Abstract
Peyronie’s disease (PD) is a benign, progressive fibrotic disorder characterized by scar or plaques within the tunica albuginea (TA) of the penis. This study provides new insights into the pathogenesis of PD based on data from different studies regarding the roles of cytokines, cell signaling pathways, biochemical mechanisms, genetic factors responsible for fibrogenesis. A growing body of literature has shown that PD is a chronically impaired, localized, wound healing process within the TA and the Smith space. It is caused by the influence of different pathological stimuli, most often the effects of mechanical stress during sexual intercourse in genetically sensitive individuals with unusual anatomical TA features, imbalanced matrix metalloproteinase/tissue inhibitor of metalloproteinase (MMP/TIMP), and suppressed antioxidant systems during chronic inflammation. Other intracellular signal cascades are activated during fibrosis along with low expression levels of their negative regulators and transforming growth factor-β1 signaling. The development of multikinase agents with minimal side effects that can block several signal cell pathways would significantly improve fibrosis in PD tissues by acting on common downstream mediators.
Keywords: Peyronie’s disease, Cell signal pathway, Penile curvature, Myofibroblast, Extracellular matrix
Introduction
Peyronie’s disease (PD) is a benign and progressive fibrotic condition of the tunica albuginea (TA). This disorder is characterized by excessive accumulation of collagen fibers and other components of the extracellular matrix (ECM). This condition also includes evading myofibroblast apoptosis and cytokine dysregulation. Eventually, it leads to the formation of inelastic penile plaques due to local inflammatory changes that progress during an abnormal wound healing process.1,2 François Gigot de la Peyronie originally described this condition in 1743, but the pathogenesis remains unclear and enigmatic.3 This disease has acute (active) and chronic stable phases with different clinical courses. During the acute phase of PD at 12–18 months after the onset, inflammatory changes occur in the TA due to repeated microtrauma during sexual intercourse. During this period, patients with PD are aware of penile pain in flaccid state, and painful erections, palpable nodes, and the development of penile deformation.4 During the chronic phase, the pain generally disappears, and plaques that form during the acute period stabilize, cause penile curvature and other deformities (such as hourglass deformities and hinge effects), and shortening of length of the penis.5, 6, 7
Despite limited epidemiologic data, the prevalence of PD ranges from 0.4% to 20.3% in men aged 40–70 years, considering concomitant pathologies such as diabetes mellitus and erectile dysfunction.8, 9, 10 Most studies over the past two decades have found that transforming growth factor (TGF)-β1 is a crucial cytokine in the pathogenesis PD. High levels of TGF-β1 expression cause the differentiation of fibroblasts into myofibroblasts, and eventually, this leads to disrupted apoptotic mechanisms and the excessive accumulation of ECM, especially, collagen I and III, IV types in penile plaques.11 The purpose of this study was to highlight new insights into PD pathogenesis and the roles of TGF-β1 in the development and stabilization of PD in the context of interactions among cell signaling pathways, as well as the expression of other key target cytokines and growth factors.
Pathogenesis of Peyronie’s disease
Roles of genetic alterations, epigenetic modifications, and chromosomal abnormalities
A few studies have investigated chromosomal abnormalities in fibroblasts derived from PD. Somers and co-authors applied karyotyping and uncovered chromosome additions (trisomy 7 and 8), deletions Y chromosome (45X,-Y), reciprocal translocations: 46XY,t(11;22) (q11;p11) and 46XY,t(l;5) (q25; q11) and chromosomal inversions: 46XY,inv(7) p22q36 and 46XY,M1.12
Other genes that are involved in the pathogenesis of PD include: 16q12.2; 20q13.12; 2p11.2; Xq21.3-q22; 11q13; 3p21.3; 17q25.3; 7q33; 21q21.3; 14q11.2; 7q11.23; 1p13.3; 1p32-p31; 5q31.1; 17q11.2-q12;12q21.33;17q12;10p11.2;5q31.3-q32;6q25.2-q27;22q13.1;1p36.21;8q24.21;19q13.3;2q37.1;15q22.1;12q24.11;Xq28;4q24;2p25;10q23.3;2q35;5q31;11p15.4;18q21.1;12q13;17q21.33 and 19q13.1. These genes transcribe factors involved in various processes of the organization and disorganization of the extracellular matrix, and the expression of multiple cytokines, growth factors, and signaling molecules during the abnormal healing process in PD tissues.11,13 Mulhall et al14,15 compared the functions of the p53 pathway between fibroblasts from plaque and from control neonatal foreskin after irradiation. They found no statistically significant differences in levels of p53, p21, and mdm2 proteins in fibroblasts derived from PD plaque before and after irradiation, indicating that the p53 signaling pathway is functionally disadvantaged. The overexpression of TGF-β1 is the key factor in many fibrotic conditions. Some studies have associated the single-nucleotide polymorphism (SNP) rs1800471 (G915C) with PD. This SNP causes the substitution of arginine with proline in TGF-β1 protein. This SNP might be the consequence of a point missense mutation in the TGF-β1 gene.16 Dolmans et al17 found a significant association between SNP rs4730775 (Wnt2) and the pathogenesis of PD.
