Abstract
The ears are air-filled structures that are directly impacted during blast exposure. In addition to hearing loss and tinnitus, blast victims often complain of vertigo, dizziness and unsteady posture, suggesting that blast exposure induces damage to the vestibular end organs in the inner ear. However, the underlying mechanisms remain to be elucidated. In this report, single vestibular afferent activity and the vestibulo-ocular reflex (VOR) were investigated before and after exposure to blast shock waves (∼20 PSI) delivered into the left external ear canals of anesthetized rats. Single vestibular afferent activity was recorded from the superior branch of the left vestibular nerves of the blast-treated and control rats one day after blast exposure. Blast exposure reduced the spontaneous discharge rates of the otolith and canal afferents. Blast exposure also reduced the sensitivity of irregular canal afferents to sinusoidal head rotation at 0.5–2Hz. Blast exposure, however, resulted in few changes in the VOR responses to sinusoidal head rotation and translation. To the best of our knowledge, this is the first study that reports blast exposure-induced damage to vestibular afferents in an animal model. These results provide insights that may be helpful in developing biomarkers for early diagnosis of blast-induced vestibular deficits in military and civilian populations.
Keywords: Primary blast injury, Vestibular end organ, Vestibulo-ocular reflex, Vestibular afferent, Vestibular hair cell
1. Introduction
Blast shock waves generated by explosive devices can damage many organ systems and produce the full range of injuries from relatively minor to lethal. Because the unprotected ears are air-filled structures that are directly exposed to the surrounding air, they are among the most frequently damaged sites during blast exposure in humans (Kronenberg et al., 1993; DePalma et al., 2005; Dougherty et al., 2013; Remenschneider et al., 2014). A recent study also showed that sound stimuli induce larger pressures in the semicircular canals than the cochlear in human cadavers (Maxwell et al., 2017). Indeed, dizziness and imbalance are frequent complaints in blast victims (for review, Fausti et al., 2009; Akin and Murnane, 2011; Franke et al., 2012). Understanding the mechanisms underlying blast-induced vestibular deficits is critical for prevention, identification and treatment of the injuries. However, in contrast to numerous animal studies on blast-induced hearing loss, few animal studies have examined blast-induced damage to vestibular peripheral end organs in the inner ear. A recent study reported that blast waves delivered to the whole body not only ruptured the tympanic membrane and disrupted the conductive structures of the middle ear (i.e., the ossicles), but also caused damage to the vestibular hair cells located in the bony labyrinth and to the VORs in mice (Lien and Dickman, 2018). However, whether the blast exposure causes deficits in vestibular afferent function needs to be demonstrated for two reasons. First, vestibular hair cell deficits may not lead to changes in afferent function because of synaptic redundancy. Second, the demonstrated VOR deficits may result from damage to other components of the VOR pathways, such as the VOR interneurons and/or motor neurons. This study was designed to investigate how blast exposure affects vestibular afferent activity in a rat model. Unlike the existing animal models, in which blast shock waves are delivered over the whole head or the whole body, in this study, we delivered blast shock waves primarily into the external ear canal. This approach has two advantages. It avoids affecting other air-filled organs (e.g., lungs) and should allow for the delivery of more consistent impacts to the inner ear structures.
2. Methods
2.1. Animals
The single unit recording experiment was performed in a group of Sprague-Dawley (SD) rats (250–350g, N = 14) (Harlan, Indianapolis, IN, USA). The VOR experiments were initiated after completion of the afferent recording experiments. It had to be performed in a different strain of rats (i.e., Long-Evans rats, 175–225g, N = 7) (Harlan Labs, Indianapolis, IN) because we found out that the pupils of the albino SD rats could not be tracked by the equipment used for eye movement measurements. The Institutional Animal Care and Use Committee at University of Mississippi Medical Center has approved all the procedures employed in this study.
2.2. Blast generator
Unlike the existing animal models of blast-induced injuries, in which blast shock waves are delivered over the whole animal or the whole head, our animal model was designed to deliver blast waves primarily into the external ear canal. The shock wave generator was constructed by modifying an air pistol (American Classic P1377 multi-pump pneumatic air pistol, Crossman Corporation, Fairport, New York, USA). Fig. 1A demonstrates how the blast generator works. High air pressure was produced in Chamber II by repeatedly pumping air in via Chamber I. Using a mechanical switch located in Chamber III, the valve of Chamber II was briefly opened to release the pressurized air through a tube (∼5 mm in diameter) that was placed at the opening of the external ear canal of an anesthetized rat. The pressurized air created a shock wave reaching a peak pressure of about 20 PSI (138 kPa or 196.8 dB SPL) at the opening of the tube, as measured by a pressure sensor (102B04, 480C02, PCB Piezotronics, Depew, NY). Fig. 1B shows the dynamics of the shock waves produced by the blast generator. The air pressure was increased rapidly (within ∼0.05ms) to a peak pressure of ∼20 PSI. The duration of the positive pressure was ∼0.16ms. This early increase in air pressure was followed by a low amplitude negative wave. Therefore, similar to the shock waves generated by shock tubes, the shock waves generated by our blast generator resembled the classic Friedlander curve (Ewert et al., 2012). It is important to note that our short duration shock wave produced by a small chamber was comparable to the shock wave produced by an automatic rifle (Saljo et al., 2008).
