Highlights
-
•
After 5 months of age, infants begin to prioritize attention to fearful over other facial expressions.
-
•
Amygdala and related early-maturing subcortical network, is important for emergence of this attentional bias.
-
•
Left amygdala volume associates positively with the emerging perceptual vigilance for fearful faces during infancy.
-
•
possible link from the prenatally defined variability in the amygdala size to social traits
Keywords: MRI, Eye tracking, Amygdala, Fear processing, Fear bias, Infant
Abstract
After 5 months of age, infants begin to prioritize attention to fearful over other facial expressions. One key proposition is that amygdala and related early-maturing subcortical network, is important for emergence of this attentional bias – however, empirical data to support these assertions are lacking. In this prospective longitudinal study, we measured amygdala volumes from MR images in 65 healthy neonates at 2–5 weeks of gestation corrected age and attention disengagement from fearful vs. non-fearful facial expressions at 8 months with eye tracking. Overall, infants were less likely to disengage from fearful than happy/neutral faces, demonstrating an age-typical bias for fear. Left, but not right, amygdala volume (corrected for intracranial volume) was positively associated with the likelihood of disengaging attention from fearful faces to a salient lateral distractor (r = .302, p = .014). No association was observed with the disengagement from neutral or happy faces in equivalent conditions (r = .166 and .125, p = .186 and .320, respectively). These results are the first to link the amygdala volume with the emerging perceptual vigilance for fearful faces during infancy. They suggest a link from the prenatally defined variability in the amygdala size to early postnatal emotional and social traits.
1. Introduction
Infants’ early-developing attentional preferences for faces may be foundational in the development of social brain networks and our sophisticated capacity to recognize various communicative signals from each other’s faces (Jack and Schyns, 2015; Johnson, 2005; Kelly et al., 2019; Leppänen, 2016). Newborn infants hold attention longer on faces than non-face patterns and by six months of age, infants begin to discriminate and categorize facial expressions of basic emotions (Johnson, 2005; Kelly et al., 2019; Leppänen, 2016). At this age, infants also start to exhibit a robust attentional bias to fearful over other facial expressions (Peltola et al., 2009, 2008). Seven-month-old infants look longer at fearful faces than at happy faces () Nelson and Dolgin, 1985 and are slower to disengage attention from fearful than happy or neutral faces when being “distracted” by a competing stimulus (Peltola et al., 2008). A similar bias is not found for angry faces vs. happy or neutral faces (Leppänen et al., 2018a). These findings suggest that the bias to look at fearful faces may arise from some specific characteristics of fearful faces, including their unique physical characteristics (e.g., wide-open eyes (Peltola et al., 2009)), the possibility that fearful faces are more novel than other facial expressions in infants’ rearing environment (Peltola et al., 2008; Somerville and Whalen, 2006), or infants’ rudimentary understanding of the unique communicative meaning of fearful faces as signals of potential danger in the environment (Peltola et al., 2009; Whalen, 1998).
While the bias to look at fearful faces is well-documented in infants, its neural bases remain poorly understood. Previous research has suggested that amygdala and related early-maturing subcortical network, is a key neural part of infants' attentional bias for faces (Johnson, 2005) and fear processing (Whalen et al., 2001). Human and primate amygdalae reach anatomical “maturity” early in development (Bachevalier et al., 1986; Berger et al., 1990; Gabard-Durnam et al., 2018; Humphrey, 1968; Kordower et al., 1992; Machado and Bachevalier, 2003). The amygdala has reciprocal connections to various cortical regions, which are important for mediating attentional processing (Amaral and Price, 1984; Vuilleumier, 2005). Recent studies have further shown that individual variations in amygdala volume and amygdalo-cortical connectivity are visible at birth and correlate with later behavioral phenotypes (Graham et al., 2018, 2016; Lyons-Ruth et al., 2016; Rifkin-Graboi et al., 2015). For instance, neonate amygdala volumes have been found to correlate positively with an escape response to a fearsome toy at 12 months among preterm and term born infants (Cismaru et al., 2016). Early amygdala volume may also be linked to environmental factors, including maternal pre- and postnatal psychological distress and fear bias (Rifkin-Graboi et al., 2013; Wen et al., 2017). However, no studies have yet examined the links between amygdala and attentional bias for fearful facial expressions in infants. Connecting the amygdalae volumes with a well-established infant cognitive phenotype, emerging at this age, may aid in the identification of normative as well as pathological fear development.
In this prospective longitudinal study, we examined whether early-appearing variations in bilateral amygdala volume, measured 2–5 weeks after birth with MRI, predict infants' emerging attentional bias to fearful facial expressions at age 8 months. We used eye tracking and an emotional overlap paradigm to assess infants’ attentional disengagement from fearful, happy and neutral facial expressions, as well as from scrambled face pattern, while a salient competing stimulus was shown in the visual periphery (an age-appropriate paradigm, see e.g. (Forssman et al., 2014; Leppänen et al., 2018a; Nakagawa and Sukigara, 2012; Peltola et al., 2018b, 2009; Videman et al., 2016)). Studies using this approach have shown that starting between 5 and 7 months, infants' disengagement times are longer for fearful compared to non-fearful facial expressions Leppänen et al., 2018b, and that this bias is heightened in infants raised in environments with higher levels of psychosocial stress (Forssman et al., 2014; Kataja et al., 2019, 2018). Given the early appearance of individual variations in amygdala volume, and the allegedly central role of this structure in perceptual vigilance for fear, the hypothesis tested in the current study was that the individual variations in amygdala volumes at birth are associated with attention to fearful, but not happy or neutral faces at 8 months. No hypothesis for the direction or the laterality of the effect was set as previous studies have reported mixed results in the associations between the amygdalae volumes and emotion processing (Cynthia M. Schumann, Melissa D. Bauman, 2011), and studies investigating the neural foundations of the developing fear systems in infancy are lacking.
2. Methods
The study was conducted according to the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Hospital District of Southwest Finland (MRI: §95, ETMK:31/180/2011; Eye tracking: §322, ETMK:107/180/2012). The mothers gave informed consent on behalf of their infant.
