Abstract
People use medicinal plants as diet, and for treatment of infectious and noninfectious diseases and they use brief procedures like frying and cooking to do so. Medicinal plants; Moringa oleifera, Azadirachta indica, and Lepidium sativum which is believed to have active components that help to treat and manage various diseases were investigated for their antibacterial activities against Staphylococcus aureus, Salmonella Typhi, Streptococcus agalactiae and Shigella boydii. Solvent methanol and aqueous were used for extraction of crudes by means of maceration. Susceptibility testing was determined by using disc diffusion method and Minimum inhibitory concentration was determined by broth dilution method. Heat treated plant material activity against test pathogen was aimed to identify resistance capacity of plant material at different interval of time and temperature. All plant extracts under study was active against all tested pathogen after exposure to 45 °C for 30 min. The antibacterial activities of the non-heat-treated extracts of Azadirachta indica were relatively low; the results of this study show that extracts of Azadirachta indica have better residual antibacterial activities. Methanol extracts of all plant leaves showed highest activity as compared to the aqueous extracts. This is probably assigning the choice of extraction solvent for extracting desired active phytochemical from plants. Many of the people in the study area were illiterate and they did not have awareness about the ways use of medicinal plants. They use the medicinal plants by cooking and frying for different purposes. In the main, plant material can be affected as the temperature of treatment is increases with respect to various times of exposures.
Keywords: Medicinal plants, Antibacterial activity, Disc diffusion, Heat treatment, Salmonella Typhi
1. Introduction
Medicinal plants play a key role in the development and advancement of modern studies by serving as a starting point for the development of novelties in drug (Wright, 2005) and various modern drugs were extracted from medicinal plants through the use of plant material as indigenous cure in folklore or traditional system of medicine (Verma and Singh, 2008). Plant materials will continue to play a major role in the primary health care as therapeutic remedies in many developing countries. Herbal remedies were known to treat many infectious diseases throughout the history of humankind. Thus, the discovery of medicinal plants as source of antimicrobial agents is useful in expanding the wide variety of antibiotics available (Zaidan et al., 2005). The majority of these herbal plants contain substances, which are precursors for the synthesis of conventional drugs, or substances that can be used for therapeutic purposes. Extracts which proved to be potentially effective can be used as natural alternative preventives to control food poisoning diseases and preserve food stuff avoiding health hazards of chemically antimicrobial agent applications (Mostafa et al., 2018). Plants are used medicinally in different countries and are a source of many potent and powerful drugs. A number of workers have approved that medicinal plants represent a rich source of antimicrobial agents. A wide range of medicinal plant parts are being used for extract as raw drug and they possess varied medicinal properties (Singh et al., 2012). These medicinal plants are used against different pathogens in raw form and as extracts. It is well known that even the most synthetic drugs have their origin from plant products (Malarkodi and Manoharan, 2013). Antibiotic resistance is a problem that continues to challenge the healthcare sector in a large part of the world in both developing and developed countries. The spread of multidrug resistant (MDR) bacteria in hospital and community settings remains a widely unresolved problem and a heavy burden to health services (Eliopoulos et al., 2003). A great efforts are being made to reverse this trend, and one of them is the wide spread screening of medicinal plants from the traditional system of medicine hoping to get some newer, safer, and more effective agents that can be used to fight infectious diseases (Natarajan et al., 2003).
1.1. Azadirachta indica
Azadirachta indica is used in folklore medicine for the treatment of diabetes, shows the potential role of antidiabetic activity (Dholi et al., 2011). The Neem plant is reported to have antimicrobial action, and is not well studied on leaves (Shravan et al., 2011). Neem leaves have antibacterial properties in controlling airborne bacterial contamination in the residential premise (Khan et al., 2008). There was great potential Neem aqueous extract as powerful chemotherapeutic and viral agent according to Amer et al. (2010). Moreover, the methanol extract of Neem was reported to have in vitro antimicrobial activities against S. aureus, E. coli, Ps. aeruginosa and C. albicans. Azadirachta indica leaves extract exhibit power of antibacterial activity. It has shown to possess significant effects on both gram-positive and gram-negative organisms and other bacteria that cause a wide array of human and animal diseases including E. coli, Streptococcus and Salmonella (Maragathavalli et al., 2012).
1.2. Lepidium sativum
Lepidium sativum are used as an aperient and in the treatment of bacterial and fungal infections (Mukhopadhyay et al., 2010). It also shows antimicrobial activity against food borne bacteria as stated by Gupta et al. (2010). Previous studies have demonstrated the protective action of Lepidium sativum against carcinogenic compounds and growth inhibition of Pseudomonas aeruginosa, a bacteria strain with a potent antibiotic resistance (Kassie et al., 2003).