Fig. 1.
The blast generator. A. Schematic illustration of the blast generator. By repeatedly pumping air into Chamber I, high pressure is built in Chamber II. By turn on the mechanical switch located in Chamber III, the valve of Chamber II is briefly opened and the pressurized air is released into tube that is positioned at the opening of the external ear canal of an anesthetized rat. B. Dynamics of the blast shock waves, which reached ∼20 PSI or 138 kPa.
2.3. Blast exposure
Under isoflurane anesthesia, a rat was laid in the right lateral decubitus position, with its head supported by a cushion and its left ear canal pointed upward. The pressure release tube of the blast generator was aimed at the left external ear canal and the opening of the tube was placed at the opening of the ear canal. In the blast-treated condition, shock waves were delivered into the ear canal. In the control condition, rats received the same dose of isoflurane anesthesia without exposure to shock waves. Before and after the blast exposure, we used a digital otoscope to examine integrity of the tympanic membrane, which was found to be ruptured in all 14 blast-treated rats. After blast exposure, rats were placed back in their cages for recovery. After recovery from anesthesia, their postures and movements were observed for 20 min. We specifically looked for body spinning and head tilts. None of the 14 rats exhibited body spinning or head tilts immediately after blast exposure or in the following days during VOR testing. In comparison to delivering shock waves over the whole body or the whole head, the ear canal-only exposure reduced damage to other organs and improved the consistency of blast impacts reaching the inner ear structures.
2.4. Surgical procedures
Vestibular afferents activity was examined one day after blast exposure in the SD rats. Details of the surgeries were described previously (Stewart et al., 2016; Zhu et al., 2011, 2014). Briefly, a rat was anesthetized initially by injection of 50 mg/kg (i.p.) sodium pentobarbital. Surgical plane was maintained by injection of 5 mg/kg as needed. A head holder was implanted on the skull to allow stabilizing the head on a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The animal’s body was restrained in a nylon jacket, which was secured on the stereotaxic plate. A heating pad (DC Temperature controller, FHC, ME, USA) was used to maintain the rat’s core body temperature at 37.0–37.4 °C. The stereotaxic instrument was mounted on a vestibular stimulator (Neuro Kinetic, Inc., Pittsburgh, PA, USA) that delivers head rotation and translation. A small piece of the left occipital bone was removed in order to access the vestibular nerve with a glass electrode. In order to assure a representative population of vestibular nerve fibers, every spontaneous discharging unit encountered was studied. Baseline firing activity was recorded for about 30 s, then we tested its responses to sinusoidal head rotations (0.5, 1, 2 Hz, peak velocity of 45°/second) (Paulin and Hoffman, 2019).
2.5. Single unit recording and analysis
Detailed data acquisition and analysis methods are described in earlier reports (Stewart et al., 2016; Zhu et al., 2011, 2014). Briefly, a CED Power 1401 system was used to sample single unit voltage signals at 20 kHz and head position signals at 1 kHz. Commercial software packages (Spike 2, Matlab and SigmaPlot) were used to analyze the data.
Based on their responses to head rotation, the vestibular nerve fibers were identified as horizontal canal afferents (HC), anterior canal afferents (AC) and superior branch otolith afferents (SO). For each afferent, the coefficient of variation (CV) of interspike intervals during spontaneous firing was calculated. We further calculated the normalized CV (CV∗) for the afferent based on the formula: CV = a· (CV∗)b using parameters of Lasker et al. (2008). Based on CV∗, afferents were further classified as regular afferents (CV∗<=0.1) or irregular afferents (CV∗>0.1) (Young et al., 1977; Goldberg et al., 1984; Goldberg and Fernandez, 1971; Baird et al., 1988). To calculate gain and phase in response to head rotation, we performed a fast Fourier transform (FFT) analysis on the averaged data. Since it was not always possible to align a canal precisely in the plane of rotation, afferent gains computed in these conditions were corrected trigonometrically according to the formula: Gc = Gm · [1/cos(90°-roll)] · [1/cos(45°-pitch)], where Gc is corrected gain and Gm is measured gain; roll and pitch are the absolute value of tilt relative to the neutral position (Hullar and Minor, 1999; Blanks and Torigoe, 1989; Daunicht and Pellionisz, 1987). This method was adopted from Hullar and Minor (1999). The corrected canal gain data allows for comparison between animals and with existing data in the literature.
2.6. Vestibulo-ocular reflex (VOR)
Detailed methods to record VOR responses to horizontal rotation and translation using an eye tracker are described in a previous study (Stewart et al., 2016). Briefly, a head holder was implanted on the skull of a LE rat. After recovery of 7 days, the rat was secured to the vestibular stimulator via the head holder after being lightly sedated with isoflurane. An eye tracker was attached to the vestibular stimulator and was focused on the left eye to record its positions at 240 frames/sec. Horizontal head rotations were delivered at 0.25, 0.5 and 1Hz (60°/s peak velocity) and horizontal translations were delivered at 0.2 Hz (0.1g peak acceleration) along the direction that was perpendicular to the visual axis of the left eye (i.e., right 45° from the nasal-occipital direction) (Paulin and Hoffman, 2019). VOR responses were measured every day for three days before blast exposure and at five intervals after blast exposure (2 h, 1day, 2 days, 3 days and 4 days). A CED Power 1401 system was used to sample eye position signals and head position signals at 1 kHz. VOR gains and phases were measured by fitting eye movement responses (velocity for rotation VOR and position for translational VOR) with a sine function (Stewart et al., 2016).