2.1. Participants
The study sample comprised of N = 65 (54 % male) infants, enrolled in the large ongoing FinnBrain Birth Cohort Study (Karlsson et al., 2018). Pregnant mothers were recruited to the study at their first ultrasound visit (gestational week [gwk] 12) at three maternal well-fare clinics performing ultrasound scans for the women giving birth at Turku University Hospital in the Southwest Finland Hospital District and the Åland Islands in Finland between December 2011 and April 2015. The inclusion criteria were (a) an ultrasound-verified pregnancy and (b) sufficient knowledge of Finnish or Swedish (the official languages of Finland). All infants (N = 65) with completed neonatal magnetic resonance imaging at 2–5 weeks gestation corrected age and valid eye-tracking assessment at 8 months were included in the current analyses. The eye-tracking assessments and imaging were conducted as part of the visits of FinnBrain Child Development and Parental Functioning Lab and Neuroimaging Lab visits at the University of Turku.
2.2. Demographics
The demographic characteristics regarding maternal education, smoking and alcohol consumption were collected with questionnaires at gwk 14 (Table 1). The data on maternal and infant age were retrieved from national birth registries (National Institute for Health and Welfare, www.thl.fi).
Table 1.
The sample characteristics.
| All (N = 65) | Female (N = 30) | Male (N = 35) | p | ||
|---|---|---|---|---|---|
| Infant characteristics, Mean(SD; Range) | |||||
| PD Scrambled | .75(.23) | .74(.22) | .76(.24) | .55 | |
| PD Neutral | .58(.25) | .56(.27) | .60(.23) | .78 | |
| PD Happy | .55(.25) | .53(.21) | .57(.28) | .45 | |
| PD Fear | .44(.29) | .40(.27) | .47(.31) | .34 | |
| Left Amygdala Volume (mm3) | 266.89(41.18) | 248.44(30.16) | 282.71(43.11) | .001 | |
| Right Amygdala Volume (mm3) | 265.45(42.75) | 243.09(34.07) | 284.62(40.39) | <.001 | |
| Intracranial Volume (mm3) | 627241.71(50211.82) | 606211.73(47216.11) | 645267.40(46039.25) | .001 | |
| Gestational Age (days) at Birth | 278.09(7.96;254−295) | 278.30(8.36) | 277.91(7.72) | .85 | |
| Age (days) at MRI from Birth | 27.08(7.74;14−54) | 27.74(7.60) | 26.30(7.95) | .46 | |
| Age (days) at MRI from Conception | 305.17(6.98;291−320) | 304.60(7.15) | 305.66(6.90) | .55 | |
| Age (months) at ET from Conception | 8.12(.20;7.80−8.73) | 8.11(.20) | 8.14(.20) | .47 | |
| Head Circumference (cm) | 35.02(1.50;5.0) | 34.66(1.54) | 35.33(1.42) | .07 | |
| Birth Weight (grams) | 3480.47(447.94;2170) | 3391.90(488.04) | 3553.86(404.25) | .15 | |
| Maternal characteristics, Mean(SD) | |||||
| Age (years) at Delivery | 30.42(4.41) | 30.07(4.59) | 30.71(4.29) | .60 | |
| Education (%) | Low | 30.2 | 37.9 | 23.5 | .25 |
| Middle | 31.7 | 34.5 | 29.4 | ||
| High | 38.1 | 27.6 | 47.1 | ||
| Smoking (%) | Yes, early pregnancy | 8.1 | 6.1 | 10.3 | .54 |
| Yes, late pregnancy | 1.6 | 0 | 3.4 | .28 | |
| Alcohol (%) | Yes, early pregnancy | 27.4 | 39.4 | 13.8 | .02 |
| Yes, late pregnancy | 12.7 | 17.6 | 6.9 | .19 | |
Abbreviations: PD = Probability of disengagement; ET = Eye tracking.
2.3. MRI acquisition
Participants were scanned with a Siemens Magnetom Verio 3 T scanner (Siemens Medical Solutions, Erlangen, Germany). The 60-minute protocol included a 2D PD-T2 TSE (dual-echo turbo spin echo) and a 3D T1 MPRAGE (T1-weighted magnetization prepared - rapid gradient echo) sequence, both providing isotropic 1.0 mm3 voxels with whole brain coverage. A repetition time (TR) of 12070 ms and effective Echo times (TE) of 13 ms and 102 ms were used to produce both PD-weighted and T2-weighted images from the same dual-echo TSE acquisition. The 3D T1 MPRAGE was acquired in sagittal plane with a TR of 1900 ms, TE of 3.26 ms, inversion time (TI) of 900 ms, and flip angle (FA) of 9 degrees. A more detailed description of the scanning protocol is provided in our prior reports (Lehtola et al., 2019; Tuulari et al., 2019).
All the brain images were assessed by a pediatric neuroradiologist for incidental findings. If those were found, parents were given a follow-up opportunity with a pediatric neurologist. Developmental status has thereafter been normal for all of the participants Kumpulainen V et al., 2020 . The incidental findings have been found to be common and clinically insignificant in previous studies (Rooks et al., 2008; Whitby et al., 2004), and were deemed not to affect the volumetric estimates of interest. Thus, these participants were kept in the sample.
2.4. Construction of an unbiased population-specific template
The measurements used in the analysis were derived using fusion-based methods that depend on achieving good registrations between the subjects and the template. This is increasingly difficult to achieve the further the template is from the subjects’ images. Thus, we constructed a population-specific dual-contrast template. All available, good quality imaging data was used for template construction following visual quality control (N = 125 / 180). Each subject’s T2 was linearly registered to their T1, and then the two together were linearly registered to the MNI 152 template. The average scaling from the native MRIs to the MNI 152 template was then computed, and the inverse used to scale the MNI 152 template to the average size of the population, which served as an initial target for construction of the population-specific template as described in (Fonov et al., 2011). This iterative procedure builds a template that minimizes the mean squared intensity difference between the template and each subject’s MRI, and minimizes the magnitude of all deformations used to map the template to each subject’s MRI. This method was applied to the T1 scans producing non-linear transformations from the template space to each scan, then these transformations were used to map the T1 scans to the template space, where they were averaged to create the T1 template; these transformations were also combined with the T2 to T1 transformations to map the T2 scans into the common space where they were averaged to create the T2 template.