1.3. Moringa oleifera
Moringa oleifera has been used extensively in traditional medicine for the treatment of several ailments, promotes digestion, skin diseases, diarrhea, as stimulant in paralytic afflictions, epilepsy and hysteria (Farooq et al., 2012). It was shown to possess antimicrobial activity against wide array of pathogens (Thilza et al., 2010). Seeds and leave extracts show activity against different bactericidal and/or bacteriostatic action against Staphylococcus aureus, Vibrio cholerae, V. parahaemolyticus, Enterococcus faecalis, Salmonella enteritidis, Aeromonas caviae, Pasteurellamultocida, Bacillus subtilis, E. coli, Pseudomonas aeruginosa, Enterobacter cloacae, Proteus vulgaris and Micrococcus kristinae (Jabeen et al., 2008). Community in the study area use medicinal plants as sole for treatment of any type of ailments from either human or animal. Therefore, the aim of this study was to assess susceptibility test of selected medicinal plant species based on their traditional medicinal uses for infectious diseases treatment in local community of Oromia, Eastern Ethiopia due to the commonness of selected strain in the area. There is a strong positive motivation for further research into the antibacterial components of Azadirachta indica, Lepidium sativum and Moringa oleifera considering the fact that plants are naturally endowed with a variety of bioactive compounds. Although some of these plants are cultivated as food and for other purposes in different parts of Ethiopia, there has been little study conducted in Ethiopia with respect to their antibacterial activities against Staphylococcus aureus, Streptococcus agalactiae, Salmonella Typhi and Shigella boydii. The objective of the study was therefore determe the antibacterial activities of non- heat and heated treated methanol and aqueous leaf extracts of Lepidium sativum, Azadirachta indica and Moringa oleifera against selected food born bacterial pathogens
2. Materials and methods
2.1. Preparation of extracts
The plants were identified and authenticated properly at the Herbarium of Haramaya University. The plant leaves were separated, washed and cleaned thoroughly with tap water and then with distilled water and air-dried in shade at botanical laboratory for several weeks. Plant extracts were prepared in accordance with the methods described in (Basri and Fan, 2005) with minor modifications. About 40 g of the powder was separately soaked in 200 mL of methanol (70%) and aqueous, in a 400 mL stoppered reagent bottle and the mixture were shaken for 72 hrs using an electrical shaker. The resulting mixture was first filtered with cheesecloth, then with Whatman No1 filter paper. The filtrates were then separately concentrated in vacuum using Rotary Evaporator at 30-40 °C. The methanol extracts were transferred carefully to labeled vials and allowed to permit evaporation of residual solvents at room temperature for 3–4 days while the aqueous extracts were dried using Lyophilizer alpha 1–2 LD plus-Martin Christ to prepare crude powder. Then the dried extracts were stored in sterile bottles and kept in refrigerator until further use. Heat treatment of plant crude extracts was done using the method described by (El-Mahmood et al., 2008, Simlai and Roy, 2012) with some modifications. Dried extracts of plant materials were kept under 45, 50, and 55 °C for both 30 and 60 min in oven dry. Then the samples were cooled at room temperature and stored in a refrigerator at 4 °C until further use. The disc diffusion method (Andrews, 2001) was used to evaluate the antibacterial activity.
2.2. Preparation of micro-organism culture
The test bacterial pathogens, i.e. Staphylococcus aureus ATCC25923, Streptococcus agalactiae ATCC 12338, Salmonella Typhi ATCC13311 and Shigella boydii ATCC9202, were obtained from Ethiopian Public Health Institution (EPHI), Addis Ababa, Ethiopia. All the bacterial strains were grown and maintained on nutrient agar slants (Basri and Fan, 2005). Fresh bacterial cultures were prepared by sub-culturing stock bacterial cultures into freshly prepared nutrient agar and incubating at 37 °C for 24 h. The colonies formed were picked up with a sterile inoculating loop and transferred into sterile saline solution and the turbidity was adjusted to the 0.5 McFarland’s standard solution (Andrews and BSAC Working Party on Susceptibility Testing, 2001, Esimone et al., 2012)
2.3. Preparation of stock solution and serial dilution
Solutions of 200 mg/mL were prepared by reconstituting 1 g of each of the dried crude powder in 5 mL of aqueous solution. From this stock solution, 150 mg/mL, 125 mg/mL and 100 mg/mL working solutions were prepared and used for the susceptibility testing. Another stock solution of crude extract 20 mg/mL was prepared by reconstituting 0.1 g of each of the dried crude powder in 5 mL of aqueous solution. Five sterile test tubes were arranged on a test tube rack and 1 mL of sterile solvent solution was dispensed into them. From the stock solution, 1 mL of extract was transferred into the first test tube and then successive two-fold serial dilutions of the extracts were carried out. The resultant concentrations in the test tubes were 10, 5, 2.5, 1.25, 0.625 mg/mL (Esimone et al., 2012). They were used, along with the stock, for determination of minimum inhibitory concentration.
