Abstract
Moringa oleifera is also known as “Miracle tree”, due to its multiple uses and adaptability. Because of nutritive and pharmacological values, it is widely cultivated across the world. M. oleifera leaves are rich source of minerals, vitamins and many health beneficial secondary metabolites, and possess significant anti-diabetic potential. Consequently, Insilco study could be noteworthy to expand effective anti-diabetic drugs from this plant. Present study was designed to find out the best bioactive compounds of M. oleifera as a potential therapeutic agent against diabetes mellitus through In-silico method. For this, structures of phytochemicals were extracted from PubChem and docked to mutated protein from PBD. Afterwards, datasets were prepared for ligand based pharmacophore and their pharmacophoric features were generated from LigandScout. Finally five phytochemicals viz. anthraquinone, 2-phenylchromenylium (Anthocyanins), hemlock tannin, sitogluside (glycoside) and A-phenolic steroid were selected, which exhibited effective binding within the active binding pocket of the targeted protein. Ligand based pharmacophore model showed the key features i.e. HBD, HBA, aromatic ring, hydrophobic, positively ionizable surface essential for receptor binding. Our findings suggest that screened phytochemicals present in M. oleifera can be used as potential therapeutic drug candidates to treat diabetes mellitus.
Keywords: Moringa oleifera, Phytochemicals anti-diabetic, In-silico, Docking, Pharmacophore
1. Introduction
Moringa oleifera Lam. (Moringaceae family), is a well-known and most broadly distributed plant species (Anwar et al., 2007). Globally, Moringa is known by different names i.e. “benzolive tree, drumstick tree, horseradish tree, mulangay, moonga, saijhan, marango, sajna, mlonge, or Ben oil tree”. Moringa tree is also called as a “miracle tree” (Fahey, 2005), of substantial socio economic importance. It has extreme nutritional components, significant pharmacological and industrial applications (Fig. 1). Greeks, Romans and Egyptians have used Moringa as herbal medicine. Moringa is widely cultivated on diverse areas in the tropical regions (Fahey, 2005). It is well known that secondary metabolites contribute significantly in the therapeutic application of plant species in traditional healing system (Torres-Castillo et al., 2013). Different parts of Moringa viz. pods, leaves and flowers used as vegetable in Pakistan, India, Hawaii, Philippines and Africa (Anwar et al., 2007). Numerous bioactive compounds have been reported from different parts of Moringa (Amaglo et al., 2010). Likewise, various parts of this plant are of high medicinal value as mentioned in (Table 1).
Fig. 1.

Different parts of Moringa oleifera tree (https://www.google.com/search).
Table 1.
Medicinal uses of different parts of M. oleifera.
| Plant parts | Medicinal uses | References |
|---|---|---|
| Root | Antilithic, rubefacient, laxative, carminative, anti-inflammatory, cardiac, circulatory tonic; used to treat rheumatism, articular pains, backache, kidney pain and constipation. Juice from the root bark relieve earaches, toothache and has anti-tubercular activity | Padmarao et al., 1996, Dahot, 1988, Ruckmani et al., 1998, Anwar et al., 2007 |
| Leaf | Purgative, applied as poultice to sores, rubbed on the temples for Headaches; used for piles, fevers, sore throat, bronchitis, eye and ear infections, scurvy and catarrh; leaf juice is believed to control glucose levels, reduces glandular swelling | Morton, 1991, Fuglie, 2001, Makonnen et al., 1997, Dahot, 1988, Anwar et al., 2007 |
| Stem bark | Rubefacient, vesicant, cure eye diseases, prevents enlargement of the spleen, tuberculosis, tumors and to heal ulcers. | Siddhuraju and Becker, 2003, Anwar et al., 2007 |
| Gum | Used for dental caries, astringent and rubefacient; relieve headaches, fevers, intestinal complaints, dysentery, asthma, abortifacient, used to treat syphilis and rheumatism | Fuglie, 2001, Anwar et al., 2007 |
| Flower | Stimulant, aphrodisiac, cure inflammations, hysteria, tumors, spleen enlargement; lowers VLDL, LDL cholesterol. | Siddhuraju and Becker, 2003, Mehta et al., 2003, Anwar et al., 2007 |
| Seed | Seed extract decreases lipid peroxide in liver | Faizi et al., 1998, Lalas and Tsaknis, 2002 |
Different parts of M. oleifera are enriched in crotenoids, alkaloids, flavonoids, glycosides, anthocyanin, anthraquinone, saponins, steroids, tannins and terpenoids (Patel et al., 2014). These phytochemicals contribute significantly in the prevention of various health disorders like cancer, diabetes, cardiovascular diseases, age related functional disorders, arthritis and inflammation. Antioxidant, anticancer, antimicrobial and anti-diabetic properties in different parts of M. oleifera are also due to above mentioned bioactive compounds (Patel et al., 2014).