2.7. Inner ear morphology
While the focus of the study was on effects of blast exposure on vestibular afferent function and the VORs, we performed morphological analyses of the inner ears in three SD rats one week after blast exposure to determine the blast-induced inner ear structural damage and allow comparison to that reported in the literature (Lien and Dickman, 2018). Under deep pentobarbital anesthesia, rats were perfused transcardially first with phosphate buffered saline with pH of 7.4, then with 10% phosphate buffered 4% paraformaldehyde. The integrity of the tympanic membrane, the ossicles, the oval window and the round window were examined under a dissecting microscope. Morphological changes of the vestibular hair cell stereocilia bundles and cuticular plate were examined by phalloidin staining (Ding et al., 2001). First, the sensory epithelium from the saccule, utricle and semicircular canals were carefully harvested after removing the surrounding temporal bone. Second, the whole mount of the cristae and maculae were immersed in Alexa Fluor® 488 Phalloidin (Life Technologies, USA) solution and then counter-stained with TOPRO-3 (Invitrogen, T3605). Finally, the tissues were mounted on glass slides and observed with a fluorescent microscope or a multiphoton laser scanning microscope.
2.8. Statistical analyses
Using software package SigmaPlot, t-test or one-way ANOVA was used to test differences between or among the groups, respectively. Multiple-comparison corrections (e.g. Tukey HSD test) were performed wherever appropriate. Statistical significance was determined based on P values of less than 0.05. Error bars are standard errors of the mean (SEM).
3. Results
3.1. Effects of blast exposure on vestibular afferents spontaneous firing rate and response to head rotation
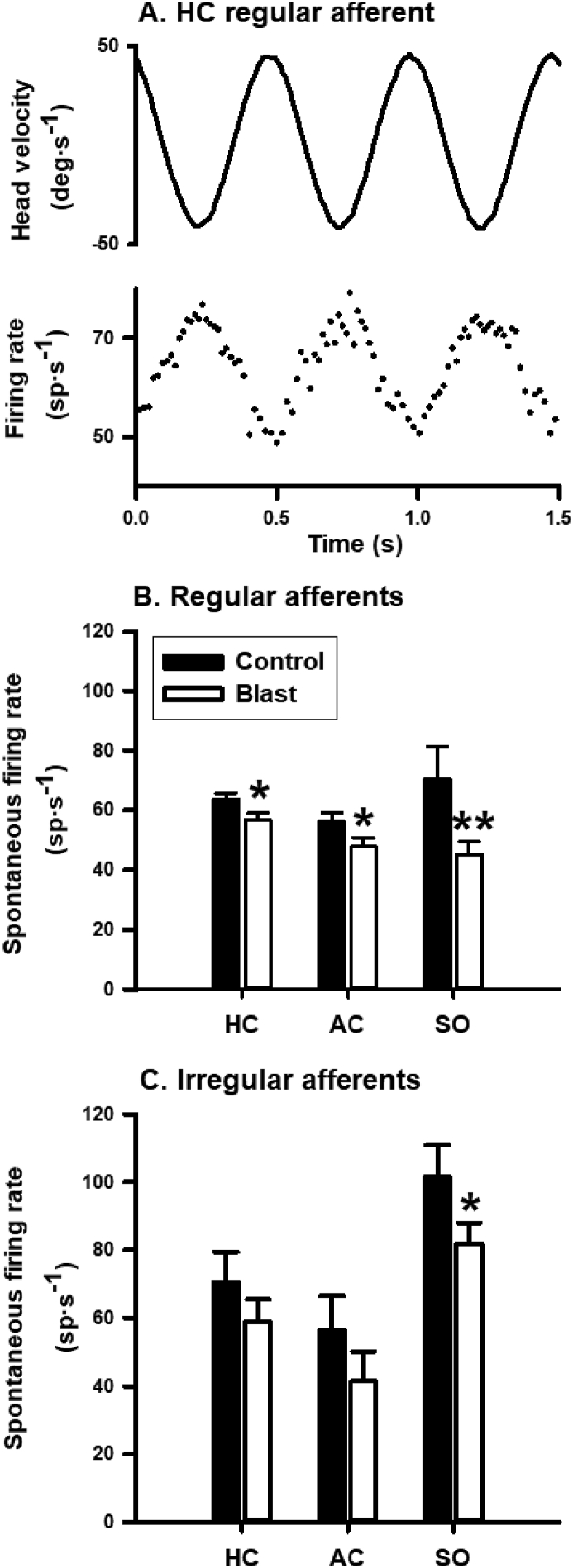
We recorded from 442 vestibular afferents from seven control rats (248) and seven blast-treated rats (194). In the afferents recorded from the control rats, there were 109 HC afferents, 49 AC afferents and 90 SO afferents. In the afferents recorded from the blast-treated rats, there were 83 HC afferents, 51 AC afferents and 60 SO afferents. Fig. 2A shows representative responses of a regular horizontal canal afferent to sinusoidal head rotation at 2Hz. The afferent had a spontaneous discharge rate of 63.4 spike/s and increased its discharge rate to leftward head rotation with a gain of 0.24 spike/s/deg/s and a phase lag of 7.2° with respect to head velocity.