2.4. Determination of the antimicrobial activities of the crude extracts
Mueller Hinton agar was prepared in the plates as the media for the test microorganisms. Sterile filter paper discs (Whatman No1, 6 mm) (Andrews, 2001) and the discs were sterilized. Each sterile disc was impregnated individually with 3 mg of 150 mg/ml, 125 mg/ml and 100 mg/ml concentration using a micropipette. Then discs impregnated with concentrations of non-heat treated and heat-treated plant extracts were placed on the spread plated Muller Hinton agar surface using sterile forceps. Disc impregnated with Amoxicillin (5 μg/mL) was used as positive control while a disc soaked with pure solvent of aqueous solution was used as a negative control. The Petri dishes were then incubated at 37 °C for 24 h. After incubation, the diameters of the zone of inhibitions were measured by using transparent ruler and the mean values of three readings were recorded.
2.5. Determination of MIC
Minimum inhibitory concentration was evaluated for extracts of non-heat-treated plant material. The MIC of each extract was determined by using concentrations (10, 5, 2.5, 1.25 and 0.625 mg/mL) of the plant extracts by using the broth dilution method (Cheruiyot et al., 2009). 4 mL of the NB was pipetted into each of 6 test tubes. 0.1 mL of the prepared successive two-fold serial dilutions of the crude extract concentrations were mixed with the nutrient broth. Thereafter, 0.1 mL of the standardized inoculum of test pathogens was dispensed into each of the test tubes containing the suspension of nutrient broth and the crude extract. Then, all test tubes were properly corked and incubated at 37 °C for 24 h. The MICs were read as least concentration that inhibited any visible growth (absence of turbidity) of the test organisms (Dike-Ndudim et al., 2016).
2.6. Percentage residual antibacterial activity
The percentage residual antibacterial activity (%RAA) was calculated using the following formula:
| (1) |
Where, % RAA = Percentage residual antibacterial activity,
ZIAHT = Mean zone of inhibition after heat treatment and,
ZIBHT = Mean Zone of inhibition before heat treatment.
3. Results and discussion
3.1. Antibacterial activity of non-heat treated crude extracts
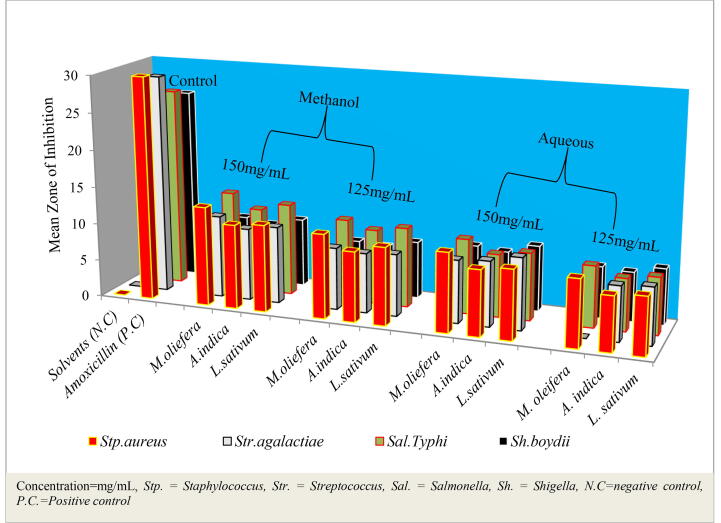
The results reveal that all plant extracts were effective in inhibiting the growth of test pathogens with varying inhibitory effects as depicted in Fig. 1. However, M. oleifera has proficiency to inhibit the growth of both gram positive and gram negative bacteria regardless of the solvent used for extraction. As stated by Dike-Ndudim et al. (2016) inhibition of both Gram-positive and Gram-negative organisms by M. oliefera extract depicts that it can serve as a source of broad spectrum antibiotics, which justified the traditional use of this plant for therapeutic purposes. In contrast methanol extracts of M. oleifera shows high zone of inhibition at 150 mg/mL against Stp. Aureus. The leaves, stems and seeds of M. oleifera could be an alternative for the control of infections caused by Stp. aureus in humans and cows with mastitis (Tirado-Torres et al., 2019).
