Abstract
Fishes of the family Lethrinidae form a considerable portion of the catch from both the Red Sea and the Arabian Gulf in Saudi Arabia, and the species Lethrinus lentjan (Lacepède, 1802) is one of the most important among these fishes. This study was conducted to evaluate the demographic structure coefficients, survival rate, and stock status of L. lentjan from the Red Sea coast of Saudi Arabia. A total of 593 samples were collected on a monthly basis for a period of one year from the landing site for fishing boats operating in Red Sea waters off Jeddah. The results indicated that 88.87% of the specimens were female; the maximum total length recorded was 43.5 cm with the most frequent length (14.67%) being 23–23.9 cm. The maximum age recorded for both males and females was 6 year-plus, and the 1 year-plus age category represented the majority of the samples (57.67%). The total mortality coefficient, natural mortality coefficient, and fishing mortality coefficient were 1.538, 0.315, and 1.223, respectively; all mortality coefficients in the female fishes were higher than those in the male fishes. The survival rate of males was higher (0.617) than that of females (0.214). The results of the present study indicate that L. lentjan is subjected to overfishing and a new management strategy is necessary to improve the stock status of this fish species.
Keywords: Demographic structure, Stock status, Lethrinus lentjan, Red Sea
1. Introduction
Fisheries are considered an important food security resource in many countries worldwide, especially coastal nations, where they constitute one of the most important sources of high-quality animal protein. In addition, fishes are a source of many other important nutrients such as lipids, which are characterized by unsaturated fatty acids, especially omega-3. Also fishes are distinguished by containing many important minerals such as phosphorous and iodine (Younis et al., 2011).
The Red Sea is a rich and diverse ecosystem (Wood, 2016). It is considered the main source of fish production in Saudi Arabia in addition to the production in both the Arabian Gulf and aquaculture (FAO, 2003). The catch composition from the Red Sea is highly varied, comprising large pelagics, small pelagics, demersal fishes, mollusks, and crustacean species (Wood, 2016).
The Lethrinids (Family Lethrinidae) are widely distributed in both tropical and subtropical areas worldwide (Carpenter and Allen, 1989). The Lethrinids, which are also known as the family of the emperor fish (Emperors), are coastal fishes found primarily on or near reefs; they are important components of many fisheries in various coastal countries (Mehanna et al., 2012). In Saudi Arabia, they form a considerable part of the catch from both the Red Sea and the Arabian Gulf (Ezzat et al., 1994).
Fisheries management depend essentially on the Knowledge of the life histories of exploited fish species which include their growth, size at maturity, spawning season, aging and mortality rates as well as the demographic structures of fish population and stock status (Tracey et al., 2006).
Despite the commercial importance and the abundance of fish species in the Red Sea, there are limited publications describing the biology and stock status of fish in the territorial waters of the Red Sea countries. The species Lethrinus lentjan, (Lacepède, 1802), known locally as Shaour, is one of the most important fishes of Lethrinus that exist in abundance in the Red Sea and the Arabian Gulf; it is considered a significant catch species in the fisheries of Saudi Arabia. Therefore, the present study was conducted to evaluate the demographic structure, mortality coefficients, survival rate, and stock status of L. lentjan from the Red Sea coast of Saudi Arabia to provide a scientific basis for appropriate management strategies toward fisheries sustainability.
2. Materials and methods
2.1. Sample collection
In total, 593 specimens of L. lentjan were collected throughout the period from January 2015 to December 2015 from the landing site for fishing boats operating in Red Sea waters off Jeddah, Saudi Arabia (21°29′24″N; 39°10′23″E). The samples included the different lengths and sizes represented in the catch; these samples were euthanized by immersion in freezing water, placed in ice-box and transported to the fisheries research laboratory at the Zoology Department, College of Science, King Saud University.
2.2. Sample processing
All samples were subjected to gross examination then dissected, where the sexes were distinguished and separated. The total length of each fish was measured to the nearest 0.1 cm, and the total body weight was determined to the nearest 0.1 g. Samples of scales from each fish were collected and stored in 70% alcohol for later age determination according to the method described by Condini et al. (2014).
2.3. Demographic structure
2.3.1. Length composition
Based on their total length, fish were classified into length categories. Length frequency and percentage of samples in each length category were determined.