2.5. Labelling the template
The structures of interest, i.e. the amygdalae, were manually labelled based on a previously described approach (Hashempour N et al., 2019) on the dual-contrast templates – noting that 0.5 mm3 resolution allowed more accurate segmentation. To ensure that these labels were accurate, we produced 21 variants of the template (each a non-linear transformation of the template to overlay one of the subjects in the population). For the creation of the 21 sub templates, the generalized conformity index (GCI) for amygdala was 0.703 (Kouwenhoven et al., 2009). Scores of 0.7–1.0 are regarded as excellent (John J. Bartko, 1991; Zijdenbos et al., 1994). The non-linear transformations derived from the template construction procedure were used to cluster the subjects into 21 clusters from which 21 targets for manual segmentation of the amygdalae were created. As the basis for clustering, the Jacobian was computed for the non-linear transform mapping each subject to the template. The values in the Jacobian were then extracted as a vector for each voxel within the template brain mask, and then clustered using an equal combination of cosine similarity and Euclidean distance with Ward’s clustering method (Ward, 1963) - with the number of clusters chosen to be 21. Then, within each cluster, the sum-squared distance from each subject to each other subject was computed, and the subject with the minimum sum-squared distance was taken as the central-most subject of the cluster. The dual-contrast template constructed in the previous step was then warped to these 21 representative subjects, and provided for manual segmentation (without those doing that segmentation being made aware that these were, in fact, 21 different versions of the same template). The manual segmentations were then warped back to the standard template, and each voxel was assigned a label based on the majority vote across all 21 manual segmentations. This yielded the final labels for the amygdalae on the standard template.
2.6. Labelling the subjects
Segmentation into left and right amygdalae for each subject was done using a label-fusion-based labeling technique based on Coupé et al. (2011), and further developed by Weier et al. (2014), and Lewis et al. (2019). The approach uses a population-specific template library. In the current work, the library was constructed by clustering (similarly to the method described above) the deformation fields from the non-linear transforms produced during construction of the template and using the central-most subject of each cluster to construct the entries in the template library. Thus, the template library represented the range of deformations found in the population. The clustering was done as described above but using a dilated mask of the amygdalae and in order to capture the anatomical context of the nonlinear registration in that area of the brain, and with the number of clusters now chosen as the square of the natural log of the number of subjects. The representative subject for each cluster was chose as described above. Importantly, this was done per hemisphere to accommodate hemispheric asymmetries.
To create the library entry for a cluster, the non-linear transform for the central-most subject was used to warp the template together with the segmentation defined on it, and this pair was added to the template library. The template library was thus a set of warped copies of the template together with their correspondingly warped segmentations. Once the template library had been created, each subject in the population was non-linearly registered to the n closest templates in the library, and the resulting transforms were used to warp their corresponding segmentations to the subject; the final labelling was then established via a per-voxel majority vote. This was also done separately for each hemisphere. The volumes of each of the final labeling was then computed and scaled to native space based on the scaling factors in the subject’s linear transforms. The output was inspected to assure the quality of the segmentations.
2.7. Eye-tracking-based assessment of infant attention disengagement from facial expressions
2.7.1. Procedure
During eye-tracking, the infant sat on his/her parent’s lap at the distance of 50–70 cm from the eye-tracker (EyeLink1000+, SR Research Ltd, Toronto, Ontario, Canada). A sampling frequency of 500 Hz was used. A five-point calibration procedure, with an audiovisual animation sequentially presented in five locations on the screen, was used before every measurement. This could be repeated at least two times before actual testing and also during measurement when necessary. Small breaks were allowed during measurement, if necessary. The researcher sat in the same, dimly lit room as the infant and parent but was separated by a curtain to avoid interference. The researcher used another independent computer to manage the measurement.
The overlap paradigm (Peltola et al., 2008) was used to study infant attention disengagement from a centrally presented face or a scrambled face control stimulus to a lateral distractor. This paradigm has been used in several previous studies to examine infants’ attentional biases for fearful vs. non-fearful facial expressions (e.g. (Nakagawa and Sukigara, 2012; Peltola et al., 2018b, 2009; Videman et al., 2016)). In the current study, the overlap paradigm was used in combination with automated eye tracking to assess infants’ disengagement times. While the spatial resolution, robustness (i.e., how fragmented the contact with the eye tracker is), and precision of eye tracking is lower in infants as compared to co-operative adults (Wass et al., 2014), previous studies have shown that disengagement times assessed by eye tracking are in high agreement with disengagement times obtained by the standard manual coding of videos of eye movements in infants (Leppänen et al., 2015), and show expected levels of test-retest reliability (Leppänen et al., 2015; Pyykkö et al., 2019).
In the overlap paradigm, the infants were shown photographs of two different women portraying happy, fearful, and neutral faces together with scrambled face control pictures (Peltola et al., 2008). Altogether, a set of 48 trials were presented, including 12 trials per condition (each emotion and the control picture) and comprising 18 photographs of each woman, and 12 non-face control pictures, in a semi-random order.
During the experiment, the infants were first shown a picture of a face (or a non-face control stimulus) in the center of the screen for 1000 ms (Fig. 1). Then, a lateral distractor (black and white checkerboard or circles) appeared on either left or right side of the face (a visual angle of 13.6°) for 3000 ms, simultaneously with the face. One trial lasted for 4000 ms. The sizes of the emotion-depicting pictures and distractor stimuli were 15.4° x 10.8° and 15.4° x 4.3°, respectively. A brief animation was shown after each trial to capture the attention of the infant to the center of the screen. Once the infant’s gaze was in the middle of the screen, the next trial was presented by the researcher. The order of the central stimuli was semi-randomized, with a constraint that the same stimulus was not presented more than three times in a row. The lateral stimulus was selected and presented randomly for each trial.
