmic secretory granules and neuronal processes. Co-secreting hypothalamic and pituitary hormone inside the tumor indicated autocrine/paracrine endocrinological stimulation.
Conclusion
Herein, we report a case of SITSH caused by an isolated pituitary gangliocytoma, expressing both TSH and TRH, which, to our best knowledge, is the first reported case of such a condition. The multidirectional differentiation and multihormonal endocrine characteristics of these tumors indicate that they are a member of neuroendocrine neoplasms, further supporting that they are derived from neural crest cells.
Keywords: pituitary gangliocytoma, inappropriate secretion of thyroid-stimulating hormone, thyroid-stimulating hormone, TSH-releasing hormone, mixed gangliocytoma-adenoma, neuroendocrine neoplasm
Pituitary gangliocytomas (GCs)/mixed gangliocytoma-adenomas (MGAs) have been categorized as neuronal and paraneuronal tumors in the fourth edition of the World Health Organization (WHO) classification of endocrine tumors in 2017 (1). GCs/MGAs are considered rare pathological entities, with only more than 150 cases of pituitary GCs/MGAs reported in the literature. Most lesions occur as mixed tumors with adenomatous and gangliocytic components, rather than isolated GCs, and approximately 75% of the patients exhibit pituitary or hypothalamic hormone hypersecretion (2). Because of the rarity of pituitary GCs, most cases are usually misdiagnosed as pituitary adenomas, as it is difficult to distinguish between the two radiographically before surgery.
Cossu et al recently reviewed 130 cases of pituitary GCs/MGAs, including their case of MGAs (3). Only 19 of these cases (14.6%) were classified as isolated GCs, whereas the others (111 cases, 85.4%) were classified as MGAs. In cases of MGAs, concomitant adenomatous components were identified as mixed somatotroph—lactotroph adenomas (43%), somatotroph adenomas (33%), lactotroph adenomas (14%), and corticotroph adenomas (6%) via immunohistochemistry. Moreover, GCs/MGAs are usually positive for growth hormone–releasing hormone (GHRH), corticotropin-releasing hormone (CRH), GH, and prolactin (PRL) in most cases (3–7). Because of the close relationships between GCs and the pituitary adenomas and the high incidence of endocrinological hypersecretion syndromes, the histogenesis of GCs/MGAs, specifically the derivation of ganglionic cells in the pituitary gland, has been extensively studied (8–11).
We present herein a first case of isolated pituitary GCs that also developed the syndrome of inappropriate secretion of thyroid-stimulating hormone (SITSH), as confirmed by extensive pathological studies. With the latest knowledge, we also reviewed “gangliocytomas of the sellar region” that were previously reported, focusing on their presumed pathogenesis.
Materials and Methods
Search strategies of literature review
We searched OVID MEDLINE and PubMed for related articles published before December 2018. The keywords were “gangliocytoma,” “ganglioneuroma,” “neuronal choristoma,” “pituitary,” and “sellar.” Relevant articles in English, German, Russian, and Japanese were also retrieved and reviewed to identify additional papers not detected in the database search. The identified articles were reviewed with a focus on “isolated” pituitary gangliocytomas without an adenomatous component.
Case presentation and pathological analysis
We describe a unique case of isolated pituitary GC presenting with SITSH. For immunohistochemical analyses, tissues were fixed in 10% formaldehyde and embedded in paraffin. The 5-µm thick sections were stained with hematoxylin and eosin (HE). The remaining serial unstained sections were used for immunohistochemistry. Immunohistochemistry was performed via an immunoperoxidase method with an ENVISION FLEX kit (Agilent/Dako, Tokyo, Japan), using horseradish peroxidase and 3,3’-diaminobenzidine tetrahydrochloride. Protein and endogen peroxidase blockage were performed. The primary antibodies used and their dilution rate are listed in Table 1.
Table 1.
Primary antibodies used in the immunohistochemistry and their dilution rate
| Categories | Antibodies | Clone | Company | Dilution rate |
|---|---|---|---|---|
| Neuronal/glial markers | Synaptophysin | 27G12 | Nichirei Bioscience | Prediluted |
| Chromogranin-A | DAK-A3 | Agilent/Dako | 1:400 | |
| Neurofilament | 2F11 | Agilent/Dako | 1:3 | |
| NCAM (CD56) | CD564 | Leica Biosystems | 1:200 | |
| NeuN | A60 | Merk Millipore | 1:200 | |
| GFAP | BSR189 | Dianova | 1:12 | |
| Epithelial markers | CAM 5.2 | CAM5.2 | BD Biosciences | 1:15 |
| CK 5/6 | D5/16B4 | Agilent/Dako | 1:100 | |
| CK 7 | OV-TL12/30 | Agilent/Dako | 1:400 | |
| CK 8 | 35βH11 | Agilent/Dako | 1:1200 | |
| CK 20 | Ks20.8 | Agilent/Dako | 1:200 | |
| CK 34βE12 | 4βE12 | Agilent/Dako | 1:100 | |
| Hormonal markers | GH | Polyclonal | Agilent/Dako | 1:4 |
| PRL | Polyclonal | Agilent/Dako | Prediluted | |
| TSH | 0042 | Agilent/Dako | 1:2 | |
| ACTH | 02A3 | Agilent/Dako | Prediluted | |
| LH | C93 | Agilent/Dako | Prediluted | |
| FSH | C10 | Agilent/Dako | Prediluted | |
| TRH | Polyclonal | BIOSS | 1:200 | |
| Transcription factors | Pit-1 | HPA050624 | Sigma-Aldrich | 1:2000 |
| GATA-2 | AF2046 | R&D systems | 1:200 | |
| SF-1 | EPR19744 | Abcam | 1:1000 | |
| Tpit | AMAB91409 | Sigma-Aldrich | 1:1000 | |
| ER | SP1 | Ventana | Prediluted | |
| TTF-1 | 8G7G3/1 | Agilent/Dako | 1:200 | |
| Others | Ki-67 | MIB-1 | Agilent/Dako | 1:100 |
| P53 | DO-7 | Leica Biosystems | 1:200 | |
| CD 3 | LN10 | Leica Biosystems | 1:300 | |
| SSTR2 | UMB1 | Abcam | 1:300 | |
| SSTR5 | UMB4 | Abcam | 1:200 |
For the ultrastructural study, small fragments of tumor tissue were fixed in 2.5% glutaraldehyde, postfixed in 1.0% osmium tetroxide, dehydrated in graded ethanol, processed through propylene oxide, and embedded in an Epon812. Ultrathin sections stained with uranyl acetate and lead citrate were studied using an H-7650 electron microscope (Hitachi, Tokyo, Japan).
Review of related literature on isolated GCs in the sellar region
Ganglion cell-containing tumors of the pituitary gland have been reported under various names (4, 6, 11). Several authors have reviewed pituitary GCs, including MGAs, under the former diagnoses of “gangliocytoma,” “ganglioneuroma,” “pituitary adenoma with neuronal choristoma,” “ganglioneuroma and adenoma,” “gangliocytoma with adenoma,” “pituitary adenoma with gangliocytic differentiation,” “adenohypophyseal neuronal choristoma,” and others (2–5, 7, 12).
In 1919, Greenfield first reported a case of a 26-year-old woman with acromegaly with complaints of a headache and visual deterioration. A postmortem examination revealed a huge ganglioneuroma in the sellar region with a maximum size of 9 cm towards the frontal base, middle fossa, and clivus. The neuroma did not infiltrate the brain, but the pituitary gland could not be found (13). Citing this case later in 1930, Courville suggested that the ganglioneuroma might have originated in the tuber cinereum (14). Alpes and Grant also reported a case of a 16-year-old man who presented with visual deterioration and feminization. This patient was found to have a large intra- and suprasellar tumor that extended up to the third ventricle that was diagnosed as a ganglioneuroma following consequent transcranial surgery and histopathological examination (15). Some cases before the advent of computed tomography (CT) and magnetic resonance imaging (MRI), including the ones discussed here, were reported to have a large extrasellar component, although their hypothalamic origin could not be ruled out (13–16). Moreover, in those days, radiation therapy was acceptable as the first-line therapy, which probably caused difficulty for obtaining an accurate pathological diagnosis because of the difference in sensitivity to radiation (17, 18).