2.3.2. Age composition
After determining the age of each fish, samples were classified into age categories. Age frequency and percentage of samples in each age category were determined.
2.4. Mortality coefficients
2.4.1. Total mortality coefficient (Z)
The Z was determined using the fishing curve analysis method, which is dependent on the length composition (Pauly, 1983). In this method, the lengths are converted to their corresponding ages using von Bertalanffy coefficients (von Bertalanffy, 1938).
2.4.2. Natural mortality coefficient (M)
The M was calculated using a method that depends on growth factors (Jensen, 1996) as follows:
Where, K is the growth factor of the von Bertalanffy equation.
2.4.3. Fishing mortality coefficient (F)
The F was calculated by calculating the difference between Z and M as
2.5. Survival rate (S)
The following formula was used to estimate the survival rate:
Where, Z is the total mortality coefficient.
2.6. Estimation of fish stock
The analysis for relative yield per recruit (Y/R) was performed by applying the model developed by Beverton and Holt (1966) as incorporated in FiSAT II software.
3. Results
3.1. Demographic structure
3.1.1. Length composition
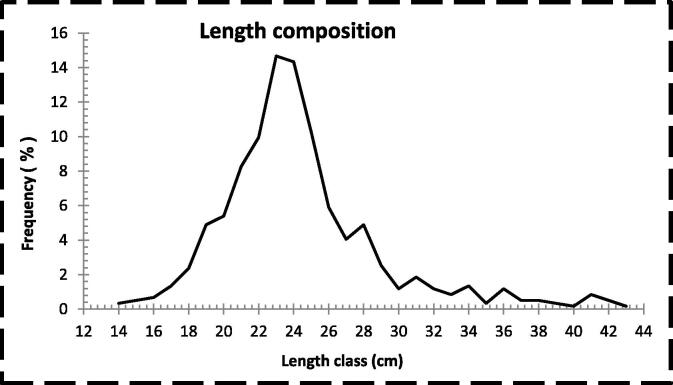
The length composition based on the 593 specimens of L. lentjan collected from the landing site for fishing boats operating in Red Sea waters off Jeddah, Saudi Arabia, during the period from January 2015 to December 2015 is presented in Table 1 and Fig. 1. The length categories for the samples were divided into 30 classes ranging from 14 to 14.9 cm as the smallest class and 43–43.9 cm as the largest. The distribution of the length composition indicated that samples of the small length categories (14–18.9 cm) constituted 4.72%, whereas a large percentage (82.63%) was recorded for the median length classes (19 to 28.9 cm). A peak of frequency (14.67%) was recorded at the length classes 23–23.9 cm followed by a lower peak (14.33%) at the length class 24–24.9 cm. The relatively large length classes (29–36.9 cm) formed 10.45% of the total samples, whereas the large length classes (37–43.9 cm) made up the lowest percentage (2.19%). Regarding to the relationship of the length composition of the samples to the sex of the fish, the results indicated that the number of female fishes was 527, which constituted 88.87% of the total fish samples. The length composition of the female fishes showed that the small length classes (14–18.9 cm) formed 4.93% and the median length classes (19–28.9 cm) represented 86.53%. The relatively large length classes of female fishes (29–36.9 cm) formed 7.78%, whereas the large classes (37–43.9 cm) constituted a very low percentage (0.76%). The number of male fishes was 66, representing 11.13% of the total fish samples and the length composition of the male fishes showed a different distribution compared to that of the female fishes. The lowest percentage of male fishes (3.03%) was recorded for small fish classes (14–18.9 cm), whereas the medium length classes (19–28.9 cm) made up 51.52% of the male fishes. Unlike female fishes, the relatively large length classes of male fishes (29–36.9 cm) accounted for about a third of the samples (31.82%), and the large classes (37–43.9 cm) constituted 13.64%.
Table 1.