Recent data from the International Diabetes Federation (IDF), show that diabetes mellitus (DM) affects more than 366 million people worldwide and is expected to grow to 552 million or even more by 2030 (Whiting et al., 2011). Diabetes mellitus type 2 (DMT2) is a clinical syndrome with abnormal glucose tolerance, universal, microangiopathia, neuropathia, insufficient insulin secretion (Hosseini and Abdollahi, 2013). The impact of deficient insulin plays an essential part in the metabolic disturbances associated to diabetes, hyperglycemia. Type 2 diabetes mellitus (T2DM) is an extending worldwide health concern. People with T2DM possess high hazard for both micro-vascular complications (retinopathy, nephropathy and neuropathy) and macro-vascular complications i.e. cardiovascular comorbidities, leading to hyperglycemia and insulin resistance (metabolic) disorder (Evans et al., 2003). In case where DMT2 is inherited, the whole cell supplement of the insulin receptor is damaged in zygote cell (Pessin and Saltiel, 2000, Hosseini and Abdollahi, 2013). The current frequency of type 2 diabetes mellitus in Pakistan is 11.77%, and it accounts 11.20% in male and 9.19% in females. Furthermore, DM accounts for 14.81% in urban zones and 10.34% in rural zones of Pakistan. To best of our knowledge, there is no comprehensive In-silico study on the different metabolites of M. oleifera as anti-diabetic agent. Therefore, we screened some major phytochemicals from this plant and docked with the mutated protein of diabetes mellitus. Our results can provide base line information that could be useful for synthetic modification of bioactive phytochemicals, de novo synthesis of structural motifs, and may lead to additional phytochemical investigations in this plant species.
2. Methodology
2.1. Disease selection
Diabetes mellitus (DM) was selected as a target disease to find out the anti-diabetic effects of M. oleifera. DM is a chronic metabolic disease, which is rapidly increasing at global level as well as in Pakistan. So far, there is no proper and effective treatment of this disease. Diabetic patients’ ends up facing other therapeutic issues too, with one common issue as interruption in insulin production. Type 2 diabetes (Non-insulin dependent diabetes) is an intricate illness that includes disabled insulin discharge by pancreatic beta cells (Kumari, 2010).
2.2. Gene identification
Protein coding gene ‘INSR’ is a member of the receptor tyrosine kinase family of proteins and was identified by GeneCard (www.genecards.org). Mutations on this gene causes the inherited severe insulin resistance syndromes. Insulin or other ligands binding to its receptor turn on the insulin signaling pathway, which regulates glucose uptake and release, in addition to the synthesis and storage of carbohydrates, lipids and protein. GeneCard has supplied concise genome, proteome, transcriptome, aliment and characteristic statistics on all recognized and anticipated human genes. GeneCard is a major information source for the bioinformatics analyses (Safran et al., 2010).
2.3. Protein selection and preparation
The insulin receptor (IR) is a trans-membrane receptor, which is activated with the assistance of insulin, IGF-I, IGF-II and belongs to the large elegance of tyrosine kinase receptors. Metabolically, the insulin receptor performs a key function in lowering glucose homeostasis, and disturbance of glucose homeostasis can bring about serious disorders such as diabetes and cancer. Protein structure of insulin receptor was taken from RCSB PDB (https://www.rcsb.org/). Test strategy was selected as X-beam crystallography for X-beam resolution with claiming 2.18 Å (Burley et al., 2018). Crystal structure of insulin receptor kinase domain in complex with cis-(R)-7-(3-(azetidin-1-ylmethyl) cyclobutyl)-5-(3-((tetrahydro-2H-pyran-2-yl) methoxy) phenyl)-7H-pyrrolo [2,3-d] pyrimidin-4-amine (5HHW) was retrieved.
2.4. Selection of plant species
Health beneficial properties of M. oleifera are well recognized. Different parts of this plant i.e. roots, leaves, flowers, green pods, and seeds are not only consumed as food but also possess significant potential to treat various diseases as discussed earlier. Numerous bioactive compounds have been identified and isolated from different parts of this plant (Amaglo et al., 2010). In addition seeds of M. oleifera are used to extract cooking oil, in biodiesel preparation and as a source of an important water sanitization agent. Extraction of proteins from Moringa seeds is easy and used for treating polluted water and as a consistent method for purifying slurry materials. Well matured roots and tree bark of Moringa is used for tanning (Anwar et al., 2007).