Fig. 1.
Illustration of the eye-tracking method used to assess infant’s attention to social signals of emotion. After the infant looked at a fixation stimulus in the center of the screen (red circle), a face or a scrambled face pattern and subsequently a high-contrast lateral distractor were presented. The probability of attention disengagement from the central to the lateral stimulus was analyzed from the eye tracking data and used as a measure of attention to scrambled face patterns and neutral, happy, and fearful faces (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
2.7.2. Extraction of disengagement probabilities
The trial data, comprised of timestamps for the onset times of central and lateral pictures and the xy coordinates of the participants’ gaze position (500 samples/second) were stored as text files, and analyzed offline using a library of Matlab 2016b (Mathworks, Natick, MA) scripts for preprocessing and analysis of SRTs from eye-tracking data (https://github.com/infant-cognition-tampere/gazeanalysislib) (Leppänen et al., 2015). Partially incomplete eye tracking data is common, and we used the following quality control criteria based on prior studies (Leppänen et al., 2015) to retain trials for the analysis. First, the infant's gaze had to stay in the area of the central stimulus >70 % of the time preceding gaze disengagement or the end of the analysis period. Second, trials had to have a sufficient number of valid samples in the gaze data (i.e., no gaps/missing eye position data >200 ms). Thirdly, trials had to have valid information about the eye movement from the central to the lateral stimulus (i.e., the eye movement did not occur during a period of missing gaze data). In order to be included in the analyses, the infant had to provide ≥3 valid trials for each stimulus condition. On average, the infants had 9.5, 9.7, 9.7, and 10.2 valid trials in the control, neutral, happy, and fearful conditions, respectively. Of these trials, the mean probability of attention disengagement from the central to the lateral stimulus was calculated for each stimulus condition and were used in statistical analyses.
2.8. Statistical analyses
Analyses were performed using IBM SPSS Statistics version 24. Parametric methods were used to analyze the associations as the distribution of the main interest variables did not significantly violate the assumptions of normality (assessed by the Kolmogorov-Smirnov statistics, skewness and kurtosis of the distribution). First, the associations between the probability of disengagement in control, neutral, happy and fearful conditions, bilateral amygdala volumes and the relevant confounders were investigated with Pearson correlations. Based on significant correlations, Hierarchical Linear Regression was used to investigate the associations between the bilateral amygdala volume (corrected for intracranial volume by dividing amygdala volume with the total intracranial volume) and the probability of disengagement from (fearful) faces, after controlling for age at MRI from conception (and age from birth). Infant sex and sex by amygdala were included in the model to probe for the possible interaction by sex. All significance testing was 2-tailed with α = .05.
For completeness, we replicated the analyses by using raw volumes.
3. Results
3.1. Potential confounders influencing the association between the amygdala volume and the probability of disengagement
Both the left and right amygdala volumes correlated positively with intracranial volume [ICV] (r = .607 and r = .580, p values < .0001, respectively), but not with age at MRI from birth (p values > .30) or age at MRI from conception (p values > .16). The correlation between the left and right amygdala volumes was high, raw volumes r = .699, p < .0001 and ICV-corrected volumes r = .532, p < .0001, which is in line with prior reports (Frühholz et al., 2017). The probability of disengagement from faces (control, neutral, happy, and fearful) did not correlate with ICV (p values > .42), age at MRI from birth (p values > .12) or age at MRI from conception (p values > .70). Consequently, both left and right amygdala volumes were adjusted for intracranial volume by dividing the amygdala volumes by ICV. Further, infant age at MRI from birth was controlled for in the regression analyses. The intercorrelations between the variables are presented in Table 2.
Table 2.
The Pearson correlations for Probability of Disengagement, Left and Right Amygdala Volumes, Intracranial volume, Age at MRI, Age at Eye Tracking, Head Circumference, and Birth Weight.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PD Sc | 1 | |||||||||||||
| 2. PD Ne | .337** | 1 | ||||||||||||
| 3. PD Ha | .506** | .717** | 1 | |||||||||||
| 4. PD Fe | .307** | .787** | .594** | 1 | ||||||||||
| 5. LAVol | -.060 | .115 | .088 | .236† | 1 | |||||||||
| 6. RAVol | -.055 | -.039 | -.038 | .008 | .699** | 1 | ||||||||
| 7. ICV | .072 | -.050 | -.026 | -.025 | .607** | .579** | 1 | |||||||
| 8. LA_ICV | -.118 | .166 | .125 | .302* | .856** | .493** | .113 | 1 | ||||||
| 9. RA_ICV | -.112 | -.021 | -.017 | .020 | .483** | .868** | .102 | .532** | 1 | |||||
| 10. MRI Age#1 | -.018 | -.133 | -.214† | -.127 | .032 | .062 | .315* | -.161 | -.112 | 1 | ||||
| 11. MRI Age#2 | .026 | -.052 | -.042 | -.014 | .120 | .175 | .346** | -.063 | .005 | .418** | 1 | |||
| 12. ET Age | -.215† | .162 | .093 | .071 | .203 | .160 | .196 | .122 | .076 | .060 | .107 | 1 | ||
| 13. HC | .045 | -.165 | -.054 | -.020 | .328** | .289* | .517** | .090 | .049 | -.258* | -.037 | .101 | 1 | |
| 14. BW | -.161 | -.289* | -.151 | -.237† | .223† | .295* | .454** | -.014 | .090 | -.112 | .314* | .096 | .557** | 1 |
PD = Probability of Disengagement; Sc = Scrambled; Ne = Neutral; Ha = Happy; Fe = Fearful; LAVol = Left amygdala (raw) volume; RAVol = Right amygdala (raw) volume; ICV = Intracranial Volume; LA_ICV = Left Amygdala adjusted for Intracranial Volume; RA_ICV = Right Amygdala adjusted for Intracranial Volume; MRI Age#1 = MRI Age (days) from Birth; MRI Age#2 = MRI Age (days) from Conception; ET Age = Age (months) at Eye Tracking; HC = Head Circumference; BW = Birth Weight.