In 1929, Kiyono reported a case of a true intrasellar tumor with a gangliocytic component detected via postmortem examination in a 59-year-old woman who had died of pulmonary tuberculosis. In his description, the gangliocytic component with 2 or multiple nuclei was found in the central part of the tumor that was predominantly composed of adenomatous cells, making it the first reported case of pituitary MGA (19). Benda briefly reported a case of a 6 × 11 mm pituitary posterior lobe tumor consisting of numerous large to small neurons, identified via postmortem examination of a 72-year-old woman (20). This was again reported by Casper 6 years later (21) and might have been the first case of a pituitary GC.
After excluding the typical MGAs, we included 32 cases of GCs in the sellar region in the review. Of these, 11 cases were “ganglioneuromas” (assumed to be of sellar origin without immunohistochemical studies) (Table 2), and 21 cases were GCs without an adenomatous component in the sellar region diagnosed by immunohistochemistry (Table 3) (5, 13–16, 18, 20–37). In this review, we avoid the use of “pituitary” GCs because the precise relationship between the tumor and the pituitary gland inside the sella had not been described for some of the cases (13–16, 29, 34). As shown in Tables 2 and 3, middle-aged women (mean age, 43.1 years; female/male, 23/9) were predisposed to develop GCs in the sellar region. Endocrinological symptoms of pituitary hypersecretion were seen in 65.6% (21/32) of all cases and 76.2% (16/21) of the recent series. These symptoms included acromegaly (8 cases), amenorrhea-galactorrhea (6 cases), Cushing’s syndrome (6 cases), and SITSH (1 case [our presented case]). The majority of these 32 cases, that spanned over a century, lacked immunohistochemical evaluation. Of the recent cases, 10 were evaluated for hypothalamic hormones, and 8 of them (80%) demonstrated immunoreactivity to GHRH, CRH, or TSH-releasing hormone (TRH). Additionally, among these 10 recent cases, 4 (40%) demonstrated immunoreactivity to pituitary hormones. In some cases, the tumor cells exhibited overlapping immunoreactivity to the same hypothalamic–pituitary hormonal axis (35, 37). Most of these pathological findings correspond to the endocrinological symptoms of the respective cases.
Table 2.
Review of 11 cases of ganglioneuromas in the sellar region diagnosed before immunohistochemistry
| Author (Ref No.) | Year | Age/Sex | Endocrinological Symptoms | Visual Symptoms | Locations | Treatment/Outcome Comment |
|---|---|---|---|---|---|---|
| Greenfield (13); Courville (14) | 1919; 1930; | 26/F | Acromegaly | Yes | Huge skull base | Patient died a few days after TCO |
|
Alpers and Grant (15) |
1931 |
16/M | Feminization | Yes | IS/SS/IIIrd | Patient died 1 day after TCO |
| Benda and Casper (20, 21) | 1933 | 72/F | Not described | No | IS* | Autopsy (died by pulmonary disease) |
| Robertson et al (22); Serebrin (23) |
1964; 1984 |
45/F | Dysmenorrhea | No | IS Sphenoid sinus |
Unrelated death 20 years after TCO; autopsy revealed recurrent tumor |
| Jakumeit et al (24) | 1974 | 41/F | Amenorrhea | Yes | IS/SS | Treated via TSO, total removal |
| 37/F | Cushing | No | IS/Sphenoid | Treated via TSO, total removal | ||
| 56/M | Acromegaly | Yes | IS/SS | Treated via TCO, total removal | ||
| Arseni et al (16) | 1975 | 5/F | Diabetes insipidus | Yes | IS/SS/IIIrd | Treated via TCO and RT |
| 7/M | Hypothyroidism | Yes | IS/SS | Treated via TCO | ||
| Ule and Waidelich (25) | 1976 | 65/F | None | No | IS | Autopsy (died by thyroid carcinoma) |
| Nikonov (26) | 1981 | 52/F | Acromegaly | Yes | IS/SS/CS | RT→ Patient died 5 days after TCO |
Abbreviations: CS, cavernous sinus; F, female; IIIrd, third ventricle; IS, intrasellar; M, male; RT, radiation therapy; SS, suprasellar; TCO, transcranial operation; TSO, transsphenoidal operation.
Table 3.
Review of 21 cases of gangliocytomas in the sellar region diagnosed by immunohistochemistry
| Immunohistochemical Results of GC | |||||||
|---|---|---|---|---|---|---|---|
| Author (Ref No.) | Year | Age/Sex | Endocrinological Symptoms | Visual Symptoms | Locations | Pituitary Hormone | Hypothalamic Hormone |
| Asa et al (27)a | 1984 | 62/F | Acromegaly | Yes | IS/SS | All negative | GHRH (+) |
| Asa et al (28) | 1984 | 58/F | Cushing | No | IS | With ACTH-AHCH(+) | CRH (+) |
| Nishio et al (29) | 1987 | 58/F | Cushing | No | IS | Not described | CRH, SST, OXT (+) |
| Yamada et al (30) | 1990 | 47/F | None | No | IS/SS | All negative | SST (+) |
| Baysefer et al (31) | 1997 | 35/M | Cushing | No | IS | Not described | Not described |
| Saeger et al (32) | 1997 | 34/F | Acromegaly | Not described | All negative | GHRH (+) | |
| McCowen et al (18) | 1999 | 36/M | Hyper-PRL | Yes | IS/SS | PRL (+) | Not described |
| Geddes et al (33) | 2000 | 53/F | Acromegaly | No | IS/SS | Not described | Not described |
| 54/M | Cushing | No | ISb | With ACTH-BI (+) | CRH negative | ||
| Ishidro et al (34) | 2005 | 66/F | Acromegaly | No | Parasellar | All negative | GHRH (+) |
| Qiao et al (5) | 2014 | Mean 34.3 y; 5/F, 2/M |
None: 3 Hyper-PRL: 3 Hyper-GH,PRL: 1 |
Not described | |||
| Domingue et al (35) | 2015 | 62/F | Cushing | No | IS/sphenoid | ACTH (+) | CRH (+) |
| Petrakakis et al (36) | 2016 | 49/F | Hyper-PRL | Yes | IS | Not described | Not described |
| Donadille et al (37) | 2017 | 59/F | None | Yes | IS/SS | GH (+) ACTH (+) | GHRH (+) |
| Present case | 2020 | 45/M | SITSH | No | IS | TSH (+) | TRH (+) |
Abbreviations: AHCH, adenohypophyseal cell hyperplasia; BI, basophil invasion; F, female; GC, gangliocytic cell; IS, intrasellar; M, male; OXT, oxytocin; SS, suprasellar; SST, somatostatin; y, years-old.
a This patient underwent radiation therapy for acromegaly 25 years ago.
b The tumor was located at the posterior pituitary.
Case Report
Presentation and examination
A 45-year-old obese man (height, 178.7 cm; weight, 97 Kg; body mass index, 30.8 kg/m2) complained of general fatigue and drowsiness at work. A blood examination revealed severe diabetes mellitus (HbA1c 10.6%), and he was accordingly referred to our university. Treatment for diabetes mellitus was started and extensive evaluations for sleep apnea syndrome were performed. Although treatment using a continuous positive airway pressure mask was initiated, general fatigue continued. Concurrently, SITSH was diagnosed based on the following findings: serum TSH, 6.890 µIU/mL; free T3, 4.9 pg/mL; and free T4, 2.29 ng/dL.
Magnetic resonance imaging of the pituitary gland revealed a poorly enhanced mass measuring 5 × 6 × 8 mm (Fig. 1A and 1B). The TRH loading test showed a low and delayed TSH response (pre-TSH, 6.89 µIU/mL; max TSH, 10.8 µIU/mL; 60 minutes after TRH loading). However, there were no abnormal responses for both GH and PRL on several other loading tests. The absence of a family history of SITSH or TRβ gene mutations prompted the diagnosis of thyrotroph adenoma.