Length composition Lethrinus lentjan in the Red Sea waters off Jeddah, Saudi Arabia.
| Length category (TL, cm) | Numberof samples | % | Number of males | % | Numberof females | % |
|---|---|---|---|---|---|---|
| 14–14.9 | 2 | 0.34 | 0 | 0 | 2 | 0.38 |
| 15–15.9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16–16.9 | 4 | 0.67 | 0 | 0 | 4 | 0.76 |
| 17–17.9 | 8 | 1.35 | 0 | 0 | 8 | 1.52 |
| 18–18.9 | 14 | 2.36 | 2 | 3.03 | 12 | 2.28 |
| 19–19.9 | 29 | 4.89 | 4 | 6.06 | 25 | 4.74 |
| 20–20.9 | 32 | 5.4 | 5 | 7.58 | 27 | 5.12 |
| 21–21.9 | 49 | 8.26 | 2 | 3.03 | 47 | 8.92 |
| 22–22.9 | 59 | 9.95 | 2 | 3.03 | 57 | 10.82 |
| 23–23.9 | 87 | 14.67 | 6 | 9.09 | 81 | 15.37 |
| 24–24.9 | 85 | 14.33 | 3 | 4.55 | 82 | 15.56 |
| 25–25.9 | 61 | 10.29 | 3 | 4.55 | 58 | 11.01 |
| 26–26.9 | 35 | 5.9 | 1 | 1.52 | 34 | 6.45 |
| 27–27.9 | 24 | 4.05 | 3 | 4.55 | 21 | 3.98 |
| 28–28.9 | 29 | 4.89 | 5 | 7.58 | 24 | 4.55 |
| 29–29.9 | 15 | 2.53 | 2 | 3.03 | 13 | 2.47 |
| 30–30.9 | 7 | 1.18 | 2 | 3.03 | 5 | 0.95 |
| 31–31.9 | 11 | 1.85 | 2 | 3.03 | 9 | 1.71 |
| 32–32.9 | 7 | 1.18 | 2 | 3.03 | 5 | 0.95 |
| 33–33.9 | 5 | 0.84 | 2 | 3.03 | 3 | 0.57 |
| 34–34.9 | 8 | 1.35 | 6 | 9.09 | 2 | 0.38 |
| 35–35.9 | 2 | 0.34 | 1 | 1.52 | 1 | 0.19 |
| 36–36.9 | 7 | 1.18 | 4 | 6.06 | 3 | 0.57 |
| 37–37.9 | 3 | 0.51 | 3 | 4.55 | 0 | 0 |
| 38–38.9 | 3 | 0.51 | 3 | 4.55 | 0 | 0 |
| 39–39.9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40–40.9 | 1 | 0.17 | 1 | 1.52 | 0 | 0 |
| 41–41.9 | 5 | 0.84 | 1 | 1.52 | 4 | 0.76 |
| 42–42.9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 43–43.9 | 1 | 0.17 | 1 | 1.52 | 0 | 0 |
| Total | 593 | 66 | 527 |
Fig. 1.
Length frequency distribution of Lethrinus lentjan in the Red Sea waters off Jeddah, Saudi Arabia.
3.1.2. Age composition
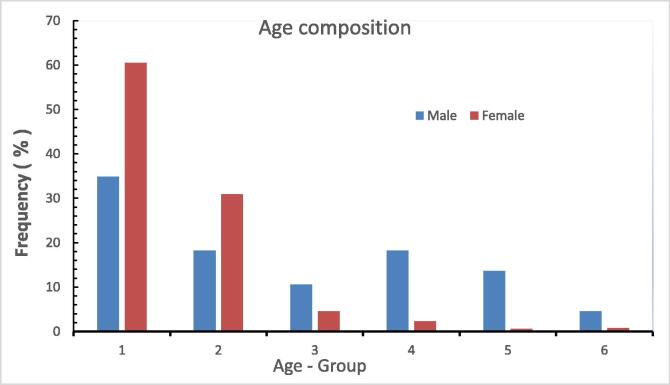
The results of age composition of L. lentjan are shown in Table 2 and Fig. 2. The age composition of the 593 specimens was classified into seven groups from less than 1 year (0+) up to 6 year-plus (VI+). The age category of 1 year-plus (I+) included the majority of fishes specimens (57.67%) followed by the 2 year-plus group (II+) (29.51%), whereas the (0+) group was represented by only two fishes (0.34%). As the female fishes accounted for the majority of the total samples (527 of 593), the results of the age distribution of the females were very similar to that of the total sample, where 60.53% of fishes belonged to the I+ age group followed by the II+ group (30.93%). In contrast, 0.38% of the females were recorded in the (0+) group. Regarding to the age composition of male fishes, no samples were recorded in the (0+) group, whereas 34.85% and 18.18% were recorded for the groups I+ and II+, respectively. The main difference in the age distribution between males and females was that the percentages of males in the older groups (III+, IV+, V+, and VI+) were higher (10.61, 18.18, 13.64, and 4.55%, respectively) than those of females (4.55, 2.28, 0.57, and 0.76%, respectively).