3.2. The associations between the left and right amygdala volumes and attention disengagement from fearful faces
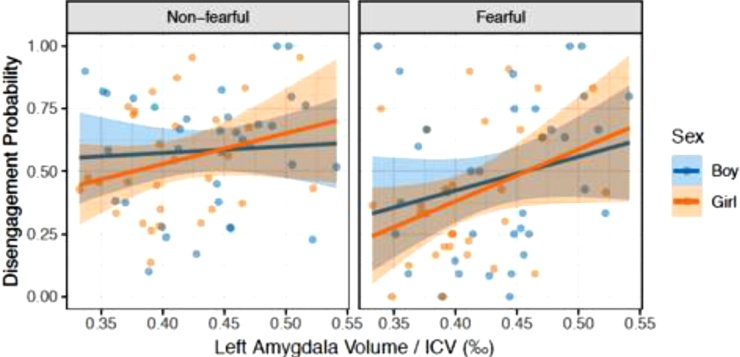
The infants were less likely to disengage from fearful (M = .44) than happy (M = .55), neutral (M = .58), or scrambled (M = .75) faces, demonstrating an age-typical bias for fear. Across the whole sample, there was a significant positive correlation between the left amygdala volumes (adjusted for ICV) and the probability of disengagement from fearful faces (r = .302, p = .014). The right amygdala did not correlate with attention disengagement (r = .020, p = .87). Results with raw volumes were similar (left amygdala, r = .236, p = .059, right amygdala, r = .008, p = .949). No significant associations were found between other facial expressions and the left or right amygdala volumes (p values > .19). The correlations between the variables of interest have been presented in Table 2. The regression lines for the associations between the left amygdala volumes and the probability of disengagement from fearful faces were similar among both sexes (Fig. 2).
Fig. 2.
The associations between the left amygdala volume (adjusted for intracranial volume) and the probability of disengagement from non-fearful (i.e. happy and neutral) and fearful faces among male and female infants.
Next, hierarchical linear regression models with the probability of disengagement from fearful faces as the dependent variable and the left amygdala volume (adjusted for ICV), age at MRI from conception and infant sex as predictors were run. Further, the interaction between infant sex and left amygdala volume was also added to the model to probe for the possible sex differences.
Across the whole sample, the left amygdala was the only significant predictor of the probability of disengagement from fearful faces after controlling for the effects of age at MRI and infant sex: the larger the left amygdala volume the higher the probability to disengage specifically from fearful faces. The interaction between infant sex and the left amygdala volume was not a significant predictor of the probability of attention disengagement (Table 3).
Table 3.
Hierarchical multiple regression for the probability of disengagement from fearful faces for the whole sample.
| R2 | ΔR2 | Unstandardized β | Standardized β | F for change in R2 | |
|---|---|---|---|---|---|
| Step 1 | .091** | .077** | 6.34** | ||
| Left amygdala (adjusted for ICV) | 1691.37** | .302** | |||
| Step 2 | .092 | .062 | .002 | ||
| Left amygdala (adjusted for ICV) | 1693.36* | .303* | |||
| Age at MRI | .000 | .006 | |||
| Step 3 | .094 | .049 | .139 | ||
| Left amygdala (adjusted for ICV) | 1624.55* | .291* | |||
| Age at MRI | 5.099 | .001 | |||
| Infant sex | -.028 | -.047 | |||
| Step 4 | .097 | .037 | .240 | ||
| Left amygdala (adjusted for ICV) | 637.67 | .114 | |||
| Age at MRI | .000 | .003 | |||
| Infant sex | -.329 | -.562 | |||
| Infant sex x Left amygdala | 715.47 | .504 |
Age at MRI = Age from conception.
p < .01.
p < .05.
4. Discussion
By demonstrating an association between amygdala volume at birth and subsequent development of attention to fearful facial expressions (i.e., larger left amygdala volume associating to higher probability to disengage from fear), this study provides a neural correlate for a well-established behavioral trait in infants and adds to the data by suggesting that prenatal and neonatal development of the amygdala may be a potential biomarker for postnatal behavioral phenotypes (Graham et al., 2018, 2016; Rifkin-Graboi et al., 2015). Attesting to the importance of these phenotypes, various sources of evidence suggest that the early-developing biases in attentional selection of visual information are foundational in the functional specialization of cortical networks (Johnson, 2005) as well as the acquisition of secure attachment (Peltola et al., 2018a; van IJzendoorn et al., 2015) and more complex social traits (Grossmann et al., 2018; Peltola et al., 2018b).
While the present study demonstrates an association between amygdala volume and attention to fearful faces in infants, it leaves open the specific mechanisms that mediate this association. Variations in the amygdala volume may arise from multiple factors, including genetic polymorphism (Satizabal et al., 2019) as well as intrauterine environmental factors, such as maternal inflammation (Graham et al., 2018) and depression (Posner et al., 2016). As an example, structural and functional connectivity within amygdala-prefrontal circuits is correlated with prenatal exposure to depression, with exposed infant showing increased bilateral, negative functional connectivity between the dorsal prefrontal areas and the amygdala, and decrease structural connectivity between the right prefrontal cortex and the right amygdala (Posner et al., 2016). The present results add to these studies by suggesting that the early-emerging structural variations in the amygdala, and possibly amygdala-cortical relations, which were not investigated here, may have important functional consequences on emotional information processing in infants. It has been speculated that such functional effects may be mediated by different mechanisms (Leppänen and Nelson, 2009), including direct feedback projections from the amygdala to visual-representation areas as well as connections from the amygdala to basal forebrain and cholinergic neurons that may transiently increase cortical excitability (Amaral et al., 2003; Bentley et al., 2003; Whalen, 1998). These possibilities can be further studied by extending the present analyses of amygdala volume to individual variations in amygdala-cortical connections, and linking these variations with attention to facial expressions of fear (Vuilleumier et al., 2004).
