Abstract
The tropical fruit sapodilla (Manilkara zapota syn. Achras zapota) is a rich source of nutrients, minerals and a myriad of bioactive phytochemicals such as flavonoids and catechins. Pharmacologically, sapodilla has been shown to exhibit anti-bacterial, anti-parasitic, anti-fungal, antiglycative, hypocholesterolemic and anti-cancer effects. However, its influence on hepatic tissue and serum lipids remains obscure. To address this, we used an in vivo model of liver damage to elucidate the effect of lyophilized sapodilla extract (LSE) treatment in carbon tetra chloride (CCl4) intoxicated rats. Exposure of CCl4 resulted in elevation of serum biomarkers of liver damage (aspartate transaminase, alanine aminotransferase, γ-glutamyl transferase and alkaline phosphatase), bilirubin and dysregulation of serum lipid profile (cholesterol and triglycerides). These effects were significantly and dose-dependently reversed by LSE treatment (250 and 500 mg/kg). Administration of LSE also reduced the structural damage caused by CCl4 in the liver. Furthermore, determination of oxidative stress parameters (malondialdehyde and non-protein sulfhydryls) revealed that LSE treatment mitigated CCl4-triggered modulation of both molecules. LSE also showed a strong antioxidant activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and β-carotene-linoleic acid assays. In conclusion, the present study discloses the hepatoprotective and lipid-lowering effects of lyophilized sapodilla extract against CCl4-induced liver damage, an effect, at least in part, mediated by its antioxidant activity.
Keywords: Manilkara zapota, Sapodilla, Hepatic damage, Carbon tetrachloride, Serum lipids, Oxidative stress
1. Introduction
The liver plays a crucial biological role in metabolism and detoxification (Dutta et al., 2018). Hepatic injury may be triggered by alcohol consumption, viral infections and a myriad of xenobiotics. The chemical agent carbon tetrachloride (CCl4) mediates redox-sensitive hepatotoxicity via generation of free radicals and subsequent lipid peroxidation (Lin et al., 2019; 20:, Suk and Kim, 2012). Carbon tetrachloride has been used to simulate hepatic injury in laboratory animals and is a widely accepted in vivo model to study the pharmacological effects of hepatoprotective molecules (Liu et al., 2014, Zhang et al., 2013). Due to various side-effects of conventional drugs, there has been a renewed interest in exploiting the biological effects of nutraceuticals. Various bioactive molecules derived from natural sources such as flavonoids and catechins possess hepatoprotective effects (Okaiyeto et al., 2018). Consumption of fruits and vegetables may, thus, be therapeutically beneficial during the course of both acute and chronic liver injury.
The tropical fruit sapodilla (Manilkara achras (Mill) Fosb., syn Achras sapota L.) family: Sapotaceae is rich in nutrients and minerals, and is widely consumed fresh but its pulp is also incorporated into various culinary preparations (Lasekan and Abbas, 2012, Singh and Bothara, 2014). Sapodilla is a good source of vitamins and minerals including vitamin A, vitamin B complex, vitamin C, carotene, calcium, potassium, magnesium and phosphorus (Srivastava et al., 2017). The leaves and fruits of sapodilla have been used in traditional medicine to treat several diseases (Kaneria et al., 2009, Lans et al., 2000, Ortiz et al., 2007). Sapodilla is enriched with phytochemicals such as catechin, epicatechin, leucocyanidin, leucodelphinidin, leucopelargonidin, and gallic acid (Ma et al., 2003, Wang et al., 2012). These bioactive constituents confer sapodilla with robust antioxidant properties (Mahattanatawee et al., 2006, Moo-Huchin et al., 2014, Shui et al., 2004). Sapodilla was shown to exhibit anti-bacterial (Rakholiya et al., 2014), anti-fungal (Nair and Chanda 2008), antiglycative (Shakthi Deve et al., 2014), hypocholesterolemic (Fayek et al., 2012), anti-cancer (Osman et al., 2011), and anti-parasitic (Rajakumar and Abdul, 2012) effects. Sapodilla was recently shown to trigger apoptosis in various cell lines by activating the mitochondrial pathway (Srivastava et al., 2014). In addition to these beneficial effects, sapodilla plums contain allergen proteins (Hegde et al., 2014), which may induce food-associated allergies (Hegde and Venkatesh 2002). Despite these pharmacological effects of sapodilla, the modulatory effects of sapodilla on liver injury remain elusive.
In the present study, we investigated the effect of lyophilized sapodilla extract (LSE) on various markers of liver damage and elucidated the role of redox mechanisms in LSE-mediated hepato-protection in CCl4-induced hepatic injury in rats.
2. Materials and methods
2.1. Plant material and dosage preparation
The fresh sapodilla fruits were purchased from a local vegetable and fruit vendor. The fruits were thoroughly washed with tap water, cut into small pieces, seeds removed and the juice obtained using an electric blender. The juice was lyophilized to get the dry powder using a lyophilizer. The freeze dried powder (LSE) was dissolved in distilled water and used in all the experiments, except for measuring the free radical scavenging and antioxidant activities, where methanolic extracts were used.
2.2. Animals
Wistar albino male rats, 180 ± 20 g were used in this study. The animals were caged individually in hygienic conditions and kept in a controlled environment with a 12 h light–dark cycle at 22 ± 3 °C for a week before starting the experiment. The animals had free access to purina chow diet and water ad libitum. The study protocol was approved by the Institutional Review Board (No. RAKMHSU-REC-08-2019-F-P).
2.3. Chemicals and reagents
All chemicals were procured from Sigma (Sigma-Aldrich, St. Louis, MO, USA). United and/or Roche Diagnostics kits (AST, ALT, ALP, Total Proteins, Cholesterol, Triglycerides, HDL, LDL, etc.) were used to determine the biochemical parameters on Reflotron Plus Analyzer (Roche Diagnostics GmbH, Mannheim, Germany) and on a Shimadzu UV mini 1240 spectrophotometer (Shimadzu Europe, Milano, Italy) for the measurement of MDA, NP-SH, and total proteins.