Table 2.
Age composition Lethrinus lentjan in the Red Sea waters off Jeddah, Saudi Arabia.
| Age - Group | Number of samples | % | Numberof Males | % | Numberof Females | % | |
|---|---|---|---|---|---|---|---|
| 0+ | 2 | 0.34 | 0 | 0.00 | 2 | 0.38 | |
| I+ | 342 | 57.67 | 23 | 34.85 | 319 | 60.53 | |
| II+ | 175 | 29.51 | 12 | 18.18 | 163 | 30.93 | |
| III+ | 31 | 5.23 | 7 | 10.61 | 24 | 4.55 | |
| IV+ | 24 | 4.05 | 12 | 18.18 | 12 | 2.28 | |
| V+ | 12 | 2.02 | 9 | 13.64 | 3 | 0.57 | |
| VI+ | 7 | 1.18 | 3 | 4.55 | 4 | 0.76 | |
| Total | 593 | 66 | 527 | ||||
Fig. 2.
Age frequency distribution of Lethrinus lentjan in the Red Sea waters off Jeddah, Saudi Arabia.
3.2. Survival and mortality
Results of mortality coefficients and survival rate are represented in Table 3. The Z, M, and F of the female fishes (1.538, 0.315, and 1.223, respectively) were higher than those of the male fishes (0.482, 0.282, and 0.201, respectively). Unlike mortality coefficients, the results of survival rate indicated that male fishes outperformed females as they recorded a survival rate of 0.617, whereas the survival rate for females was 0.214.
Table 3.
Survival rate and mortality coefficients of Lethrinus lentjan in the Red Sea waters off Jeddah, Saudi Arabia.
| Samples | Total mortality coefficient (Z) | Natural mortality coefficient (M) | Fishing mortality Coefficient (F) | Survival rate (S) |
|---|---|---|---|---|
| Total sample | 1.08 | 0.30 | 0.78 | 0.338 |
| Males | 0.482 | 0.282 | 0.201 | 0.617 |
| Females | 1.538 | 0.315 | 1.223 | 0.214 |
3.3. Estimation of fish stock
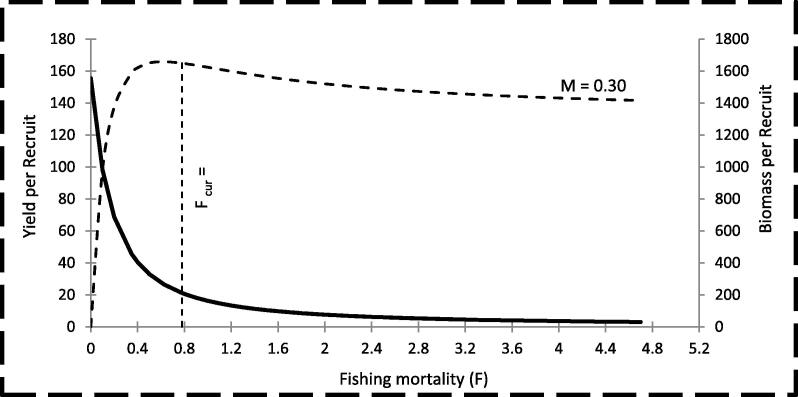
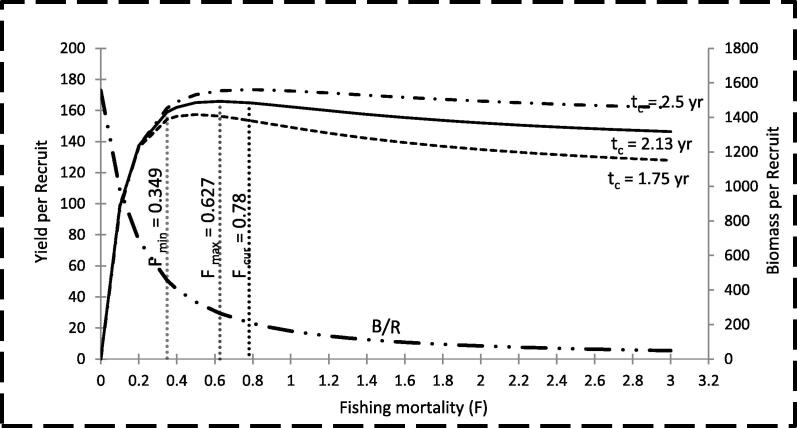
The relative Y/R was calculated as a function of mortality coefficients M and F with the age of fish at the first catch (tc). The results are shown in Fig. 3, which indicated that, under the current conditions of M = 0.3 and tc = 2.13 yr, the maximum Y/R was 165.77 and can be obtained at F = 0.627. This means that the current level of F (0.78) is higher than the level required to obtain the maximum Y/R. The relative Y/R was also calculated as a function of F (0.78) at different values for tc. The results are shown in Fig. 4 and indicate that, an increase in tc from 2.13 yr (present value) to 2.51 yr will result in an increase in the Y/R from 164.92 to 173.38 at the current level of F (0.78). In addition, decreasing tc from 2.13 yr to 1.75 yr will lead to a decrease in the Y/R from 164.92 to 153.56.
Fig. 3.
Yield per recruit of Lethrinus lentjan in the Red Sea waters off Jeddah, Saudi Arabia.
Fig. 4.
Yield per recruit of Lethrinus lentjan in the Red Sea waters off Jeddah, Saudi Arabia.
4. Discussion
Studies of the age of fishes is an important aspect for the programs of fisheries research and essential for the evaluation and management of fish stock in order to maintain the sustainability of fish resources (Schneider, 2001), and the use the hard parts of the fish (scales and otoliths) to determine age is a well-known and agreed practice among scholars in this field. Schneider (2001) is among those who relied on the use of scales to determine the age of fish; it was acknowledged that the scales are reliable indicators of age and that they are easier to use and do not cause harm to the fish, whereas the use of otoliths and their extraction requires the dissection of the fish.