2.5. Phytochemicals’ structures
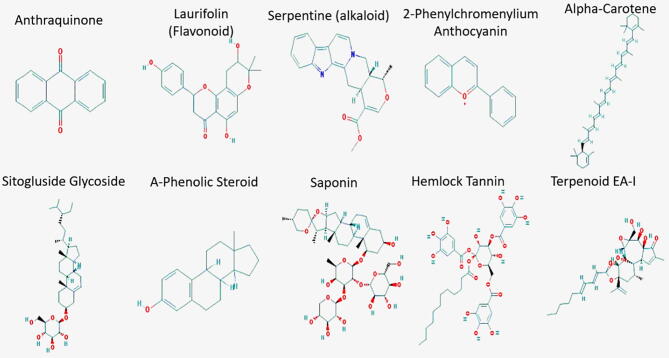
Structures of different phytochemicals present in M. oleifera such as Anthraquinone, Laurifolin, Serpentine, Sitogluside, 2-Phenylchromenylium, Hemlock Tannin, A-Phenolic Steroid, Saponin, Terpenoid, Alpha-Carotene were extracted and analyzed using PubChem (https://pubchem.ncbi.nlm.nih.gov). PubChem provides open access for information on chemical substances and their biological functions. The Substance database comprises of chemical information reported by individual data suppliers to PubChem, and the compound database stores distinctive chemical structures extracted from the Substance database (Kim et al., 2016).
2.6. Toxicity identification
Toxic properties of the extracted phytochemicals of M. oleifera were estimated by ProTox (tox.charite.de/tox). And out of 10 phytochemicals, only 5 i.e. Anthraquinone, Sitogluside (glycoside), Hemlock Tannin, A-Phenolic Steroid, 2-Phenylchromenylium (Anthocyanins) were selected for further analysis. Assortment was based on the toxicity class, and bioactive compounds having 5, 6 toxicity class were selected. The ProTox web server has a simple interface and mainly based on 2D structure of atoms for which risk is to be anticipated (Drwal et al., 2014).
2.7. Potent phytochemicals’ docking complex with marker protein
We selected top five phytochemicals that were fulfilling the Lipinski rule of 5 and docked with the mutated target protein of diabetes mellitus in Patch dock server. PatchDock is an online server which is a geometry based molecular docking algorithm. And the docking results were analyzed with discovery studio. It is intended at finding docking transformations that produce good molecular shape (Schneidman-Duhovny et al., 2005).
2.8. Training datasets preparation for pharmacophore
The 3D structures of five phytochemicals of M. oleifera were retrieved as explained earlier (Kim et al., 2016). Explicit hydrogen atoms were brought to each inhibitor to ensure that each of them has all-atom structures, accompanied by energy minimization (Dhanjal et al., 2015). Due to the fact that maximum of the ligands are bendy, considering a variety of various conformations for every molecule is a vital requisite for developing a pharmacophore so that it will get a shape similar to the actual experimental molecular orientation.
2.9. Pharmacophore model generation
M. oleifera is an important drug target for the treatment of diabetes mellitus. We performed a ligand based molecular docking method to identify novel structural characteristics and scaffolds. The pharmacophoric feature of every ligand was checked and all of the phytochemical compounds (ligands) were imported in ligand based perspective of LigandScout to generate pharmacophore (Munir et al., 2016). The software LigandScout was used for identification and visualization of protein ligand interaction sites and pharmacophore model generation. It extracts ligands and their protein environment from PDB files and assigns bond characteristics to small molecule (ligands) in a fully automated and distributed way. Furthermore, this software creates and visualizes pharmacophore models that represent the interaction between protein and ligand in a universal way.
3. Results
Mutated protein insulin receptor kinase has structural weight of 35448.66, Atom Count 2480, Residue Count 307, and only 1 unique protein chain. Fig. 2, shows crystal structure of insulin receptor kinase domain in complex with cis-(R)-7-(3-(azetidin-1-ylmethyl) cyclobutyl)-5-(3-((tetrahydro-2H-pyran-2-yl) methoxy) phenyl)-7H-pyrrolo [2,3-d] pyrimidin-4-amine. The 5HHW protein depicted two mutations i.e. C5S, D156N. This protein structure was refined by removing nonstandard residues. Predocking file was submitted to the docking server PatchDock to analyze the protein ligand interactions and structure of the targeted protein with ligand.
Fig. 2.
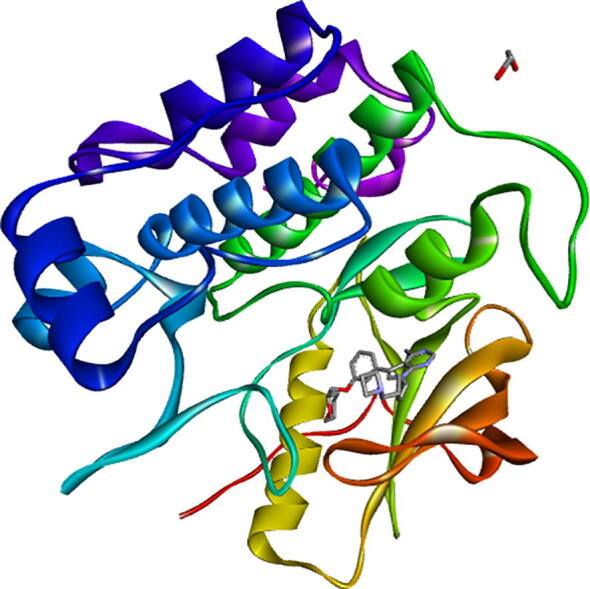
Crystal structure of insulin receptor kinase (https://www.rcsb.org/).