Fig. 1.
The antibacterial activities of untreated leaf extracts on pathogenic bacteria.
On other way aqueous extracts of M. oleifera had no inhibitory activity against Str.agalactiae at 125 mg/mL concentration. According to (Taylor et al., 2001, Manikkuwadura et al., 2019) presence of inadequate quantities of active constituent or constituents in the extract to exhibit the antimicrobial activity can be the reason for the negative results. Absence of antimicrobial activity does not mean that the bioactive compounds are not present in the plant or the plant has no antimicrobial activity against microorganisms. The present study denotes that antimicrobial activities was dose dependent and influence of solvent on extraction of metabolites required for antibacterial activity, thus the availability of plant crude extracts is depending on type of solvent used for extraction and concentration of the plant material. The aqueous extract had minimum antibacterial activity against the test organisms. This for the reason that, activities of water extracted plant material has reduced due to the fact that time used during rotary evaporator has influence on crude extracts when compared to low boiling point solvents. Hence, the aqueous extracted crude extract has lower zone of inhibition than methanol crude extracts as in the results obtained by (Dewangan et al., 2010).
According to this study, various factors such as rotary evaporator used during extraction of crude also trigger influence on the activities of crudes based on boiling point of solvent used for extraction. For instance, the temperature used for methanol and water is different (i.e. the temperature used during Rotary evaporator is 45 °C for both solvents; because of water require high temperature than methanol, it require long duration during extraction) and this can adversely affect the activity of plant material. El-Mahmood et al. (2010) states that, several factors are known to influence yield and biological activities of plant based products, including the age of the plant, time of harvest, drying and processing of the materials, methods of extraction and the solvents used.
Methanol crude extracts of M. oleifera shown the highest antibacterial activities against Stp.aureus, and followed by methanol crude extracts of M. oleifera and L.sativum against Sal.Typhi at 150 mg/mL concentration as presented by figure. As from the figure, aqueous solution used as negative control was not shown antimicrobial activities while Amoxicillin which used as positive control was shown the highest antibacterial activities against all tested pathogenic bacteria. Methanol crude extracts at high concentration show better antibacterial activities to reliable points when compared with positive control as depicted on the figure.
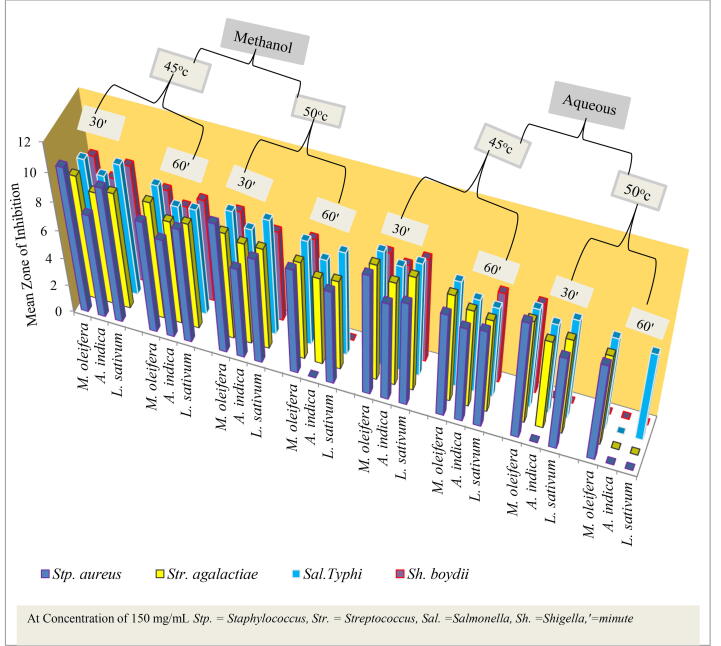
3.2. Antibacterial activities of heat-treated crude extracts
The results showed that antibacterial activities were reduced with heat treatment from temperatures of 45–55 °C for both 30 and 60 min. Relatively higher retention of antibacterial activity was observed for 30 min of heat treatment. Previous researchers had also reported that heat treatment (including pasteurization and sterilization) could lead to decomposition of active phytochemicals (Negi, 2012). The heat-treated antibacterial activities of crude extracts were observed in Fig. 2. The activities of all crude extracts were reduced to zero by heat treatment at a temperature of 55 °C for both 30 and 60 min. Community in the study area used medicinal plants like moringa oliefera, leaf for consumption by cooking; thus using the same procedure for cooking cabbage. In case of Lepidium sativum they use the seeds for treatment of many stomach and intestinal diseases by frying on frying pan and leaves by cooking even if they need to treat any types of diseases either infectious or noninfectious diseases. In case of Azadirachta indica leaf first they ground into small peace and then they boil it very well. They do this for the sake of reducing the bad smell of the leaves because the leaves of Neem have bad smell during grinding. This is due to having no further concern with the nature of plant material and lack of awareness regarding to usage of medicinal plants. It is important to explore the nature of active constituents of plant crude extracts, including their stability to heat treatment. As heat used for treatment exposed to even less time of exposure, the value of inhibition zone was come to be zero, thus at 55 °C there were no antibacterial activities because it depreciate the antibacterial activity of crude extracts of plants. The higher the heating temperature, the weaker was the antibacterial effect (Kim et al., 2002).