In the current study, scales were used to determine the age of L. lentjan as they are large in size and the age can be detected clearly and easily. The maximum age recorded for both males and females was 6 years or more (VI+). This result is in agreement with the findings of Zaahkouk et al (2017) who mentioned that, the maximum life span of L. lentjan in the Egyptian Red Sea was 6 years. Also the results of the present study are consistent with those reported by Wassef (1991) that the age of L. lentjan obtained from the Red Sea is 7 years; the result is also very close to the 8 years reported by Currey et al. (2013) for L. lentjan obtained from the Great Barrier Reef. Grandcourt et al. (2011) reported that the age of L. lentjan obtained from the southern Arabian Gulf was 11 years. This difference may be attributed to several reasons, such as the differences in the environment as the current study was conducted in the Red Sea, whereas that by Grandcourt et al. (2011) was in the Arabian Gulf. Also, the difference in results may be due to the use of different fishing methods and tools, which in turn affects the size of the fish caught and thus, its age. The maximum total length recorded in this study was 43.5 cm in the length category of 43–43.9 cm which is largely consistent with the maximum total length (42 cm) recorded by Wassef (1991) for L. lentjan obtained from the Red Sea. Grandcourt et al. (2011) reported a maximum fork length of 38.9 cm for the same fish in the southern Arabian Gulf, which is of course less than the total length.
Mortality coefficients (Z, M, and F) are of great importance in studying the dynamic composition of fish populations, and they indicate the amount of biomass loss of fish in the sea owing to environmental factors (e.g., diseases and aging) in the case of natural mortality and fishing activity in the case of fishing mortality. The current study on L. lentjan recorded Z, M, and F as 1.8, 0.3, and 0.78, respectively, these results are in accordance with Zaahkouk et al (2017) who recorded total mortality (Z = 1.52) and natural mortality (M = 0.35) for L. lentjan in the Egyptian Red Sea., while the fishing mortality in the study of Zaahkouk et al (2017) was greater (F = 1.17) than the F in the current study. Grandcourt et al. (2011) reported lower mortality coefficients (0.44, 0.22, and 0.22) for Z, M, and F, respectively, for the same species in the southern Arabian Gulf. This difference in results with Grandcourt et al. (2011) indicates that the environmental factors in the Red Sea have a greater influence on the life of this species than those in the Arabian Gulf and the fishing effort for this fish in the Red Sea is also larger than that in the southern Arabian Gulf.