Fig. 2.
Effects of blast exposure on vestibular afferent spontaneous firing rate. A. Responses of a representative horizontal canal afferent to 2 Hz sinusoidal horizontal head rotation. The upper trace is head velocity with positive values for rightward head movement. The lower trace is firing rate. B and C. Effects of blast exposure on spontaneous firing rates of regular and irregular vestibular afferents innervating the horizontal canal (HC), the anterior canal (AC) and the otoliths in the superior vestibular nerve (SO). ∗, P < 0.05; ∗∗, P < 0.01.
Blast exposure did not change the proportions of regular and irregular afferents in the blast-treated rats (Chi-square test, P = 0.307). Blast exposure resulted in lower spontaneous firing rates in the regular and irregular HC/AC/SO afferents (Fig. 2B and 2C). The reductions reached statistical significance in regular HC afferents (63.6 ± 2.1 vs 56.7 ± 2.4 spike/s, P = 0.019, t = 2.11, df = 85), regular AC afferents (56.2 ± 2.9 vs 47.9 ± 2.9 spike/s, P = 0.025, t = 2.0, df = 60), regular SO afferents (70.5 ± 10.8 vs 45.2 ± 4.4 spike/s, P = 0.0097, t = 2.52, df = 22), and irregular SO afferents (101.5 ± 9.4 vs 81.7 ± 6.2 spike/s, P = 0.04, t = 1.82, df = 117).
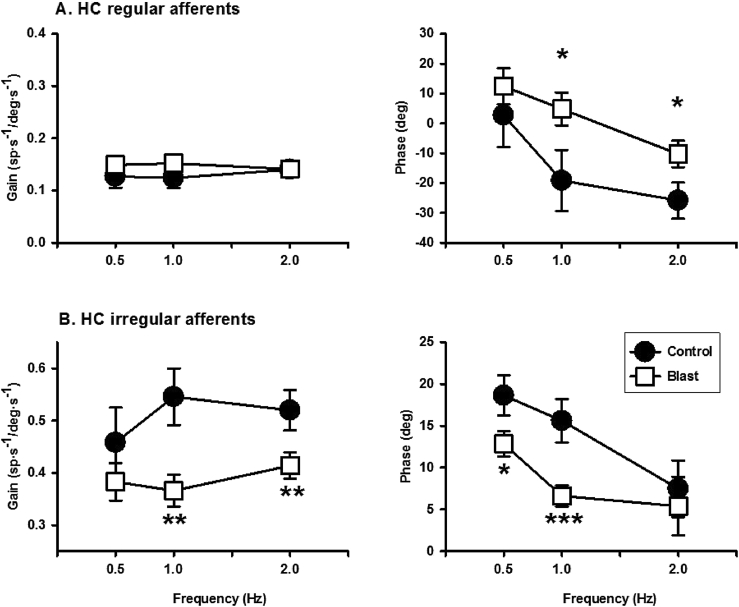
Blast exposure resulted in changes in gain and phase of HC and AC afferents (Fig. 3, Fig. 4). For regular HC afferents, blast exposure did not affect their gains, but reduced their phase lags at 1 Hz (Control:−19.16 ± 10.25 vs. Blast: 4.80 ± 5.49 deg, P = 0.017, t = 2.18, df = 50) and at 2 Hz (Control:−25.85 ± 6.04 vs. Blast: -10.28 ± 4.47 deg, P = 0.019, t = 2.11, df = 85). For irregular HC afferents, blast exposure significantly reduced their gains at 1 Hz (Control: 0.546 ± 0.05 vs Blast: 0.366 ± 0.03 spike/s/deg/s, p = 0.0015, t = 2.072, df = 92) and 2 Hz (Control: 0.52 ± 0.04 vs. Blast: 0.41 ± 0.03 spike/s/deg/s, t = 2.398, df = 89, P = 0.009), and reduced their phase leads at 0.5 Hz (Control: 18.64 ± 2.4 vs. Blast: 12.85 ± 1.51 deg, t = 2.10, df = 27, P = 0.023) and 1 Hz (Control: 15.62 ± 2.62 vs. Blast: 6.62 ± 1.31 deg, t = 2.18, df = 50, P = 0.0006). For regular AC afferents, blast exposure significantly reduced their gains at 2 Hz (Control: 0.19 ± 0.02 vs Blast: 0.12 ± 0.01 spike/s/deg/s, p = 0.002, t = 2.98, df = 59) and increased phase lags at 2 Hz (Control: −6.41 ± 6.53 vs. Blast: −31.88 ± 9.54 deg; t = 2.24, df = 59, P = 0.014). Compared to the control condition, the irregular AC afferents exhibited a decrease in phase lead at 2 Hz following blast exposure (Control: 16.65 ± 7.89 vs. Blast: −21.75 ±11.88 deg, t = 2.61, df = 26, P = 0.0075).
