Abstract
miR-18a is a member of primary transcript called miR-17-92a (C13orf25 or MIR17HG) which also contains five other miRNAs: miR-17, miR-19a, miR-20a, miR-19b and miR-92a. This cluster as a whole shows specific characteristics, where miR-18a seems to be unique. In contrast to the other members, the expression of miR-18a is additionally controlled and probably functions as its own internal controller of the cluster. miR-18a regulates many genes involved in proliferation, cell cycle, apoptosis, response to different kinds of stress, autophagy and differentiation. The disturbances of miR-18a expression are observed in cancer as well as in different diseases or pathological states. The miR-17-92a cluster is commonly described as oncogenic and it is known as ‘oncomiR-1’, but this statement is a simplification because miR-18a can act both as an oncogene and a suppressor.
In this review we summarize the current knowledge about miR-18a focusing on its regulation, role in cancer biology and utility as a potential biomarker.
Abbreviations: 5-FU, 5-fluorouracyl; ACVR2A, activin A receptor type 2A; AKT, AKT serine/threonine kinase; AR, androgen receptor; ATG7, autophagy related 7; ATM, ATM serine/threonine kinase; BAX, BCL2 associated Xapoptosis regulator; BCL2, BCL2 apoptosis regulator; BCL2L10, BCL2 like 10; BDNF, brain derived neurotrophic factor; Bp, base pair; C-myc (MYCBP), MYC binding protein; CASC2, cancer susceptibility 2; CD133 (PROM1), prominin 1; CDC42, cell division cycle 42; CDKN1, Bcyclin dependent kinase inhibitor 1B; ceRNA, competitive endogenous RNA; cncRNA, protein coding and non-coding RNA; DDR, DNA damage repair; E2F family (E2F1, E2F2, E2F3), E2F transcription factors; EBV, Epstein-Barr virus; EMT, epithelial-to-mesenchymal transition; ER, estrogen receptor; ERBB (EGFR), epidermal growth factor receptor; FENDRR, FOXF1 adjacent non-coding developmental regulatory RNA; FER1L4, fer-1 like family member 4 (pseudogene); GAS5, growth arrest–specific 5; HIF-1α (HIF1A), hypoxia inducible factor 1 subunit alpha; HNRNPA1, heterogeneous nuclear ribonucleoprotein A1; HRR, homologous recombination-based DNA repair; IFN-γ (IFNG), interferon gamma; IGF1, insulin like growth factor 1; IL6, interleukin 6; IPMK, inositol phosphate multikinase; KRAS, KRAS proto-oncogene, GTPase; LMP1, latent membrane protein 1; lncRNA, long-non coding RNA; MAPK, mitogen-activated protein kinase; MCM7, minichromosome maintenance complex component 7; MET, mesenchymal-to-epithelial transition; MTOR, mechanistic target of rapamycin kinase; N-myc (MYCN), MYCN proto-oncogene, bHLH transcription factor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOTCH2, notch receptor 2; PERK (EIF2AK3), eukaryotic translation initiation factor 2 alpha kinase 3; PI3K (PIK3CA), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PIAS3, protein inhibitor of activated STAT 3; RISC, RNA-induced silencing complex; SMAD2, SMAD family member 2; SMG1, SMG1 nonsense mediated mRNA decay associated PI3K related kinase; SNHG1, small nucleolar RNA host gene 1; SOCS5, suppressor of cytokine signaling 5; STAT3, signal transducer and activator of transcription 3; STK4, serine/threonine kinase 4; TCGA, The Cancer Genome Atlas; TGF-β (TGFB1), transforming growth factor beta 1; TGFBR2, transforming growth factor beta receptor 2; TNM, Classification of Malignant Tumors: T - tumor / N - lymph nodes / M – metastasis; TP53, tumor protein p53; TP53TG1, TP53 target 1; TRIAP1, p53-regulating inhibitor of apoptosis gene; TSC1, TSC complex subunit 1; UCA1, urothelial cancer associated 1; UTR, untranslated region; WDFY3-AS2, WDFY3 antisense RNA 2; WEE1, WEE1 G2 checkpoint kinase; WNT family, Wingless-type MMTV integration site family/Wnt family ligands; ZEB1/ZEB2, zinc finger E-box binding homeobox 1 and 2; COAD, colon adenocarcinoma; UCEC, uterine corpus endometrial carcinoma; BLCA, bladder urothelial carcinoma; HNSC, head and neck squamous cell carcinoma; LUAD, lung adenocarcinoma; STAD, stomach adenocarcinoma; KIRC, clear cell kidney carcinoma; PRAD, prostate adenocarcinoma; BRCA, breast cancer; ESCA, esophageal carcinoma; LUSC, lung squamous cell carcinoma; LIHC, liver hepatocellular carcinoma; KIRP, kidney renal papillary cell carcinoma; PAAD, pancreatic adenocarcinoma; THCA, papillary thyroid carcinoma
Keywords: miR-18a, miR-17-92a, miRNA, Cancer, TCGA, Oncogene, Suppressor, Biomarker, Circulating miRNA, Liquid biopsy
1. miRNA
miRNAs are a group of short (22 nucleotides long) non-coding RNAs. These small RNAs are associated with cell cycle, apoptosis, proliferation, differentiation, metabolic pathways and cell response to various types of stress.1, 2, 3 It is estimated that miRNAs regulate from 30% to 60% of human genes.4 miRNA genes are situated in external or internal space of genes, in introns or exons of coding and non-coding protein genes and they have a transcription promoter, either their own or common with mRNAs. Some of them are encoded as a single, simple miRNA, and other, such as the multiple miRNAs, create clusters. About 30% of miRNAs in genomes of vertebrates are found as part of a polycistronic cluster,5,6 and miRNAs from the same cluster are preferentially co-expressed and the expression levels are varied in different tissues in the same organism.7
miRNA genes are transcribed from genome by RNA polymerase II.8 The canonical biogenesis of miRNAs is divided into two main steps; the first one occurs in the nucleus and the other one in the cytoplasm, where immature forms of miRNAs’ harpins are changed to about 22 nts duplexes by Drosha and Dicer enzymes. In the non-canonical pathway, mirtrons are spliced out of mRNA transcripts in a Drosha-independent manner and further steps are similar to those in the canonical pathway.9,10 One of the strands from the duplex, called the guide strand, is incorporated into the RISC complex and the miRNA in this complex can bind with the 3’UTR region of target mRNA. miRNAs regulate gene expression via three pathways of blocking proteins translations: i) repression of mRNA translation; ii) cleavage of mRNA and iii) mRNA deadenylation.5,11
2. miR-17-92a cluster
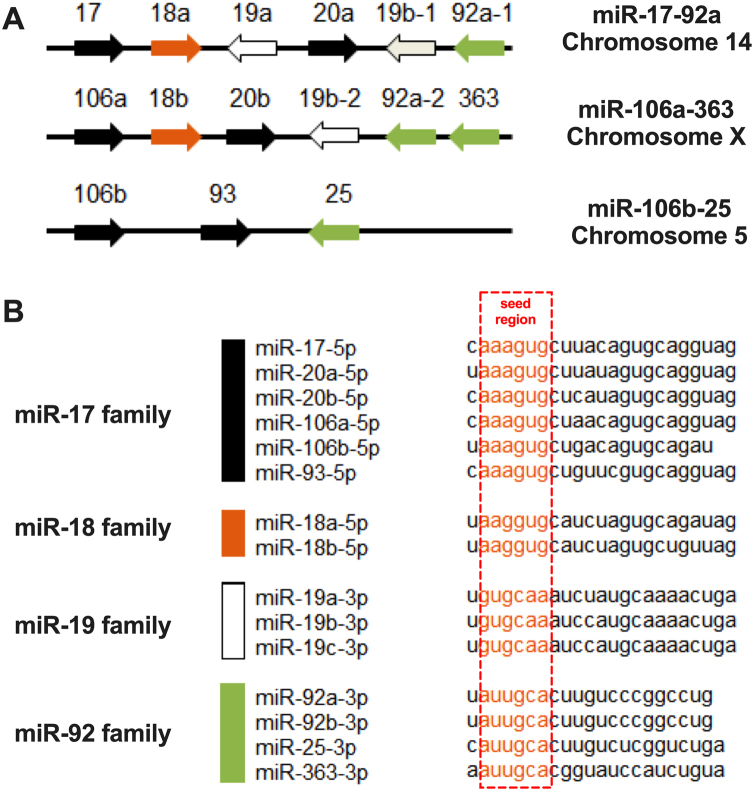
miR-18a belongs to the miR-17-92a cluster and it is transcribed from a polycistronic non-protein-coding gene. This gene named C13orf25 or MIR17HG (also as the miR-17/92 cluster host gene) is located on chromosome 13 (13q31.3) and contains miR-17-92a cluster, 800 nts long, with six mature miRNAs: miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92a.12 The characteristic of the miR-17-92a cluster is its organization and sequence of miRNAs which are highly conserved across vertebrates.13,14 Probably, the ancestral miR-17 cluster contained miRNAs belonging to the early miR-17 group, miR-19 group and miR-92. The miR-18a was created by its duplication from the miR-17 group and previous duplication of the whole cluster that created the ancestors of the miR-17-18a-19a-20a-19b-92a family.13 It should be noted that there are two more paralogs of the miR-17-92a cluster: miR-106a-363 and miR-106b-25. They were created due to duplication and gene deletion in evolution changes.13 The miR-106b-25 cluster is situated on chromosome 7, inside the intron of the MCM7 gene, and encodes three miRNAs: miR-106b, miR-93 and miR-25; whereas the miR-106a-363 cluster is located on chromosome X and contains six miRNAs: miR-106a, miR-18b, miR-20b, miR-19b-2, miR-92a-2 and miR-363.15,16
These three human clusters: miR-17-92a, miR-106b-25 and miR-106a-363, include 15 miRNAs that share four “seed” regions: the miR-17, miR-18, miR-19, and miR-92 families (Fig. 1).14 It was indicated that orthologs of the miR-17, miR-18 and miR-19 seed families are not found outside the vertebrates, but homological for miR-92 seed family miRNAs are found in D. melanogaster and C. elegans.13 The expression of these three similar clusters is not at the same level; miR-17-92a and miR-106b-25 clusters are highly expressed in many types of tissues, and the miR-106a-363 cluster is expressed at a lower level.15,16
Fig. 1.
(A) The localization of the members within the miR-17-92a cluster and paralogs miR-106a-363 and miR-106b-25. (B) The sequence similarities and differences among 15 miRNAs that share four “seed” regions: the miR-17, miR-18, miR-19, and miR-92 families; framed nucleotides indicate the “seed” regions.