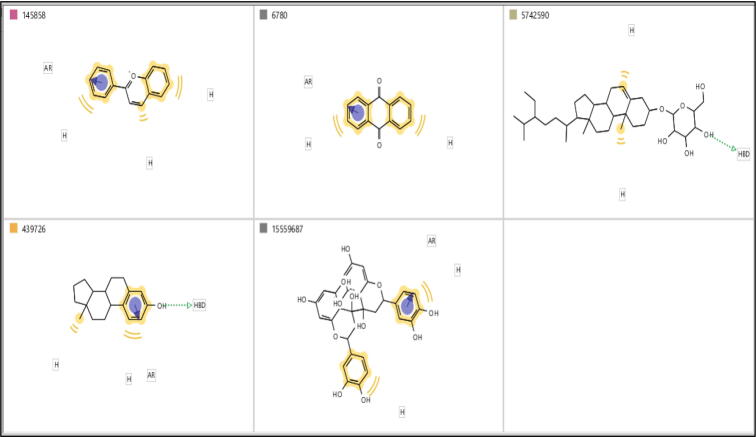
Our model is based on the ligand based pharmacophoric features of already known active compounds of the plant species. Structures of health beneficial phytochemicals i.e. Anthraquinone, Laurifolin, Serpentine, Sitogluside, 2-Phenylchromenylium, Hemlock Tannin, A-Phenolic Steroid, Saponin, Terpenoid, Alpha-Carotene (Fig. 3), present in M. oleifera were extracted and analyzed using PubChem (https://pubchem.ncbi.nlm.nih.gov).
Fig. 3.
2D structures of phytochemical compounds of M. oleifera.
Out of ten phytochemical constituents of M. oleifera, only five fulfilled the Lipinski rule of 5, and were selected to generate pharmacophoric models. The selected phytochemical constituents along with their chemical properties are shown in Table 2.
Table 2.
Phytochemicals of M. oleifera following Lipinski rule of 5 and toxicity class.
| Phytochemicals | HBD | HBA | MW (g/Mol) | RB | Cp | TC |
|---|---|---|---|---|---|---|
| Anthraquinone | 0 | 2 | 208.2 | 0 | 261 | 5 |
| Sitogluside (glycoside) | 4 | 6 | 576.8 | 9 | 920 | 6 |
| Hemlock Tannin | 10 | 12 | 578.5 | 3 | 881 | 5 |
| A Phenolic Steroid | 1 | 1 | 256.3 | 0 | 352 | 5 |
| 2-Phenylchromenylium (Anthocyanins) | 0 | 0 | 207.2 | 1 | 220 | 5 |
HBD. Hydrogen Bond Donor, HBA. Hydrogen Bond Accepter, MW. Molecular weight, RB. Rotatable bonds, Cp. Complexity, TC. Toxicity class.
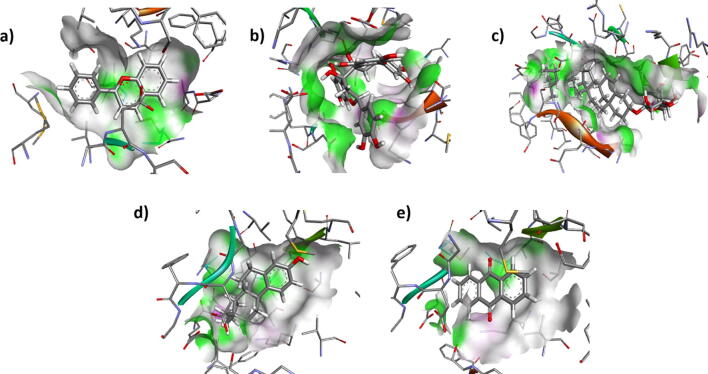
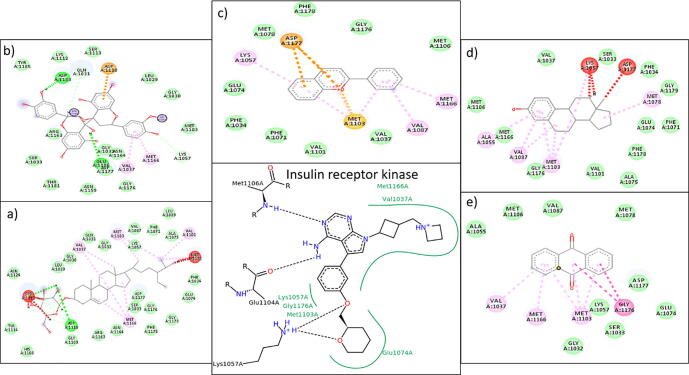
Protein ligand docking have the ability to select the promising compounds at an early level of the drug discovery pipeline. Consequently, to validate the proposed model, molecular docking was executed from software PATCHDOCK to focus on the critical binding interactions. The molecular interaction pattern between mutated protein and each phytochemical was studied to investigate the site to which ligand was binding. Docking results were analyzed and compared with discovery studio. It was observed that all of five ligands (solutions) are successfully bind within the active binding pocket of the targeted protein as shown in Fig. 4(a–e). The protein ligands interactions shows all the interacting residues of the proteins and strong van der Waals forces.