In addition, among similar studies, Mehanna et al. (2012) reported mortality coefficients of 1.22, 0.34, and 0.88 for Z, M, and F, respectively, for male L. nebulosus from the Arabian Sea coast of Oman, whereas the results of the current study indicated lower mortality coefficients for male L. lentjan (0.48 for Z, 0.28 for M, and 0.20 for F). In contrast, mortality coefficients for female L. lentjan in the present study (1.54 for Z and 0.32 for M) were greater than those reported by Mehanna et al. (2012) for female L. nebulosus (1.32 for Z and 0.29 for M), except for the F which was 1.22 for L. lentjan versus 1.03 for L. nebulosus. In contrast, Grandcourt et al. (2006) mentioned different mortality coefficients, 0.56, 0.20, and 0.36 for Z, M, and F, respectively, for L. nebulosus in the southern Arabian Gulf. These differences clearly indicate that the mortality coefficients also differ according to the fish species in addition to the difference owing to environmental factors and fishing activity between locations.
Calculating the relative Y/R is one of the important methods for assessing fish stocks, wherein productivity can be determined under the conditions of the current situation of the exploitation level compared to that of the continuous maximum Y/R and the required level of exploitation to achieve it. The results of the current study indicated that, at the current M, the maximum Y/R can be achieved at a lower F than the current one, which means that L. lentjan is subjected to overfishing and that the F should be reduced by 19.62% of its current level to maintain the stock of this fish species. In contrast, the continuation of the F at its present value with an increase in tc will lead to an increase in Y/R and this requires the regulation of fishing operations and its tools. The results of this study are consistent with the findings of Mehanna et al. (2012) that L. nebulosus in the Arabian Sea coast of Oman is being overexploited and that fishing pressure should be reduced by 50 to 75% for rational exploitation. In contrast, the study by Grandcourt et al. (2011) on L. lentjan in the southern Arabian Gulf indicated that the F was much lower than the level necessary to obtain the maximum Y/R; therefore, the current F (0.22) should be raised to 0.56 to achieve optimal Y/R.
5. Conclusion
It can be concluded that the stock of L. lentjan in the Red Sea coast of Saudi Arabia is overexploited as the F was higher than that required to achieve the maximum Y/R. Therefore, the fishing pressure should be reduced through the regulation of fishing operations by decreasing the number of fishing trips or fishing days in addition to the promotion of the use of suitable fishing tools for catching larger sized fish.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work and would like to thank the Researchers Support Services Unit for their technical support.
Funding
This work was supported by the Deanship of Scientific Research at King Saud University through research group no (RGP-1440-002).
Footnotes
Peer review under responsibility of King Saud University.
References
- Beverton, R.J., Holt, S.J., 1966. Manual of methods for fish stock assessment. Part 2. Tables of yield functions. FAO Fisheries Technical Paper, No. 38 (Rev.1). Food and Agriculture Organization of the United Nations, Rome.
- Carpenter, K., Allen, G., 1989. Emperor fishes and large eye breams of the world (Family Lethrinidae). FAO Fisheries Synopsis No. 125, Vol 9. Food and Agriculture Organization of the United Nations, Rome.