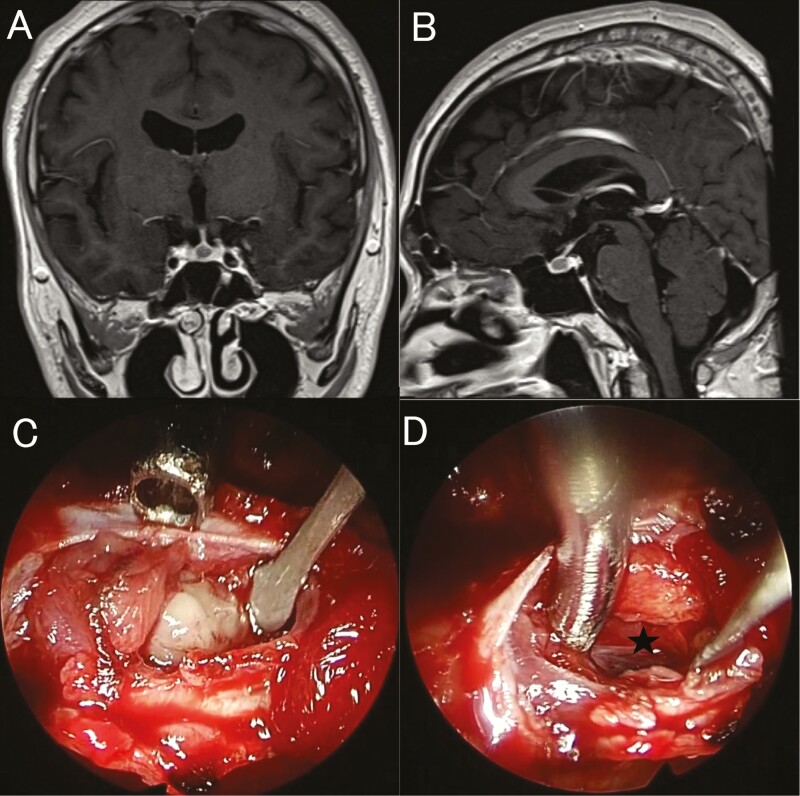
Figure 1.
Magnetic resonance imaging (MRI). Preoperative contrast-enhanced coronal (A) and sagittal (B) MRIs showing a 5 × 6 × 8 mm less-enhanced mass lesion inside the pituitary gland. Intraoperative images show a well-circumscribed whitish tumor after splitting the pituitary gland (C), which was completely removed via a fine dissection plane. D: The arachnoid membrane can be seen over the resection cavity (star).
Initial treatment with the somatostatin analog (SSA) did not yield any response. Further, the free T4 levels remained over 2 ng/dL after 3 courses of lanreotide autogel (90 mg). Since his diabetes mellitus was already under control, we decided to remove the tumor surgically.
Operative findings and postoperative course
The surgery was performed using the standard endoscopic endonasal transsphenoidal approach. The pituitary gland appeared normal on the surface. However, a midline split revealed a well-circumscribed whitish tumor inside the pituitary gland (Fig. 1C). Complete tumor resection was achieved (Fig. 1D), and tumor tissues were collected wherever possible. As the intraoperative pathological diagnosis ruled out a pituitary adenoma, tissue samples for electron microscopy were obtained.
His serum TSH levels decreased drastically to 0.320 µIU/mL on postoperative day 1. However, the patient developed transient diabetes insipidus that was treated with oral desmopressin acetate tablets. Three days after the operation, he was diagnosed with influenza type A, followed by hyponatremia with a minimum serum Na level of 118 mEq/L. Electrolyte levels were closely monitored during the treatment for influenza, and he was discharged 18 days after the operation without any electrolyte management. Oral administration of hydrocortisone and levothyroxine was started after the operation, which was tapered and ceased completely after 3 months.
The patient’s complaint of fatigue improved, and his cardiac heart rate was reduced by 10 bpm. Blood examination 6 months after the operation revealed an improvement in endocrinological parameters, including serum TSH (0.808 µIU/mL), free T3 (2.9 pg/mL), and free T4 (1.35 ng/dL). The TRH loading test showed a normal TSH response (pre-TSH, 0.589 µIU/mL; max TSH, 7.830 µIU/mL) 30 minutes after TRH loading. The patient has remained healthy for 2 years without tumor recurrence.
Pathological and radiographical findings
Postoperative MRI revealed complete tumor resection. Hematoxylin and eosin staining showed that the tumor was composed of small- to large-sized neuronal or ganglionic cells containing abundant acidophilic cytoplasm and nuclei with a prominent nucleolus against a background of fine, fibrillar, neuropil-like matrix (Fig. 2A and 2B), which were diffusely immunoreactive to synaptophysin, chromogranin A, neurofilament, and NCAM (CD56), and partially immunoreactive to NeuN (Fig. 3A–3C). Interjacent small cells were considered to be reactive lymphocytes and not adenomatous cells based on their immunoreactivity to CD3. The cytoplasm of the tumor cells with peripheral displacement of the nucleus was immunoreactive for low-molecular-weight keratins, CAM 5.2, and patchy reactive for CK7 (Fig. 3D and 3E), but not for CK5/6, CK 8, CK 20, and CK 34βE12, supporting the exclusion of paragangliomas. Further, the absence of immunoreactivity to glial fibrillary acidic protein confirmed the exclusion of ganglioglioma (Fig. 3F). Although Ki-67 staining revealed a labeling index of 2.6%, no other atypical features were detected. Only 0.4% of the entire tumor showed P53 immunopositivity. Based on these findings, a final pathological diagnosis of isolated GC was made.
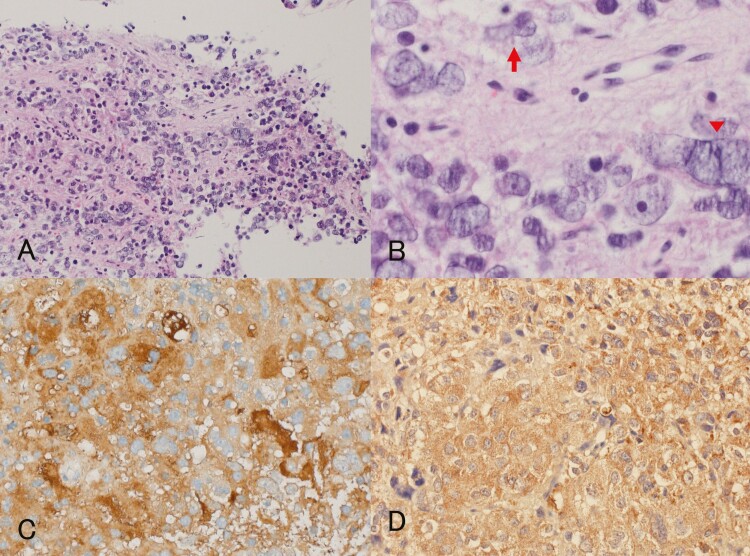
Figure 2.
Hematoxylin and eosin staining shows tumor cells with small to large bizarre nuclei against a background of finely, fibrillar, neuropil-like matrix (magnification ×100) (A). B: Individual tumor cells with irregular shapes and dysplastic nuclei containing a prominent nucleolus can be seen (magnification ×400). Some cells display cleaved nuclei (arrow), and some are multinucleated (arrow head). Immunohistochemical staining for pituitary and hypothalamic hormones revealed a diffuse co-expression of both TSH (C) and TRH (D) for the cytoplasm of tumor cells (magnification ×200).
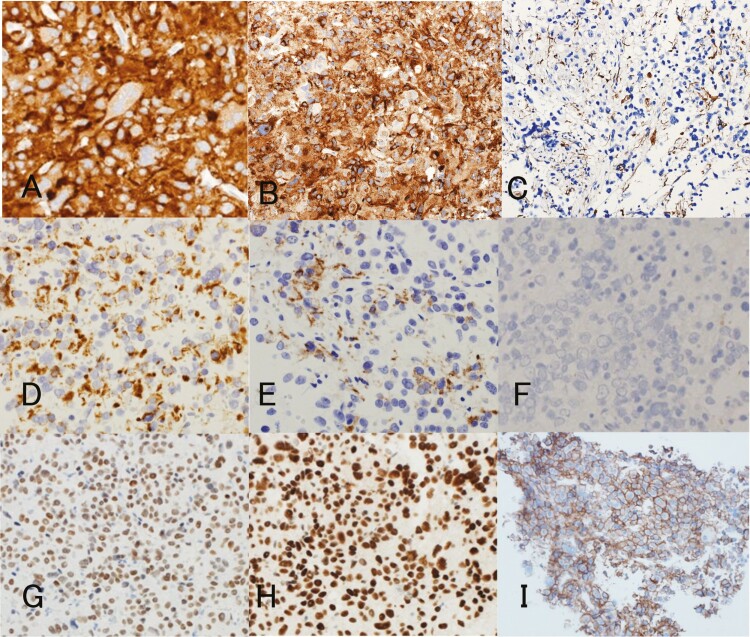
Figure 3.