2.4. Carbon tetrachloride-induced hepatic toxicity and drug treatment
Five groups (I–V) of animals (N = 6) were used. Group I served as normal control and received all the vehicles via respective routes. Group II received only carbon tetrachloride (CCl4) as intoxicated control. Groups III and IV were assigned as test groups. Group III and IV were pre-treated with LSE at doses of 250 and 500 mg/kg orally, daily for 17 days, whereas group V was pre-treated with silymarin (SIL) at a dose of 10 mg/kg b.w. orally, for 17 days; this group was used as a positive control. A dosage of 250 mg/kg for rat will be equivalent to 43 mg/kg for human according to dose conversion based on metabolically active mass of animals (Khan 2003). Silymarin is a mixture of flavonolignans extracted from the milk thistle [Silybum marianum (L.) Gaertneri] and is a potent hepatoprotective agent (Féher and Lengyel 2012). At the 16th day, groups II–V received CCl4 in liquid paraffin (1:1) at a dose of 1.25 mL/kg/rat intraperitoneally. After 24 h, following CCl4 challenge, rats were sacrificed and blood was collected by cardiac puncture, serum separated and stored at −80 °C until analysis. The liver was removed for biochemical and histological assessment.
2.5. Analysis of biomarkers for liver function
The serum biomarkers of liver function including alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), and bilirubin were analysed by commercial kits supplied by United Diagnostic Industry, KSA.
2.6. Determination of serum lipids
Serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL), and triglycerides (TG) were determined by the Roche diagnostics kits (Roche Diagnostics GmbH, Mannheim, Germany) using the previously reported protocols. The levels of low-density lipoprotein cholesterol (LDL) and very low-density lipoprotein cholesterol (VLDL) were computed using the following equations: LDLC = TC-HDL-VLDL; VLDL = TG/5.27.
2.7. Determination of malondialdehyde (MDA)
A modified method of Utley et al. (1967) was used. The liver was homogenized (10% w/v, 1 mL) in 0.15 M KCl at 4 °C. After incubating (37 °C for 3 h) the homogenate, equal volume of trichloroacetic acid (TCA, 10%) was added and the mixture was centrifuged at 800g for 10 min. An aliquot (1 mL) was then mixed with equal volume of 0.67% thiobarbituric acid (TBA) and the tubes were kept in a boiling water bath for 10 min. After cooling at room temperature, the contents were diluted with 1 mL distilled water, and the absorbance was recorded at 535 nm. The level of MDA (nmol/g wet tissue) was calculated using the equation:
2.8. Analysis of nonprotein sulfhydryls (NP-SH)
The method of Sedlak and Lindsay (1968) was used for the estimation of NP-SH levels in rat liver. The tissue was homogenized in ice-cold ethylenediaminetetraacetic acid (EDTA, 0.02 M). The homogenate (1 mL) was mixed with equal volume of distilled water and 0.25 mL of trichloroacetic acid (50%), contents shaken for 10 min and centrifuged. An aliquot of supernatant (0.5 mL) was mixed with 1.0 mL ofTris buffer (0.4 M, pH 8.9) and 0.25 mL of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB). After shaking the mixture, the absorbance was read at 412 nm against a reagent blank. The level of NP-SH in liver tissue was calculated according to the following equation:
2.9. Analysis of total proteins (TP)
Serum TP was measured using a commercial kit (Crescent Diagnostics, Jeddah, Saudi Arabia) according to manufacturer’s instructions. The Absorbance of coloured complex at 546 nm was directly proportional to total protein levels in the sample, which were calculated using the equation: Absorbance of sample/Absorbance of standard) × concentration of standard.
2.10. Free radical scavenging assay
The ability of LSE to scavenge the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was evaluated as reported earlier (Brand et al., 1995). The LSE was re-dissolved in methanol and serial concentrations (10–1000 μg/mL) of the extract, 125 μL DPPH (1.0 mM) and 375 μL solvent (methanol) were added. The contents were incubated at 25 °C for 30 min and the decrease in absorbance was measured at 517 nm. The free radical scavenging activity of LSE was calculated from the following equation:
2.11. β-Carotene-linoleic acid assay
We used β-carotene bleaching method (Mothana et al., 2012) for measuring the antioxidant activity of LSE. One milliliter of a 0.2 mg/mL β-carotene solution was mixed with 0.02 mL of linoleic acid and 0.2 mL of Tween-20 followed by removal of organic solvent (chloroform) by heating at 40 °C in a rotary evaporator. The residual mixture was diluted with 10 mL of distilled water and briefly mixed to form an emulsion. The blank was prepared without using β-carotene. The control contained 0.2 mL of 80% methanol instead of extract. An aliquot (5 mL) of the emulsion was mixed with 0.2 mL of sample at the concentration of 1.0 mg/mL. Rutin (1.0 mg/mL) was used as a positive control. The tubes were kept in a water bath at 40 °C for 2 h and the absorbance was recorded at 470 nm at 15 min intervals, using a UV–visible spectrophotometer (UV mini-1240, Shimadzu, Japan). The following equation was used to calculate the percent antioxidant activity:
% antioxidant activity = 1 − (Abs0 − Abst)/(Abs°0 − Abs°t) × 100
where Abs0 and Abs°0 are absorbance values recorded at zero time for sample and control, respectively. Abst and Abs°t are the absorbance readings for sample and control, respectively, measured at 120 min.
2.12. Histopathology
The liver tissue samples were fixed in 10% neutral buffered formalin for 24 h and processed using a tissue processor. The processed tissue was then embedded in paraffin blocks and sections (5 µm thickness) were cut by a rotary microtome. Sections were stained with haematoxylin and eosin (H & E) and examined under a light microscope for histopathological changes.
2.13. Statistical analysis
The data were analysed by one-way analysis of variance (ANOVA) followed by post-hoc Dunnett’s multiple comparison test, using the statistical package, SPSS version 10. P values less than 0.05 were considered significant.
3. Results
We explored the effect of LSE on serum biomarkers indicative of hepatic injury. Administration of CCl4 (1.25 mL/kg) induced a significant elevation of the serum biomarkers AST, ALT, GGT, ALP and bilirubin (Table 1). This effect was significantly blunted in rats administered with LSE (250 and 500 mg/kg) in a dose-dependent manner (Table 1). The positive control silymarin-treated group similarly showed a significant reduction in CCl4-triggered upregulation of serum biomarkers of liver injury (Table 1). These data suggest that LSE administration mediates hepatoprotective effects against chemically-induced liver damage.
Table 1.