- Condini M.V., Albuquerque C.Q., Garcia A.M. Age and growth of dusky grouper (Epinephelus marginatus) (Perciformes: Epinephelidae) in the southwestern Atlantic, with a size comparison of offshore and littoral habitats. Fish. Bull. 2014;112:311–321. doi: 10.7755/fb.112.4.7. [DOI] [Google Scholar]
- Currey L.M., Williams A.J., Mapstone B.D., Davies C.R., Carlos G., Welch D.J., Simpfendorfer C.A., Ballagh A.C., Penny A.L., Grandcourt E.M., Mapleston A., Wiebkin A.S., Bean K. Comparative biology of tropical Lethrinus species (Lethrinidae): challenges for multi-species management. J. Fish Biol. 2013;82:764–788. doi: 10.1111/jfb.3495. [DOI] [PubMed] [Google Scholar]
- Ezzat A.A., Wassef E.A., Bawazeer F.A. Histological studies of the developing gonads of red spot emperor, Lethrinus lentjan (Lacepède), from Jeddah waters of the Red Sea. J. KAU Mar. Sci. 1994;7:215–232. doi: 10.4197/mar.7-1.19. [DOI] [Google Scholar]
- FAO Fisheries statistics of Saudi Arabia . Ministry of Agriculture; Kingdom of Saudi Arabia: 2003. Marine Fisheries Department. [Google Scholar]
- Grandcourt E.M., Al Abdessalaam T.Z., Al Shamsi A.T., Francis F. Biology and assessment of the painted sweetlips (Diagramma pictum (Thunberg, 1792)) and the spangled emperor (Lethrinus nebulosus (Forsskål, 1775)) in the southern Arabian Gulf. Fish. Bull. 2006;104:75–88. doi: 10.1111/j.1095-8649.2011.03044.x. [DOI] [Google Scholar]
- Grandcourt, E., Al Abdessalaam., T.Z., Francis, F., AlShamsi, A., 2011. Demographic parameters and status assessments of Lutjanus ehrenbergii, Lethrinus lentjan, Plectorhinchus sordidus and Rhabdosargus sarba in the southern Arabian Gulf. J. Appl. Ichthyol. 27, 1203–1211.
- Jensen A.L. Beverton and Holt life history invariants result from optimal trade-off of reproduction and survival. Can. J. Fish. Aquat. Sci. 1996;53:820–822. doi: 10.1139/f95-233. [DOI] [Google Scholar]
- Mehanna, S., Zaki, S., Al-Kiuymi, F., Al-Kharusi, L., Al-Bimani, S., 2012. Biology and fisheries management of spangled Emperor Lethrinus nebulosus from the Arabian Sea coast of Oman. INOC-CNRS, International Conference on Land-Sea Interactions in the Coastal Zone, Jounieh, Lebanon, 6–8 November 2012. https://doi.org/10.4172/2150-3508.1000040.
- Pauly D. Length-converted catch curves. A powerful tool for fisheries research in the tropics. Part 1. Fishbyte. 1983;1:9–13. [Google Scholar]
- Schneider J.C. Fisheries technical report; Michigan Department of Natural Resources, Fisheries Division: 2001. Aging scales of walleye, yellow perch and northern pike. [Google Scholar]
- Tracey S.R., Lyle J.M., Haddom M. Reproductive biology and per-recruit analyses of striped trumpeter (Latris lineata) from Tasmania, Australia: implications for management. Fish. Res. 2006;84:358–367. doi: 10.1016/j.fishres.2006.11.025. [DOI] [Google Scholar]
- von Bertalanffy L. A quantitative theory of organic growth (Inquiries on growth laws II) Hum. Biol. 1938;10:181–213. [Google Scholar]
- Wassef E.A. Comparative growth studies on Lethrinus lentjan, Lacepède 1802 and Lethrinus mahsena, Forsskål 1775 (Pisces, Lethrinidae) in the Red Sea. Fish. Res. 1991;11:75–92. [Google Scholar]
- Wood L. Jhon Beaufoy Publishing Limited; UK: 2016. Underwater Guide to THE RED SEA. [Google Scholar]
- Younis E.M., Abdel-Warith A.A., Ali A., Al-Asgah N.A., AlShayia A.S. Chemical Composition and Mineral Contents of Six commercial fish Species from the Arabian Gulf coast of Saudi Arabia. J. Anim. Vet. Adv. 2011;10(23):3053–3059. [Google Scholar]
- Zaahkouk, S.A., Khalaf-Allah, H. M., Mehanna, S.F., El-Gammal, F.I., Makkey, A.F., 2017.
- Studies on age, growth, and mortality rates for management of the redspot emperor, Lethrinus lentjan (Lacepède, 1802) in the Egyptian sector of Red Sea. Egyptian Journal of Aquatic Biology and Fisheries, 21(1), 63–72. http://doi.org/10.21608/ejabf.2017.2384.