The expression of the miR-17-92a cluster is regulated at two major levels: transcriptional and post-transcriptional. Transcriptional regulation is connected with C-myc and N-myc, E2F and cyclin D1.17 MYC factors bind to a conserved non-canonical E-box sequence located 1480 bp upstream of miR-17-92a. On the other hand, miR-17-5p and miR-20a inhibit C-myc-mediated induction of E2F1 expression.17 Similarly, the E2F family of transcription factors, with the main role of E2F3, can directly bind to the promoter of miR-17-92a and regulate its expression. Moreover, two members of this cluster, miR-17 and miR-20, regulate the translation of E2F1, E2F2, and E2F3, creating an auto-regulatory loop.18, 19, 20 The regulatory mechanisms among C-myc, E2F family and miR-17-92a create the cellular balance between apoptosis and proliferation of the cell.18,20 Sylvestre et al. proposed that the deregulation of the E2F/miR-17-92a autoregulatory feedback loop may occur during the transformation of normal cells into tumor cells.19 In the case of paralog miR-106a-363, putative C-myc binding sites are indicated in the proximity of the cluster, but direct regulation was not demonstrated. For miR-106b-25, there is no information about the transcriptional regulation.14
The transcription of the miR-17-92a cluster is also regulated by the androgen receptor (AR). AR, through its binding sites, can up-regulate the expression of cluster members.21 Moreover, Brock et al. demonstrated that the expression of miR-17-92a in primary hepatocytes and HepG2 cells is modulated by IL6. Immune cells secrete IL6 activating STAT3 in hepatocytes, which stimulates the acute-phase genes and miR-17-92a cluster. In turn, miR-18a targets the PIAS3 (inhibitor of STAT3), which enhances the activation of STAT3.22 The similar STAT3-dependent activation of miR-17-92a transcription after endothelial cells stimulation with IL6 was observed, and it could be indicated as a cell type independent way of activation.23 Moreover, about 100 bp upstream from the start codon of C13orf25 an evolutionarily conserved palindromic TT-AA motif with 4-bp of space is situated. It is responsible for STAT3 binding and regulation of the miR-17-92a cluster.23
The post-transcriptional regulation of the miR-17-92a cluster causes miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92a to be often expressed at different levels. They are processed with different efficiency or their stability is varied.14 Probably, a specific globular tertiary structure of the primary transcript where the 3'-end functions as the core domain, has the main role in the regulation of expression of miR-17-92a cluster members has. Other internal well-defined secondary structures and other elements also play a significant role.24,25 The miRNAs inside the core are processed less efficiently than outer miRNAs due to their different accessibility to the Drosha enzyme. Moreover, the disruption of the cluster structure leads to an overall increase of miRNA expression from the 3'-end of the cluster; especially miR-92a, which has an opposite biological function compared to the miRNAs from the 5'-end of the cluster.24,26 It should be noted that other cellular factors, such as C-myc, could also modify miRNAs expression and lead to a higher level of transcription from the 5'-end and lower level miRNAs situated at the 3'-end of the cluster’s core.18
Diosdado et al. indicated that, unlike the other members of the miR-17-92a cluster, expression of miR-18a is significantly lower. Moreover, increased expression of the cluster during progression from colorectal adenoma to adenocarcinoma is associated with the DNA copy number gain of the miR-17-92a locus on 13q31 and with C-myc expression.27 It should be noted that miR-18a requires the presence of HNRNPA1 protein.28 The low expression of miR-18a may be caused by the specific tertiary structure of pri-miR-17-92a, which affects the miR-18a biogenesis process.29 It was indicated that HNRNPA1 binds to pri-miR-17-92a, locally remodels RNA secondary structure and facilitates its biogenesis.30 The study based on animal models showed the important role of HNRNPA1 in miR-18a biogenesis and its connection with disease development. miR-18a down-regulation is the result of phosphorylation and destabilization of HNRNPA1 by phosphorylated PERK as the consequence of endoplasmic reticulum stress.31
The expression levels of individual miRNAs from the miR-17-92a cluster depends on tissue type and external factors.17 One of the newly described and observed processes in the cellular regulatory network is an interaction between miRNA and lncRNA (long-non coding RNA) (Table 1).32 The interactions between miR-18a and specific lncRNAs were observed, e.g.: UCA1 as well as CASC2 in breast cancer,33, 34, 35, 36 CASC2 in colorectal cancer37 and in malignant melanoma,38 GAS5 in malignant glioma39 and prostate,40 FER1L4 in osteosarcoma,41 WDFY3-AS2 in ovarian cancer,42 TP53TG1 in non-small cell lung cancer,43 FENDRR in prostate cancer,44 and lncRNA SNHG1 in the case of ischemic stroke.45
Table 1.
Molecular interaction between miR-18a and lncRNAs in different types of cancers.
| lncRNA | Cancer type | Mechanism of action | Ref. |
|---|---|---|---|
| GAS5 | Malignant glioma | miR-18a-5p negatively regulates GAS5 and GAS5 represses miR-18a-5p in vitro and in vivo | 39 |
| FER1L4 | Osteosarcoma | FER1L4 directly inhibits miR-18a-5p | 41 |
| FER1L4 knockdown suppresses apoptosis and promotes EMT, stemness markers and PI3K/AKT pathway via upregulating the ratio of miR-18a-5p/SOCS5 in osteosarcoma cells | |||
| CASC2 | Breast cancer | Increased expression of CSC2 causes reduced expression of miR-18a-5p | 36 |
| WDFY3-AS2 | Ovarian cancer | WDFY3-AS2 acts as molecular sponge miR-18a-5p | 42 |
| Upregulated miR-18a-5p reverses the anticancer effect of WDFY3-AS2 (inhibition of proliferation, invasion, EMT and tumorigenesis) | |||
| GAS5 | Prostate cancer | GAS5 acts as molecular sponge miR-18a-5p | 40 |
| TP53TG1 | Non-small cell lung cancer | TP53TG1 acts as molecular sponge miR-18a-5p | 43 |
| TP53TG1 enhances cisplatin sensitivity via sponging miR-18a-5p and regulating PTEN | |||
| FENDRR | Prostate cancer | FENDRR acts as molecular sponge for miR-18a-5p | 44 |
| FENDRR inhibits prostate cancer malignacy through regulation of miR-18a-5p | |||
| CASC2 | Malignant melanoma | CASC2 may inhibit malignant melanoma development through regulating miR-18a-5p via molecular sponging mechanism | 38 |
| UCA1 | Breast cancer | Mir-18a-5p and YAP1 (miR-18a-5p target gene) regulate UCA1 expression in breast cancer cells resulting in sensitizing them to trastuzumab | 33 |
| UCA1 | Breast cancer | UCA1 acts as molecular sponge miR-18a-5p and enhances breast cancer cells resistance to tamoxifen | 34,35 |
| CASC2 | Colorectal cancer | CASC2 acts as molecular sponge miR-18a-5p and overexpression of CASC2 causes proliferation and tumour growth inhibition | 37 |
For example, Liu et al. described that lncRNA GAS5 (growth arrest–specific 5) functions as a competitive endogenous RNA (ceRNA) and regulates the cellular level of mature miR-18a-5p in glioma. The interaction between GAS5:miR-18a-5p has functional consequences by influence on expression level of miR-18a-5p target neogenin. Moreover, miR-18a-5p reduces the level of GAS5, so the feed-back loop between oncmiR and lncRNA suppressor keeps specific balance in the cell.39 Another study, performed by Sanchez-Mejias et al., indicated SOCS5/miR-18a/miR-25 axis and its regulation on MTOR signaling and tumor suppressor TSC1 in liver cancer.46 Suppressor of cytokine signaling 5 (SOCS5) gene encodes 4 transcripts (spliced variants) with protein as well as non-protein (lncRNA) potential47 and it is the example of bi-functional, protein coding and non-coding RNAs (cncRNAs).48 SOCS5 can function as the molecular sponge and competing for binding to miR-18a and miR-25 and prevents the interaction of miRNAs with their targets.46 These examples, which involved miR-18a and RNAs with cncRNA potential, underline the complexity of the regulatory network in the cell, which is based on the bioavailability of a particular transcript.
Rodriguez-Aguayo et al. indicated that miR-15a-5p and miR-25-3p are important regulators of miR-18a-3p expression by direct modulation of HNRNPA1 expression. miR-18a-3p is the direct target of the well known oncogene KRAS and miR-18a-3p/KRAS ratio can be used as a prognostic factor for chemotherapy-resistant ovarian cancer.49
Moreover, the miR-18a regulates the expression of the Dicer enzyme, which has the effect on the biogenesis of the miR-17-92a cluster members.50 It should be noted that one of the strands of the premature transcript, -5p or -3p, could be favored as stronger and incorporated into the RISC complex. One member could also manifest co-expression with other members of the cluster or with different miRNAs.51 However, the biological role of this phenomenon is not clear.