Fig. 3.
Effects of blast exposure on gains and phases of HC vestibular afferents. A. Regular HC afferents. B. Irregular HC afferents. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. Some error bars are within the symbols.
Fig. 4.
Effects of blast exposure on gains and phases of AC vestibular afferents. A. Regular AC afferents. B. Irregular AC afferents. ∗, P < 0.05; ∗∗, P < 0.01. Some error bars are within the symbols.
3.2. Effects of blast overpressure waves on the middle and inner ear structures
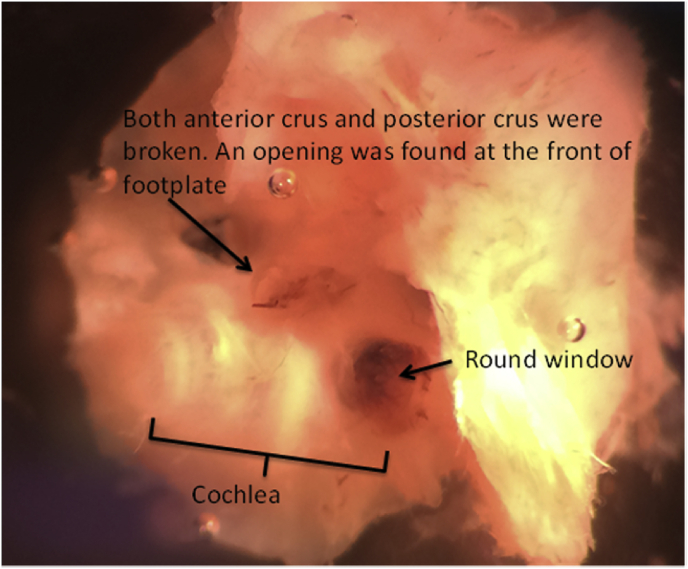
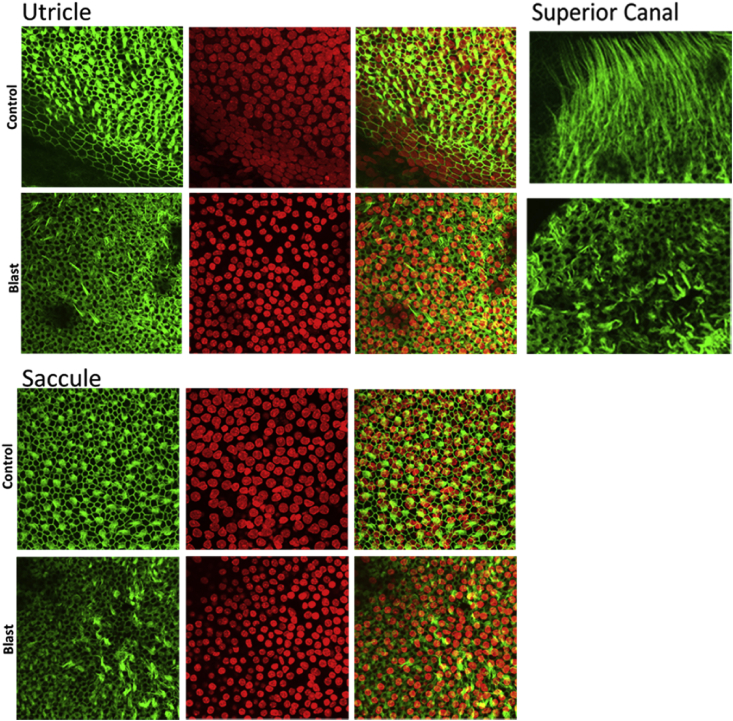
Effects of blast shock waves on the middle and inner ear structures were examined. First, consistent with earlier reports (Lien and Dickman, 2018 in mice; Hickman et al., 2018 in chinchillas), blast exposure ruptured the tympanic membranes of all the 14 blast treated ears, but not the untreated ears. Blast shocks also disrupted the ossicular chain and damaged both the anterior and posterior crus and the front of the footplate of the stapes (Fig. 5). Blood clots were seen on the surfaces of both the oval and round windows, indicating rupture of both of the labyrinth openings (not shown). These observations suggest that blast shock waves impact the vestibular end organs via the middle ear sound transduction pathway. Although the blast shock waves were delivered primarily into the ear canal and few damage was observed in the untreated ear, a role of bone transduction cannot be ruled out (Greene et al., 2018). In addition to blast-induced structural damage to the middle ear, we also confirmed stereocilia bundle damage to the vestibular hair cells. A representative phalloidin staining of vestibular hair cell stereocilia bundles shown in Fig. 6 exhibits substantial disruptions of stereocilia bundles in central regions of a macula (utricle) and a crista (AC) 7 days post blast exposure. While preliminary, these results verified that the ear-only blast approach produced middle ear and inner ear structural damage similar to that produced by the whole body blast approach (Lien and Dickman, 2018; Hickman et al., 2018).