In addition to its neural mechanisms, the hemispheric specificity and developmental significance of the association between the amygdala volume and attention to fearful faces require further research. There is currently no clear interpretation for the hemispheric specificity of the amygdala (Buss et al., 2012; Acosta et al.,2019; Ortiz-Mantilla et al., 2010), which is also the case for the observed direction of the association between amygdala volume and attention to fearful faces. If increased amygdala volume signifies relatively greater risk (Graham et al., 2018; Howell et al., 2014), it may be expected to result in an exacerbated bias for fear (Forssman et al., 2014; Kataja et al., 2019, 2018), contrary to the direction of the correlation in this study. However, the extant evidence is also open to the alternative possibility that the "at-risk" phenotype, as indicated by larger amygdala, reduces infants' engagement with fearful faces - e.g., due to an avoidance of this cue or enhanced vigilance towards other competing inputs (Nakagawa and Sukigara, 2019) - reflecting a less optimal trajectory of early social development(Grossmann et al., 2018; Peltola et al., 2018b). Addressing these questions requires larger scale studies that examine amygdala volume and attention to fear in relation to pre- and postnatal environment (e.g., parental depression) and link early variations with long-term developmental outcomes. Future studies are also likely to capture the normative amygdalar growth in more detail and will be relevant for interpreting the findings of the current study (Ouyang et al., 2019).
In conclusion, the current results provide the first piece of evidence for a link between amygdala volumes and the well-established attentional bias for fearful facial expressions in infants. This finding suggest a connection from the prenatally defined variability in the amygdala size to early postnatal emotional and social traits, although further research is needed to examine the stability and mechanisms of this association, as well as its long-term significance.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
JJT was supported by the Hospital District of Southwest Finland, Turku University Foundation, State research grant and Emil Aaltonen Foundation, Alfred Kordellin Foundation (data collection and data analysis) as well as and Sigrid Jusélius Foundation (interpretation of the data and writing the manuscript). ELK was supported by Finnish Cultural Foundation and Alli Paasikivi Foundation. JML was supported by Sigrid Jusélius foundation. JDL was supported by grant ANRP-MIRI13-3388 from the Azrieli Neurodevelopmental Research Program in partnership with the Brain Canada Multi-Investigator Research Initiative. SN was supported by Yrjö Jahnsson Foundation, Emil Aaltonen Foundation, Signe & Ane Gyllenberg Foundation, Finnish Cultural Foundation and Alexander von Humboldt Foundation. SJL received research grants from Maire Taponen Foundation, Juho Vainio Foundation and the Hospital District of Southwest Finland (State research grant). NH was supported by the Juho Vainio Foundation and the Hospital District of Southwest Finland. NMS was supported by State Research grants (EVO) of the South-Western Hospital District and the Signe and Ane Gyllenberg Foundation. RK was supported by Ane & Signe Gyllenberg Foundation ja Academy of Finland (308252). LK was supported by NARSAD Brain and Behavior Research Foundation YI Grant 1956, the Academy of Finland (#308176), Signe and Ane Gyllenberg Foundation, Yrjö Jahnsson Foundation, Finnish State Grant for Clinical Research, Jalmari and Rauha Ahokas Foundation and the Waterloo Foundation (2110-3601). HK was supported by Finnish Academy No. 264363, 253270, 134950; and Signe and Ane Gyllenberg Foundation and Jane and Aatos Erkko Foundation.
The research also benefited from computational resources provided by Compute Canada (www.computecanada.ca) and Calcul Quebec (www.calculquebec.ca).
We would like to thank Kristiina Kuvaja for carrying out the scans with the investigators and especially all FinnBrain families that participated to the measurements.
References
- Acosta H. Maternal Pregnancy-Related Anxiety Is Associated With Sexually Dimorphic Alterations in Amygdala Volume in 4-Year-Old Children. Frontiers in Behavioral Neuroscience. 2019 doi: 10.3389/fnbeh.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Price J.L. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Behniea H., Kelly J.L. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/S0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Ungerleider L.G., Blanche O’Neill J., Friedman D.P. Regional distribution of [3H]naloxone binding in the brain of a newborn rhesus monkey. Brain Res. 1986;390:302–308. doi: 10.1016/S0006-8993(86)80240-2. [DOI] [PubMed] [Google Scholar]
- Bartko John J. Measurement and reliability: statistical thinking considerations. Schizophr. Bull. 1991;17:483–489. doi: 10.1093/schbul/17.3.483. [DOI] [PubMed] [Google Scholar]
- Bentley P., Vuilleumier P., Thiel C.M., Driver J., Dolan R.J. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003;20:58–70. doi: 10.1016/S1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- Berger B., Febvret A., Greengard P., Goldman‐Rakic P.S. DARPP‐32, a phosphoprotein enriched in dopaminoceptive neurons bearing dopamine D1 receptors: DIstribution in the cerebral cortex of the newborn and adult rhesus monkey. J. Comp. Neurol. 1990;299:327–348. doi: 10.1002/cne.902990306. [DOI] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismaru A.L., Gui L., Vasung L., Lejeune F., Barisnikov K., Truttmann A., Borradori Tolsa C., Hüppi P.S. Altered amygdala development and fear processing in prematurely born infants. Front. Neuroanat. 2016;10:1–10. doi: 10.3389/fnana.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé P., Manjón J.V., Fonov V., Pruessner J., Robles M., Collins D.L. Patch-based segmentation using expert priors: application to hippocampus and ventricle segmentation. Neuroimage. 2011;54:940–954. doi: 10.1016/j.neuroimage.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssman L., Peltola M.J., Yrttiaho S., Puura K., Mononen N., Lehtimäki T., Leppänen J.M. Regulatory variant of the TPH2 gene and early life stress are associated with heightened attention to social signals of fear in infants. J. Child Psychol. Psychiatry Allied Discip. 