Effect of lyophilized sapodilla extract (LSE) on serum marker enzymes in control and experimental rats.
| Treatment | AST (U/L) | ALT (U/L) | GGT (U/L) | ALP (U/L) | Bilirubin (mg/dL) |
|---|---|---|---|---|---|
| Control | 91.71 ± 4.20 | 33.86 ± 2.30 | 3.48 ± 0.34 | 332.1 ± 10.06 | 0.54 ± 0.01 |
| CCl4 | 296.0 ± 9.76** | 236.33 ± 7.85** | 11.71 ± 0.75** | 560.1 ± 10.28** | 2.42 ± 0.05** |
| LSE-250 + CCl4 | 287.0 ± 8.00** | 233.50 ± 2.88** | 12.03 ± 0.41** | 522.0 ± 7.04**# | 2.11 ± 0.12**# |
| LSE-500 + CCl4 | 291.8 ± 5.51** | 202.16 ± 4.17**# | 9.46 ± 0.24* | 516.5 ± 8.92**# | 2.06 ± 0.14**# |
| Silymarin-10 + CCl4 | 196.6 ± 6.44*## | 107.46 ± 4.79*## | 5.10 ± 0.13## | 413.8 ± 9.20*## | 1.02 ± 0.06*## |
All values represent mean ± SEM. *P < 0.01 and **P < 0.001 verses control; #P < 0.05 and ##P < 0.01 versus CCl4 only group.
Administration of CCl4 significantly increased serum cholesterol, triglycerides, LDL and VLDL while reducing serum HDL (Table 2). These effects were significantly and dose-dependently mitigated by LSE. Similar to LSE, the positive control silymarin significantly curtailed CCl4-induced changes in serum lipid profile (Table 2), thus, providing additional evidence of the hepatoprotective effects of LSE on CCl4–triggered hepatocellular damage.
Table 2.
Effect of lyophilized sapodilla extract (LSE) on serum lipoproteins in control and experimental rats.
| Treatment | Cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) |
|---|---|---|---|---|---|
| Control | 89.6 ± 3.03 | 65.9 ± 3.36 | 53.40 ± 2.44 | 23.00 ± 2.44 | 13.19 ± 0.67 |
| CCl4 | 200.3 ± 8.63** | 157.1 ± 4.23** | 26.80 ± 1.01* | 142.10 ± 8.69** | 31.43 ± 0.84* |
| LSE-250 + CCl4 | 151.5 ± 5.59*## | 129.8 ± 4.26*# | 39.85 ± 2.22## | 86.68 ± 6.81*## | 25.96 ± 0.85*# |
| LSE-500 + CCl4 | 123.0 ± 3.72## | 101.4 ± 5.02*## | 42.03 ± 1.45## | 60.68 ± 5.01*## | 20.28 ± 1.00## |
| Silymarin-10 + CCl4 | 130.8 ± 6.17## | 131.6 ± 5.05*## | 36.91 ± 1.13## | 67.58 ± 6.48*## | 26.33 ± 1.01*# |
All values represent mean ± SEM. *P < 0.01 and **P < 0.001 verses control; #P < 0.05 and ##P < 0.01 versus CCl4 only group.
In view of the modulatory effect of LSE on CCl4-induced hepatic injury, an additional series of experiments was performed to elucidate the underlying mechanism. We determined whether LSE treatment affected CCl4-stimulated redox imbalance in the rat liver. We estimated hepatic MDA levels. As illustrated in Fig. 1, CCl4 treatment significantly increased hepatic MDA levels, an effect that was significantly and dose-dependently curtailed by LSE and the positive control silymarin. These results suggest the participation of antioxidant effects of LSE in ameliorating CCl4-induced liver damage.
Fig. 1.
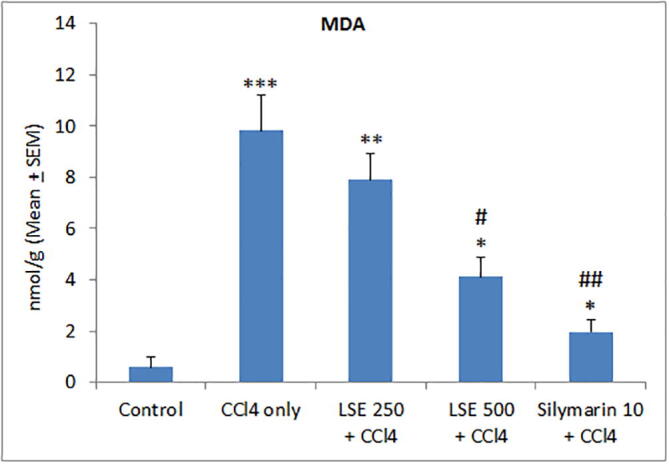
Effect of lyophilized sapodilla extract (LSE) on liver tissue lipid peroxidation (MDA) levels in carbon tetrachloride (CCl4) intoxicated rats. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control; #P < 0.01 and ##P < 0.001 versus CCl4 only group.
To corroborate the aforementioned antioxidant effects of LSE on the hepatic tissue, we determined hepatic non-protein sulfhydryl levels. As depicted in Fig. 2, CCl4 treatment significantly reduced NP-SH levels in rat liver, an effect significantly attenuated by both LSE and silymarin, suggesting that LSE-induced hepatoprotection in CCl4-treated rats is mediated by its antioxidant effects. Further experiments addressed the effect of LSE on CCl4–induced decrease in total hepatic protein. As shown in Fig. 3, CCl4-triggered decreased hepatic protein levels were significantly and dose-dependently reversed by both LSE and silymarin treatment.
Fig. 2.
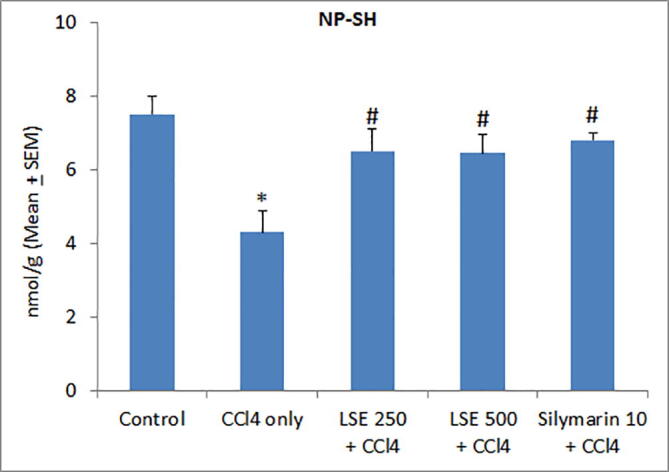
Effect of lyophilized sapodilla extract (LSE) on liver tissue non-protein sulfhydryl (NP-SH) levels in carbon tetrachloride (CCl4) intoxicated rats. *P < 0.01 versus control; #P < 0.05 versus CCl4 only group.