The expression of miR-18a can also be regulated by interaction with different substances such as SiO2 leading to lung damage52 or phenanthrene (polycyclic aromatic hydrocarbon) which interacts with hnRNPA1 and contributes to the suppression of miR-18a and hepatocyte apoptosis.53
What is interesting, the biological function of miR-18a can also be regulated by other members of the miR-17-92a cluster. For example, miR-18a alone can activate the autophagy process, but the co-expression of miR-18a with miR-17 and miR-20a causes that miR-18a changes its authophagy activation function.21,54
3. miR-18a and cancer
Single miRNA can function as a tumor suppressor, oncogene (oncomiR) or it can have a dual function. Tumor suppressor miRNAs are down-regulated in cancers and the lack of them causes the translation of oncogenic proteins. In contrast, onkomiRs are up-regulated in cancers and a high expression of these miRNAs blocks tumor suppressor mRNAs. Down-regulation of suppressor miRNAs and up-regulation of oncomiRs initiate proliferation, invasion, angiogenesis and metastasis of a tumor.55, 56, 57, 58 It should be noted that half of the known miRNA genes are located close to or inside the regions of chromosomes which are usually mutated in cancers.59, 60, 61
The first evidence of an involvement of miR-17-92a cluster in cancer progress was suggested by Ota et al. They observed an amplification of this cluster in diffuse large B cell lymphomas of recurrent focal amplifications.62 A lot of studies suggested that miR-18a is involved in cancer progression. In the case of nasopharyngeal carcinoma (NPC) miR-18a is overexpressed and positively correlated with tumor size and TNM stage. miR-18a targets SMG1 and causes activation of the MTOR pathway and influence on cell survival, epithelial-to-mesenchymal transition (EMT) and invasion. Additional evidence of miR-18a involvement in early steps of NPC tumorigenesis is activation of this miRNA through latent membrane protein 1 (LMP1) encodes by Epstein-Barr virus (EBV) or NF-κB activation and its downstream genes.63 Luo et al. indicated that expression of miR-18a in NPC is correlated with the clinical stage and tumor metastasis. Studies in a mouse model showed that up-regulation of miR-18a increases tumor growth and lung metastases.50 In the case of patients with lung cancer, there is also a correlation between the level of tumor differentiation and lymph node metastases and the expression of miR-18a in tumor tissue.64 Li et al. examined the process of miRNAs changes from normal through adenoma to colorectal carcinoma transition and observed that miR-18a-5p is up-regulated during this process and involved in the regulation of important cellular processes.65 Another evidence comes from a study based on gastric cancer where up-regulation of miR-17–92a cluster members (including miR-18a) was observed in patients’ plasma compared to healthy individuals.66 Rammer et al. proposed that miR-18a may be involved only in the initial steps in tumor progression such as invasion beyond the submucosa, and its role in further progression is limited. They indicated that the level of miR-18a depends on the T-stage of colon carcinoma. The highest level of miR-18a is shown for T3 samples, then it decreases and, finally for T4 and T1-stage cancers, it is similar. Moreover, the expression of miR-18a is not connected with lymph node metastasis.67 In contrast to this, gastric cancer cell metastasis may be caused by down-regulation SMAD2 and up-regulation of WNT/β-catenin signaling by miR-18a and miR-19a. Moreover, IRF-1 regulates the expression of miRNAs from the miR-17-92a cluster by binding to the MIR17HG promoter site, and the use of IFN-γ to regulate miR-18a and miR-19a expression causes inhibition of cancer metastasis.68
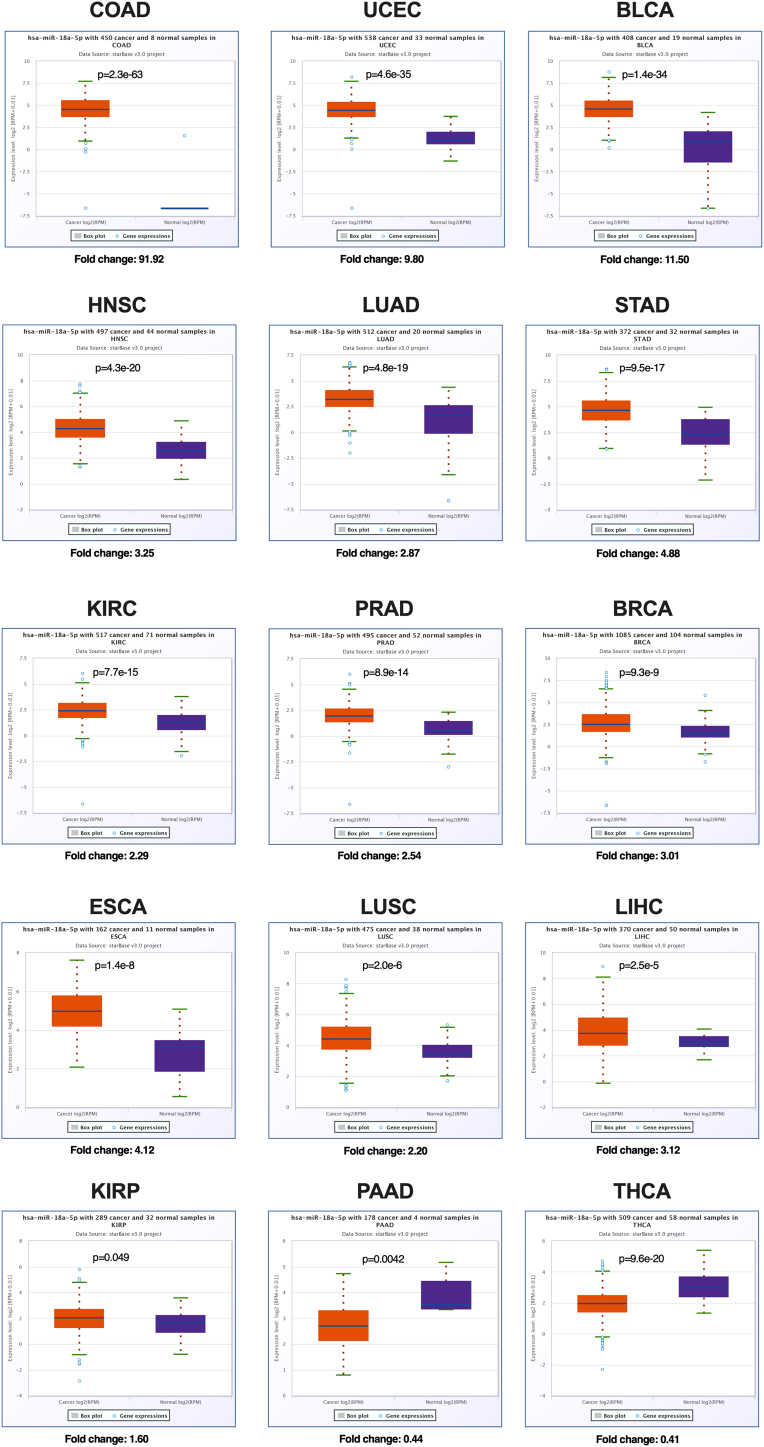
In spite of this evidence of the specific role of miR-18a, which comes from different cancer models, the role of miR-18a in cancerogenesis is indisputable. miR-18a is up-regulated in many cancers, such as: ovarian,69 breast,70, 71, 72 intestine,73 gastric cancer,68,74 nasopharynx,50,75 lung,64,76 osteosarcoma,77 glioblastoma,78 colorectal,79 prostate,80,81 pancreatic cancer,82 esophagus adenocarcinoma,83 head and neck cancers,84 retinoblastoma,85 embryonic hepatoblastoma.86 Down-regulation of miR-18a was reported in epithelial ovarian cancer,87 in gastric cancer88 and in exosomes isolated from multiple myeloma patients.89 According to The Cancer Genome Atlas (TCGA) project (data available by starBase v3.0), miR-18a-5p is significantly up-regulated in 13 different cancers, COAD, UCEC, BLCA, HNSC, LUAD, STAD, KIRC, PRAD, BRCA, ESCA, LUSC, LICH, KIRP with fold change from 91.92 to 1.60. Only for two types of cancer, PAAD and THCA, a significant down-regulation of miR-18a-5p is indicated, with fold change of 0.44 and 0.41, respectively, Fig. 2. However, the observed difference between single, independent studies and TCGA project may be due to the fact that in the TCGA project only a small part of analyzed samples were normal (no-cancer tissue) and they were not matched-adjacent normal samples (from the same patient) in contrast to independent studies.
Fig. 2.
Expression of miR-18a-5p in different types of human cancer according to TCGA database. StarBase v3.0 data - modified; COAD (colon adenocarcinoma), UCEC (uterine corpus endometrial carcinoma), BLCA (bladder urothelial carcinoma), HNSC (head and neck squamous cell carcinoma), LUAD (lung adenocarcinoma), STAD (stomach adenocarcinoma), KIRC (clear cell kidney carcinoma), PRAD (prostate adenocarcinoma), BRCA (breast cancer), ESCA (esophageal carcinoma), LUSC (lung squamous cell carcinoma), LIHC (liver hepatocellular carcinoma), KIRP (kidney renal papillary cell carcinoma), PAAD (pancreatic adenocarcinoma), THCA (papillary thyroid carcinoma).
The role of individual members of the miR-17-92a cluster in the process of tumor formation is unclear. This cluster can act both as a suppressor or an oncogene. Likewise, miR-18a can take both roles, Table 2. The suppressor role of miR-17-92 was shown in prostate cancer. It was indicated that the restoration of all members of this cluster induces changes in cell morphology, MET (mesenchymal-to-epithelial transition) process, reduction of cell proliferation, inhibition of cell migration in vitro, delay tumorigenicity and reduction of tumor growth in vivo. Moreover, according to Ottman et al. studies, miR-17-92a induces prostate cell sensitivity and its inhibition could be used as a new therapeutic approach.90 Similar results were indicated by Liu et al., where over-expression of miR-18a induces cell cycle arrest, apoptosis and reduces tumor growth in vivo. miR-18a directly targets the tumor protein TP53-regulating inhibitor of apoptosis gene 1 (TRIAP1) and inositol phosphate multikinase (IPMK).91 The suppressor role of miR-18a was also indicated in lung cancer cells and cancer initiating cells. The over-expression of miR-18a causes radiosensitivity, reduces tumor growth in vivo and slightly influences overall survival in an animal model.92
Table 2.
Characteristic of miR-18a as a cellular regulator.