Only one of nine loci is positively associated with Wnt signaling, and it is located on chromosome 7, a frequent site of genetic abnormalities in PD plaques.17,18 Zorba et al19 showed that elevated levels of anti-apoptotic Bcl-2 gene expression enhance proliferative activity. They also showed that Fas gene receptors are not upregulated in TA and PD plaques, compared with Fas ligand. Levels of caspase 3 and 8 are also decreased in both TA and PD plaques. The amount of Fas expression plays an important role in sensitizing fibroblasts to Fas ligand-induced apoptosis with different cytokines. Increased cell-surface Fas expression is necessary to sensitize fibroblasts to Fas ligand-induced apoptosis, and to overcome non-Fas-mediated anti-apoptotic mechanisms induced by TGF-β1.20,21 Many fibrotic diseases have epigenetic modifications without changes in the DNA sequence. The epigenetic regulators, histone deacetylases (HDAC), have been studied in detail. They alleviate the TGF-β1-induced nuclear translocation of Smad2/3 and its further transcription effects in fibroblasts in many organs.22,23 Ryu et al24 showed that inhibiting histone deacetylase 2 with a specific small interfering (si) RNA significantly decreases ECM production. They also showed a decrease in myofibroblastic differentiation, and the phosphorylation of Smad2/3 in TGF-β1-induced fibroblasts derived from PD plaques. Kang et al25 showed a similar effect by inhibiting histone deacetylase 7 with siRNA. They also demonstrated that the expression of mRNA of HDAC 2,3,4,5,7,8,10, and 11 isoforms was higher in fibroblasts derived from PD-plaque than in normal TA. Some studies of animal models have shown that inhibitors of HDAC such as trichostatin A (TSA) and valproic acid (VPA) significantly decrease the level of Smad3/Smad4 complexes in TGF-β1/Smad signaling of fibroblasts that rely on the epigenetic modulation of collagen I type.26,27 Choi et al28 showed that in a mouse model of unilateral ureteral obstruction (UUO), CG200745, an inhibitor of HDAC attenuated oxidative stress, inflammatory cytokines and also markedly reduced the expression of α-SMA, fibronectin, collagen I, and TGF-β. Choi et al29 showed that the overexpression gene of Smad7 inhibited the TGF-β1 induced production of ECM, myofibroblastic differentiation of PD-derived fibroblasts, phosphorylation, and nuclear translocation of Smad2/3. They also found that Smad7 induced apoptosis and blocked cell cycle entry in fibroblasts from PD plaque. Similar results were generated by Wu et al30 who co-cultivated bone mesenchymal stem cells (MSC) transfected with a lentiviral vector carrying a Smad7 gene, with hepatic stellate cells in a rat model of hepatic fibrosis. Numerous transcriptomic studies have shown that the human genome is transcribed with multiple non-coding RNA (ncRNA), including microRNA (miRNA, miR) and long non-coding RNAs (lncRNA).
These RNA participate in diverse cellular processes, such as gene regulation, gene expression, cell proliferation, cell differentiation, and apoptosis. They might also be involved in the development and progression of fibrotic diseases. Overexpression of miR-29b significantly reduces the expression of α-SMA and COL1A1, inhibits the proliferation of myofibroblasts and induce their apoptosis, indicating that the miR-29b might play a significant anti-fibrotic role in hypertrophic scars.31,32 Inhibiting miR-148b enhances the TNF-α/IL-1β-mediated endothelial-to-mesenchymal transition (EndMT) of endothelial cells during wound healing with the loss of its markers CD31 and VE-cadherin. On the other hand, miR-148b overexpression promotes angiogenesis, accelerates wound closure, and attenuates cytokine-mediated EndMT.33
Influence of the alterations of ECM in Peyronie's disease and other fibrotic disorders
The cell microenvironment greatly influences the growth, survival, and behavior of cells. The ECM provides not only structural and mechanical support for cells and tissues but also binds transmembrane receptors and soluble ligands to coordinate several signal cell pathways. A plethora of studies has demonstrated fibroblast differentiation into myofibroblasts and excessive ECM accumulation in many fibrotic states.34,35 The ECM serves as a reservoir for several latent growth factors. Changes in the organization of ECM might contribute to the disrupted activation of signaling molecules and harm tissue homeostasis. Several mass-spectrometry(MS)-based proteomic studies have found predominant ECM components in connective tissues and basal membranes, including collagen I, III, IV, VIII, XII, XIV, and XXI types, laminins, nidogens, fibronectin, vimentin, perlecan, prolargin, versican, matriline, tenascins, thrombospondin, decorin, and fibromodulin.36,37 The maintenance of homeostasis and ECM turnover is involved in several factors and mechanisms. This comprises complex interactions between matrix metalloproteinases (MMP) and tissue inhibitors of matrix metalloproteinases (TIMP). The MMP are involved in the reinforcement and debilitation of many processes that influence the development of fibrosis. Metalloproteinases might possess both profibrotic and antifibrotic properties. Stromelysin-1 (MMP-3) and gelatinase B (MMP-9) can activate latent TGF-β1, and thus manifest a pro-fibrotic effect. Matrilysin (MMP-7) can indirectly mediate fibrosis by splitting syndecan-1 carrying the chemokine CXCL1, which is required for neutrophil influx. Each of MMP-2, MMP-3, and MMP-7 can degrade the ECM proteoglycan decorin and release latent TGF-β1.