Fig. 3.
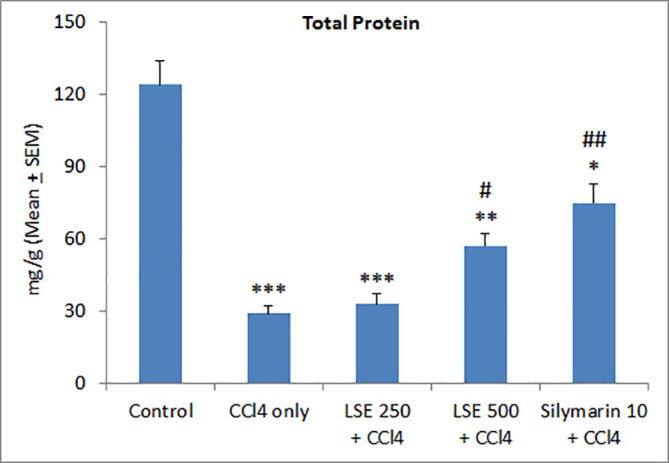
Effect of lyophilized sapodilla extract (LSE) on liver tissue total protein levels in carbon tetrachloride (CCl4) intoxicated rats. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control; #P < 0.05 and ##P < 0.01 versus CCl4 only group.
The potential antioxidant activity of the LSE was investigated on the basis of DPPH radical scavenging activity and of inhibition of linoleic acid oxidation. As demonstrated in Table 3, LSE was able to reduce the stable free radical DPPH (purple) to the yellow-colored DPPH-H in a concentration dependent manner. In β-carotene/linoleic acid antioxidant potential test, LSE was also able to inhibit the discoloration of β-carotene at a concentration of 1.0 mg/mL, showing the total antioxidant capacity as 74.4% as compared with the positive control, rutin (90.9%) (Table 3).
Table 3.
Free radical scavenging activity and antioxidant activity of the methanolic extract of sapodilla as compared to other antioxidants.
| Radical scavenging activity (%) |
Total antioxidant activity (%) | |||||
|---|---|---|---|---|---|---|
| 10 (µg/mL) | 50 (µg/mL) | 100 (µg/mL) | 500 (µg/mL) | 1000 (µg/mL) | 1000 (µg/mL) | |
| Sapota | 7.8 | 28.5 | 54.1 | 71.2 | 85.5 | 74.4 |
| Ascorbic acid | 16.8 | 80.2 | 90.1 | 92.8 | 94.9 | – |
| Rutin* | – | – | – | – | – | 90.9 |
*Rutin is a plant pigment (flavonoid) with potent antioxidant properties. It was used as a positive control.
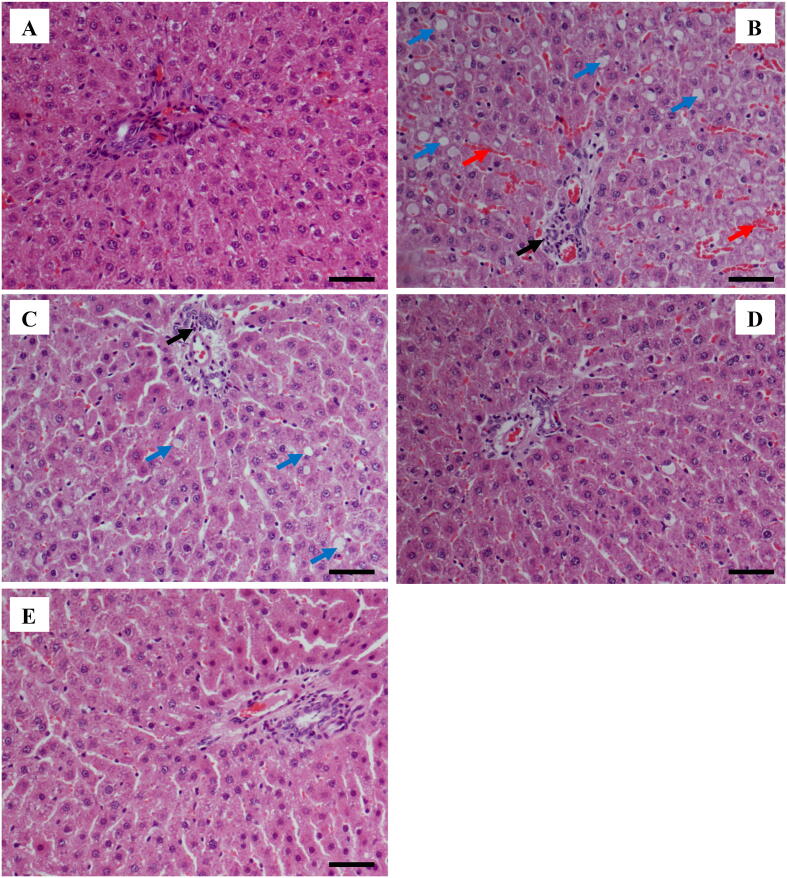
The results of histopathological assessment of hepatic tissues (Fig. 4) correlated with the above mentioned biochemical findings. Group A (control group) showed normal hepatic tissues. Group B (CCl4-intoxicated rat) revealed severe fatty degeneration, ballooning, and inflammatory cell infiltration in addition to massive hemorrhages. Group C (250 mg/kg LSE) showed focal degeneration concomitant with vacuolization and patchy inflammatory cells infiltrations were observed. Group D (500 mg/kg LSE) showed insignificant change in the form of mild cellular degeneration. Group E (10 mg/kg silymarin) had normal appearance of hepatocytes population and portal triad architecture.
Fig. 4.
Histopathology of livers of rats from different treatment groups. (A) Control group showing a normal structure; (B) CCl4-intoxicated rat showing abundant fatty deposition and ballooning (blue arrow), inflammatory cells infiltration (black arrow) and massive hemorrhage (red arrow); (C) LSE (250 mg/kg) group showing mild steatosis, focal vacuolization (blue arrow) and inflammatory cells (black arrow); (D) LSE (500 mg/kg) group showing some mild pathological changes; (E) silymarin (10 mg/kg) showing normal appearance of hepatocytes. Scale bar = 100 µm.