| Positive role | Negative role |
|---|---|
| • Induction of changes in cell morphology by mesenchymal-to-epithelial transition process and influence on cancer initiating cells | • Induction of changes in cell morphology by epithelial-to-mesenchymal transition process |
| • Reduction of cell proliferation | • Involvement in initial steps of tumor progression |
| • Induction of cell cycle arrest | • Induction of cell proliferation and influence on higher cell viability |
| • Induction of apoptosis | • Higher expression in advanced and higher grade tumors |
| • Negatively modulation of autophagy process | • Increasing of tumor growth and metastases |
| • Inhibition of cell migration | • Induction of cell resistance to chemotherapeutic agents |
| • Tumorigenicity delay | • Positive modulation of autophagy process |
| • Reduction of tumor growth in vivo | |
| • Induction of cell sensitivity to irradiation and drugs | |
| • Homeostatic function, prevention of the oncogenic effect of miR-17-92a cluster |
However, not all members of the miR-17-92a cluster can act as suppressors. Guo et al. checked the role of the miR-17-92a cluster as a whole transcript as well as individual members from the cluster. The authors indicated that the miR-17-92a cluster is down-regulated after celastrol therapy inducing autophagy in prostate cancer, but only miR-17 and miR-20a, through targeting ATG7 mRNA, are responsible for the inhibition of autophagy. miR-18a seems to act as a negative modulator of the autophagy process and miR-17 together with miR-20a can overcome this miR-18a-blocking effect.21 However, it was also noted by others that miR-18a can induce the autophagy process.54 Humphreys et al. underlined that probably miR-18a has an adverse role to other miRNAs from the miR-17-92a cluster. It was assumed that miR-18a may have a homeostatic function and prevent the oncogenic effect of the miR-17-92a cluster in colorectal cancer. The increased miR-17-92a cluster expression without increased miR-18a may promote tumor progression.93
Tumor growth and proliferation. Generally, miR-18a induces cell proliferation, for example by regulating WEE1, a cell cycle inhibitor.94 However, in colorectal cancer, the increased expression of miR-18a results in decreased proliferation and migration of tumor cells and increases their apoptosis. Humphreys et al. indicated that the cell cycle regulator, CDC42, is the direct target of miR-18a.93 On the other hand, it was shown that miR-18a suppresses cell proliferation in the T24 (human bladder carcinoma) cell line. In this case, the Dicer enzyme is regulated by miR-18a and there is a feedback loop that controls the expression of the miR-17-92a cluster as a whole.95 It was also shown that miR-18a regulates K-RAS and suppresses the development of squamous cell carcinoma and other tumors.96 In contrast to this, Liu et al. indicated that miR-18a has no effect on either the proliferation ratio or apoptosis of cervical cancer cells.97
In glioblastoma cancers, the reduction of miR-18a causes suppression of cell proliferation, migration and invasion as well as induces cell cycle arrest and apoptosis. These effects are connected with the expression of neogenin, which is the direct target of miR-18a.78 Similarly, the reduction of miR-18a decreases cell growth in prostate cancer cells by directly targeting STK4, which in turn influences the dephosphorylation of AKT.80
Cellular phenotype. miR-18a also plays a role in the regulation of cellular phenotype, the EMT process and the metastasis of cancer cells. The first evidence of miR-18a participation in phenotype regulation processes is a correlation between a higher level of miR-18a and more advanced and higher grade tumors.78 Luo et al. observed that miR-18a reduces the expression of e-cadherin and increases the expression of vimentin, ZEB1, ZEB2 and fibronectin. This demonstrates the role of miR-18a in the transition from epithelial to mesenchymal phenotype.50
Response to stress and apoptosis. Analysis of the irradiation influence on breast cancer cells showed that the expression of the miR-17-92a cluster is dysregulated after irradiation, which depends on the irradiation method and the dose. Functional annotation indicated that the miR-17-92a cluster’s target genes are involved in the regulation of radiation-associated pathways, such as MAPK, ERBB, TP53, WNT, transforming the growth factor-β (TGF-β), MTOR signaling pathways, and regulate the cell cycle.70 Liu et al. observed that miR-18a down-regulates ATM expression by directly targeting 3’UTR of ATM mRNA.97 Mutations within the ATM gene or the repression of the ATM protein were found in many types of cancers.98 It was demonstrated that over-expression of miR-18a reduces the DNA damage repair (DDR) process and the efficiency of homologous recombination-based DNA repair (HRR) which causes cells sensitization to radiation treatment in breast cancer cell lines. Generally, the inhibition of miR-18a reduces cellular radiosensitivity.97 Similarly, miR-18a is down-regulated in irradiated lung cancer cells and the over-expression of miR-18a causes cellular radiosensitivity by targeting ATM and HIF-1α in lung cancer cells, as well as in CD133+ stem-like cells.92
Based on the colon cancer model, it was indicated that up-regulation of miR-18a induces apoptosis. The direct binding of miR-18a to HNRNPA1 in the cytoplasm causes inhibition of the oncogenic functions of the ribonucleoprotein. Moreover, miR-18a and HNRNPA1 form a complex that is degraded through the autophagolysosomal pathway leading to cellular death.99
In gastric carcinoma cell lines, under hypoxic conditions, the expression of miR-18a is decreased which promotes apoptosis and the inhibition of cell invasion. The hypoxia-inducible factor (HIF)-1α was identified as a direct target of miR-18a, which induces apoptosis via the HIF-1α/mitochondrial apoptosis pathway through changes in the expression of BCL-2, BAX, caspase-3 and caspase-9.100 Uhr et al., based on 36 breast cancer cell lines models, checked different drugs and the relation between cells sensitivity and changes in expression levels of 411 miRNAs. Taking into account different molecular subtypes of breast cancer, they observed a connection between 11 miRNAs and 8 different drugs, where miR-18a together with let-7d was connected with sensitivity to Tivantinib (highly selective inhibitor of tyrosine-protein kinase Met).101 In the case of paclitaxel resistance triple-negative breast cancer patients, the over-expression of miR-18a directly leads to Dicer repression at mRNA and protein levels and increases the cell viability and chemotherapy resistance in vitro.71 Different models of induced apoptosis and paclitaxel resistant triple-negative breast cancer cells were presented by Fan and co-workers. They showed that the enforced expression of miR-18a directly leads to increased autophagy via inhibition of the MTOR signaling pathway.102 In pancreatic cancer cells miR-18a probably also plays a role in the apoptosis and autophagy processes, but this statement was not directly shown.103 Ottley et al. demonstrated that Activin A, which is a member of the TGF-β superfamily and is an inhibitor of tumor growth, down-regulates the miR-18a expression in prostate cells, influences the expression of ACVR2A, TGFBR2, CDKN1B and correlates with Activin A-mediated growth arrest.104
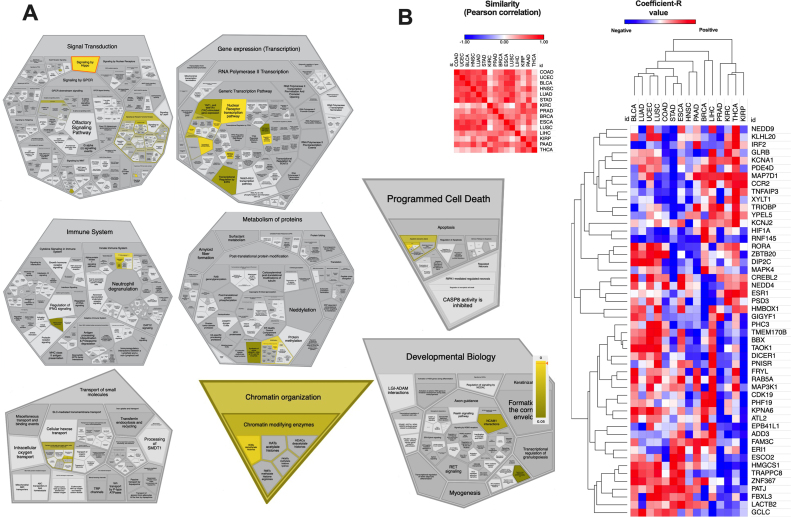
Based on miRDB database for miRNA target prediction potential miR-18a-5p targets were analyzed. Over 380 targets with target score > = 50 were indicated and these targets are involved in important cellular processes such as signal transduction, gene expression (transcription), immune system, metabolism of proteins, programed cell death, transport of small molecules, chromatin organization or involvement in developmental biology processes indicated by the PANTHER (Protein ANalysis THrough Evolutionary Relationships) classification system, Fig. 3A. Using starBase v3.0 tool, the correlation between miR-18a-5p and 50 of the top predicted targets for 15 types of cancer (TCGA) were analyzed. Our analysis indicated that COAD, UCEC, BLCA, HNSC, LUAD, STAD, ESCA and LUSC are the most similar cancers in terms of the expression of miR-18a-5p targets, Fig. 3B.
Fig. 3.
Predicted miR-18a-5p targets according to miRDB database. (A) Participation of miR-18a-5p targets in specific biological processes. (B) Similarity of cancers in terms of miR-18a-5p targets expression and heat-map and clustering of the top 50 miR-18a-5p targets in 15 different types of cancers. Based on miRDB, PANTHER and StarBase v3.0 databases.
4. miR-18a and other diseases and pathological conditions
It should be noted that the expression of miR-18a is also changed in other diseases and pathological conditions. The up-regulation is observed in drug-induced cutaneous reaction (toxic epidermal necrolysis),105 or in inflammation conditions such as mucosal of the bowel.106
Zhang et al. indicated that in the case of idiopathic pulmonary fibrosis miR-18a is down-regulated in the pleural mesothelial cell after bleomycin treatment and miR-18a-5p reduction contributes to the EMT process. miR-18a directly regulates the mRNA of TGF-βRII (transforming the growth factor β receptor II) and suppresses the TGF-β-SMAD2/3 signaling pathway, causing changes in the cell phenotype. The over-expression of miR-18a can prevent this process and overcome the negative bleomycin-induced sub-pleural fibrosis in mouse models.107 Similar results were observed by Geng et al. Based on an in vitro model of human aortic valvular endothelial cells (HAVECs), they observed that up-regulation of miR-18a-5p prevents endothelial-mesenchymal transition and cardiac fibrosis induced by high glucose concentration. miR-18a was identified as a direct regulator of NOTCH2 expression, which in turn regulates cellular phenotype. Molecular modulation of miR-18a-5p/NOTCH2 axis can be a promising approach to treatment of diabetic cardiomyopathy.108 The prominent role of miR-18a was also described in the case of muscle physiology. Higher expression of miR-18a causes negative changes in the differentiation process and leads to myotubes atrophy. miR-18a targets 3’UTR of IGF1 and through modulation of IGF1/PI3K/AKT signaling negatively influences on the protein expression in muscle cells.109 Lin et al. proposed that miR-18a could be used as an important molecular target in the diagnosis as well as treatment of acute myocardial infarction. They observed that down-regulation of miR-18a can prevent the cellular senescence and induced autophagy of cardiomyocytes through up-regulation of BDNF (brain derived neurotrophic factor) expression and inhibition of the AKT/MTOR axis.110
In the case of toxic epidermal necrolysis, the over-expression of miR-18a causes an increase of caspase-9 activity, directly down-regulates the BCL2L10 and mediates intrinsic keratinocyte apoptosis.105
5. miR-18a as potential biomarker
miRNAs seem to be good diagnostic, prognostic and predictive biomarkers. It turns out that miRNAs potentially show all the features of a classic biomarker, such as: i) characterization of specific conditions and differentiation groups; ii) simplicity of acquisition from a variety of biological materials; iii) stability and iv) easy and inexpensive analysis process.111
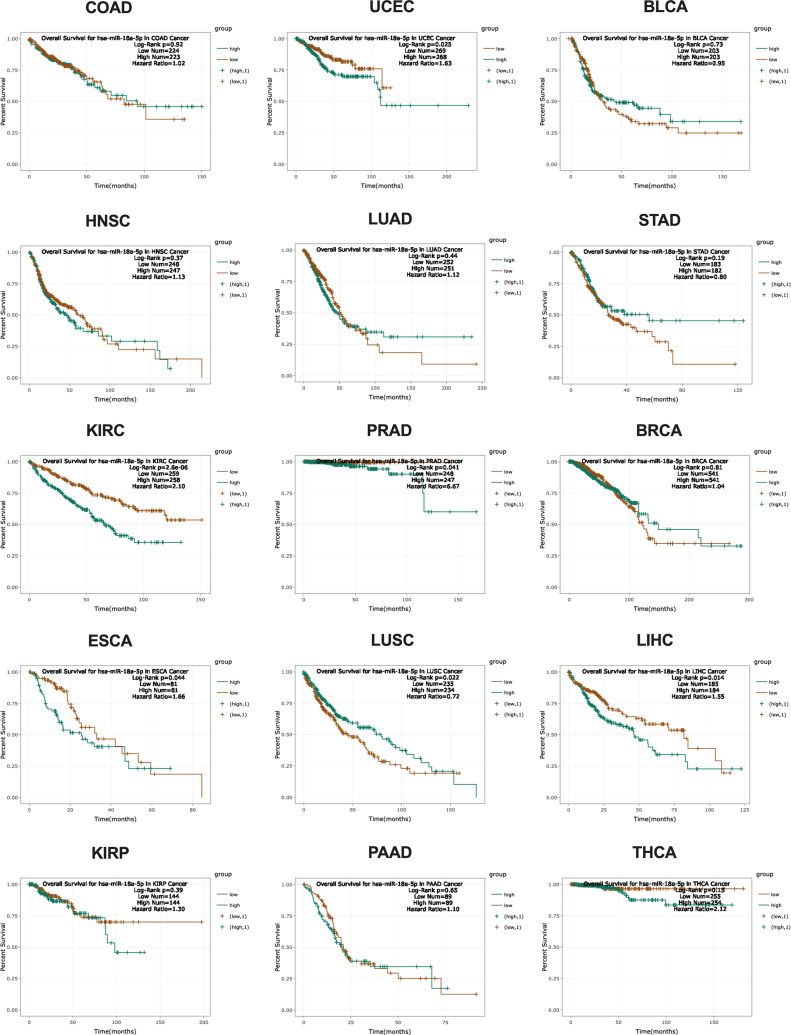
miR-18a is a good marker with a diagnostic potential to discriminate the cancer and healthy tissue in the case of many cancers.81 Moreover, miRNA can define specific histological subtypes of cancer. For example, a high expression of miR-18a as well as miR-18b is strongly associated with basal-like breast cancer type.112 Similarly, the level of miR-18a can be used to distinguish the subtypes of neuroendocrine tumors of the lung. A low level of miR-18a is characteristic of typical and increasing for atypical carcinoids and large cell neuroendocrine lung cancer, and a high expression is characteristic for small cell lung cancer, especially for high-grade neuroendocrine pulmonary tumors.113 For diagnostic and prognostic assessment of NPC patients, Zhuo et al. defined miR-18a and miR-135b as connected with metastasis and useful as potential biomarkers. It should be noted that miR-18a has a lower diagnostic accuracy, but the best prognostic ability, and patients with a low level of miR-18a have a longer overall survival rate.114 Grassi et al. indicated that miR-18a in combination with miR-182 or miR-183 can be used as a predictive marker where a high level of miRNAs ratio is observed in the case of relapse after resection of localized colon cancer.79 In the case of esophageal squamous cell carcinoma Xu et al. noted that miR-18a can be used as a prognostic marker for overall survival - patients with a lower expression of miR-18a have better prognosis.115 In spite of those results, our TCGA analysis, based on starBase v3.0 website, indicated that patients with a lower expression of miR-18a-5p have a significantly better overall survival in the case of KIRC, PRAD, ESCA and LIHC. However, patients with a low expression of miR-18a-5p have a worse prognosis only in the case of lung squamous cell carcinoma (LUSC). For the rest of the 10 types of cancers no significant correlation between survival and expression level of miR-18a-5p is observed, Fig. 4.