Immunohistochemical study reveals strong reactivity for neuronal markers such as synaptophysin (A), chromogranin A (B), and neurofilament (C). Scattered expression of cytokeratins for cytoplasm of the tumor cells are revealed using CAM 5.2 (D) and CK7 (E). Negative for GFAP rules out ganglioglioma (F). Immunostaining for the transcription factor Pit-1 (G) and GATA-2 (H) display strong and diffuse nuclear staining in the whole tumor cell component. The cytomembranes of the tumor cells stained positively for SSTR2A (I), which is 1 of the 5 subtype receptor families for the ligand somatostatin (magnification ×200 [A, B, D–F], and magnification ×100 [C, G–I]).
With respect to the pituitary and hypothalamic hormones, the cytoplasm of the tumor cells showed diffuse but strong immunoreactivity for TSH (Fig. 2C) and equivocal faint positivity for gonadotropin; however, it was negative for GH, PRL, and ACTH. Meanwhile, it also showed diffuse but strong immunoreactivity to TRH (Fig. 2D). Transcription factors such as the acidophilic cell lineage transcription factor Pit-1, SF-1, Tpit, ER, and GATA-2 are important to pituitary cytodifferentiation from the Rathke pouch stem cell. Further immunohistochemical studies for these transcription factors revealed diffuse and strong nuclear immunoreactivity for Pit-1 and GATA-2 (Fig. 3G and 3H), but not for SF-1, Tpit, and ER, confirming thyrotropic cell differentiation of tumor cells. Thyroid transcription factor-1 (TTF-1) is a tissue-specific transcription factor that regulates the expression of selected genes in the thyroid, lung, and diencephalon for embryonic development and differentiation; it is well known that it aids in nuclear expression, specifically that of posterior pituitary, thyroid, and lung tumors (1, 38). All tumor cells stained negatively for TTF-1. Somatostatin receptor (SSTR) status may predict treatment response to first- and second-generation SSAs. With respect to SSTR membranous immunopositivity proposed by Volante et al (39), more than 50% of tumor cells stained positively for SSTR2 (Score 3 (Fig. 3I)); however, none of the cells stained positively for SSTR5 (Score 0).
Based on these immunohistochemical findings, this tumor was considered to be differentiated into an entirely neuronal lineage rather than mixed or interjacent with 2 components. Electron microscopy revealed the tumor cell has a light nucleus with a prominent nucleolus, which is surrounded by many secretary granules, synaptic vesicles, and some lysosomes in the cytoplasm. Typical neuronal processes contained both dense core vesicles and clear vesicles (Fig. 4).
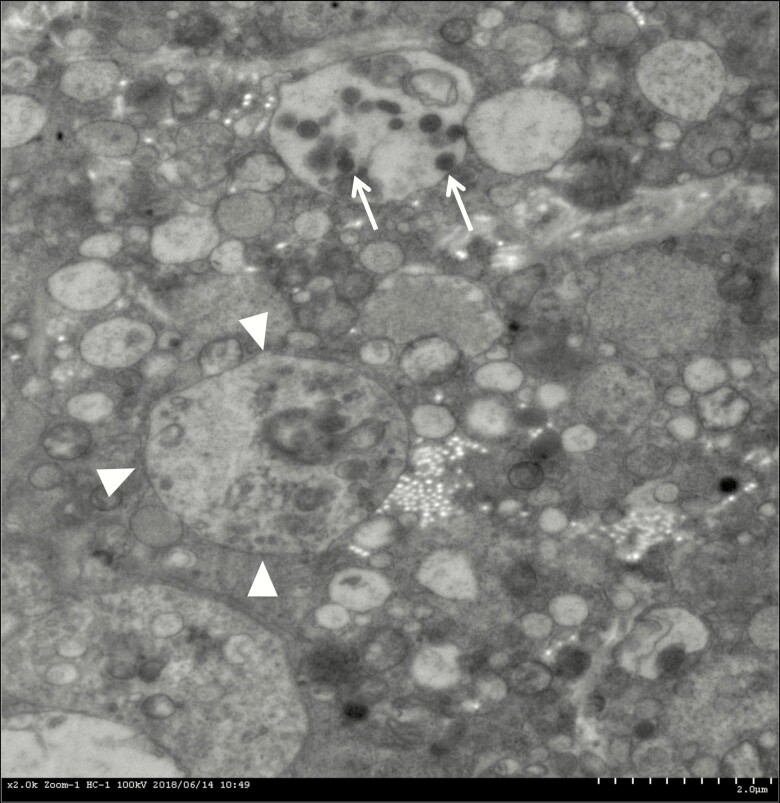
Figure 4.
Electron microscopy revealed that the tumor cell has a light nucleus with a prominent nucleolus (arrow head), which are surrounded by many secretary granules, synaptic vesicles, and some lysosomes in the cytoplasm. Typical neuronal process contains both dense core vesicles (white arrow) and clear vesicles.
Discussion
Incidence of pituitary GCs/MGAs and hypersecretion of TSH
The origin and histogenesis of pituitary gangliocytic tumors have remained an enigma. Gangliocytomas have the potential to manifest anywhere within the central nervous system, but it is well known that the majority of pituitary gangliocytic tumors occur in combination with pituitary adenomas. However, pituitary gangliocytic tumors are rare, accounting for only 0.14%–1.42% of all sellar lesions (2, 9, 10). Therefore, isolated GCs are considered rather as cases that could not be identified as having an adenomatous component in microscopic and immunohistochemical studies (6). Meanwhile, gangliocytic cells may secrete pituitary or hypothalamic hormones, thus directly manifesting syndromes of pituitary hypersecretion such as acromegaly, Cushing’s disease, and amenorrhea-galactorrhea syndrome (18, 27–29, 32, 34–37).
In 1960, Jailer and Holub first reported a case of a patient with symptoms of hyperthyroidism and expanded sella on X-ray imaging. They called this condition SITSH, which was presumably due to a pituitary adenoma (40). Since then, more than 450 cases of thyrotroph adenomas have been published. These adenomas account for 0.5%–3% of all pituitary tumors, and their age-standardized national incidence is 0.15 per 1 million population annually (41). Although the incidence of SITSH is low, it has neither been reported with isolated GCs nor with MGAs. The majority of functioning thyrotroph adenomas and somatotroph adenomas are sensitive to SSA treatment, correlating with expression of SSTR2 and SSTR5 (42). In the current study, the patient did not respond to preoperative SSA treatment, and the reason is unknown. However, in thyrotroph adenomas, it has been reported that the presence of SSTR5 is correlated with both in vivo and in vitro response to SSA (43, 44). In addition, the intracellular downstream mechanisms after SSTRs, including the tumor microenvironment, may be different between thyrotroph adenomas and this tumor (45, 46).
Pathogenesis of GC/MGA development
The simultaneous occurrence of GCs and pituitary adenomas at high rates is unlikely to be a simple coincidental finding and is more likely to involve a causative relationship between the 2 lesions, which needs to be understood. The pituitary gland is composed of the anterior, intermediate, and posterior lobes. The first 2 are derived from Rathke’s pouch, an invagination of the oral ectoderm, whereas the last is derived from the overlying diencephalic neural ectoderm.
Immunohistochemical studies of GCs/MGAs have led to several hypotheses explaining the pathogenesis of these tumors (2–4, 6, 8–12, 27, 47). First, abnormal migration of hypothalamic neurons within the adenohypophyseal parenchyma may occur during embryogenesis (47). Secretion of hormones by the ganglionic cells of hypothalamic origin could lead to the development of adenomas. However, this hypothesis was contradicted because of a lack of correlation between the released hypothalamic hormones and the adenoma cell secretions in some cases (12, 27). Moreover, the hypothalamic-releasing hormones secreted by the ganglionic cells seem to promote proliferation and not initiate tumorigenesis of pituitary adenomas.
Second is the widely accepted theory that pre-existing adenoma cells transdifferentiate into gangliocytic cells with the acquisition of neural differentiation (2, 8). Ultrastructural studies by Horvath et al showed the presence of transitional cell forms between neurons and adenohypophyseal cells (8). Additionally, there were some clinical cases that exhibited neuronal components inside adenomas that could be denominated as neuronal metaplasia (48–51). Although it may be impossible for neoplastic pituitary cells to transform into well-differentiated mature neuronal tumors with the dominating embryological concepts (9), “metaplasia” may arise not only as a result of proliferation and transformation of immature cells, but also of the transformation of mature cells under various conditions such as sublethal injury, chronic inflammation, and vitamin A deficiency (52). However, because a pre-existing adenoma is an absolute condition, it is difficult to explain the occurrence of isolated GCs or other neuronal tumors, including neurocytomas and neuroblastomas (1).