MMP-8 blocks the IL-10 signal and leads to fibroblast activation and collagen overproduction. Therefore, MMP-dependent functions during fibrosis are not limited by effects on ECM turnover, and they also influence the proliferation, gene expression, and many aspects of inflammation. Notably, matrixins (MMP) require Zn2+ ions for adequate functioning and catalytic activity.38,39 Gunes et al40 recently applied atomic absorption spectrophotometry and found significantly lower levels of the trace elements Mn, Cu, Zn, and Fe, whereas levels of Cd and Co remained unchanged in patients with PD. A zinc deficiency might contribute to the progression of fibrotic changes. Sakiyama et al41 showed that Zn and Cu deficiencies diminish the activity of superoxide dismutase 1 (SOD1), which increases collagen accumulation in the livers of SOD1 knockout mice. They also found that levels of TIMP-1 and collagen-specific molecular chaperone-heat shock protein-47 were increased due to the actions of an excessive amount of superoxide anions in SOD1 knock-out mice. Zhang et al42 showed that zinc deficiency in a mouse model of streptozotocin-induced diabetes resulted in increased synthesis of type I collagen, fibronectin, and an increased number of α-SMA positive renal fibroblasts. All these activities lead to an MMP/TIMP imbalance and further progression of fibrosis. Some studies have found that TIMP 1–4 accumulation increases in plaques, whereas MMP levels remain unaltered during the pathogenesis of PD. Del Carlo et al43 demonstrated that TGF-β induces the excessive accumulation of all four TIMP, but no changes in the levels of collagenases (MMP1, 8, and 13). On the other hand, interleukin (IL)-1β significantly induces the production of MMP 1, 3, 10, and 13 via a mechanism that is independent of Ca2+. Notably, TGF1β reduces the activity of MMP by increasing the expression of TIMP. Watanabe et al44 found no changes in the levels of MMP-2 and MMP-9 expression between patients with PD and healthy men. They also found high levels of heparanase-1 and -2 expression compared with controls, while levels of dermatan sulfate, hyaluronic acid synthases (HAS1 and HAS2) and hyaluronidases (Hyl1 and Hyl2) did not change in PD plaques. Concentrations of hyaluronic acid are high in the TA. Owing to its hydrating action, the distribution of nutrients in connective tissue structures is controlled and the inflammatory cytokine pool is influenced. These studies also uncovered the decreased apoptosis and fewer blood vessels in PD tissues. Heparan sulfate proteoglycans interact with other matrix components to form a type of shelter for growth factors and cytokines. Accordingly, the enzymatic destruction of the heparan sulfate structure caused by heparanase (HPSE) accompanies the release of these molecules and of low molecular weight fragments of heparan sulfate. The latter increases the production of interleukins and other proinflammatory cytokines by leukocytes.45
Yue et al46 also found sulfatase-1 and -2 overexpression in idiopathic pulmonary fibrosis, and that these enzymes are regulated by TGF-β1. Heparan sulfate protects the ECM from migrants and free radicals, but heparinase affects this function.47 ProMMP mainly exists in an inactive form since they their structures contain a “cysteine switch,” in which the cysteine residue coordinates with the Zn2+ ion in the catalytic domain. Activation of MMP might occur through several mechanisms: (1) the extracellular catalytic cleavage of ProMMP by another active MMP or MT-MMP through TIMP-2; (2) intracellular activation of MT-MMP (MMP-11, MMP-23, MMP-28) by furin protein; (3) oxidation and nitrosylation of the thiol group of the cysteine switch in ProMMP by reactive oxygen species (ROS) and nitric oxide (NO), respectively; (4) low pH and warm temperature; and (5) proteolytic cleavage by other proteases such as plasmin, α2-macroglobulin.39,48, 49, 50 Several proteoglycans in the ECM serve as a depot for latent profibrotic cytokines. Akman et al51 found PD-like alterations in PD model rats treated with decorin than those treated with TGFβ. Decorin belongs to the small leucine-rich proteoglycan (SLRP) family, which binds to TGF-β and neutralizes its activity. It also modulates TGFβ-dependent cell growth, stimulation, or inhibition. Zhang et al52 in a scar contracture model modified by the incorporation of recombinant human decorin into collagen gel, showed that decorin