Fig. 4.
(a–e) Protein ligand interactions within active binding pocket of mutated protein of diabetes mellitus.
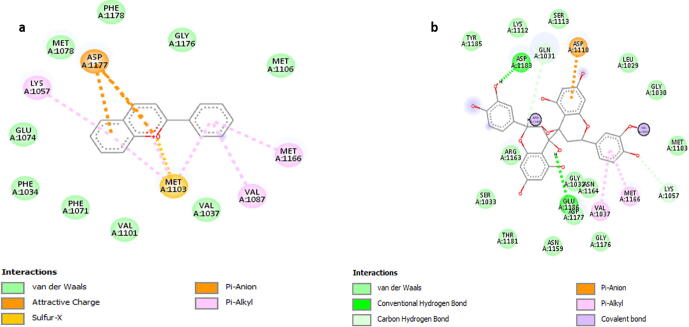
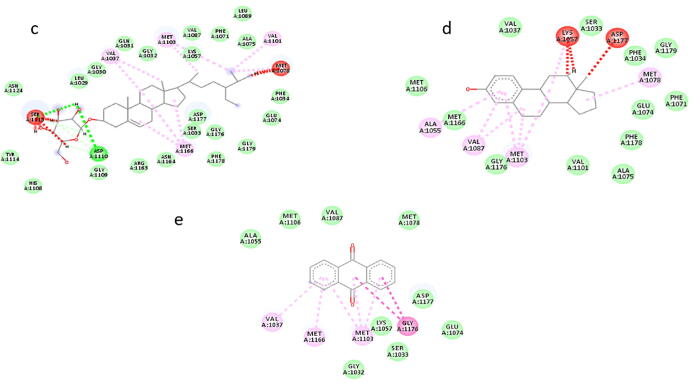
The 2d diagrams of the protein ligand interactions showed all the interacting residues of protein and strong van der Waal forces as shown in Fig. 5. Results of docking of 5 shortlisted phytochemical constituents of M. oleifera with the binding region of INSR targeted protein (Fig. 5 and 6) indicate that; 2-Phenylchromenylium (Anthocyanins) was interacting with ASP A: 1177, LYS A: 1057, MET A: 1103, VAL A: 1087, MET A: 1166 (Fig. 5a), while Hemlock Tannin was found to be interacting with ASP A: 1183, GLN A: 1031, ASP A: 1110, GLU A: 1186, ASP A: 1177, VAL A: 1037, MET A: 1166, LYS A: 1057 (Fig. 5b). Likewise, Sitogluside (Glucoside) showed strong interaction with VAL A: 1037, MET A: 1103, LYS A: 1057, MET A: 1078 VAL A: 1101, SER A: 1113, ASP A: 1110, MET A: 1166 (Fig. 6c), Phenolic Steroid had interaction with ALA A: 1055, VAL A: 1087, MET A: 1103, LYS A: 1057, ASP A: 1177 (Fig. 6d), Anthraquinone was found to be interacting with VAL A: 1037, MET A: 1166, MET A: 1103, GLY A: 1176 (Fig. 6e). In docking results of 5 phytochemical constituents with mutated protein of diabetes mellitus, there were one or two unfavorable bumps or errors which show poor interactions and binding between interacting amino acids and drug atoms. However, fewer number of bumps or errors i.e. one or two are negligible (Zainab et al., 2020).
Fig. 5.
2D diagram of docking result of (a) 2-Phenylchromenylium (Anthocyanins) and (b) Hemlock Tannin with binding site region with mutated protein.
Fig. 6.
(c–e) 2D diagram of docking result of (c) Sitogluside (glycoside), (d) Phenolic Steroid and (e) Anthraquinone with binding site region with mutated protein..