4. Discussion
Nonalcoholic fatty liver disease (NAFLD) refers to a wide spectrum of liver damage that spans from mild steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis (Lewis and Mohanty 2010). This disease is a major cause of elevation of liver enzymes. The global prevalence of NAFLD is about 25% and continues to raise worldwide (Mundi et al., 2019). A recent Swiss study has reported that the incidence of advanced liver disease is estimated to increase by approximately 40% by 2030 (Goossens et al., 2019). It is important to note that NAFLD is an independent risk factor for drug-related acute hepatitis; that means NAFLD patients are at higher risk for acute hepatotoxicity due to medications for other illnesses (Tarantino et al., 2007). An animal study also showed that nonalcoholic fatty liver sensitizes rats to CCl4 hepatotoxicity (Donthamsetty et al., 2007). Thus, searching for novel prophylactic and therapeutic hepato-protective agents with no side effects is of prime concern.
In this study we used CCl4 treated rat model of liver damage. Besides elevating the levels of liver enzymes, blood glucose, non-esterified fatty acids in the serum and glycogen in liver, CCl4 also significantly decreases phosphorylase activity in liver and increases carbohydrate intolerance and insulin resistance (Sadek et al., 2016). Administration of CCl4 causes liver steatosis (Cui et al., 2017) and therefor it has been used to develop experimental models of NAFLD (Kanai et al., 2016, Chheda et al., 2014) and NASH (Tsuchida et al., 2018, Owada et al., 2018); the latter condition is a type of NAFLD with inflammation and liver cell damage along with fat accumulation. A mass spectrometry study showed that the damage to rat liver tissue by CCl4 is accompanied with the alterations in diacylglycerols, cholesterol and fatty acids, such as linoleic and oleic acids (Otrubova et al., 2018).
This study showed that exposure of CCl4 significantly elevated the levels of serum aminotransferases and other liver enzymes indicating the hepatic damage and severe liver dysfunction caused by CCl4 (Table 1). Both AST and ALT are important biomarkers of liver function (Alwelaie et al., 2019, Al Asmari et al., 2018). The impairment of liver function in CCl4 alone treated rats was accompanied with dyslipidemia (Table 2). Treatment with sapodilla significantly reversed the adverse effects of CCl4 on liver function as well as serum lipid profile suggesting the pharmacological potential of sapodilla extract in liver disease. The flavonoids were previously shown to possess robust lipid-lowering effects (Assini et al., 2013). It is likely that the hypocholesterolmic effects of sapodilla are mediated by bioactive constituents such as flavonoids.
The hepatoprotective effect of sapodilla was also confirmed by histopathology. Administration of CCl4 caused severe fatty degeneration, ballooning and inflammatory cell infiltration in rat liver (Fig. 4B). Fat accumulation is a hallmark of NAFLD that may lead to fibrosis, necrosis or even cirrhosis. Hepatocellular ballooning is considered as an important histological parameter in the diagnosis of NASH and is indicative of a greater risk of disease progression (Caldwell et al., 2010). Observation of human liver biopsies has confirmed that accumulation of inflammatory cells such as macrophages is associated with progressive NAFLD (Wehr et al., 2014). Treatment of rats with sapodilla extract reversed the CCl4-induced structural changes, indicating the regeneration of hepatic tissue.
Administration of CCl4 alone significantly increased MDA (Fig. 1) and depleted NP-SH (Fig. 2) levels in rat liver. Both MDA and NP-SH are important indicators of oxidative stress (Reddy et al., 2015, Khan et al., 2012, Al Asmari et al., 2006, Tariq et al., 2002, Al Deeb et al., 2000). Treatment of rats with sapodilla extract as well as the antioxidant silymarin significantly reversed CCl4-induced oxidative stress. Previous studies have shown that CCl4 is capable of generating highly reactive free radicals that lead to oxidative stress (Jayesh et al., 2019) and inflammation (Torres et al., 2016). Several investigators have implicated oxidative stress (Chatterjee et al., 2013) and proinflammatory cytokines (Park et al., 2017, Mehta et al., 2013) in fatty liver disease. Whereas, compounds with antioxidant (Yang et al., 2015, Shah et al., 2015) and anti-inflammatory (Oró et al., 2016) properties have shown beneficial effects in protecting animals against chemical-induced liver damage. Treatment with ASB14780, a phospholipase A2 (PLA2) inhibitor, markedly ameliorated liver injury and hepatic fibrosis in CCl4 treated mice (Kanai et al., 2016). Reduced production of eicosanoids in the liver suppressed the inflammation and oxidative stress resulting in the protection against chemical-induced liver injury in mice (Cao et al., 2013). In mouse models of acute CCl4 and chronic methionine-choline-deficient (MCD) diet-induced hepatic injuries, treatment with anti-CXCL16 chemokine not only significantly decreased the infiltration of inflammatory macrophages into the liver but also reduced the levels of proinflammatory cytokines and reversed steatosis (Wehr et al., 2014).
Our results showed a dose-dependent free radical scavenging activity of sapodilla extract (Table 3). The anti-inflammatory activity of sapodilla has been reported earlier (Liu et al., 2019). Mechanistically, the reported biological effects of sapodilla are mediated by its bioactive constituents. Of the various polyphenols characterized from sapodilla extracts, methyl 4-O-galloylchlorogenate was shown to possess the highest antioxidant activity (Ma et al., 2003). Sapodilla is also rich in proanthocyanidins (Wang et al., 2012), which have been shown to possess a variety of beneficial health effects including hepatoprotection (Deng et al., 2012, Shin et al., 2010), an effect that could be attributed to its anti-apoptotic effect in hepatic cells (Ray et al., 1999). Ozturk et al. (2009) have shown the beneficial effects of apricot on CCl4-induced liver steatosis and damage, due to its high radical-scavenging capacity antioxidant contents (beta-carotene and vitamin), suggesting that dietary intake of apricot can reduce the risk of liver steatosis and degeneration caused by free radicals. Exposure of CCl4 caused significant decrease in total protein levels (Fig. 3) that may be attributed to CCl4-induced decrease in several amino acids including the essential amino acids, leucine and isoleucine (Li et al., 2014). Supplementation of branched chain amino acids (BCAA) has been shown to modify CCl4-induced cirrhosis in rats (Jia et al., 2013).