2014;55:793–801. doi: 10.1111/jcpp.12181. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Schlegel K., Grandjean D. Amygdala structure and core dimensions of the affective personality. Brain Struct. Funct. 2017;222:3915–3925. doi: 10.1007/s00429-017-1444-9. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., O’Muircheartaigh J., Dirks H., Dean D.C., Tottenham N., Deoni S. Human amygdala functional network development: a cross-sectional study from 3 months to 5 years of age. Dev. Cogn. Neurosci. 2018;34:63–74. doi: 10.1016/j.dcn.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Buss C., Rasmussen J.M., Rudolph M.D., Demeter D.V., Gilmore J.H., Styner M., Entringer S., Wadhwa P.D., Fair D.A. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev. Cogn. Neurosci. 2016;18:12–25. doi: 10.1016/j.dcn.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Rudolph M.D., Heim C.M., Gilmore J.H., Styner M., Potkin S.G., Entringer S., Wadhwa P.D., Fair D.A., Buss C. Maternal systemic Interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry. 2018;83:109–119. doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Missana M., Krol K.M. The neurodevelopmental precursors of altruistic behavior in infancy. PLoS Biol. 2018;16:1–22. doi: 10.1371/journal.pbio.2005281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashempour Niloofar. A Novel Approach for Manual Segmentation of the Amygdala and Hippocampus in Neonate MRI. Frontiers in Neuroscience. 2019 doi: 10.3389/fnins.2019.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., Grand A.P., Mccormack K.M., Shi Y., Laprarie J.L., Maestripieri D., Styner M.A., Sanchez M.M. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Dev. Psychobiol. 2014;56:1735–1746. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. J. Comp. Neurol. 1968;132:135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Jack R.E., Schyns P.G. The human face as a dynamic tool for social communication. Curr. Biol. 2015;25:R621–R634. doi: 10.1016/j.cub.2015.05.052. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Subcortical face processing. Nat. Rev. Neurosci. 2005;6:766. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Karlsson L., Tolvanen M., Scheinin N.M., Uusitupa H.M., Korja R., Ekholm E., Tuulari J.J., Pajulo M., Huotilainen M., Paunio T., Karlsson H. Cohort profile: the FinnBrain birth cohort study (FinnBrain) Int. J. Epidemiol. 2018;47:15–16. doi: 10.1093/ije/dyx173. [DOI] [PubMed] [Google Scholar]
- Kataja E.L., Karlsson L., Leppänen J.M., Pelto J., Häikiö T., Nolvi S., Pesonen H., Parsons C.E., Hyönä J., Karlsson H. Maternal depressive symptoms during the pre- and postnatal periods and infant attention to emotional faces. Child Dev. 2018;00:1–16. doi: 10.1111/cdev.13152. [DOI] [PubMed] [Google Scholar]
- Kataja E.L., Karlsson L., Parsons C.E., Pelto J., Pesonen H., Häikiö T., Hyönä J., Nolvi S., Korja R., Karlsson H. Maternal pre- and postnatal anxiety symptoms and infant attention disengagement from emotional faces. J. Affect. Disord. 2019;243:280–289. doi: 10.1016/j.jad.2018.09.064. [DOI] [PubMed] [Google Scholar]
- Kelly D.J., Duarte S., Meary D., Bindemann M., Pascalis O. Infants rapidly detect human faces in complex naturalistic visual scenes. Dev. Sci. 2019:1–10. doi: 10.1111/desc.12829. [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Piecinski P., Rakic P. Neurogenesis of the amygdaloid nuclear complex in the Rhesus monkey. Dev. Brain Res. 1992;68:9–15. doi: 10.1016/0165-3806(92)90242-O. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven E., Giezen M., Struikmans H. Measuring the similarity of target volume delineations independent of the number of observers. Phys. Med. Biol. 2009;54:2863–2873. doi: 10.1088/0031-9155/54/9/018. [DOI] [PubMed] [Google Scholar]
- Kumpulainen V. Prevalence and Risk Factors of Incidental Findings in Brain MRIs of Healthy Neonates-The FinnBrain Birth Cohort Study. Frontiers in Neurology. 2020 doi: 10.3389/fneur.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtola S.J., Tuulari J.J., Karlsson L., Parkkola R., Merisaari H., Saunavaara J., Lähdesmäki T., Scheinin N.M., Karlsson H. Associations of age and sex with brain volumes and asymmetry in 2–5-week-old infants. Brain Struct. Funct. 2019 doi: 10.1007/s00429-018-1787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen J.M. Using eye tracking to understand infants’ attentional Bias for faces. Child Dev. Perspect. 2016;10:161–165. doi: 10.1111/cdep.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen J.M., Forssman L., Kaatiala J., Yrttiaho S., Wass S. Widely applicable MATLAB routines for automated analysis of saccadic reaction times. Behav. Res. Methods. 2015;47:538–548. doi: 10.3758/s13428-014-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen J.M., Cataldo J.K., Bosquet Enlow M., Nelson C.A. Early development of attention to threat-related facial expressions. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen J.M., Cataldo J.K., Enlow M.B., Nelson C.A. Early development of attention to threat-related facial expressions. PLoS One. 2018;13:1–13. doi: 10.1371/journal.pone.0197424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.D., Fonov V.S., Collins D.L., Evans A.C., Tohka J. Cortical and subcortical T1 white/gray contrast, chronological age, and cognitive performance. Neuroimage. 2019;196:276–288. doi: 10.1016/j.neuroimage.2019.04.022. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K., Pechtel P., Yoon S.A., Anderson C.M., Teicher M.H. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav. Brain Res. 2016;308:83–93. doi: 10.1016/j.bbr.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C.J., Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J. Child Psychol. Psychiatry Allied Discip. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Nakagawa A., Sukigara M. Difficulty in disengaging from threat and temperamental negative affectivity in early life: a longitudinal study of infants aged 12-36 months. Behav. Brain Funct. 2012;8:1–8. doi: 10.1186/1744-9081-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A., Sukigara M. Attentional bias assessed by a facial expression cuing paradigm in infants. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-018-36806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Dolgin K. The Generalized Discrimination of Facial Expressions by Seven-Month-Old Infants. Child Dev. 1985;56:58–61. doi: 10.2307/1130173. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Choe Msun, Flax J., Grant P.E., Benasich A.A. Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage. 2010;49:2791–2799. doi: 10.1016/j.neuroimage.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Ouyang M., Dubois J., Yu Q., Mukherjee P., Huang H. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage. 2019;185:836–850. doi: 10.1016/j.neuroimage.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola M.J., Leppänen J.M., Palokangas T., Hietanen J.K. Fearful faces modulate looking duration and attention disengagement in 7-month-old infants. Dev. Sci. 2008;11:60–68. doi: 10.1111/j.1467-7687.2007.00659.x. [DOI] [PubMed] [Google Scholar]
- Peltola M.J., Leppänen J.M., Mäki S., Hietanen J.K. Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Soc. Cogn. Affect. Neurosci. 2009;4:134–142. doi: 10.1093/scan/nsn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola M.J., van IJzendoorn M.H., Yrttiaho S. Attachment security and cortical responses to fearful faces in infants. Attach. Hum. Dev. 2018:0–3. doi: 10.1080/14616734.2018.1530684. [DOI] [PubMed] [Google Scholar]
- Peltola M.J., Yrttiaho S., Leppänen J.M. Infants’ attention bias to faces as an early marker of social development. Dev. Sci. 2018;21:1–14. doi: 10.1111/desc.12687. [DOI] [PubMed] [Google Scholar]
- Posner J., Cha J., Roy A.K., Peterson B.S., Bansal R., Gustafsson H.C., Raffanello E., Gingrich J., Monk C. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry. 2016;6:4–11. doi: 10.1038/tp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyykkö J., Ashorn P., Ashorn U., Niehaus D.J.H., Leppänen J.M. 2019. Cross-cultural Analysis of Attention Disengagement Times Supports the Dissociation of Faces and Patterns in the Infant Brain; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Tint M.T., Hameed W.B., Meaney M.J., Gluckman P.D., Qiu A., Chong Y.-S., Bai J., Sim L.W., Leutscher-Broekman B., Fortier M.V., Chen H. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol. Psychiatry. 2013;74:837–844. doi: 10.1016/j.biopsych.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Kong L., Sim L.W., Sanmugam S., Broekman B.F.P., Chen H., Wong E., Kwek K., Saw S.M., Chong Y.S., Gluckman P.D., Fortier M.V., Pederson D., Meaney M.J., Qiu A. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry. 2015;5:e668–12. doi: 10.1038/tp.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks V.J., Eaton J.P., Ruess L., Petermann G.W., Keck-Wherley J., Pedersen R.C. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. Am. J. Neuroradiol. 2008;29:1082–1089. doi: 10.3174/ajnr.A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal C.L., Adams H.H.H., Hibar D.P., White C.C., Knol M.J., Stein J.L. Genetic architecture of subcortical brain structures in 38,851 individuals. Nat. Genet. 2019;51:1624–1636. doi: 10.1038/s41588-019-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann Cynthia M., Melissa D. Bauman D.G.A. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia2. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Whalen P.J. Prior experience as a stimulus category confound: an example using facial expressions of emotion. Soc. Cogn. Affect. Neurosci. 2006;1:271–274. doi: 10.1093/scan/nsl040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari J.J., Scheinin N.M., Lehtola S., Merisaari H., Saunavaara J., Parkkola R., Sehlstedt I., Karlsson L., Karlsson H., Björnsdotter M. Neural correlates of gentle skin stroking in early infancy. Dev. Cogn. Neurosci. 2019;35:36–41. doi: 10.1016/j.dcn.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn M.H., Puura K., Forssman L., Leppänen J.M., Peltola M.J. Attention to faces expressing negative emotion at 7 months predicts attachment security at 14 months. Child Dev. 2015;86:1321–1332. doi: 10.1111/cdev.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videman M., Stjerna S., Roivainen R., Nybo T., Vanhatalo S., Gaily E., Leppänen J.M. Evidence for spared attention to faces in 7-month-old infants after prenatal exposure to antiepileptic drugs. Epilepsy Behav. 2016;64:62–68. doi: 10.1016/j.yebeh.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Richardson M.P., Armony J.L., Driver J., Dolan R.J. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat. Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Ward J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963;58:236–244. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
- Wass S.V., Forssman L., Leppänen J. Robustness and precision: how data quality may influence key dependent variables in infant eye-tracker analyses. Infancy. 2014;19:427–460. doi: 10.1111/infa.12055. [DOI] [Google Scholar]
- Weier K., Fonov V., Lavoie K., Doyon J., Louis Collins D. Rapid automatic segmentation of the human cerebellum and its lobules (RASCAL)-Implementation and application of the patch-based label-fusion technique with a template library to segment the human cerebellum. Hum. Brain Mapp. 2014;35:5026–5039. doi: 10.1002/hbm.22529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D.J., Poh J.S., Ni S.N., Chong Y., Chen H., Kwek K., Shek L.P., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. 2017. In Fluences of Prenatal and Postnatal Maternal Depression on Amygdala Volume and Microstructure in Young Children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr. Dir. Psychol. Sci. 1998;7:177–188. [Google Scholar]
- Whalen P.J., Shin L.M., McInerney S.C., Fischer H., Wright C.I., R.S A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Whitby E.H., Griffiths P.D., Rutter S., Smith M.F., Sprigg A., Ohadike P., Davies N.P., Rigby A.S., Paley M.N. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet. 2004;363:846–851. doi: 10.1016/S0140-6736(04)15730-9. [DOI] [PubMed] [Google Scholar]
- Zijdenbos A.P., Dawant B.M., Margolin R.A., Palmer A.C. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans. Med. Imaging. 1994;13:716–724. doi: 10.1109/42.363096. [DOI] [PubMed] [Google Scholar]