Third is an alternative theory advocated by Kontogeorgos et al that stipulates that neuronal and adenohypophyseal cells may derive from a common pituitary stem/progenitor cell (9, 53). Transdifferentiation of adenoma cells into neurons is yet to be established, but adenoma cells are known to express some neuronal epitopes, particularly synaptophysin, and may, therefore, have the potential to transdifferentiate (51, 54). Some studies have demonstrated that neuronal markers, including NeuN and neurofilament, are expressed in the adenomatous cell component in MGAs, suggesting a common origin for the gangliocytic and adenomatous cells (9, 10, 51, 55). This hypothesis can explain both the differences in the hormonal profiles between adenomatous and gangliocytic components as well as the development of isolated GCs and MGAs.
However, not all gangliocytic tumors within a sellar lesion follow the same pathobiological process, as can be seen from 2 cases of isolated posterior pituitary GCs that did not associate to the adenohypophyseal cells (21, 33). Meanwhile, Horvath et al have demonstrated that the ganglionic cells in the posterior pituitary could be the result of the ectopic migration of neuronal cells (47), and these migrated cells could give rise to such tumors.
Stem cell theory and transcription factors in pituitary GCs/MGAs
It is interesting to note that although the tumor did not have an adenomatous component, the present case had comparable specific-lineage transcription factors to that of thyrotroph adenomas such as Pit-1 and GATA-2; this case also exhibited specific neuronal differentiation. Several cell-restricted transcription factors such as PROP1, Pit-1, SF-1, Tpit, ER, and GATA-2 during cytodifferentiation from the pituitary stem cells (PSCs) have been identified, and PSCs can generate each differentiated cell within pituitary tissues to support organ repair and regeneration. However, it is still unknown whether PSCs are a single population with multipotent differentiation capacity or distinct populations with a more restricted lineage commitment (56–58). Alternatively, a theory has been proposed that tumor stem cells (TSCs) arising from tissue-specific stem cells following an oncogenic transformation may initiate the tumorigenesis and possess stem cell characteristics of self-renewal, maintenance, and the potential to differentiate and grow into a heterogeneous tumor (57–59). Many studies have shown the presence of populations of undifferentiated cells with clonogenic capability within pituitary tumors, suggesting that the TSC model may be relevant to pituitary neoplasms (57). Chen et al found that isolated cells that expressed neural stem/progenitor cell markers formed neurospheres in vitro and generated daughter cells with the capability to differentiate into multiple neural lineages (60). However, they failed to demonstrate the production of pituitary hormone cell types, which may be derived from neural crest stem cells and form pericytes associated with the pituitary vasculature (58, 61).
Some authors have reported that the acidophilic lineage transcription factor Pit-1 is expressed in both adenomatous and gangliocytic cells in MGAs and speculated a common ectodermal origin for the 2 components or transdifferentiation of neuroendocrine cells to a more neuronal phenotype (55, 62). The more likely scenario involves transdifferentiation or differentiation from a progenitor cell originating in the neuroendocrine component that has common transcription factors, rather than neuronal differentiation from well-differentiated adenoma cells, and may generate MGAs and other neuronal tumors in the sellar region.
Proposal for categorization as neuroendocrine neoplasms
A new classification of neoplasms of adenohypophyseal cells proposed to rename them to “pituitary neuroendocrine tumors (PitNETs)” for comprehensive cognizance of their wide pathological spectrum and clinical behavior (63, 64). However, the 2017 WHO classification categorized pituitary tumors according to pituitary cytogenetic lineage and morphofunctional subtypes (1, 64). Concurrently, the WHO International Agency for Research on Cancer has also classified neuroendocrine neoplasms (NENs) across different organ systems, including PitNETs, which have been previously classified using site-specific terminologies and chaotic criteria under a common framework (65).
Although it has long been debated about their triploblastic origin (66), neuroendocrine cells that have acquired neuroendocrine integration from various stem/progenitor cells are found in almost every individual’s organs and are known to form the diffused neuroendocrine system. Neuroendocrine neoplasms express a variable spectrum of proteins shared with their normal cell counterparts at specific anatomical locations, including markers of general neuroendocrine differentiation as well as site-specific markers such as hormones and transcription factors (65, 67).
Furthermore, these arguments concerning the pathogenesis of mixed tumors are also seen in adrenal or gastroenteropancreatic NENs (68–71). “Mixed neuroendocrine–non-NENs (MiNENs)” are defined by the association of at least 2 morphologically different neoplastic components, with the requirement that the minor component comprise ≥ 30% of the tumor, which have been introduced in the fifth edition of the WHO classification of the digestive system tumors published in 2019 (69, 70, 72). Many NENs may show evidence of non-neuroendocrine differentiation, whereas many non-neuroendocrine tumors may contain subpopulations of neuroendocrine cells. These phenomena, including multidirectional differentiation and multihormonal endocrine characteristics, have been observed in NENs of undisputed neural crest origin and in tumors of other origin (73).
The clinical implication of cytokeratin expression in the cytoplasm of gangliocytic cells in MGAs, which has been considered evidence of transdifferentiation of adenoma epithelial cells into gangliocytic cells, is still debated to date (6, 8, 9, 11). However, some authors implied a participation of neural crest-derived cells (9, 11). The immunohistochemical results of positivity for CAM5.2 and CK7 but negativity for CK20 were confirmed in a subpopulation of NENs as well as that of pituitary adenomas, which may indicate organ-related differentiation in neoplasms (38, 74). While the European Pituitary Pathology Group has proposed the diagnostic algorithms for PitNETs, it is interesting to note that the pathological features of GCs/MGAs are more similar to those of NENs than to those of functional adenomas (67, 75).
Pituitary tumors co-secreting both hypothalamic and pituitary hormones
The phenomena in pituitary tumors exhibiting co-secretion of hypothalamic upstream hormones and pituitary downstream hormones in the same tumor cell have been reported on rare occasions. In acromegaly, co-secreting GH-GHRH-producing adenoma has been reported in cases of both pure pituitary adenoma and adenomatous components in MGA (76, 77). Moreover, some investigations have demonstrated that GHRH can synthesize not only in the normal pituitary gland, but also concurrently in somatotroph adenoma cells (78, 79).
In addition, co-secretion of both CRH and ACTH in the same tumor cell of both pure pituitary adenoma and isolated GC have also been reported in patients with Cushing’s disease (35, 80). Domingue et al suggested that these tumor cells can induce adrenal hypersecretion both directly thorough ACTH secretion and indirectly via paracrine stimulation of the normal corticotroph cells (35). Asa et al also reported corticotroph hyperplasia of the surrounding pituitary cells but not corticotroph adenomas in CRH-producing GC (28), supporting the autocrine/paracrine mechanisms. It is interesting that tumors exhibiting co-secretion of both CRH and ACTH are occasionally seen in gastroenteropancreatic NENs rather than in pituitary tumors (81–83).
In our case, diffuse co-secretion of both TSH and TRH was seen in the tumor cells, which is an undisputed cause of SITSH. In general, most thyrotroph adenomas are reported to be large and invasive at diagnosis, and about 30% of the TSH-immunopositive adenomas are clinically silent (41, 44, 84). Moreover, 2 cases of MGAs wherein the gangliocytic component exhibited TSH immunoreactivity have been reported; however, these patients did not clinically manifest hyperthyroidism or SITSH (6, 85). Although our case resembled a microadenoma, the SITSH may have resulted in continuous activation across the hypothalamic–pituitary–thyroid axis. More specifically, autocrine/paracrine stimulation can occur inside and/or outside the tumor cells. Some studies have suggested that the TRH gene is expressed not only in tumoral anterior pituitary cells, but also in normal anterior pituitary cells. Further, TRH signaling induces TSHβ expression through GATA-2 as a principal mediator, which may act as an autocrine/paracrine regulator of TSH secretion (86–90).