In conclusion, the present study reveals hepatoprotective effect of lyophilized sapodilla extracts against CCl4 induced hepatic damage, an effect, at least in part, mediated by its antioxidant activity. Since the liver injury caused by CCl4 in rats mimics the NAFLD seen in humans, the dietary consumption of sapodilla fruit may have beneficial effects in prophylaxis or regeneration of hepatic tissue. This study was conducted in experimental animals and its relevance to humans requires additional clinical studies.
Acknowledgments
This study was supported by Researchers Supporting Project (No. RSP-2019/103), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al Asmari A., Al Moutaery K., Manthari R.A., Khan H.A. Time-course of lipid peroxidation in different organs of mice treated with Echis pyramidum snake venom. J. Biochem. Mol. Toxicol. 2006;20:93–95. doi: 10.1002/jbt.20121. [DOI] [PubMed] [Google Scholar]
- Al Asmari A.K., Khan H.A., Manthiri R.A., Al-Khlaiwi A.A., Al-Asmari B.A., Ibrahim K.E. Protective effect of the natural herbal compound quercetin against snake venom-induced hepatic and renal toxicities in rats. Food Chem. Toxicol. 2018;118:105–110. doi: 10.1016/j.fct.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Al Deeb S., Al Moutaery K., Khan H.A., Tariq M. Exacerbation of iminodipropionitrile-induced behavioral toxicity, oxidative stress and vestibular hair cell degeneration by gentamicin. Neurotoxicol. Teratol. 2000;22:213–220. doi: 10.1016/s0892-0362(99)00075-6. [DOI] [PubMed] [Google Scholar]
- Alwelaie M.A., Al Mutary M.G., Siddiqi N.J., Arafah M.M., Alhomida A.S., Khan H.A. Time-course evaluation of iminodipropionitrile-induced liver and kidney toxicities in rats: a biochemical, molecular and histopathological study. Dose Response. 2019;17 doi: 10.1177/1559325819852233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assini J.M., Mulvihill E.E., Huff M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013;24:34–40. doi: 10.1097/MOL.0b013e32835c07fd. [DOI] [PubMed] [Google Scholar]
- Brand W.W., Cuvelier H.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995;82:25–30. [Google Scholar]
- Caldwell S., Ikura Y., Dias D., Isomoto K., Yabu A., Moskaluk C. Hepatocellular ballooning in NASH. J. Hepatol. 2010;53:719–723. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Mulvihill M.M., Mukhopadhyay P. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology. 2013;144:808–817. doi: 10.1053/j.gastro.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda T.K., Shivakumar P., Sadasivan S.K. Fast food diet with CCl4 micro-dose induced hepatic-fibrosis-a novel animal model. BMC Gastroenterol. 2014;14:89. doi: 10.1186/1471-230X-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M.X., Jiang J.F., Min G.N., Han W., Wu Y.J. Ciliary neurotrophic factor analogue aggravates CCl4-induced acute hepatic injury in rats. Can. J. Physiol. Pharmacol. 2017;95:620–623. doi: 10.1139/cjpp-2016-0564. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Ganini D., Tokar E.J. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J. Hepatol. 2013;58:778–784. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Xu Z., Liu W., Yang H., Xu B., Wei Y. Effects of lycopene and proanthocyanidins on hepatotoxicity induced by mercuric chloride in rats. Biol. Trace Elem. Res. 2012;146:213–223. doi: 10.1007/s12011-011-9242-3. [DOI] [PubMed] [Google Scholar]
- Donthamsetty S., Bhave V.S., Mitra M.S., Latendresse J.R., Mehendale H.M. Nonalcoholic fatty liver sensitizes rats to carbon tetrachloride hepatotoxicity. Hepatology. 2007;45:391–403. doi: 10.1002/hep.21530. [DOI] [PubMed] [Google Scholar]
- Dutta S., Chakraborty A.K., Dey P., Kar P., Guha P., Sen S., Kumar A., Sen A., Chaudhuri T.K. Amelioration of CCl4 induced liver injury in swiss albino mice by antioxidant rich leaf extract of Croton bonplandianus Baill. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0196411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayek N.M., Monem A.R., Mossa M.Y., Meselhy M.R., Shazly A.H. Chemical and biological study of Manilkara zapota (L.) Van Royen leaves (Sapotaceae) cultivated in Egypt. Pharmacognosy Res. 2012;4:85–91. doi: 10.4103/0974-8490.94723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féher J., Lengyel G. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr. Pharm. Biotechnol. 2012;13:210–217. doi: 10.2174/138920112798868818. [DOI] [PubMed] [Google Scholar]
- Goossens N., Bellentani S., Cerny A. Nonalcoholic fatty liver disease burden - Switzerland 2018–2030. Swiss Med. Wkly. 2019;149 doi: 10.4414/smw.2019.20152. [DOI] [PubMed] [Google Scholar]
- Hegde V.L., Ashok Kumar H.G., Sreenath K., Hegde M.L., Venkatesh Y.P. Identification and characterization of a basic thaumatin-like protein (TLP 2) as an allergen in sapodilla plum (Manilkara zapota) Mol. Nutr. Food Res. 2014;58:894–902. doi: 10.1002/mnfr.201300261. [DOI] [PubMed] [Google Scholar]
- Hegde V.L., Venkatesh Y.P. Oral allergy syndrome to sapodilla (Achras zapota) J. Allergy Clin. Immunol. 2002;110:533–534. doi: 10.1067/mai.2002.126816. [DOI] [PubMed] [Google Scholar]
- Jayesh K., Helen L.R., Vysakh A., Binil E., Latha M.S. Protective role of Terminalia bellirica (Gaertn.) Roxb fruits against CCl4 induced oxidative stress and liver injury in rodent model. Indian. J. Clin. Biochem. 2019;34:155–163. doi: 10.1007/s12291-017-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Takahashi S., Saito K., Kato H. DNA microarray analysis identified molecular pathways mediating the effects of supplementation of branched-chain amino acids on CCl4-induced cirrhosis in rats. Mol. Nutr. Food Res. 2013;57:291–306. doi: 10.1002/mnfr.201200538. [DOI] [PubMed] [Google Scholar]