Fig. 4.
Overall survival depending on miR-18a-5p expression level in different types of human cancer according to TCGA database. StarBase v3.0 data - modified; COAD (colon adenocarcinoma), UCEC (uterine corpus endometrial carcinoma), BLCA (bladder urothelial carcinoma), HNSC (head and neck squamous cell carcinoma), LUAD (lung adenocarcinoma), STAD (stomach adenocarcinoma), KIRC (clear cell kidney carcinoma), PRAD (prostate adenocarcinoma), BRCA (breast cancer), ESCA (esophageal carcinoma), LUSC (lung squamous cell carcinoma), LIHC (liver hepatocellular carcinoma), KIRP (kidney renal papillary cell carcinoma), PAAD (pancreatic adenocarcinoma), THCA (papillary thyroid carcinoma).
Potentially, miR-18a can be used as a marker of resistance to specific drugs. In the case of diffuse large B-cell lymphoma patients treated with an anthracyclin-based immuno-chemotherapy regimen scheme, the expression of miR-18a was correlated with patient's' overall survival.116 Hummel et al., based on the in vitro model of cisplatin and 5-FU-resistant esophageal adeno- and squamous cell carcinoma cells lines, indicated that miR-18a-3p is down-regulated compared to sensitive cell lines. It is proposed that miR-18a-3p influences chemotherapy response via regulating the expression of the K-RAS oncogene.117 Study based on analysis of residual tumors after neoadjuvant chemotherapy in breast cancer patients indicated that miR-18a could serve as a prognostic marker. In the case of luminal type of breast tumors, a high expression of miR-18a observed after neoadjuvant chemotherapy was linked with a worse overall survival. Moreover, authors indicated that miR-18a is responsible for proliferation of cancer cells and directly targets the estrogen receptor (ER) mRNA.118,119 Up-regulation of miR-18a in an in vitro model causes low sensitivity to tamoxifen as well as up-regulation of genes characteristic for luminal B and endocrine resistance breast cancers.118
Wang et al. checked the expression level of miR-34c-3p and miR-18a-5p in tumor-educated platelet (blood platelets that contain tumor RNAs), which are released into the bloodstream and can easily be obtained by a liquid biopsy method. They observed that both of these miRNAs are significantly up-regulated in NPC patients, regardless of patients’ demographic variables and their TNM stages, and may be used as a diagnostic marker.120 It should be noted that members of the miR-17-92a cluster are present not only in tissue or inside the cell. For example, miR-18a is found in exosomes and as free circulating RNAs in plasma or serum.121, 122, 123 It was indicated that exosomal miR-18a is significantly associated with both progression free survival and overall survival in the case of multiple myeloma patients.89 In the case of hepatocellular carcinoma, the exosomal level of miR-18a is significantly higher in cancer patients compared to chronic hepatitis B and liver cirrhosis.124
Many studies indicated a free circulating miR-18a as a potential biomarker for colon cancer,125, 126, 127 gastric cancer,128, 129, 130 breast cancer,131,132 esophageal squamous cell carcinoma,133 retinoblastoma,134 pancreatic cancer82 and endometriosis.123 In most of the studies the expression level of miR-18a is up-regulated in patients’ plasma/serum.
Circulating miR-18a (with 8 other miRNAs) can be used as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer with a high discrimination potential between cancer and healthy individuals.132 Similar to these results, Marcuello et al. proposed to use miR-18a as a component of a miRNA-based panel in diagnostic of colorectal cancer using serum samples. Moreover, the authors observed that using the assessment of miRNAs with estimation of faecal hemoglobin f(Hb) concentration improved sensitivity and specificity of diagnostics and could be used as a non-invasive method for early detection of colorectal cancer.135 Al-Kafaji et al. observed that the expression of miR-18a is significantly increased in peripheral blood of prostate cancer patients compared to benign prostatic hyperplasia as well as healthy controls. Moreover, a higher level of miR-18a associated with cancer progression was noticed.136 It should be noted that this study was based on a very small group of patients. However, Ibrahim et al. proposed the use of plasma miR-21, miR-141, miR-18a and miR-221 as non-invasive biomarkers for diagnosis of prostate cancer patients. miR-18a was found to have the best clinical value with a high discrimination ability to distinguish cancer patients from healthy controls, and miR-221, which may serve as a marker of metastasis. Authors underline that the combination of miR-18a and miR-221 assessment gives the best results and recommended further validation in another group of patients.137
In a similar study, miR-18a taken from whole blood of non-small cell lung cancer was examined. In this case miR-18a was up-regulated in cancer patients compared to healthy donors. Unfortunately, in spite of good discrimination ability, miR-18a cannot be taken into consideration as a marker in the diagnostic panel proposed by the authors.138 Fan et al. in the preliminary study based on plasma from gastric cancer patients, indicated that expression of miR-17-92a cluster is connected with disease progression and response to oxaliplatin/capecitabine chemotherapy treatment. Patients with response to treatment had a decreased level of these plasma miRNAs in contrast to the group of non-responders, which characterized a high expression level of miR-17-92a cluster after chemotherapy.139
Cancer cells are likely to be the source of circulating miR-18a. Tsujiura et al., based on the in vitro model of gastric cancer cells, showed that miR-18a is released from cells to media.130 However, they did not indicate if miR-18a is actively released as free-RNA (or in a complex with proteins or lipids) or as exosomal miRNA. It should be noted that nucleic acids can also be released as a result of apoptosis or necrosis as well as manual destruction of the cell can highly influence this phenomenon.140 In spite of this, Tsujiura et al. indicated that plasma miR-18a in gastric patients may not be derived from peripheral blood cells and there is no correlation between different types of blood cells and miR-18a levels.130
Circulating miR-18a is significantly down-regulated in non-small cell lung cancer patients compared to healthy donors and high plasma miR-18a expression levels correlate with worse disease free survival and shorter overall survival.141 Meta-analyses confirmed that miR-18a could be a promising noninvasive biomarker in gastric carcinoma diagnosis.142 Morimura et al. and Hirajima et al. indicated that the level of miR-18a plasma is significantly higher in pancreatic cancer and in esophageal squamous cell carcinoma patients, while in controls and after surgery its expression is reduced. They also suspected that disease-recurred patients probably regain a high level of miR-18a.82,133 Unfortunately, in both studies, pre- and postoperative observations are based on very small number of cases and they need to be verified to draw far-reaching conclusions.
What is important, some studies indicated that panels of circulating miRNAs may have a higher diagnostic value than that of traditional biomarkers.128 In contrast to this statement, other authors pointed out that miR-18a, as well as other miRNAs, are not reliable biomarkers and can only be used to a minimal extent.64,143 In spite of many studies indicating the potential of miRNAs as biomarkers in colon cancer, there are difficulties to establish a comprehensive diagnostic panel. None of the studies used the prediagnostic samples to compare the existing miRNA panel.125 Wikberg et al. checked the expression level of selected miRNAs and indicated that circulating miR‐18a, indeed, can be used as a marker in colon cancer diagnostics, but it is not useful for estimating the prediagnostic samples.125 Liu et al. indicated that miR-18a is not significantly changed in patients’ serum with advanced adenoma of colorectal cancer compared to control samples, and only miR-21 and miR-92a have a diagnostic and prognostic value.144
6. Conclusions
miR-18a is a member of the miR-17-92a cluster, which is a unique miRNA from this cluster because of its biological role and regulation of its transcription. Many studies, as well as the TCGA project, indicated that miR-18a is mostly up-regulated in different types of cancer.
The behavior of miR-18a probably depends on the genetic background of the cell and it may act both as a suppressor and an oncogene. The expression of specific members of the miR-17-92a cluster is not balanced and the members are transcribed as mature miRNAs with different efficiency, stability and miR-18a is likely to function as a regulator of the whole transcript. miR-18a has an effect on a lot of important cellular processes, such as cell proliferation, migration or cancer metastasis. It should be noted that miR-18a can also regulate the cellular phenotype. Through negative/positive influence on processes associated with apoptosis, miR-18a regulates the cell response to stress caused by ionizing radiation or chemical exposure.
miR-18a can probably be used as a biomarker in the future. A lot of studies indicated the potential role of this miRNA as a diagnostic, predictive or prognostic tool in clinical practice. Moreover, the aforementioned studies focused mainly on circulating miR-18a highlighting its great usability as a miRNA, which could be potentially selected for designing a tumor genes panel used for liquid biopsy diagnostics in the future.
To sum up, miR-18a seems to play a dual, suppressive as well as oncogenic role, in the miR-17-92a cluster, which is referred to as oncomir-1. The exact role of miR-18a depends on many factors. We suspect, however, that the most important ones include transcriptional and posttranscriptional regulation. In spite of this, miR-18a has a significant impact on many cellular processes and its exact role is still mysterious.
Conflict of interest
Concerning publication of an article titled: Good or not good: role of miR-18a in cancer biology written by Tomasz Kolenda, Kacper Guglas, Magda Kopczyńska, Joanna Sobocińska, Anna Teresiak, Renata Bliźniak, Katarzyna Lamperska
The authors declare:
-
1)
The contents of this manuscript have not been copyrighted or published previously.
-
2)
The contents of the manuscript are not now under consideration for publication elsewhere.
-
3)
There is no conflict of interest including any financial, personal or other relationships with other people or organizations regarding the publication of this paper.