Fig. 5.
Blast exposure ruptured both the oval window and the round window.
Fig. 6.
Blast-induced losses and disruptions of hair cell stereocilia bundles in the otolith and semicircular canal end organs.
3.3. Effects of blast exposure on the VOR
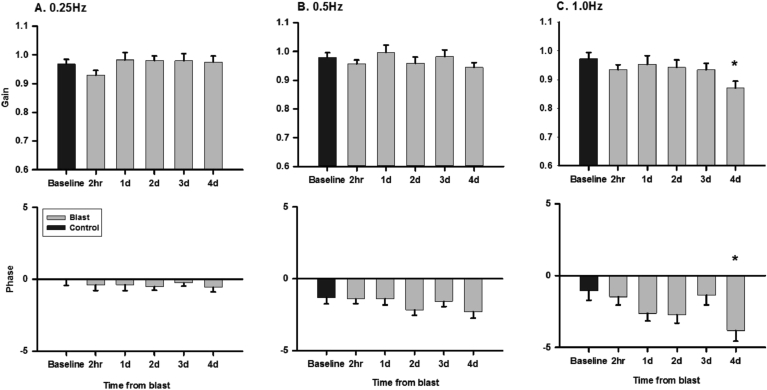
After blast exposure, rats did not exhibit spinning or head tilts. We measured gain and phase of the VOR to head rotation and translation in seven LE rats for four days after blast exposure. While the rotational VOR in control rats is expected to have a gain of ∼1 and a phase of 0° with respect to the reversed head velocity, the translational VOR in rats has been shown to be less robust (Stewart et al., 2016). On day 4 post blast exposure, the rotational VOR at 1 Hz exhibited a small decrease in gain (0.97 ± 0.02 vs. 0.87 ± 0.02, t = 3.582, P = 0.006) and phase lag (−1.5 ± 0.6 deg vs −3.8 ± 0.8 deg, t = 3.773, P = 0.003) (Fig. 7); and the translational VOR at 0.2 Hz exhibited a small decrease in gain (0.087 ± 0.006deg/cm vs 0.076 ± 0.006 deg/cm, t = 3.231, P = 0.014) (Fig. 8).
Fig. 7.
Effects of blast exposure on gains and phases of the rotational VORs at 0.25 Hz (A), 0.5 Hz (B) and 1 Hz (C). ∗, P < 0.05.
Fig. 8.
Effects of blast exposure on gain (A) and phase (B) of the translational VOR of the left eye at 0.2 Hz in the direction of 45° right of the nasal occipital axis. ∗, P < 0.05.
4. Discussion
4.1. Exposure to blast shock waves via the ear canal produced damage to vestibular afferent function
The present study examined the effects of blast exposure on vestibular afferent function and on the VOR. The main finding was that one day after blast exposure, vestibular afferents exhibited a reduction in spontaneous firing and in sensitivity to head rotation (Fig. 2, Fig. 3, Fig. 4). For the canal afferents, blast exposure reduced spontaneous firing rate by 12.8% and 21.5% in regular and irregular afferents, respectively. For the otolith afferents, blast exposure reduced spontaneous firing rate by 35.9% and 19.5% in regular and irregular afferents, respectively. Reduced spontaneous firing rates in vestibular afferents would lead to decreased tonic levels of the secondary neurons in the vestibular nuclei and in the neurons that they project to, such as motoneurons of the extraocular muscles. Since the tonic level of VOR interneurons and motoneurons affects their sensitivity to inputs (Zhou et al., 2007), a reduction in vestibular afferent spontaneous firing rate would result in a lower VOR gain. A blast-induced reduction in vestibular afferent sensitivity to head rotation would decrease dynamic input to the VOR interneurons and motoneurons, also resulting in a reduced VOR gain. Changes found in vestibular afferent phase response to head rotation were opposite in the regular and irregular afferent populations, i.e., blast exposure increased phase lead in the regular afferents, but decreased phase lead in the irregular afferents. Thus, it is possible that the effect of blast exposure on VOR phase is dependent on the summed phase changes in the two afferent populations.
The vestibular sensory epithelium is located on the maculae of the saccule/utricle and the cristae of the semicircular canals and has two types of hair cells (Baird et al., 1988). Whereas Type I hair cells are in the central regions and are innervated by irregular afferents with calyx terminals, Type II hair cells are in the peripheral regions and are innervated by regular afferents with bouton terminals (Baird et al., 1988). Since blast exposure reduced the spontaneous firing rate and the sensitivity of both regular and irregular vestibular afferent, damage to both Type I and Type II hair cells is expected.