- Kanai S., Ishihara K., Kawashita E. ASB14780, an orally active inhibitor of group IVA phospholipase A2, is a pharmacotherapeutic candidate for nonalcoholic fatty liver disease. J. Pharmacol. Exp. Ther. 2016;356:604–614. doi: 10.1124/jpet.115.229906. [DOI] [PubMed] [Google Scholar]
- Kaneria M., Baravalia Y., Vaghasiya Y., Chanda S. Determination of antibacterial and antioxidant potential of some medicinal plants from saurashtra region, India. Indian J. Pharm. Sci. 2009;71:406–412. doi: 10.4103/0250-474X.57289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H.A. CalcDose: a software for drug dosage conversion using metabolically active mass of animals. Drug Chem. Toxicol. 2003;26:51–58. doi: 10.1081/dct-120017557. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Abdelhalim M.A., Al Ayed M.S., Alhomida A.S. Effect of gold nanoparticles on glutathione and malondialdehyde levels in liver, lung and heart of rats. Saudi J. Biol Sci. 2012;19:461–464. doi: 10.1016/j.sjbs.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans C., Harper T., Georges K., Bridgewater E. Medicinal plants used for dogs in Trinidad and Tobago. Prev. Vet. Med. 2000;45:201–220. doi: 10.1016/s0167-5877(00)00123-9. [DOI] [PubMed] [Google Scholar]
- Lasekan O., Abbas K.A. Distinctive exotic flavor and aroma compounds of some exotic tropical fruits and berries: a review. Crit. Rev. Food Sci. Nutr. 2012;52:726–735. doi: 10.1080/10408398.2010.507910. [DOI] [PubMed] [Google Scholar]
- Lewis J.R., Mohanty S.R. Nonalcoholic fatty liver disease: a review and update. Dig. Dis. Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang F., Wang D., Li Z., Qin X., Du G. NMR-based metabonomic and quantitative real-time PCR in the profiling of metabolic changes in carbon tetrachloride-induced rat liver injury. J. Pharm. Biomed. Anal. 2014;89:42–49. doi: 10.1016/j.jpba.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Lin S.Y., Dan X., Du X.X. Protective effects of salidroside against carbon tetrachloride (CCl4)-induced liver injury by initiating mitochondria to resist oxidative stress in mice. Int. J. Mol. Sci. 2019;20:E3187. doi: 10.3390/ijms20133187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Tian G., Yan H., Geng X., Cao Q., Wang H., Ng T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the wild edible mushroom russula vinosa lindblad. J. Agric. Food Chem. 2014;62:8858–8866. doi: 10.1021/jf502632c. [DOI] [PubMed] [Google Scholar]
- Liu Y.P., Yan G., Guo J.M., Liu Y.Y., Li Y.J., Zhao Y.Y., Qiang L., Fu Y.H. Prenylated coumarins from the fruits of Manilkara zapota with potential anti-inflammatory effects and anti-HIV activities. J. Agric. Food Chem. 2019;67:11942–11947. doi: 10.1021/acs.jafc.9b04326. [DOI] [PubMed] [Google Scholar]
- Ma J., Luo X.D., Protiva P., Yang H., Ma C., Basile M.J., Kennelly E.J. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla) J. Nat. Prod. 2003;66:983–986. doi: 10.1021/np020576x. [DOI] [PubMed] [Google Scholar]
- Mahattanatawee K., Manthey J.A., Luzio G., Talcott S.T., Goodner K., Baldwin E.A. Total antioxidant activity and fiber content of select Florida-grown tropical fruits. J. Agric. Food Chem. 2006;54:7355–7363. doi: 10.1021/jf060566s. [DOI] [PubMed] [Google Scholar]
- Mehta R., Birerdinc A., Neupane A. Expression of inflammation-related genes is altered in gastric tissue of patients with advanced stages of NAFLD. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/684237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moo-Huchin V.M., Estrada-Mota I., Estrada-Leon R., Cuevas-Glory L., Ortiz-Vazquez E., Vargas y Vargas M.deL., Sauri-Duch E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014;152:508–515. doi: 10.1016/j.foodchem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Mothana R.A.A., Al-Said M.S., Al-Rehaily A.J. Anti-inflammatory, antinociceptive, antipyretic and antioxidant activities and phenolic constituents from Loranthus regularis Steud. exSprague. Food Chem. 2012;130:344–349. [Google Scholar]
- Mundi M.S., Velapati S., Patel J., Kellogg T.A., Abu Dayyeh B.K., Hurt R.T. Evolution of NAFLD and its management. Nutr. Clin. Pract. 2019 doi: 10.1002/ncp.10449. [DOI] [PubMed] [Google Scholar]
- Nair R., Chanda S. Antimicrobial activity of Terminalia catappa, Manilkara zapota and Piper betel leaf extract. Indian J. Pharm. Sci. 2008;70:390–393. doi: 10.4103/0250-474X.43012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaiyeto K., Nwodo U.U., Mabinya L.V., Okoh A.I. A review on some medicinal plants with hepatoprotective effects. Phcog. Rev. 2018;12:186–199. [Google Scholar]
- Oró D., Yudina T., Fernández-Varo G. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 2016;64:691–698. doi: 10.1016/j.jhep.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Ortiz B.I., Shields K.M., Clauson K.A., Clay P.G. Complementary and alternative medicine use among Hispanics in the United States. Ann. Pharmacother. 2007;41:994–1004. doi: 10.1345/aph.1H600. [DOI] [PubMed] [Google Scholar]
- Osman M.A., Rashid M.M., Aziz M.A., Habib M.R., Karim M.R. Inhibition of Ehrlich ascites carcinoma by Manilkara zapota L. stem bark in Swiss albino mice. Asian Pac. J Trop. Biomed. 2011;1:448–451. doi: 10.1016/S2221-1691(11)60098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otrubova O., Jerigova M., Halaszova S., Turecky L., Muchova J., Velic D. Rat liver intoxication with CCl4: biochemistry, histology, and mass spectrometry. Gen. Physiol. Biophys. 2018;37:527–535. doi: 10.4149/gpb_2018011. [DOI] [PubMed] [Google Scholar]
- Owada Y., Tamura T., Tanoi T. Novel non-alcoholic steatohepatitis model with histopathological and insulin-resistant features. Pathol. Int. 2018;68:12–22. doi: 10.1111/pin.12612. [DOI] [PubMed] [Google Scholar]
- Ozturk F., Gul M., Ates B., Ozturk I.C., Cetin A., Vardi N., Otlu A. Yilmaz I. Protective effect of apricot (Prunus armeniaca L.) on hepatic steatosis and damage induced by carbon tetrachloride in wistar rats. Br. J. Nutr. 2009;102:1767–1775. doi: 10.1017/S0007114509991322. [DOI] [PubMed] [Google Scholar]