-
4)
This work was supported by Greater Poland Cancer Center – grant No.: 12/2011 and grant No.: 13/2016.
Financial disclosure
Article titled: Good or not good: role of miR-18a in cancer biology written by Tomasz Kolenda, Kacper Guglas, Magda Kopczyńska, Joanna Sobocińska, Anna Teresiak, Renata Bliźniak, Katarzyna Lamperska was supported by Greater Poland Cancer Center – grant No.: 12/2011 and grant No.: 13/2016.
Acknowledgment
This work was supported by Greater Poland Cancer Centre – grant No.: 12/2011 and grant No.: 13/2016.
References
- 1.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Bueno M.J., Malumbres M. MicroRNAs and the cell cycle. Biochim Biophys Acta. 2011;1812(5):592–601. doi: 10.1016/j.bbadis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Hummel R., Hussey D.J., Haier J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer. 2010;46(2):298–311. doi: 10.1016/j.ejca.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Muljo S.A., Kanellopoulou C., Aravind L. MicroRNA targeting in mammalian genomes: Genes and mechanisms. Wiley Interdiscip Rev Syst Biol Med. 2010;2(2):148–161. doi: 10.1002/wsbm.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ul Hussain M. Micro-RNAs (miRNAs): genomic organisation, biogenesis and mode of action. Cell Tissue Res. 2012;349(2):405–413. doi: 10.1007/s00441-012-1438-0. [DOI] [PubMed] [Google Scholar]
- 6.Olena A.F., Patton J.G. Genomic organization of microRNAs. nJournal of cellular physiology. 2010;222(3):540–545. doi: 10.1002/jcp.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang Y., Ridzon D., Wong L., Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y., Kim M., Han J. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu S., Jin L., Zhang Y. The loop position of shRNAs and pre-miRNAs is critical for the accuracy of dicer processing in vivo. Cell. 2012;151(4):900–911. doi: 10.1016/j.cell.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starega-Roslan J., Koscianska E., Kozlowski P., Krzyzosiak W.J. The role of the precursor structure in the biogenesis of microRNA. Cell Mol Life Sci. 2011;68(17):2859–2871. doi: 10.1007/s00018-011-0726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L., Thomson J.M., Hemann M.T. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogilyansky E., Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20(12):1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanzer A., Stadler P.F. Molecular evolution of a microRNA cluster. J Mol Biol. 2004;339(2):327–335. doi: 10.1016/j.jmb.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 14.Concepcion C.P., Bonetti C., Ventura A. The miR-17-92 family of microRNA clusters in development and disease. Cancer J. 2012;18(3):262–267. doi: 10.1097/PPO.0b013e318258b60a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houbaviy H.B., Murray M.F., Sharp P.A. Embryonic stem cell-specific micrornas. Dev Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 16.Ventura A., Young A.G., Winslow M.M. Targeted deletion reveals essential and overlapping functions of the mir-17 through 92 family of mirna clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguda B.D., Kim Y., Piper-Hunter M.G., Friedman A., Marsh C.B. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc Natl Acad Sci U S A. 2008;105(50):19678–19683. doi: 10.1073/pnas.0811166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell K.A., Wentzel E.A., Zeller K.I., Dang C.V., Mendell J.T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(Jun 9(7043)):839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 19.Sylvestre Y., De Guire V., Querido E. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282(4):2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 20.Woods K., Thomson J.M., Hammond S.M. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J Biol Chem. 2007;282(4):2130–2134. doi: 10.1074/jbc.C600252200. [DOI] [PubMed] [Google Scholar]
- 21.Guo J., Mei Y., Li K., Huang X., Yang H. Downregulation of miR-17-92a cluster promotes autophagy induction in response to celastrol treatment in prostate cancer cells. Biochem Biophys Res Commun. 2016;478(2):804–810. doi: 10.1016/j.bbrc.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Brock M., Trenkmann M., Gay R.E. MicroRNA-18a enhances the interleukin-6-mediated production of the acute-phase proteins fibrinogen and haptoglobin in human hepatocytes. J Biol Chem. 2011;286:40142–40150. doi: 10.1074/jbc.M111.251793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brock M., Trenkmann M., Gay R.E. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104(10):1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 24.Chaulk S.G., Thede G.L., Kent O.A. Role of pri-miRNA tertiary structure in miR-17∼92 miRNA biogenesis. RNA Biol. 2011;8(6):1105–1114. doi: 10.4161/rna.8.6.17410. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty S., Mehtab S., Patwardhan A., Krishnan Y. Pri-miR-17-92a transcript folds into a tertiary structure and autoregulates its processing. RNA. 2012;18(5):1014–1028. doi: 10.1261/rna.031039.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonauer A., Carmona G., Iwasaki M. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 27.Diosdado B., van de Wiel M.A., Terhaar Sive Droste J.S. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br J Cancer. 2009;101(4):707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guil S., Cáceres J.F. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat Struct Mol Biol. 2007;14(7):591–596. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- 29.Chaulk S.G., Fahlman R.P. Tertiary structure mapping of the pri-miRNA miR-17∼92. Methods Mol Biol. 2014;1182:43–55. doi: 10.1007/978-1-4939-1062-5_5. [DOI] [PubMed] [Google Scholar]
- 30.Michlewski G., Guil S., Semple C.A., Cáceres J.F. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol Cell. 2008;32(3):383–393. doi: 10.1016/j.molcel.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo J.H., Lee H.J., Kim W., Kim S.G. Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-Mediated degradation of HNRNPA1 and up-regulation of SMAD2. Gastroenterology. 2016;150(1):181–193. doi: 10.1053/j.gastro.2015.09.039. e8. [DOI] [PubMed] [Google Scholar]
- 32.Kolenda T., Guglas K., Ryś M. Biological role of long non-coding RNA in head and neck cancers. Rep Pract Oncol Radiother. 2017;22(5):378–388. doi: 10.1016/j.rpor.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H.Y., Bai W.D., Ye X.M., Yang A.G., Jia L.T. Long non-coding RNA UCA1 desensitizes breast cancer cells to trastuzumab by impreding miR-18a repression of Yes-associated protein 1. Biochem Biophys Res Commun. 2018;496(Feb 19(4)):1308–1313. doi: 10.1016/j.bbrc.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Wu Y., Liu A., Tang X. Long non-coding RNA UCA1 enhances tamoxifen resistance in breast cancer cells through a miR-18a-HIF1α feedback regulatory loop. Tumour Biol. 2016;37(11):14733–14743. doi: 10.1007/s13277-016-5348-8. [DOI] [PubMed] [Google Scholar]
- 35.Li X.N., Liu A.H., Tang X., Ren Y. Urothelial carcinoma-associated 1 enhances tamoxifen resistance in breast cancer cells through competitively inhibiting miR-18a. Beijing Da Xue Xue Bao. 2017;49(Apr 18(2)):295–302. [PubMed] [Google Scholar]
- 36.Zheng P., Dong L., Zhang B. Long noncoding RNA CASC2 promotes paclitaxel resistance in breast cancer through regulation of miR-18a-5p/CDK19. Histochem Cell Biol. 2019;152(Oct (4)):281–291. doi: 10.1007/s00418-019-01794-4. [DOI] [PubMed] [Google Scholar]
- 37.Huang G., Wu X., Li S., Xu X., Zhu H., Chen X. The long noncoding RNA CASC2 functions as competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep. 2016;6(May 20):26524. doi: 10.1038/srep26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Qian W., Feng F. Upregulated lncRNA CASC2 may inhibit malignant melanoma development through regulating miR-18a-5p/RUNX1. Oncol Res. 2019;27(Feb 21(3)):371–377. doi: 10.3727/096504018X15178740729367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q., Yu W., Zhu S. Long noncoding RNA GAS5 regulates the proliferation, migration and invasion glioma cells by negatively regulating miR-18a-5p. J Cell Physiol. 2018;234(Jan (1)):757–768. doi: 10.1002/jcp.26889. [DOI] [PubMed] [Google Scholar]
- 40.Yang J., Hao T., Sun J., Wei P., Zhang H. Long noncoding RNA GAS5 modulates α-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed Pharmacother. 2019;112(Apr) doi: 10.1016/j.biopha.2019.108656. [DOI] [PubMed] [Google Scholar]
- 41.Ye F., Tian L., Zhou Q., Feng D. LncRNA FER1L4 induces apoptosis and suppresses EMT and the activation of PI3K/AKT pathway in osteosarcoma cells via inhibiting miR-18a-5p to promote SOCS5. Gene. 2019;721(Aug 29):144093. doi: 10.1016/j.gene.2019.144093. [DOI] [PubMed] [Google Scholar]
- 42.Li W., Ma S., Bai X., Pan W., Ai L., Tan W. Long noncoding RNA WDFY3-AS2 suppresses tumor progression by acting as a competing endogenous RNA of microRNA-18a in ovarian cancer. J Cell Physiol. 2019;(Jul 25) doi: 10.1002/jcp.29028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Xiao H., Liu Y., Liang P. TP53TG1 enhances cisplatin sensitivity of non-small cell lung cancer cells through regulating miR-18a/PTEN axis. Cell Biosci. 2018;8(Mar 22):23. doi: 10.1186/s13578-018-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhan G., Han G., Zhang X. Long non-coding RNA FENDRR reduces prostate cancer malignacy by competitively binding miR-18a-5p with RUNX1. Biomarkers. 2018;23(Jul (5)):435–445. doi: 10.1080/1354750X.2018.1443509. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L., Luo X., Chen F. LncRNA SNHG1 regulates cerebrovascular pathologies as a competing endogenous RNA through HIF-1α/VEGF signaling in ischemic stroke. J Cell Biochem. 2018;119(7):5460–5472. doi: 10.1002/jcb.26705. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez-Mejias A., Kwon J., Chew X.H. A novel SOCS5/miR-18/miR-25 axis promotes tumorigenesis in liver cancer. Int J Cancer. 2019;144(Jan 15(2)):311–321. doi: 10.1002/ijc.31857. [DOI] [PubMed] [Google Scholar]
- 47.https://www.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000171150;r=2:46698952-46780245.