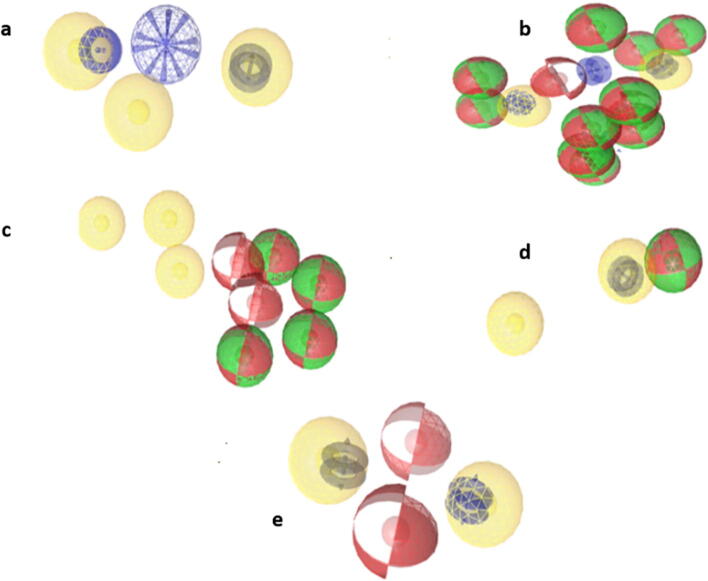
Pharmacophore analysis was measured as an essential part of the drug layout. The pharmacophore generated with the aid of LigandScout for the selected chemical constituents confirmed few features such as Hydrogen bond acceptor (HBA), Hydrogen bond donor (HBD), Hydrophobic (H), positive ionizable (I) and aromatic rings (AR). In every pharmacophore model of selected chemical constituents. The red color represents Hydrogen bond acceptor, green color represents Hydrogen bond donor and yellow spheres represent hydrophobic, blue rings represents an aromatic ring and blue spheres represents positive ionizable as shown in Fig. 7.
Fig. 7.
Ligand based pharmacophore of (a) 2-Phenylchromenylium (Anthocyanins), (b) Hemlock Tannin, (c) Sitogluside (Glucoside), (d) A Phenolic Steroid and (e) Anthraquinone respectively
Several excluded volumes have been additionally produced inside the models to illustrate the distance balancing. All of the five models were comprised of aromatic rings, hydrophobic as a common feature but hydrogen bond acceptors, hydrogen bond donors were not presents in all five models as common feature. Such as 3D structure of 2-phenylchromenylium (anthocyanins), anthraquinone and hemlock tannin contains aromatic ring, positive ionizable and hydrophobic, while sitogluside (glucoside) have hydrophobic, hydrogen bond acceptors, hydrogen bond donors. Pharmacophoric features of the shortlisted phytochemical constituents are shown in Fig. 8.
Fig. 8.
Pharmacophoric features of shortlisted phytochemicals of M. oleifera.
Comparative analysis of insulin receptor kinase domain in complex with cis-(R)-7-(3-(azetidin-1-ylmethyl) cyclobutyl)-5-(3-((tetrahydro-2H-pyran-2-yl) methoxy) phenyl)-7H-pyrrolo [2,3-d] pyrimidin-4-amine and other five phytochemicals is shown in Fig. 9. This analysis is the validation step that all screened phytochemicals docked within the active binding pocket of the protein likewise its original ligand as shown in Fig. 9.
Fig. 9.
2d comparison of protein and five phytochemicals
4. Discussion
Several medicinal plants have been used as therapeutic agent for various diseases. M. oleifera is a very important medicinal plant, as it is traditionally used to treat various health disorders including type 2 diabetes (Farooq et al., 2012). The most prevailing form of diabetes is non-insulin dependent diabetes mellitus (type II), which is usually caused by high postprandial glucose levels. Glucosidases, a class of enzymes (α-amylase and α-glucosidase) that helps in the breakdown of complex carbohydrates (starch and oligosaccharides) into simple sugars such as maltose and glucose. Despite of impaired secretion of insulin or insulin action, the fast uptake of glucose in the intestine by the action of α- amylase and α-glucosidase activities is another important factor that results in a hyperglycemia. Therefore leaves of M. oleifera showed high inhibitory action on α- amylase and α-glucosidase activities (Jaiswal et al., 2009).
Therefore, a number of plants and plant derived compounds having lesser/or no side effects have been used to manage postprandial hyperglycemia through mild inhibitory effect against α-amylase and strong inhibitory activity against α-glucosidase. Plant based foods can improve glucose metabolism as well as it can enhance the overall health of diabetic patients, as it improves lipid metabolism, antioxidant status, improving capillary function, and lowering blood pressure and cholesterol level (Chinwe and Isitua, 2010). The anti-hyperglycemic activity of the Moringa oleifera leaves is reported in a controlled study with untreated T2DM patients (William et al., 1993). Its dietary consumption in T2DM patients, significantly reduced the glucose level in bloodstreams (Kumari, 2010). Leaves and seeds of M. oleifera have been recognized to have relatively high antioxidant activity. The in vivo studies shows that aqueous extract of M. oleifera leaves reduces the blood glucose level in regular rats and normalizes the high blood glucose stages in sub, moderate and significantly diabetic rats (Villarruel-López et al., 2018). It additionally improves glucose tolerance in ordinary, sub and moderate diabetic animals. It is interesting to notice that the extract was more effective than reference drug (Jaiswal et al., 2009). This significant reduction in blood glucose level could be due to presence of phytochemicals: anthraquinone, anthocyanins, hemlock tannin, and glycoside and A-phenolic steroid. It was previously reported that aqueous extract shows higher anthraquinone concentration (11.68 ± 0.04) which are a group of naturally occurring phenolic compounds, found in M. oleifera leaves. It is quite possible that other than its anti-microbial effects it has potential as therapeutic agents for diabetes. In this research work, activity of the screened phytochemicals were computationally analyzed. In-silico molecular docking reveals that these screened phytochemicals were highly selective and have shown strong interactions with mutated protein of diabetes.