Fig. 2.
The antibacterial activities of heat-treated leaf extracts against pathogenic bacteria.
The extracts of all tested plant species (M. oleifera, A. indica and L. sativum) retained their antibacterial activities after 30 min of heat treatment at 45 °C against all test pathogen. The data showed that some of the plant crude extracts were more resistant to heat treatment and shows antibacterial activities regardless of time of exposure than others as from the result. According to (Ginovyan, 2017) some of the plant crude extracts retained their antimicrobial activity even after 121 °C heat exposures. This was indicated by methanol extracts of M. oleifera and L. sativum, persist there antibacterial activities against all tested pathogen after exposure for 60 min at temperature of 50 °C except crude extracts of L. sativum against Sh. Boydii. However, almost all aqueous extracts of plant material lost their antibacterial activities against after just 60 min of exposure to 50 °C except aqueous extracts of M. oleifera which retain its antibacterial activities against Sal. Typhi, Stp.aureus and Str.agalactiae. Similarly, the methanol extract of A. indica and L. sativum lost its antibacterial activity against Sh. boydii after 60 min of exposure to 50 °C, while aqueous extracts A. indica lost its activities against all test pathogens after 60 min of exposure to 50 °C. The result was observed the same result for the control experiments as in non-heat treated plant material in Fig. 1. Aqueous solution used as negative control was not shown antimicrobial activities while Amoxicillin which used as positive control was shown the highest antibacterial activities against all tested pathogenic bacteria.
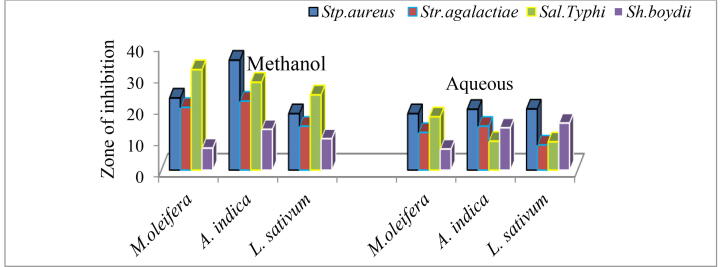
3.3. Percentage residual antibacterial activity
In this study, the percentage residual antibacterial activities were calculated only for result having the highest inhibition zone (i.e. for 150 mg/mL and after heat treatment at 45 °C for 30 min). In this study, the non-heat-treated plant extracts showed a better antibacterial activity than heat-treated ones. This is used to look thoroughly in order to understand which plant is sensitive to heat and which is resistant by comparing the percentage calculated from the result obtained before and after heat treatment. As can be seen from the Fig. 3, the percentage residual antibacterial activities of crude extracts ranged from 6.7 to 35% for both solvents. The lowest values of the percentage indicate that the inhibition zone of the crude extract of heat treated and non-heat-treated plant material was more or less similar and vice versa. Although the antibacterial activities of the non-heat-treated extracts of A. indica were relatively low, the results of this study show that extracts of A. indica have better residual antibacterial activities than the other two plant extracts against all tested pathogens except Sal. Typhi. This implies that this plant has sensitive to heat as compared to the remaining two test plants.
Fig. 3.
Percentages mean residual antibacterial activity.