- 48.Kumari P., Sampath K. cncRNAs: Bi-functional RNAs with protein coding and non-coding functions [published correction appears in Semin Cell Dev Biol. 2016 May;53:168] Semin Cell Dev Biol. 2015;47-48:40–51. doi: 10.1016/j.semcdb.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Aguayo C., Monroig P.D.C., Redis R.S. Regulation of hnRNPA1 by microRNAs controls the miR-18a-K-RAS axis in chemotherapy-resistant ovarian cancer. Cell Discov. 2017;3:17029. doi: 10.1038/celldisc.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo Z., Dai Y., Zhang L. miR-18a promotes malignant progression by impairing microRNA biogenesis in nasopharyngeal carcinoma. Carcinogenesis. 2013;34(2):415–425. doi: 10.1093/carcin/bgs329. [DOI] [PubMed] [Google Scholar]
- 51.Sand M., Hessam S., Amur S. Expression of oncogenic miR-17-92 and tumor suppressive miR-143-145 clusters in basal cell carcinoma and cutaneous squamous cell carcinoma. J Dermatol Sci. 2017;86(2):142–148. doi: 10.1016/j.jdermsci.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Yang H., Zhang Y., Li W., Lao C., Li M., Zheng Y. Altered microRNA expression profiles in lung damage induced by nanosized SiO2. Bioengineered. 2017;8(1):45–54. doi: 10.1080/21655979.2016.1227578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong X., Qin J., Chen R. Phenanthrene-induced apoptosis and its underlying mechanism. Environ Sci Technol. 2017;51(24):14397–14405. doi: 10.1021/acs.est.7b04045. [DOI] [PubMed] [Google Scholar]
- 54.Qased A.B., Yi H., Liang N., Ma S., Qiao S., Liu X. MicroRNA-18a upregulates autophagy and ataxia telangiectasia mutated gene expression in HCT116 colon cancer cells. Mol Med Rep. 2013;7(2):559–564. doi: 10.3892/mmr.2012.1214. [DOI] [PubMed] [Google Scholar]
- 55.Deng S., Calin G.A., Croce C.M., Coukos G., Zhang L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle. 2008;7(17):2643–2646. doi: 10.4161/cc.7.17.6597. [DOI] [PubMed] [Google Scholar]
- 56.Esquela-Kerscher A., Slack F.J. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 57.Lotterman C.D., Kent O.A., Mendell J.T. Functional integration of microRNAs into oncogenic and tumor suppressor pathways. Cell Cycle. 2008;7(16):2493–2499. doi: 10.4161/cc.7.16.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang B., Pan X., Cobb G.P. Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol (Basel) 2007;302(1):1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 59.Calin G.A., Sevignani C., Dumitru C.D. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serrano N.A., Xu C., Liu Y. Integrative analysis in oral squamous cell carcinoma reveals DNA copy number-associated miRNAs dysregulating target genes. Otolaryngol Head Neck Surg. 2012;9:143. doi: 10.1177/0194599812442490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L., Huang J., Yang N. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci U S A. 2006;103(24):9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ota A., Tagawa H., Karnan S. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64(9):3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 63.Mai S., Xiao R., Shi L. MicroRNA-18a promotes cancer progression through SMG1 suppression and mTOR pathway activation in nasopharyngeal carcinoma. Cell Death Dis. 2019;10(11):819. doi: 10.1038/s41419-019-2060-9. Published 2019 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen Z., Wu X., Wang Z., Li B., Zhu X. Effect of miR-18a overexpression on the radiosensitivity of non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(1):643–648. [PMC free article] [PubMed] [Google Scholar]
- 65.Li J., Zhong Y., Cai S., Zhou P., Yao L. MicroRNA expression profiling in the colorectal normal-adenoma-carcinoma transition. Oncol Lett. 2019;18(2):2013–2018. doi: 10.3892/ol.2019.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan B., Shen C., Wu M., Zhao J., Guo Q., Luo Y. miR-17-92 cluster is connected with disease progression and oxaliplatin/capecitabine chemotherapy efficacy in advanced gastric cancer patients: a preliminary study. Bull Sch Med Univ Md. 2018;97(35) doi: 10.1097/MD.0000000000012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rammer M., Webersinke G., Haitchi-Petnehazy S. MicroRNAs and their role for T stage determination and lymph node metastasis in early colon carcinoma. Clin Exp Metastasis. 2017;34(6-7):431–440. doi: 10.1007/s10585-017-9863-9. [DOI] [PubMed] [Google Scholar]
- 68.Yuan J., Tan L., Yin Z. MIR17HG-miR-18a/19a axis, regulated by interferon regulatory factor-1, promotes gastric cancer metastasis via Wnt/β-catenin signalling. Cell Death Dis. 2019;10(6):454. doi: 10.1038/s41419-019-1685-z. Published 2019 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L., Wang B., Fang M., Guo F., Cui M. Identification of microRNAs and target genes involved in serous ovarian carcinoma and their influence on survival. Eur J Gynaecol Oncol. 2014;35(6):655–661. [PubMed] [Google Scholar]
- 70.Leung C.M., Chen T.W., Li S.C. MicroRNA expression profiles in human breast cancer cells after multifraction and single-dose radiation treatment. Oncol Rep. 2014;31(5):2147–2156. doi: 10.3892/or.2014.3089. [DOI] [PubMed] [Google Scholar]
- 71.Sha L.Y., Zhang Y., Wang W. MiR-18a upregulation decreases Dicer expression and confers paclitaxel resistance in triple negative breast cancer. Eur Rev Med Pharmacol Sci. 2016;20(11):2201–2208. [PubMed] [Google Scholar]
- 72.Castellano L., Giamas G., Jacob J. The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci U S A. 2009;106(37):15732–15737. doi: 10.1073/pnas.0906947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yau T.O., Wu C.W., Dong Y. microRNA-221 and microRNA-18a identification in stool as potential biomarkers for the non-invasive diagnosis of colorectal carcinoma. Br J Cancer. 2014;111(9):1765–1771. doi: 10.1038/bjc.2014.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan C., Zhang Y., Tu W., Guo Y. Integrated miRNA profiling and bioinformatics analyses reveal upregulated miRNAs in gastric cancer. Oncol Lett. 2019;18(2):1979–1988. doi: 10.3892/ol.2019.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wan X.X., Yi H., Qu J.Q., He Q.Y., Xiao Z.Q. Integrated analysis of the differential cellular and EBV miRNA expression profiles in microdissected nasopharyngeal carcinoma and non-cancerous nasopharyngeal tissues. Oncol Rep. 2015;34(5):2585–2601. doi: 10.3892/or.2015.4237. [DOI] [PubMed] [Google Scholar]
- 76.Gao W., Shen H., Liu L., Xu J., Xu J., Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137(4):557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 77.Namløs H.M., Meza-Zepeda L.A., Barøy T. Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song Y., Wang P., Zhao W. MiR-18a regulates the proliferation, migration and invasion of human glioblastoma cell by targeting neogenin. Exp Cell Res. 2014;324(1):54–64. doi: 10.1016/j.yexcr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Grassi A., Perilli L., Albertoni L. A coordinate deregulation of microRNAs expressed in mucosa adjacent to tumor predicts relapse after resection in localized colon cancer. Mol Cancer. 2018;17(1):17. doi: 10.1186/s12943-018-0770-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsu T.I., Hsu C.H., Lee K.H. MicroRNA-18a is elevated in prostate cancer and promotes tumorigenesis through suppressing STK4 in vitro and in vivo. Oncogenesis. 2014;3:e99. doi: 10.1038/oncsis.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song C.J., Chen H., Chen L.Z., Ru G.M., Guo J.J., Ding Q.N. The potential of microRNAs as human prostate cancer biomarkers: a meta-analysis of related studies. J Cell Biochem. 2018;119(3):2763–2786. doi: 10.1002/jcb.26445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morimura R., Komatsu S., Ichikawa D. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br J Cancer. 2011;105(11):1733–1740. doi: 10.1038/bjc.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xi T., Zhang G. Epigenetic regulation on the gene expression signature in esophagus adenocarcinoma. Pathol Res Pract. 2017;213(2):83–88. doi: 10.1016/j.prp.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Zeljic K., Jovanovic I., Jovanovic J., Magic Z., Stankovic A., Supic G. MicroRNA meta-signature of oral cancer: evidence from a meta-analysis. Ups J Med Sci. 2018;123(1):43–49. doi: 10.1080/03009734.2018.1439551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y., Mei Q. miRNA signature identification of retinoblastoma and the correlations between differentially expressed miRNAs during retinoblastoma progression. Mol Vis. 2015;21:1307–1317. [PMC free article] [PubMed] [Google Scholar]
- 86.Gyugos M., Lendvai G., Kenessey I. MicroRNA expression might predict prognosis of epithelial hepatoblastoma. Virchows Arch. 2014;464(4):419–427. doi: 10.1007/s00428-014-1549-y. [DOI] [PubMed] [Google Scholar]
- 87.Liu P., Qi X., Bian C. MicroRNA-18a inhibits ovarian cancer growth via directly targeting TRIAP1 and IPMK. Oncol Lett. 2017;13(6):4039–4046. doi: 10.3892/ol.2017.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo J., Miao Y., Xiao B. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24(4):652–657. doi: 10.1111/j.1440-1746.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 89.Manier S., Liu C.J., Avet-Loiseau H. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129(17):2429–2436. doi: 10.1182/blood-2016-09-742296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ottman R., Levy J., Grizzle W.E., Chakrabarti R. The other face of miR-17-92a cluster, exhibiting tumor suppressor effects in prostate cancer. Oncotarget. 2016;7(45):73739–73753. doi: 10.18632/oncotarget.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu P., Qi X., Bian C. MicroRNA-18a inhibits ovarian cancer growth via directly targeting TRIAP1 and IPMK. Oncol Lett. 2017;13(6):4039–4046. doi: 10.3892/ol.2017.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen X., Wu L., Li D. Radiosensitizing effects of miR-18a-5p on lung cancer stem-like cells via downregulating both ATM and HIF-1α. Cancer Med. 2018;7(8):3834–3847. doi: 10.1002/cam4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Humphreys K.J., McKinnon R.A. Michael MZ. miR-18a inhibits CDC42 and plays a tumour suppressor role in colorectal cancer cells. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brockway S., Zeleznik-Le N.J. WEE1 is a validated target of the microRNA miR-17-92 cluster in leukemia. Cancer Genet. 2015;208(5):279–287. doi: 10.1016/j.cancergen.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tao J., Wu D., Li P., Xu B., Lu Q., Zhang W. microRNA-18a, a member of the oncogenic miR-17-92 cluster, targets Dicer and suppresses cell proliferation in bladder cancer T24 cells. Mol Med Rep. 2012;5(1):167–172. doi: 10.3892/mmr.2011.591. [DOI] [PubMed] [Google Scholar]
- 96.Tsang W.P., Kwok T.T. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30(6):953–959. doi: 10.1093/carcin/bgp094. [DOI] [PubMed] [Google Scholar]
- 97.Liu S., Pan X., Yang Q. MicroRNA-18a enhances the radiosensitivity of cervical cancer cells by promoting radiation-induced apoptosis. Oncol Rep. 2015;33(6):2853–2862. doi: 10.3892/or.2015.3929. [DOI] [PubMed] [Google Scholar]
- 98.Choi M., Kipps T., Kurzrock R. ATM mutations in cancer: therapeutic implications. Mol Cancer Ther. 2016;15(8):1781–1791. doi: 10.1158/1535-7163.MCT-15-0945. [DOI] [PubMed] [Google Scholar]