Study strengths and limitations
The features of multipotential cells in pituitary GCs/MGAs that lead to mature neuronal differentiation or to hypothalamic–pituitary axis hormonal synthesis could be explained by concepts of systemic NENs. Importantly, it may support the theory that pituitary GCs/MGAs arise from common stem/progenitor cells, specifically from specialized neuroendocrine cells derived from the neural crest in the embryo. Interestingly, some investigators have recently demonstrated that the mesenchyme on the rostral side of Rathke’s pouch originates from the neural crest. Moreover, neural crest cells invade the embryonic anterior pituitary, which is believed to derive solely from the adenohypophyseal placode in a stepwise manner and differentiate into all hormone-producing cell lineages and vascular pericytes (91–94). The presence of such stem/progenitor cells can be explained by the pathogenesis of GCs/MGAs, including wide-ranging differentiation from a subpopulation of pure pituitary adenomas to pure gangliocytomas, including these mixed tumors with various proportions.
In this literature review, only 21 cases of GCs in the sellar region with immunohistochemical studies were identified during the past 35 years, thus limiting the generalizability of our findings. Further research and collection of data on these tumors, including MGAs, are necessary.
Conclusions
We report herein a rare case of pituitary GC-manifesting SITSH. Immunohistochemical studies revealed diffuse co-secretion of both TRH and TSH by the tumor, with diffuse expression of lineage-restricted transcription factors (ie, Pit-1 and GATA-2), which may have resulted in a continuous autocrine/paracrine regulation of TSH secretion. The high frequency of co-occurrence of gangliocytic and adenomatous components within a neuroendocrine tumor suggests that they arise from multipotent progenitor cells that can differentiate into multidirectional morphology and have the capacity for multihormonal synthesis; these abovementioned aspects result in syndromes of pituitary hypersecretion in an autocrine/paracrine manner.
Acknowledgments
We thank our helpful and efficient nursing, ENT, and anesthesiology teams for the perioperative management of the endoscopic endonasal pituitary surgery.
Glossary
Abbreviations
- GC
gangliocytoma
- MGA
mixed gangliocytoma-adenoma
- NET
neuroendocrine tumors
- PitNETs
pituitary neuroendocrine tumor
- PRL
prolactin
- PSC
pituitary stem cell
- SITSH
syndrome of inappropriate secretion of thyroid-stimulating hormone
- SSA
somatostatin analog
- SSTR
somatostatin receptor
- TSC
tumor stem cell
- WHO
World Health Organization
Additional Information
Disclosure Summary: The authors have no personal financial and institutional interest in any of the drugs, materials, or devices described in this article.
Data Availability
The datasets and analyzed data in this current study are available from the corresponding author upon reasonable request.
References
- 1. Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134(4):521–535. [DOI] [PubMed] [Google Scholar]
- 2. Puchner MJ, Lüdecke DK, Saeger W, Riedel M, Asa SL. Gangliocytomas of the sellar region–a review. Exp Clin Endocrinol Diabetes. 1995;103(3):129–149. [DOI] [PubMed] [Google Scholar]
- 3. Cossu G, Daniel RT, Messerer M. Gangliocytomas of the sellar region: a challenging diagnosis. Clin Neurol Neurosurg. 2016;149:122–135. [DOI] [PubMed] [Google Scholar]
- 4. Towfighi J, Salam MM, McLendon RE, Powers S, Page RB. Ganglion cell-containing tumors of the pituitary gland. Arch Pathol Lab Med. 1996;120(4):369–377. [PubMed] [Google Scholar]
- 5. Qiao N, Ye Z, Wang Y, et al. Gangliocytomas in the sellar region. Clin Neurol Neurosurg. 2014;126:156–161. [DOI] [PubMed] [Google Scholar]
- 6. Balci S, Saglam A, Oruckaptan H, Erbas T, Soylemezoglu F. Pituitary adenoma with gangliocytic component: report of 5 cases with focus on immunoprofile of gangliocytic component. Pituitary. 2015;18(1):23–30. [DOI] [PubMed] [Google Scholar]
- 7. Shepard MJ, Elzoghby MA, Ghanim D, Lopes MBS, Jane JA Jr. Transsphenoidal surgery for mixed pituitary gangliocytoma-adenomas. World Neurosurg. 2017;108:310–316. [DOI] [PubMed] [Google Scholar]
- 8. Horvath E, Kovacs K, Scheithauer BW, Lloyd RV, Smyth HS. Pituitary adenoma with neuronal choristoma (PANCH): composite lesion or lineage infidelity? Ultrastruct Pathol. 1994;18(6):565–574. [DOI] [PubMed] [Google Scholar]
- 9. Kontogeorgos G, Mourouti G, Kyrodimou E, Liapi-Avgeri G, Parasi E. Ganglion cell containing pituitary adenomas: signs of neuronal differentiation in adenoma cells. Acta Neuropathol. 2006;112(1):21–28. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen MT, Lavi E. Pituitary adenoma-neuronal choristoma is a pituitary adenoma with ganglionic differentiation. Exp Mol Pathol. 2015;99(3):628–631. [DOI] [PubMed] [Google Scholar]
- 11. Novello M, Gessi M, Doglietto F, Anile C, Lauriola L, Coli A. Characteristics of ganglion cells in pituitary gangliocytomas. Neuropathology. 2017;37(1):64–68. [DOI] [PubMed] [Google Scholar]
- 12. Fischer EG, Morris JH, Kettyle WM. Intrasellar gangliocytoma and syndromes of pituitary hypersecretion. Case report. J Neurosurg. 1983;59(6):1071–1075. [DOI] [PubMed] [Google Scholar]
- 13. Greenfield JG. The pathological examination of forty intracranial neoplasms. Brain. 1919;42(1):29–85. [Google Scholar]
- 14. Courville CB. Ganglioglioma. Tumor of the central nervous system; review of the literature and report of two cases. Arch Neurol Psychiatry. 1930;24(3):439–491. [Google Scholar]
- 15. Alpers BJ, Grant FC. The ganglioneuromas of the central nervous system. Arch Neurol Psychiatry. 1931;26(3):501–523. [Google Scholar]
- 16. Arseni C, Horvath L, Carp N, Ciurea V. Intracranial ganglioneuromas in children. Acta Neurochir (Wien). 1975;32(3-4):279–286. [DOI] [PubMed] [Google Scholar]
- 17. Rhodes RH, Dusseau JJ, Boyd AS Jr, Knigge KM. Intrasellar neural-adenohypophyseal choristoma. A morphological and immunocytochemical study. J Neuropathol Exp Neurol. 1982;41(3):267–280. [DOI] [PubMed] [Google Scholar]
- 18. McCowen KC, Glickman JN, Black PM, Zervas NT, Lidov HG, Garber JR. Gangliocytoma masquerading as a prolactinoma. Case report. J Neurosurg. 1999;91(3):490–495. [DOI] [PubMed] [Google Scholar]
- 19. Kiyono H. Die Histopathologie der hypophyse. Virchows Arch (Pathol Anat). 1926;259:388–465. [Google Scholar]
- 20. Benda HC. Beiträge zur normalen und pathologischen Morphologie der Hypophyse. Verh Dtsch Ges Pathol. 1927;22:185–190. [Google Scholar]
- 21. Casper J. Ueber neurogene geschwülste im hinterlappen der Hypophyse. Zentralb Allg Pathol. 1932/1933;56:404–411. [Google Scholar]
- 22. Robertson DM, Hetherington RF. A case of ganglioneuroma arising in the pituitary fossa. J Neurol Neurosurg Psychiatry. 1964;27(3):268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serebrin R, Robertson DM. Ganglioneuroma arising in the pituitary fossa: a twenty year follow-up. J Neurol Neurosurg Psychiatry. 1984;47(1):97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jakumeit HD, Zimmermann V, Guiot G. Intrasellar gangliocytomas. Report of four cases. J Neurosurg. 1974;40(5):626–630. [DOI] [PubMed] [Google Scholar]
- 25. Ule G, Waidelich FW. Neurosekretorisches ganglienzell-choristom in der adenohypophyse. [Neurosekretorisches Ganglienzell-choristom in der adenohypophyse]. Acta Neuropath (Berl). 1976;36(1):81–84. [DOI] [PubMed] [Google Scholar]