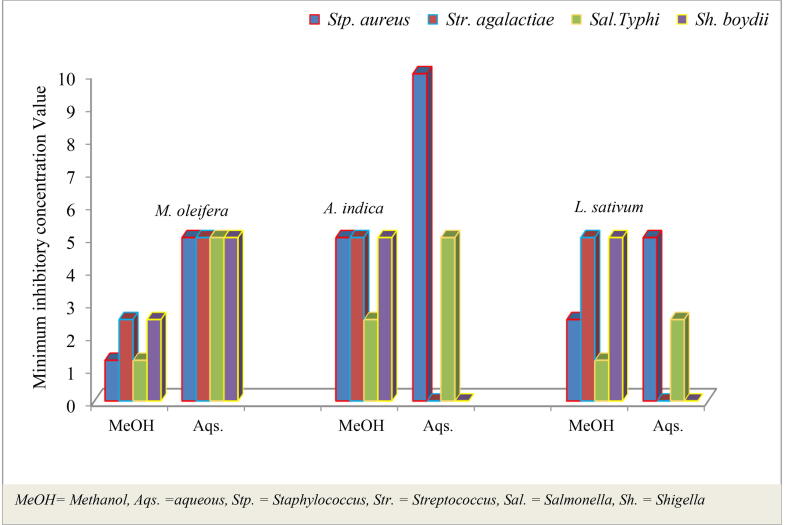
3.4. Determination of (MIC)
In the present study, the MIC of the extracts ranged from 1.25 mg/ml −10 mg/mL. The MIC was determined by selecting the lowest concentration of plant extract that completely inhibited the growth of the organism in the broth medium as detected by the unaided eye. The results of the MIC determination for the different plant extracts against the test pathogens are shown in Fig. 4. The result of this study directs that, methanol crude extracts shows better antibacterial activities. The same result with Kinsalin et al. (2014) states that methanol extract possesses potent antimicrobial activity which in turn may be due to the presence of biologically active ingredients with antimicrobial activity in the medicinal plants. Hence, aqueous crude extracts have lower MIC as compared to methanol extracts because the plant material may contain the non-polar parts of molecules. This is due to the fact that, the polar components frequently do not dissolve non-polar components, therefore aqueous crude extracts lose some of their antibacterial activities. Whereas using water mixable solvents such as methanol were used as optional solvent to enhance the extraction of non-water soluble material, because methanol has potential to dissolve both polarities. The higher MIC indicates that the plant extracts had weaker activities of killing or inhibiting the test pathogens and vice versa. According to (Okeke et al., 1999) the extract was more active against Gram-positive bacteria and had MIC values against different strains of like Stp. Aureus. However, the present study shows that, the strongest activity was exhibited against both gram negative and gram positive bacteria. Specially, the methanol extracts of M. oliefera against Stp. Aureus and Sal. Typhi, and methanol extracts of L. sativum against Sal. Typhi shows higher antibacterial activity as compared to the remaining plant crude extracts.
Fig. 4.
The MIC of crude leaf extracts against four pathogenic bacteria.
4. Conclusion
Crude extracts of M. oleifera, A. indica and L. sativum has satisfactory antibacterial effects against all test pathogen before exposed to heat. Heat treatment of the crude extracts of medicinal plants to various times and temperatures can be gradually reduces the effectiveness of the plant material. All plants lost their antibacterial activities after exposure to 55 °C as observed from the present study. Methanol extracts of all plant leaves showed highest activity as compared to the aqueous extracts. This is probably assigning the choice of extraction solvent for extracting desired active phytochemical from plants. The minimum inhibitory concentration value was ranged from 2.5 to 10 mg/ML and is the lowest concentration of crude extract that completely inhibited the growth of test organism in the broth medium. In contrast to this, the minimum inhibitory concentration was evaluated for heat treated crude extracts and didn’t show any inhibition against pathogenic growth. Generally, biological molecules can be affected as the temperature of treatment is increases with respect to various times of exposures. Accordingly, the community who need to consume medicinal plants either as nutrients or treatment of different ailments should not subject the plant material to temperature, especially above 50 °C even to make the plants texture decent for use.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thankful to School of Biological science and Biotechnology, and Haramaya University for providing financial and Institutional support. Finally, hearty and special thanks are due to the dear family for their constant appreciation and encouragement.
Footnotes
Peer review under responsibility of King Saud University.
Appendix.
See Fig. A1, Fig. A2, Fig. A3.
Fig. A1.
Crude sample preparation.
Fig. A2.
Crude powder preparation by using Lyophilizer.
Fig. A3.
Antibacterial test and recorded results.