The phytochemical investigation of M. oleifera showed the presence of bio flavonoids that can stimulates the glucose uptake in peripheral tissues and regulates the activity of the enzymes involved in carbohydrate metabolism (Gupta et al., 2012). Flavonoids helps to secrete insulin, possibly by influencing the pleiotropic mechanisms to reduce diabetic complications (Gupta et al., 2012). In present work Insilico study was conducted to investigate the anti-diabetic effects of phytochemicals of M. oleifera. Structure of flavonoids was extracted from PubChem and its toxicity class was checked. Docking analysis of flavonoid with mutated protein shows that it binds within pocket but there were unfavorable bumps detected which reveals poor binding between interacting amino acids and drug atoms.
This study indicates that all ligands (phytochemicals) were highly selective to their targeted protein. Anthraquinone, 2-Phenylchromenylium (anthocyanins), Hemlock tannin docked strongly to the mutated diabetic protein but Sitogluside (glycoside) and A-Phenolic steroid showed two unfavorable bumps respectively. The 3D structure conformation of ligand based pharmacophore model showing the key features i.e. HBD, HBA, Aromatic ring, Hydrophobic, positive ionizable essential for receptor binding. In future, these pharmacophoric features of phytochemicals will assist to discover new anti-diabetic drugs. Moreover, in-silico elucidation revealed that such studies contribute significantly in drug designing and selection of appropriate chemical compounds against various health disorders such as cancer, cardiovascular diseases, neuro-generative problems, pathogenic infections, viral diseases and genetic disorders. In addition, efficacy of those phytochemicals that exhibited significant potential against diabetes could be confirmed by in-vivo and in-vitro assays.
5. Conclusion
The In silico molecular docking and pharmacophoric studies of M. oleifera has shown potential bio-molecular target phytochemicals of the plant and determined that anthraquinone, 2-phenylchromenylium (anthocyanins), hemlock tannin, sitogluside (glycoside) and A-phenolic steroid phytochemical classes and structural manifolds likely target mutated protein of diabetes mellitus. These results can provide the outline for synthetic modification of bioactive phytochemicals, de novo synthesis of structural motifs, and lead to additional phytochemical investigations. The simulated complexes revealed stability and ligands remained inside the active binding pocket of the mutated protein. This study concluded that these screened phytochemicals can be used as potential therapeutic drug candidate to prevent diabetes mellitus.
Acknowledgments
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this work through the Research group Project no RG-1435-086. In addition, we are grateful to the Department of Bioinformatics Post Graduate College Mandian Abbottabad, Pakistan for providing research facilities.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amaglo N.K., Bennett R.N., Lo Curto R.B., Rosa E.A.S., Lo Turco V., Giuffrida A., Timpo G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010;122(4):1047–1054. doi: 10.1016/j.foodchem.2010.03.073. [DOI] [Google Scholar]
- Anwar F., Latif S., Ashraf M., Gilani A.H. Moringa oleifera: a food plant with multiple medicinal uses. Phytotherapy Res. 2007;21(1):17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Burley S.K., Berman H.M., Christie C., Duarte J.M., Feng Z., Westbrook J., Zardecki C. RCSB Protein Data Bank: Sustaining a living digital data resource that enables breakthroughs in scientific research and biomedical education: RCSB Protein Data Bank. Protein Sci. 2018;27(1):316–330. doi: 10.1002/pro.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinwe C., Isitua N. A Poster Presented at 2nd International Conference on Applied Biotechnology, October 2010, 25–27, Khartoum, Sudan. 2010. Studies on the haematological impact of Moringa oleifera in rabbits. [Google Scholar]
- Dahot M.U. Vitamin contents of flowers and seeds of Moringa oleifera. Pak. J. Biochem. 1988;21:1–24. [Google Scholar]
- Dhanjal J.K., Sharma S., Grover A., Das A. Use of ligand-based pharmacophore modeling and docking approach to find novel acetylcholinesterase inhibitors for treating Alzheimer’s. Biomed. Pharmacother. 2015;71:146–152. doi: 10.1016/j.biopha.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Drwal M.N., Banerjee P., Dunkel M., Wettig M.R., Preissner R. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014;42(W1):W53–W58. doi: 10.1093/nar/gku401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress- activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52(1):1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Fahey J.W. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005;1(5) [Google Scholar]