- 26. Nikonov AA. [Hormonally active (somatotropin) hypophyseal ganglioneuroma]. Arkh Patol. 1981;43(12):48–52. [PubMed] [Google Scholar]
- 27. Asa SL, Scheithauer BW, Bilbao JM, et al. A case for hypothalamic acromegaly: a clinicopathological study of six patients with hypothalamic gangliocytomas producing growth hormone-releasing factor. J Clin Endocrinol Metab. 1984;58(5):796–803. [DOI] [PubMed] [Google Scholar]
- 28. Asa SL, Kovacs K, Tindall GT, Barrow DL, Horvath E, Vecsei P. Cushing’s disease associated with an intrasellar gangliocytoma producing corticotrophin-releasing factor. Ann Intern Med. 1984;101(6):789–793. [DOI] [PubMed] [Google Scholar]
- 29. Nishio S, Takei Y, Fukui M. Immunoreactivity with “hypothalamic” neuropeptides in neuronal neoplasms of the central nervous system. With special reference to the histogenesis of neuronal neoplasms. Neurol Med Chir (Tokyo). 1987;27(2):105–109. [DOI] [PubMed] [Google Scholar]
- 30. Yamada S, Stefaneanu L, Kovacs K, Aiba T, Shishiba Y, Hara M. Intrasellar gangliocytoma with multiple immunoreactivities. Endocr Pathol. 1990;1(1):58. [DOI] [PubMed] [Google Scholar]
- 31. Baysefer A, Gezen F, Kayali H, Erdoğan E, Timurkaynak E, Celasun B. Intrasellar gangliocytoma resembling pituitary adenoma. Minim Invasive Neurosurg. 1997;40(3):107–109. [DOI] [PubMed] [Google Scholar]
- 32. Saeger W, Lüdecke DK, Losa M. Combined neuronal and endocrine tumors of the sellar region. Pathologe. 1997;18(6):419–424. [DOI] [PubMed] [Google Scholar]
- 33. Geddes JF, Jansen GH, Robinson SF, et al. “Gangliocytomas” of the pituitary: a heterogeneous group of lesions with differing histogenesis. Am J Surg Pathol. 2000;24(4):607–613. [DOI] [PubMed] [Google Scholar]
- 34. Isidro ML, Iglesias Díaz P, Matías-Guiu X, Cordido F. Acromegaly due to a growth hormone-releasing hormone-secreting intracranial gangliocytoma. J Endocrinol Invest. 2005;28(2):162–165. [DOI] [PubMed] [Google Scholar]
- 35. Domingue ME, Marbaix E, Do Rego JL, et al. Infrasellar pituitary gangliocytoma causing Cushing’s syndrome. Pituitary. 2015;18(5):738–744. [DOI] [PubMed] [Google Scholar]
- 36. Petrakakis I, Pirayesh A, Krauss JK, Raab P, Hartmann C, Nakamura M. The sellar and suprasellar region: a “hideaway” of rare lesions. Clinical aspects, imaging findings, surgical outcome and comparative analysis. Clin Neurol Neurosurg. 2016;149:154–165. [DOI] [PubMed] [Google Scholar]
- 37. Donadille B, Villa C, Gaillard S, Christin-Maitre S. Gangliocytoma: outcome of a rare silent pituitary tumour. BMJ Case Rep. 2017;2017:bcr2016218859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cai YC, Banner B, Glickman J, Odze RD. Cytokeratin 7 and 20 and thyroid transcription factor 1 can help distinguish pulmonary from gastrointestinal carcinoid and pancreatic endocrine tumors. Hum Pathol. 2001;32(10):1087–1093. [DOI] [PubMed] [Google Scholar]
- 39. Volante M, Brizzi MP, Faggiano A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol. 2007;20(11):1172–1182. [DOI] [PubMed] [Google Scholar]
- 40. Jailer JW, Holub DA. Remission of Graves’ disease following radiotherapy of a pituitary neoplasm. Am J Med. 1960;28(3):497–500. [DOI] [PubMed] [Google Scholar]
- 41. Amlashi FG, Tritos NA. Thyrotropin-secreting pituitary adenomas: epidemiology, diagnosis, and management. Endocrine. 2016;52(3):427–440. [DOI] [PubMed] [Google Scholar]
- 42. Thodou E, Kontogeorgos G. Somatostatin receptor profile in pituitary thyrotroph adenomas. Clin Neurol Neurosurg. 2020;195:105865. [DOI] [PubMed] [Google Scholar]
- 43. Gatto F, Barbieri F, Castelletti L, et al. In vivo and in vitro response to octreotide LAR in a TSH-secreting adenoma: characterization of somatostatin receptor expression and role of subtype 5. Pituitary. 2011;14(2):141–147. [DOI] [PubMed] [Google Scholar]
- 44. Wang EL, Qian ZR, Yamada S, et al. Clinicopathological characterization of TSH-producing adenomas: special reference to TSH-immunoreactive but clinically non-functioning adenomas. Endocr Pathol. 2009;20(4):209–220. [DOI] [PubMed] [Google Scholar]
- 45. Chalabi M, Duluc C, Caron P, et al. Somatostatin analogs: does pharmacology impact antitumor efficacy? Trends Endocrinol Metab. 2014;25(3):115–127. [DOI] [PubMed] [Google Scholar]
- 46. Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol. 2013;34(3):228–252. [DOI] [PubMed] [Google Scholar]
- 47. Horvath E, Kovacs K, Tran A, Scheithauer BW. Ganglion cells in the posterior pituitary: result of ectopia or transdifferentiation? Acta Neuropathol. 2000;100(1):106–110. [DOI] [PubMed] [Google Scholar]
- 48. Scheithauer BW, Horvath E, Kovacs K, et al. Prolactin-producing pituitary adenoma and carcinoma with neuronal components–a metaplastic lesion. Pituitary. 1999;1(3-4):197–205. [DOI] [PubMed] [Google Scholar]
- 49. Rotondo F, Bernardo MC, Scheithauer BW, et al. Atypical pituitary adenoma with neurocytic transformation. Appl Immunohistochem Mol Morphol. 2014;22(1):72–76. [DOI] [PubMed] [Google Scholar]
- 50. Thodou E, Kontogeorgos G, Horvath E, Kovacs K. Prolactin-producing pituitary adenoma with incomplete neuronal transformation: an intermediate adenoma-neuronal tumor. Acta Neuropathol. 2004;108(2):115–120. [DOI] [PubMed] [Google Scholar]
- 51. Johnson MD, Fan X, Bourne P, Walters D. Neuronal differentiation and expression of neural epitopes in pituitary adenomas. J Histochem Cytochem. 2007;55(12):1265–1271. [DOI] [PubMed] [Google Scholar]
- 52. Lugo M, Putong PB. Metaplasia. An overview. Arch Pathol Lab Med. 1984;108(3):185–189. [PubMed] [Google Scholar]
- 53. Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146(9):3985–3998. [DOI] [PubMed] [Google Scholar]
- 54. Lloyd RV, Scheithauer BW, Kovacs K, Roche PC. The immunophenotype of pituitary adenomas. Endocr Pathol. 1996;7(2):145–150. [DOI] [PubMed] [Google Scholar]
- 55. Lopes MB, Sloan E, Polder J. Mixed gangliocytoma-pituitary adenoma: insights on the pathogenesis of a rare sellar tumor. Am J Surg Pathol. 2017;41(5):586–595. [DOI] [PubMed] [Google Scholar]
- 56. Vankelecom H, Chen J. Pituitary stem cells: where do we stand? Mol Cell Endocrinol. 2014;385(1-2):2–17. [DOI] [PubMed] [Google Scholar]
- 57. Haston S, Manshaei S, Martinez-Barbera JP. Stem/progenitor cells in pituitary organ homeostasis and tumourigenesis. J Endocrinol. 2018;236(1):R1–R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Caffarini M, Orciani M, Trementino L, Di Primio R, Arnaldi G. Pituitary adenomas, stem cells, and cancer stem cells: what’s new? J Endocrinol Invest. 2018;41(7):745–753. [DOI] [PubMed] [Google Scholar]
- 59. Würth R, Thellung S, Corsaro A, Barbieri F, Florio T. Experimental evidence and clinical implications of pituitary adenoma stem cells. Front Endocrinol (Lausanne). 2020;11(2):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen L, Ye H, Wang X, et al. Evidence of brain tumor stem progenitor-like cells with low proliferative capacity in human benign pituitary adenoma. Cancer Lett. 2014;349(1):61–66. [DOI] [PubMed] [Google Scholar]
- 61. Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128(7):1059–1068. [DOI] [PubMed] [Google Scholar]
- 62. Sergeant C, Jublanc C, Leclercq D, et al. Transdifferentiation of neuroendocrine cells: gangliocytoma associated with two pituitary adenomas of different lineage in MEN1. Am J Surg Pathol. 2017;41(6):849–853. [DOI] [PubMed] [Google Scholar]