References
- Amer H., Helmy W.A., Taie H.A. In vitro antitumor and antiviral activities of seeds and leaves Neem (Azadirachta indica) extracts. Int. J. Acad. Res. 2010;2(2):47–51. [Google Scholar]
- Andrews, J.M., BSAC Working Party on Susceptibility Testing, F.T., 2001. BSAC standardized disc susceptibility testing method. J. Antimicrobial Chemotherapy 48(suppl_1), 43-57. [DOI] [PubMed]
- Basri D.F., Fan S.H. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J. Pharmacol. 2005;37(1):26. [Google Scholar]
- Cheruiyot K.R., Olila D., Kateregga J. In-vitro antibacterial activity of selected medicinal plants from Longisa region of Bomet district, Kenya. African Health Sci. 2009;9(2) [PMC free article] [PubMed] [Google Scholar]
- Dewangan G., Koley K.M., Vadlamudi V.P., Mishra A., Poddar A., Hirpurkar S.D. Antibacterial activity of Moringa oleifera (drumstick) root bark. J. Chem. Pharmaceutical Res. 2010;2(6):424–428. [Google Scholar]
- Dholi S.K., Raparla R., Mankala S.K., Nagappan K. In vivo Antidiabetic evaluation of Neem leaf extract in alloxan induced rats. J. Appl. Pharmaceutical Sci. 2011;01(04):100–105. [Google Scholar]
- Dike-Ndudim J.N., Anyanwu G.O., Egbuobi R.C., Okorie H.M., Udujih H.I., Nwosu D.C., Okolie N.J.C. Anti-bacterial and Phytochemical Potential of Moringa oleifera leaf extracts on some wound and enteric pathogenic Bacteria. European J. Botany, Plant Sci. Phytol. 2016;3(1):50–60. [Google Scholar]
- Eliopoulos G.M., Cosgrove S.E., Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin. Infect. Dis. 2003;36(11):1433–1437. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]
- El-Mahmood A.M., Doughari J.H., Ladan N. Antimicrobial screening of stem bark extracts of Vitellaria paradoxa against some enteric pathogenic microorganisms. African J. Pharmacy Pharmacol. 2008;2(5):089–094. [Google Scholar]
- El-Mahmood A.M., Ogbonna O.B., Raji M. The antibacterial activity of Azadarichta indica (neem) seeds extracts against bacterial pathogens associated with eye and ear infections. J. Med. Plant Res. 2010;4(14):1414–1421. [Google Scholar]
- Esimone C.O., Attama A.A., Mundi K.S., Ibekwe N.N., Chah K.F. Antimicrobial activity of Psidium guajava Linn. Stem extracts against methicillin-resistant Staphylococcus aureus. Afr. J. Biotechnol. 2012;11(89):15556–15559. [Google Scholar]
- Farooq F., Rai M., Tiwari A., Khan A.A., Farooq S. Medicinal properties of Moringa oleifera: an overview of promising healer. J. Med. Plants Res. 2012;6(27):4368–4374. [Google Scholar]
- Ginovyan, 2017. Effect of heat treatment on antimicrobial activity of crude extracts of some Armenian herbs. Chem. Biol. 51(2), 113–117.
- Gupta P.C., Pant D., Joshi P., Lohar D.R. Evaluation of antibacterial activity of Lepidium sativum L. seeds against food borne pathogens. Int. J. Chem. Anal. Sci. 2010;1:74–75. [Google Scholar]
- Jabeen R., Shahid M., Jamil A., Ashraf M. Microscopic evaluation of the antimicrobial activity of seed extracts of Moringa oleifera. Pak. J. Bot. 2008;40(4):1349–1358. [Google Scholar]
- Kassie F., Laky B., Gminski R., Mersch-Sundermann V., Scharf G., Lhoste E., Kansmüller S. Effects of garden and water cress juices and their constituents, benzyl and phenethyl isothiocyanates, towards benzo (a) pyrene-induced DNA damage: a model study with the single cell gel electrophoresis/Hep G2 assay. Chem. Biol. Interact. 2003;142(3):285–296. doi: 10.1016/s0009-2797(02)00123-0. [DOI] [PubMed] [Google Scholar]
- Khan S.A., Aslam J. Study on the effect of Neem (Azadirachta indica) leaves smoke in controlling airborne bacteria in residential premises. Curr. Res. Bacteriol. 2008;1:64–66. [Google Scholar]
- Kim Jeong-Youn, Lee Young-Chun, Kim Keun-Sung. Effect of heat treatments on the antimicrobial activities of Garlic (Allium sativum) J. Microbiol. Biotechnol. 2002;12(2):331–335. [Google Scholar]
- Kinsalin, V.A., KUMAR, P.S., Duraipandiyan, V., Ignacimuthu, S., Al-Dhabi, N.A., 2014. Antimicrobial activity of methanol extracts of some traditional medicinal plants from Tamil Nadu, India. Asian J. Pharmaceutical Clin. Res. 7(1), 38-42.