- Faizi S., Siddiqui B.S., Saleem R., Aftab K., Shaheen F., Gilani A.H. Hypotensive constituents from the pods of Moringa oleifera. Planta Med. 1998;64:225–228. doi: 10.1055/s-2006-957414. [DOI] [PubMed] [Google Scholar]
- Farooq F., Rai M., Tiwari A., Khan A.A., Farooq S. Medicinal properties of Moringa oleifera: An overview of promising healer. J. Med. Plants Res. 2012;6(27):4368–4374. [Google Scholar]
- Fuglie L.J. CTA Publication; Wageningen, the Netherlands: 2001. Combating Malnutrition with Moringa. The Miracle Tree: The Multiple Attributes of Moringa; pp. 117–136. [Google Scholar]
- Gupta R., Mathur M., Bajaj V.K., Katariya P., Yadav S., Kamal R., Gupta R.S. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes: Antidiabetic activity of M. oleifera. J. Diabetes. 2012;4(2):164–171. doi: 10.1111/j.1753-0407.2011.00173.x. [DOI] [PubMed] [Google Scholar]
- Hosseini A., Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid. Med. Cell. Longevity. 2013;2013:1–15. doi: 10.1155/2013/168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal D., Kumar Rai P., Kumar A., Mehta S., Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009;123(3):392–396. doi: 10.1016/j.jep.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Kim S., Thiessen P.A., Bolton E.E., Chen J., Fu G., Gindulyte A., Bryant S.H. PubChem substance and compound databases. Nucleic Acids Res. 2016;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari D.J. Hypoglycaemic effect of Moringa oleifera and Azadirachta indica in type 2 diabetes mellitus. Bioscan. 2010;5(20):211–214. [Google Scholar]
- Lalas S., Tsaknis J. Extraction and identification of natural antioxidants from the seeds of Moringa oleifera tree variety of Malavi. J. Am. Oil Chem. Soc. 2002;79:677–683. [Google Scholar]
- Makonnen E., Hunde A., Damecha G. Hypoglycaemic effect of Moringa stenopetala aqueous extract in rabbits. Phytother. Res. 1997;11:147–148. [Google Scholar]
- Mehta L.K., Balaraman R., Amin A.H., Bafna P.A., Gulati O.D. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003;86:191–195. doi: 10.1016/s0378-8741(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Morton J.F. The horseradish tree, Moringa pterigosperma (Moringaceae). A boon to arid lands. Econ. Bot. 1991;45:318–333. [Google Scholar]
- Munir A., Azam S., Mehmood A. Structure-based pharmacophore modeling, virtual screening and molecular docking for the treatment of ESR1 mutations in breast cancer. Drug Design.: Open Access. 2016;05(03) doi: 10.4172/2169-0138.1000137. [DOI] [Google Scholar]
- Padmarao P., Acharya B.M., Dennis T.J. Pharmacognostic study on stembark of Moringa oleifera Lam. Bull. Medico-Ethno-Botan. Res. 1996;17:141–151. [Google Scholar]
- Patel P., Patel N., Patel D., Desai S., Meshram D. Phytochemical analysis and antifungal activity of Moringa oleifera. Int. J. Pharm. Pharm. Sci. 2014;6(5):144–147. [Google Scholar]
- Pessin J.E., Saltiel A.R. Signaling pathways in insulin action: molecular targets of insulin resistance. J. Clin. Investig. 2000;106(2):165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckmani K., Kavimani S., Anandan R., Jaykar B. Effect of Moringa oleifera Lam on paracetamol-induced hepatoxicity. Indian J. Pharm. Sci. 1998;60:33–35. [Google Scholar]
- Safran M., Dalah I., Alexander J., Rosen N., Iny Stein T., Shmoish M., Lancet D. GeneCards Version 3: the human gene integrator. Database. 2010;2010 doi: 10.1093/database/baq020. baq020–baq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H.J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33(Web Server):W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.) J. Agric. Food Chem. 2003;15:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Torres-Castillo J.A., Sinagawa-García S.R., Martínez-Ávila G.C.G., López-Flores A.B., Sánchez-González E.I., Aguirre-Arzola V.E., Gutiérrez-Díez A. Moringa oleifera: phytochemical detection, antioxidants, enzymes and antifungal properties. Int. J. Exp. Bot. 2013;82:193–202. [Google Scholar]
- Villarruel-López A., López-de la Mora D.A., Vázquez-Paulino O.D., Puebla-Mora A.G., Torres-Vitela M.R., Guerrero-Quiroz L.A., Nuño K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Alternat. Med. 2018;18(1):127. doi: 10.1186/s12906-018-2180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William F., Lakshminarayanan S., Chegu H. Effect of some Indian vegetables on the glucose and insulin response in diabetic subjects. Int. J. Food Sci. Nutr. 1993;44(5):191–196. [Google Scholar]
- Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabet. Res. Clin. Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Zainab B., Ayaz Z., Munir A., Mahmoud A.H., Elsheikh M.S., Mehmood A., Khan S., Rizwan M., Jahangir K., Abbasi A.M. Repositioning of strongly integrated drugs against achromatopsia (CNGB3) J. King Saud Univ.-Sci. 2020 doi: 10.1016/j.jksus.2020.01.021. [DOI] [Google Scholar]