Although blast exposure reduced vestibular afferent spontaneous discharge activity (static) and responses to head rotation (dynamic), we found few deficits in the steady state VOR. One potential mechanism to explain this is central compensation (For a review, Ris et al., 1995; Lacour et al., 2016). Extensive studies have shown that multiple molecular, cellular and circuit-level processes are involved. These events rebalance the neuronal activity of the vestibular nuclei via neural protection and structural reorganization mechanisms and help restore static and dynamic responses. Thus, within a few days following blast exposure, the resulting peripheral vestibular damage may be masked by these robust compensatory mechanisms. The masking of peripheral damage by central compensation may have serious consequences for blast victims. Because they may not show deficits in the steady state VOR tests commonly used as a part of the medical evaluation following blast exposure, their blast-induced vestibular peripheral deficits may be overlooked and they may not receive needed medical care. The effects of peripheral damage to the vestibular hair cells and the vestibular afferents may progress over time as central compensation processes become less effective due to aging. Unfortunately, at this stage, the best time for treatment has passed. This highlights the need to develop more sensitive clinical vestibular tests for early diagnosis of blast-induced vestibular deficits so that victims can get appropriate management and avoid long-term complications. Because central compensation is incomplete for dynamic vestibular responses (For a review, Lacour et al., 2016; Ris et al., 1995), one potential solution is to use diagnostic vestibular tests that demand high frequency dynamic responses, such as the head impulse thrust tests (HIT) and the vestibular-evoked myogenic potentials (VEMPs). It is also important to check vestibular function as soon as possible following the blast exposure.
4.2. Future directions
While this study provides the first evidence of blast-induced deficits in the function of vestibular afferents, there are limitations that need to be addressed by future studies. First, the present study only recorded vestibular afferent activity 1 day post blast exposure and VOR response 4 days post blast exposure. Future studies need to examine vestibular afferents and the VOR at additional time points post blast exposure so that effects of direct mechanical assault and lesion progression can be investigated. Secondly, the VOR was only measured in response to sinusoidal head movements. The steady state VOR is mediated by both direct and indirect pathways and the blast-induced deficits in the vestibular end organs may be masked by central compensation via the indirect pathways. Thus, to reveal blast-induced peripheral vestibular deficits, future studies need to employ high acceleration high frequency head rotations to test angular VOR (Minor and Lasker, 2009) as well as VEMPs to test the otolithic function (Sheykholeslami et al., 2009). Finally, the preliminary morphological analysis used in this study needs to be expanded to include assessment of hair cell morphology and synaptic ribbons at multiple time points post blast exposure. Despite the limitations, this study established a novel animal model of blast-induced vestibular deficits by delivering blast shock waves primarily into the ear canal. This new model lays the foundation for future studies to thoroughly investigate the neural mechanisms of blast exposure-induced vestibular disorders, and to develop effective prevention, diagnosis and treatment programs for military and civilian populations who are at risk of exposure to blast shock waves.
Declaration of competing interest
None of the authors has any conflicts of interests.
Acknowledgement
This work was supported by NIH R01DC012060 and R21DC017293.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Wu Zhou, Email: wzhou@umc.edu.
Hong Zhu, Email: hozhu@umc.edu.
References
- Akin F.W., Murnane O.D. Head injury and blast exposure: vestibular consequences. Otolaryngol. Clin. 2011;44:323–334. doi: 10.1016/j.otc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Baird R.A., Desmadryl G.L., Femandez C., Goldberg J.M. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J. Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Blanks R.H., Torigoe Y. Orientation of the semicircular canals in rat. Brain Res. 1989;487(2):278–287. doi: 10.1016/0006-8993(89)90832-9. [DOI] [PubMed] [Google Scholar]
- Daunicht W.J., Pellionisz A.J. Spatial arrangement of the vestibular and the oculomotor system in the rat. Brain Res. 1987;435:48–56. doi: 10.1016/0006-8993(87)91585-X. [DOI] [PubMed] [Google Scholar]
- DePalma R.G., Burris D.G., Champion H.R., Hodgson M.J. Blast injuries. N. Engl. J. Med. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- Ding D., McFadden S.L., Salvi R.J. James F. Willott. Handbook of Mouse Auditory Research. CRS Press; Florida: 2001. Cochlear hair cell densities and inner-ear staining techniques; pp. 189–204. [DOI] [Google Scholar]
- Dougherty A.L., MacGregor A.J., Han P.P., Viirre E., Heltemes K.J., Galarneau M.R. Blast-related ear injuries among U.S. military personnel. J. Rehabil. Res. Dev. 2013;50(6):893–904. doi: 10.1682/JRRD.2012.02.0024. [DOI] [PubMed] [Google Scholar]