- Malarkodi E., Manoharan A. Study on antibacterial activity of Parthenium hysterophorus L. J. Chem. Pharmaceutical Res. 2013;5(1):134–136. [Google Scholar]
- Manikkuwadura, Hasara Nethmini De Zoysa, Hasanga, Rathnayake, Ruwani, Punyakanthi Hewawasam, Weerasinghe, Mudiyanselage Dilip Gaya Bandara Wijayaratne, 2019. Determination of In Vitro Antimicrobial Activity of five Sri Lankan medicinal plants against selected human pathogenic bacteria. Int. J. Microbiol. 2019, 8. [DOI] [PMC free article] [PubMed]
- Maragathavalli, S., Brindha, S., Kaviyarasi, N.S., Annadurai, B., Gangwar, S.K., 2012. Antimicrobial activity in leaf extract of Neem (Azadirachta indica Linn.). Int. J. Sci. Nature, 3(1), 110-113.
- Mostafa A.A., Al-Askar A.A., Almaary K.S., Dawoud T.M., Sholkamy E.N., Bakri M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biological Sci. 2018;25(2):361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, D., Parihar, S.S., Chauhan, J.S., Preeti Joshi, S.C., 2010.Effect of temperature and desiccation on seed viability of Lepidium sativum L. New York Sci. J., 3, 34-36.
- Natarajan V., Venugopal P.V., Menon T. Effect of Azadirachta indica (neem) on the growth pattern of dermatophytes. Indian J. Med. Microbiol. 2003;21(2):98. [PubMed] [Google Scholar]
- Negi P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012;156(1):7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Okeke I.N., Ogundaini A.O., Ogungbamila F.O., Lamikanra A. Antimicrobial spectrum of Alchornea cordifolia leaf extract. Phytotherapy Res.: Int. J. Devoted Pharmacol. Toxicol. Evaluat. Natural Prod. Derivatives. 1999;13(1):67–69. doi: 10.1002/(SICI)1099-1573(199902)13:1<67::AID-PTR366>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Shravan, Kumar Dholi, Ramakrishna, Raparla, Santhosh, Kumar Mankala, Kannappan, Nagappa, 2011. J. Appl. Pharmaceutical Sci. 01(04), 100-105.
- Simlai A., Roy A. Analysis of and correlation between phytochemical and antimicrobial constituents of Ceriops decandra, a medicinal mangrove plant, from Indian Sundarban estuary. J. Med. Plants Res. 2012;6(32):4755–4765. [Google Scholar]
- Singh S.K., Vishnoi R., Dhingra G.K., Kishor K. Antibacterial activity of leaf extracts of some selected traditional medicinal plants of Uttarakhand, North East India. J. Appl. Natural Sci. 2012;4(1):47–50. [Google Scholar]
- Taylor J.L.S., Rabe T., McGaw L.J., Jäger A.K., Van Staden J. Towards the scientific validation of traditional medicinal plants. Plant Growth Regul. 2001;34(1):23–37. [Google Scholar]
- Thilza I.B., Sanni S., Zakari A.I., Sanni F.S., Muhammed T., Musa B.J. In vitro antimicrobial activity of water extract of Moringa oleifera leaf stalk on bacteria normally implicated in eye diseases. Academia arena. 2010;2(6):80–82. [Google Scholar]
- Tirado-Torres D., Chan-Keb C.A., Pérez-Balán R.A., Ake-Canché B., Gómez Solano M.I., Aragón-Gastélum J.L., Gómez-López I., Aguirre-Crespo F.J., López-Ramos M.C., Gutiérrez-Alcántara E.J. Antimicrobial activity of Moringa oleifera against multidrug-resistant Staphylococcus aureus isolated from raw milk. Appl. Ecol. Environ. Res. 2019;17(1):587–599. [Google Scholar]
- Verma S., Singh S.P. Current and future status of herbal medicines. Veterinary World. 2008;1(11):347. [Google Scholar]
- Wright C.W. Plant derived antimalarial agents: new leads and challenges. Phytochem. Rev. 2005;4(1):55–61. [Google Scholar]
- Zaidan M.R., Noor Rain A., Badrul A.R., Adlin A., Norazah A., Zakiah I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop. Biomed. 2005;22(2):165–170. [PubMed] [Google Scholar]