- 63. Asa SL, Casar-Borota O, Chanson P, et al. ; attendees of 14th Meeting of the International Pituitary Pathology Club, Annecy, France, November 2016. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an International Pituitary Pathology Club proposal. Endocr Relat Cancer. 2017;24(4):C5–C8. [DOI] [PubMed] [Google Scholar]
- 64. Trouillas J, Jaffrain-Rea ML, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers (Basel). 2020;12(2):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosai J. The origin of neuroendocrine tumors and the neural crest saga. Mod Pathol. 2011;24(Suppl 2):S53–S57. [DOI] [PubMed] [Google Scholar]
- 67. Duan K, Mete O. Algorithmic approach to neuroendocrine tumors in targeted biopsies: practical applications of immunohistochemical markers. Cancer Cytopathol. 2016;124(12):871–884. [DOI] [PubMed] [Google Scholar]
- 68. Gupta S, Zhang J, Erickson LA. Composite pheochromocytoma/paraganglioma-ganglioneuroma: a clinicopathologic study of eight cases with analysis of succinate dehydrogenase. Endocr Pathol. 2017;28(3):269–275. [DOI] [PubMed] [Google Scholar]
- 69. La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol. 2016;27(4):284–311. [DOI] [PubMed] [Google Scholar]
- 70. La Rosa S, Uccella S, Molinari F, et al. Mixed adenoma well-differentiated neuroendocrine tumor (MANET) of the digestive system: an indolent subtype of mixed neuroendocrine-nonneuroendocrine neoplasm (MiNEN). Am J Surg Pathol. 2018;42(11):1503–1512. [DOI] [PubMed] [Google Scholar]
- 71. Lau SK, Chu PG, Weiss LM. Mixed cortical adenoma and composite pheochromocytoma-ganglioneuroma: an unusual corticomedullary tumor of the adrenal gland. Ann Diagn Pathol. 2011;15(3):185–189. [DOI] [PubMed] [Google Scholar]
- 72. Bellizzi AM. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol. 2020;96(2):8–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. DeLellis RA, Tischler AS, Wolfe HJ. Multidirectional differentiation in neuroendocrine neoplasms. J Histochem Cytochem. 1984;32(8):899–904. [DOI] [PubMed] [Google Scholar]
- 74. Cykowski MD, Takei H, Baskin DS, Rivera AL, Powell SZ. Epithelial and organ-related marker expression in pituitary adenomas. Neuropathology. 2016;36(4):354–364. [DOI] [PubMed] [Google Scholar]
- 75. Villa C, Vasiljevic A, Jaffrain-Rea ML, et al. A standardised diagnostic approach to pituitary neuroendocrine tumours (PitNETs): a European Pituitary Pathology Group (EPPG) proposal. Virchows Arch. 2019;475(6):687–692. [DOI] [PubMed] [Google Scholar]
- 76. Matsuno A, Katakami H, Sanno N, et al. Pituitary somatotroph adenoma producing growth hormone (GH)-releasing hormone (GHRH) with an elevated plasma GHRH concentration: a model case for autocrine and paracrine regulation of GH secretion by GHRH. J Clin Endocrinol Metab. 1999;84(9):3241–3247. [DOI] [PubMed] [Google Scholar]
- 77. Teramoto S, Tange Y, Ishii H, Goto H, Ogino I, Arai H. Mixed gangliocytoma-pituitary adenoma containing GH and GHRH co-secreting adenoma cells. Endocrinol Diabetes Metab Case Rep. 2019;2019(2019):EDM190099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Joubert D, Benlot C, Lagoguey A, et al. Normal and growth hormone (GH)-secreting adenomatous human pituitaries release somatostatin and GH-releasing hormone. J Clin Endocrinol Metab. 1989;68(3):572–577. [DOI] [PubMed] [Google Scholar]
- 79. Matsuno A, Katakami H, Nagashima T, Teramoto A, Osamura RY, Kirino T. Growth hormone-releasing hormone expression in pituitary somatotroph adenomas, studied by immunohistochemistry and in situ hybridization using catalyzed signal amplification system. Hum Pathol. 2000;31(7):789–794. [DOI] [PubMed] [Google Scholar]
- 80. Yamada Y, Ohashi A, Inoue T, et al. Cushing’s syndrome with a large pituitary adenoma producing both corticotropin-releasing hormone (CRH) and adrenocorticotropin (ACTH). Intern Med. 2002;41(7):549–554. [DOI] [PubMed] [Google Scholar]
- 81. Karageorgiadis AS, Papadakis GZ, Biro J, et al. Ectopic adrenocorticotropic hormone and corticotropin-releasing hormone co-secreting tumors in children and adolescents causing cushing syndrome: a diagnostic dilemma and how to solve it. J Clin Endocrinol Metab. 2015;100(1):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park SY, Rhee Y, Youn JC, et al. Ectopic Cushing’s syndrome due to concurrent corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secreted by malignant gastrinoma. Exp Clin Endocrinol Diabetes. 2007;115(1):13–16. [DOI] [PubMed] [Google Scholar]
- 83. Sauer N, zur Wiesch CS, Flitsch J, et al. Cushing’s syndrome due to a corticotropin-releasing hormone- and adrenocorticotrophic hormone-producing neuroendocrine pancreatic tumor. Endocr Pract. 2014;20(4):e53–e57. [DOI] [PubMed] [Google Scholar]
- 84. Clarke MJ, Erickson D, Castro MR, Atkinson JL. Thyroid-stimulating hormone pituitary adenomas. J Neurosurg. 2008;109(1):17–22. [DOI] [PubMed] [Google Scholar]
- 85. Yang B, Yang C, Sun Y, et al. Mixed gangliocytoma-pituitary adenoma in the sellar region: a large-scale single-center experience. Acta Neurochir (Wien). 2018;160(10):1989–1999. [DOI] [PubMed] [Google Scholar]
- 86. Bruhn TO, Rondeel JM, Jackson IM. Thyrotropin-releasing hormone gene expression in the anterior pituitary. IV. Evidence for paracrine and autocrine regulation. Endocrinology. 1998;139(8):3416–3422. [DOI] [PubMed] [Google Scholar]
- 87. Joseph-Bravo P, Jaimes-Hoy L, Charli JL. Advances in TRH signaling. Rev Endocr Metab Disord. 2016;17(4): 545–558. [DOI] [PubMed] [Google Scholar]
- 88. Ohba K, Sasaki S, Matsushita A, et al. GATA2 mediates thyrotropin-releasing hormone-induced transcriptional activation of the thyrotropin β gene. Plos One. 2011;6(4): e18667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pagesy P, Croissandeau G, Le Dafniet M, Peillon F, Li JY. Detection of thyrotropin-releasing hormone (TRH) mRNA by the reverse transcription-polymerase chain reaction in the human normal and tumoral anterior pituitary. Biochem Biophys Res Commun. 1992;182(1):182–187. [DOI] [PubMed] [Google Scholar]
- 90. Pazos-Moura CC, Ortiga-Carvalho TM, Gaspar de Moura E. The autocrine/paracrine regulation of thyrotropin secretion. Thyroid. 2003;13(2):167–175. [DOI] [PubMed] [Google Scholar]
- 91. Cheung LY, Davis SW, Brinkmeier ML, Camper SA, Pérez-Millán MI. Regulation of pituitary stem cells by epithelial to mesenchymal transition events and signaling pathways. Mol Cell Endocrinol. 2017;445:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Davis SW, Mortensen AH, Keisler JL, et al. β-catenin is required in the neural crest and mesencephalon for pituitary gland organogenesis. BMC Dev Biol. 2016;16(1):16. doi: 10.1186/s12861-016-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ueharu H, Yoshida S, Kikkawa T, et al. Gene tracing analysis reveals the contribution of neural crest-derived cells in pituitary development. J Anat. 2017;230(3):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ueharu H, Yoshida S, Kanno N, et al. SOX10-positive cells emerge in the rat pituitary gland during late embryogenesis and start to express S100β. Cell Tissue Res. 2018;372(1):77–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and analyzed data in this current study are available from the corresponding author upon reasonable request.