- Ewert D.L., Lu J., Li W., Du X., Floyd R., Kopke R. Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hear. Res. 2012;285(1–2):29–39. doi: 10.1016/j.heares.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Fausti S.A., Wilmington D.J., Gallun F.J., Myers P.J., Henry J.A. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J. Rehabil. Res. Dev. 2009;46(6):797–810. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Franke L.M., Walker W.C., Cifu D.X., Ochs A.L., Lew H.L. Sensorintegrative dysfunction underlying vestibular disorders after traumatic brain injury: a review. J. Rehabil. Res. Dev. 2012;49(7):985–994. doi: 10.1682/JRRD.2011.12.0250. [DOI] [PubMed] [Google Scholar]
- Greene N.T., Alhussaini M.A., Easter J.R., Argo T.F., Walilko T., Tollin D.J. Intracochlear pressure measurements during acoustic shock wave exposure. Hear. Res. 2018;365:149–164. doi: 10.1016/j.heares.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J.M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. 3. Variations among units in their discharge properties. J. Neurophysiol. 1971;34(4):676–684. doi: 10.1152/jn.1971.34.4.676. [DOI] [PubMed] [Google Scholar]
- Goldberg J.M., Smith C.E., Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J. Neurophysiol. 1984;51(6):1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Hickman T.T., Smalt C., Bobrow J., M.C. Liberman Blast-induced cochlear synaptopathy in chinchillas. Sci. Rep. 2018;8:10740. doi: 10.1038/s41598-018-28924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar T.E., Minor L.B. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibuloocular reflexes. J. Neurophysiol. 1999;82:2000–2005. doi: 10.1152/jn.1999.82.4.2000. [DOI] [PubMed] [Google Scholar]
- Kronenberg J., Ben-Shoshan J., Wolf M. Perforated tympanic membrane after blast injury. Am. J. Otol. 1993;14(1):92–94. PMID: 8424486. [PubMed] [Google Scholar]
- Lacour M., Helmchen C., Vidal P.P. Vestibular compensation: the neuro-otologist’s best friend. J. Neurol. 2016;263(Suppl. 1):S54–S64. doi: 10.1007/s00415-015-7903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker D.M., Han G.C., Park H.J., Minor L.B. Rotational responses of vestibular-nerve afferents innervating the semicircular canals in the C57BL/6 mouse. J Assoc Res Otolaryngol. 2008;9(3):334–348. doi: 10.1007/s10162-008-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien S., Dickman J.D. Vestibular injury after low-intensity blast Exposure. Front. Neurol. 2018;9:297. doi: 10.3389/fneur.2018.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell A.K., Banakis Hartl R.M., Greene N.T., Benichoux V., Mattingly J.K., Cass S.P., Tollin D.J. Semicircular canal pressure changes during high-intensity acoustic stimulation. Otol. Neurotol. 2017;38(7):1043–1051. doi: 10.1097/MAO.0000000000001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor L.B., Lasker D.M. Tonic and phasic contributions to the pathways mediating compensation and adaptation of the vestibulo-ocular reflex. J. Vestib. Res. 2009;19(5–6):159–170. doi: 10.3233/VES-2009-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin M.G., Hoffman L.F. Models of vestibular semicircular canal afferent neuron firing activity. J. Neurophysiol. 2019;122:2548–2567. doi: 10.1152/jn.00087.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenschneider A.K., Lookabaugh S., Aliphas A., Brodsky J.R., Devaiah A.K., Dagher W., Grundfast K.M., Heman-Ackah S.E., Rubin S., Sillman J., Tsai A.C., Vecchiotti M., Kujawa S.G., Lee D.J., Quesnel A.M. Otologic outcomes after blast injury: the Boston Marathon experience. Otol. Neurotol. 2014;35(10):1825–1834. doi: 10.1097/MAO.0000000000000616. [DOI] [PubMed] [Google Scholar]
- Ris L., de Waele C., Serafin M., Vidal P.P., Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert Guinea pig. J. Neurophysiol. 1995;74(5):2087–2099. doi: 10.1152/jn.1995.74.5.2087. [DOI] [PubMed] [Google Scholar]
- Saljo A., Arrhén F., Bolouri H., Mayorga M., Hamberger A. Neuropathology and pressure in the pig brain resulting from low-Impulse noise exposure. J. Neurotrauma. 2008;25:1397–1406. doi: 10.1089/neu.2008.0602. [DOI] [PubMed] [Google Scholar]
- Sheykholeslami K., Megerian C.A., Zheng Q.Y. Vestibular evoked myogenic potentials in normal mice and Phex mice with spontaneous endolymphatic hydrops. Otol. Neurotol. 2009 doi: 10.1097/MAO.0b013e31819bda13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C., Yu Y., Huang J., Maklad A., Tang X., Allison J., Mustain M., Zhou W., Zhu H. Effects of high intensity noise on the vestibular system in rats. Hear. Res. 2016;335:118–127. doi: 10.1016/j.heares.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E.D., Fernandez C., Goldberg J.M. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol. 1977;84:352–360. doi: 10.3109/00016487709123977. [DOI] [PubMed] [Google Scholar]
- Zhou W., Xu Y., Simpson I., Cai Y. Multiplicative computation in the vestibulo-ocular reflex (VOR) J. Neurophysiol. 2007;97:2780–2789. doi: 10.1152/jn.00812.2006. [DOI] [PubMed] [Google Scholar]
- Zhu H., Tang X., Wei W., Maklad A., Mustain M., Rabbitt R., Highstein S., Zhou W. Input-output functions of vestibular afferent responses to air-conducted clicks in rats. J Assoc Res Otolaryngol. 2014;15(1):73–86. doi: 10.1007/s10162-013-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Tang X., Wei W., Xu Y., Mustain W., Zhou W. Click-evoked responses in vestibular afferents in rats. J. Neurophysiol. 2011;106(2):754–763. doi: 10.1152/jn.00003.2011. [DOI] [PubMed] [Google Scholar]