- 99.Fujiya M., Konishi H., Mohamed Kamel M.K. microRNA-18a induces apoptosis in colon cancer cells via the autophagolysosomal degradation of oncogenic heterogeneous nuclear ribonucleoprotein A1. Oncogene. 2014;33(40):4847–4856. doi: 10.1038/onc.2013.429. [DOI] [PubMed] [Google Scholar]
- 100.Wu F., Huang W., Wang X. microRNA-18a regulates gastric carcinoma cell apoptosis and invasion by suppressing hypoxia-inducible factor-1α expression. Exp Ther Med. 2015;10(2):717–722. doi: 10.3892/etm.2015.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uhr K., Prager-van der Smissen W.J.C., Heine A.A.J. MicroRNAs as possible indicators of drug sensitivity in breast cancer cell lines. PLoS One. 2019;14(5):e0216400. doi: 10.1371/journal.pone.0216400. Published 2019 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan Y.X., Dai Y.Z., Wang X.L., Ren Y.Q., Han J.J., Zhang H. MiR-18a upregulation enhances autophagy in triple negative cancer cells via inhibiting mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20(11):2194–2200. [PubMed] [Google Scholar]
- 103.Kim M., Chin Y.W., Lee E.J. α, γ-Mangostins induce autophagy and show synergistic effect with gemcitabine in pancreatic cancer cell lines. Biomol Ther (Seoul) 2017;25(6):609–617. doi: 10.4062/biomolther.2017.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ottley E.C., Nicholson H.D., Gold E.J. Activin A regulates microRNAs and gene expression in LNCaP cells. Prostate. 2016;76(11):951–963. doi: 10.1002/pros.23184. [DOI] [PubMed] [Google Scholar]
- 105.Ichihara A., Wang Z., Jinnin M. Upregulation of miR-18a-5p contributes to epidermal necrolysis in severe drug eruptions. J Allergy Clin Immunol. 2014;133(4):1065–1074. doi: 10.1016/j.jaci.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 106.Béres N.J., Kiss Z., Sztupinszki Z. Altered mucosal expression of microRNAs in pediatric patients with inflammatory bowel disease. Dig Liver Dis. 2017;49(4):378–387. doi: 10.1016/j.dld.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Q., Ye H., Xiang F. miR-18a-5p inhibits sub-pleural pulmonary fibrosis by targeting TGF-β receptor II. Mol Ther. 2017;25(3):728–738. doi: 10.1016/j.ymthe.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geng H., Guan J. MiR-18a-5p inhibits endothelial-mesenchymal transition and cardiac fibrosis through the Notch2 pathway. Biochem Biophys Res Commun. 2017;491(Sep 16(2)):329–336. doi: 10.1016/j.bbrc.2017.07.101. [DOI] [PubMed] [Google Scholar]
- 109.Liu C., Wang M., Chen M. miR-18a induces myotubes atrophy by down-regulating IgfI. Int J Biochem Cell Biol. 2017;90(Sep):145–154. doi: 10.1016/j.biocel.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 110.Lin B., Feng D., Xu J. Cardioprotective effects of microRNA-18a on acute myocardial infarction by promoting cardiomyocyte autophagy and suppressing cellular senescence via brain derived neurotrophic factor. Cell Biosci. 2019;9(38) doi: 10.1186/s13578-019-0297-8. Published 2019 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kolenda T., Teresiak A., Kapałczyńska M. Let-7d and miR-18a as biomarkers of head and neck cancers. Lett Oncol Sci. 2015;12:37–47. [Google Scholar]
- 112.Jonsdottir K., Janssen S.R., Da Rosa F.C. Validation of expression patterns for nine miRNAs in 204 lymph-node negative breast cancers. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mairinger F.D., Ting S., Werner R. Different micro-RNA expression profiles distinguish subtypes of neuroendocrine tumors of the lung: results of a profiling study. Mod Pathol. 2014;27(12):1632–1640. doi: 10.1038/modpathol.2014.74. [DOI] [PubMed] [Google Scholar]
- 114.Zhuo X., Zhou W., Ye H. Screening of key miRNAs and evaluation of their diagnostic and prognostic values in nasopharyngeal carcinoma. Oncol Lett. 2019;17(6):5803–5810. doi: 10.3892/ol.2019.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu X.L., Jiang Y.H., Feng J.G., Su D., Chen P.C., Mao W.M. MicroRNA-17, microRNA-18a, and microRNA-19a are prognostic indicators in esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;97(3):1037–1045. doi: 10.1016/j.athoracsur.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 116.Alencar A.J., Malumbres R., Kozloski G.A. MicroRNAs are independent predictors of outcome in diffuse large B-cell lymphoma patients treated with R-CHOP. Clin Cancer Res. 2011;17(12):4125–4135. doi: 10.1158/1078-0432.CCR-11-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hummel R., Sie C., Watson D.I. MicroRNA signatures in chemotherapy resistant esophageal cancer cell lines. World J Gastroenterol. 2014;20(40):14904–14912. doi: 10.3748/wjg.v20.i40.14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luengo-Gil G., García-Martínez E., Chaves-Benito A. Clinical and biological impact of miR-18a expression in breast cancer after neoadjuvant chemotherapy. Cell Oncol (Dordr). 2019;42(Oct (5)):627–644. doi: 10.1007/s13402-019-00450-2. [DOI] [PubMed] [Google Scholar]
- 119.Leivonen S.K., Mäkelä R., Ostling P. Protein lysate microarray analysis to identify microRNAs regulating estrogen receptor signaling in breast cancer cell lines. Oncogene. 2009;28(Nov 5(44)):3926–3936. doi: 10.1038/onc.2009.241. [DOI] [PubMed] [Google Scholar]
- 120.Wang H., Wei X., Wu B., Su J., Tan W., Yang K. Tumor-educated platelet miR-34c-3p and miR-18a-5p as potential liquid biopsy biomarkers for nasopharyngeal carcinoma diagnosis. Cancer Manag Res. 2019;11:3351–3360. doi: 10.2147/CMAR.S195654. Published 2019 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsumura T., Sugimachi K., Iinuma H. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113(2):275–281. doi: 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou X., Wen W., Zhu J. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8(21):34468–34480. doi: 10.18632/oncotarget.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cosar E., Mamillapalli R., Ersoy G.S., Cho S., Seifer B., Taylor H.S. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402–409. doi: 10.1016/j.fertnstert.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 124.Sohn W., Kim J., Kang S.H. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184. doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wikberg M.L., Myte R., Palmqvist R., van Guelpen B., Ljuslinder I. Plasma miRNA can detect colorectal cancer, but how early? Cancer Med. 2018;7(5):1697–1705. doi: 10.1002/cam4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo X., Stock C., Burwinkel B., Brenner H. Identification and evaluation of plasma microRNAs for early detection of colorectal cancer. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zekri A.R., Youssef A.S., Lotfy M.M. Circulating serum miRNAs as diagnostic markers for colorectal Cancer. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu H.N., Wu H., Tseng Y.J. Serum microRNA signatures and metabolomics have high diagnostic value in gastric cancer. BMC Cancer. 2018;18(1):415. doi: 10.1186/s12885-018-4343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shin V.Y., Ng E.K., Chan V.W., Kwong A., Chu K.M. A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol Cancer. 2015;14:202. doi: 10.1186/s12943-015-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsujiura M., Komatsu S., Ichikawa D. Circulating miR-18a in plasma contributes to cancer detection and monitoring in patients with gastric cancer. Gastric Cancer. 2015;18(2):271–279. doi: 10.1007/s10120-014-0363-1. [DOI] [PubMed] [Google Scholar]
- 131.Godfrey A.C., Xu Z., Weinberg C.R. Serum microRNA expression as an early marker for breast cancer risk in prospectively collected samples from the Sister Study cohort. Breast Cancer Res. 2013;15(3):R42. doi: 10.1186/bcr3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kodahl A.R., Lyng M.B., Binder H. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: a case control study. Mol Oncol. 2014;8(5):874–883. doi: 10.1016/j.molonc.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirajima S., Komatsu S., Ichikawa D. Clinical impact of circulating miR-18a in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2013;108(9):1822–1829. doi: 10.1038/bjc.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beta M., Venkatesan N., Vasudevan M., Vetrivel U., Khetan V., Krishnakumar S. Identification and in silico analysis of retinoblastoma serum microRNA profile and gene targets towards prediction of novel serum biomarkers. Bioinform Biol Insights. 2013;7:21–34. doi: 10.4137/BBI.S10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marcuello M., Duran-Sanchon S., Moreno L. Analysis of a 6-Mirna signature in serum from colorectal Cancer Screening participants as non-invasive biomarkers for advanced adenoma and colorectal Cancer detection. Cancers (Basel). 2019;11(10):1542. doi: 10.3390/cancers11101542. Published 2019 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Al-Kafaji G., Al-Naieb Z.T., Bakhiet M. Increased oncogenic microRNA-18a expression in the peripheral blood of patients with prostate cancer: a potential novel non-invasive biomarker. Oncol Lett. 2016;11(2):1201–1206. doi: 10.3892/ol.2015.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ibrahim N.H., Abdellateif M.S., Kassem S.H., Abd El Salam M.A., El Gammal M.M. Diagnostic significance of miR-21, miR-141, miR-18a and miR-221 as novel biomarkers in prostate cancer among Egyptian patients. Andrologia. 2019;51(Nov (10)) doi: 10.1111/and.13384. [DOI] [PubMed] [Google Scholar]
- 138.Ulivi P., Foschi G., Mengozzi M. Peripheral blood miR-328 expression as a potential biomarker for the early diagnosis of NSCLC. Int J Mol Sci. 2013;14(5):10332–10342. doi: 10.3390/ijms140510332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fan B., Shen C., Wu M., Zhao J., Guo Q., Luo Y. miR-17-92 cluster is connected with disease progression and oxaliplatin/capecitabine chemotherapy efficacy in advanced gastric cancer patients: a preliminary study. Bull Sch Med Univ Md. 2018;97(35) doi: 10.1097/MD.0000000000012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fernandez-Mercado M., Manterola L., Larrea E. The circulating transcriptome as a source of non-invasive cancer biomarkers: Concepts and controversies of non-coding and coding RNA in body fluids. J Cell Mol Med. 2015;19(10):2307–2323. doi: 10.1111/jcmm.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu X., Zhu S., Tao Z., Ye S. High circulating miR-18a, miR-20a, and miR-92a expression correlates with poor prognosis in patients with non-small cell lung cancer. Cancer Med. 2018;7(1):21–31. doi: 10.1002/cam4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liang Q., Zhang G., Wang J., Sheng S. Diagnostic value of MicroRNA-18a for gastric cancer: a meta-analysis. Clin Lab. 2018;64(1):177–184. doi: 10.7754/Clin.Lab.2017.170827. [DOI] [PubMed] [Google Scholar]
- 143.Scheffer A.R., Holdenrieder S., Kristiansen G., von Ruecker A., Müller S.C., Ellinger J. Circulating microRNAs in serum: novel biomarkers for patients with bladder cancer? World J Urol. 2014;32(2):353–358. doi: 10.1007/s00345-012-1010-2. [DOI] [PubMed] [Google Scholar]
- 144.Liu G.H., Zhou Z.G., Chen R. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol. 2013;34(4):2175–2181. doi: 10.1007/s13277-013-0753-8. [DOI] [PubMed] [Google Scholar]