- Park J.H., Lee D.H., Park M.S., Jung Y.S., Hong J.T. C-C chemokine receptor type 5 deficiency exacerbates alcoholic fatty liver disease through pro-inflammatory cytokines and chemokines-induced hepatic inflammation. J. Gastroenterol. Hepatol. 2017;32:1258–1264. doi: 10.1111/jgh.13657. [DOI] [PubMed] [Google Scholar]
- Rajakumar G., Abdul Rahuman A. Acaricidal activity of aqueous extract and synthesized silver nanoparticles from Manilkara zapota against Rhipicephalus (Boophilus) microplus. Res. Vet. Sci. 2012;93:303–309. doi: 10.1016/j.rvsc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Rakholiya K., Kaneria M., Chanda S. Inhibition of microbial pathogens using fruit and vegetable peel extracts. Int. J. Food Sci. Nutr. 2014;65:733–739. doi: 10.3109/09637486.2014.908167. [DOI] [PubMed] [Google Scholar]
- Ray S.D., Kumar M.A., Bagchi D. A novel proanthocyanidin IH636 grape seed extract increases in vivo Bcl-XL expression and prevents acetaminophen-induced programmed and unprogrammed cell death in mouse liver. Arch. Biochem. Biophys. 1999;369:42–58. doi: 10.1006/abbi.1999.1333. [DOI] [PubMed] [Google Scholar]
- Reddy U.A., Prabhakar P.V., Rao G.S., Rao P.R., Rahman M.F., Kumari S.I., Grover P., Khan H.A., Mahboob M. Biomarkers of oxidative stress in rat for assessing toxicological effects of heavy metal pollution in river water. Env. Sci. Poll. Res. 2015;22:13453–13463. doi: 10.1007/s11356-015-4381-2. [DOI] [PubMed] [Google Scholar]
- Sadek K., Beltagy D., Saleh E., Abouelkhair R. Camel milk and bee honey regulate profibrotic cytokine gene transcripts in liver cirrhosis induced by carbon tetrachloride. Can. J. Physiol. Pharmacol. 2016;94:1141–1150. doi: 10.1139/cjpp-2015-0596. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Shah M.D., Gnanaraj C., Haque A.T., Iqbal M. Antioxidative and chemopreventive effects of Nephrolepis biserrata against carbon tetrachloride (CCl4)-induced oxidative stress and hepatic dysfunction in rats. Pharm. Biol. 2015;53:31–39. doi: 10.3109/13880209.2014.909502. [DOI] [PubMed] [Google Scholar]
- Shakthi Deve A., Sathish Kumar T., Kumaresan K., Rapheal V.S. Extraction process optimization of polyphenols from Indian Citrus sinensis - as novel antiglycative agents in the management of diabetes mellitus. J. Diab. Metab. Disord. 2014;13:11. doi: 10.1186/2251-6581-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M.O., Yoon S., Moon J.O. The proanthocyanidins inhibit dimethylnitrosamine-induced liver damage in rats. Arch. Pharm. Res. 2010;33:167–173. doi: 10.1007/s12272-010-2239-1. [DOI] [PubMed] [Google Scholar]
- Shui G., Wong S.P., Leong L.P. Characterization of antioxidants and change of antioxidant levels during storage of Manilkara zapota L. J. Agric. Food Chem. 2004;52:7834–7841. doi: 10.1021/jf0488357. [DOI] [PubMed] [Google Scholar]
- Singh S., Bothara S.B. Manilkara zapota (Linn.) seeds: a potential source of natural gum. ISRN Pharm. 2014 doi: 10.1155/2014/647174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, A.K., Kashyap, P., Meena, V.S., Verma, N., Singh, S.P., 2017. Sapota [(Manilkara achras (Mill.) Fosberg (Syn: Achras zapota L.)]. In: Ghosh, S.N., Singh, A., Thakur, A. (Eds.), Underutilized Fruit Crops: Importance and Cultivation. Jaya Publishing House, New Delhi, India.
- Srivastava M., Hegde M., Chiruvella K.K., Koroth J., Bhattacharya S., Choudhary B., Raghavan S.C. Sapodilla plum (Achras sapota) induces apoptosis in cancer cell lines and inhibits tumor progression in mice. Sci. Rep. 2014;4:6147. doi: 10.1038/srep06147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk K.T., Kim D.J. Drug-induced liver injury: present and future. Clin. Mol. Hepatol. 2012;18:249–257. doi: 10.3350/cmh.2012.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino G., Conca P., Basile V., Gentile A., Capone D., Polichetti G. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol. Res. 2007;37:410–415. doi: 10.1111/j.1872-034X.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- Tariq M., Khan H.A., Al Moutaery K., Al Deeb S. Attenuation of iminodipropionitrile-induced behavioral syndrome by sodium salicylate in rats. Pharmacol. Biochem. Behav. 2002;73:647–654. doi: 10.1016/s0091-3057(02)00858-4. [DOI] [PubMed] [Google Scholar]
- Torres L.R., Santana F.C., Torres-Leal F.L. Pequi (Caryocar brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016;97:205–216. doi: 10.1016/j.fct.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Tsuchida T., Lee Y.A., Fujiwara N. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018;69:385–395. doi: 10.1016/j.jhep.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utley H.C., Bernheim F., Hochslein P. Effect of sulfhydryl reagent onperoxidation in microsome. Arch. Biochem. Biophys. 1967;260:521–531. [Google Scholar]
- Wang H., Liu T., Song L., Huang D. Profiles and alpha-amylase inhibition activity of proanthocyanidins in unripe Manilkara zapota (chiku) J. Agric. Food Chem. 2012;60:3098–3104. doi: 10.1021/jf204715q. [DOI] [PubMed] [Google Scholar]
- Wehr A., Baeck C., Ulmer F. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0112327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Sung S.H., Kim Y.C. The ethanolic extract of Juglans sinensis leaves and twigs attenuates CCl4-induced hepatic oxidative stress in rats. Pharmacogn. Mag. 2015;11:533–539. doi: 10.4103/0973-1296.160463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zhao Y., Sun Y., Lu X., Yang X. Isolation, characterization, and hepatoprotective effects of the raffinose family oligosaccharides from Rehmannia glutinosa Libosch. J. Agric. Food Chem. 2013;61:7786–7793. doi: 10.1021/jf4018492. [DOI] [PubMed] [Google Scholar]