inhibited TGF-β1-induced α-SMA and F-actin expression and enhanced the contraction of collagen gel in hypertrophic scar fibroblasts. Jiang et al53 showed that decorin decreases levels of TGF-β1, p38 MAPK, Akt/PI3K in human peritoneal mesothelial cells. Along with decorin in the ECM, TGF-β might be in the form of large latent complexes (LLC), in which latent TGF-β binding protein (LTBP) is covalently bound to dimeric TGF-β through its latency-associated peptide (LAP).54 Fibrin as a possible inducer of fibrogenesis is found in PD plaques in humans and in animal models. Fibrin is a key inducer of collagen synthesis and a major promoter of fibroblast differentiation into myofibroblasts.55 Davila et al56 showed that fibrin introduced into the TA of the rat penis, acts as a potential profibrotic protein, perhaps via the local release of TGF-β1. Currently prevalent is the concept that trauma during sexual contact is a trigger for the development of PD. Considering this, the internal and external layers of the TA might undergo splitting, thus creating a milieu for inflammation due to the accumulation of extravascular blood. A provisional matrix might arise and undergo remarkable changes such as the formation of a collagen-rich scar from a fibrin-rich “scaffold”. Any disruption or shift of ECM structures during inflammation, or mechanical damage would result in changes to intracellular signaling pathways.
All these processes probably occur between the inner layer of the TA and the vascular, loose, areolar connective tissue sleeve without elastic fibers in Smith space.57 Bernau et al58 found that tensin 1 (TNS1) overexpression in primary cultures of human pulmonary fibroblasts contributes to the excessive accumulation of ECM due to the formation of fibrillar adhesions that bind extracellular fibronectin fibrils. The status of hyaluronan/versican complexes within a provisional matrix plays important roles in maintaining many fibrotic conditions and probably also PD. During the inflammatory phase of PD, leukocyte infiltration can lead to the formation of small fragments of versican and hyaluronan that have pro-inflammatory properties and stimulate myeloid cells to release inflammatory cytokines.
Moreover, low molecular weight fragments of hyaluronic acid promote collagen production.59 A role of mechanical stress has also been shown in several models of PD. The TA of the corpora cavernosa consists of inner and outer layers. Bundles of the inner layer are oriented in a circular fashion and support cavernous tissues. Bundles of the outer layer are longitudinally oriented, and extend from the glans penis to the proximal crura and insert into the inferior pubic ramus. Gefen et al60 created a model that shows stress concentrated around blood vessels and nerve roots due to plaques forming in PD. This irritates nerve endings or compresses the vascular bed and eventually leads to penile deformities and/or painful erections. Herwig and Bayerl61 developed a physical simulation of buckling stress in PD. This model showed that a fiber-load angle of buckling between 1.2° and 6.4°in the inner layer of the penis can lead to significant rupture of the outer layer. Other factors in the ECM besides decorin, MMP, fibrin, and mechanical stress such as integrin αvβ6, thrombospondin-1, furin-convertase, can contribute to TGF-β activation. Integrin αvβ6 binds to latent TGF-β complexes in the ECM via LAP, and active TGF-β is released via the intracellular link of the receptor to the cell cytoskeleton and forces arising from mechanical stress for example, during sexual intercourse.62
Integrin αvβ6 is not expressed under normal conditions but it is upregulated during injury. Thrombospondin-1 also activates TGF-β1 by binding to LAP in latent TGF-β1 complexes. Wu et al showed that integrin αvβ6 knockout reduces levels of thrombospondin-1 and αSMA in mouse models of corneal wound healing.63,64 Among other components of the extracellular matrix, the proteoglycan perlecan plays an important role in the development of fibrotic processes and thus perhaps in PD. Perlecan is a ubiquitous heparan sulfate proteoglycan that localizes in connective tissues.
Most cells in the body synthesize perlecan, which manifests profibrotic properties by inhibiting fibroblast apoptosis by binding to its C-terminal fragment via PI3K. Perlecan also promotes collagen fibrillogenesis via pendant glycosaminoglycan chains (Fig. 1).65
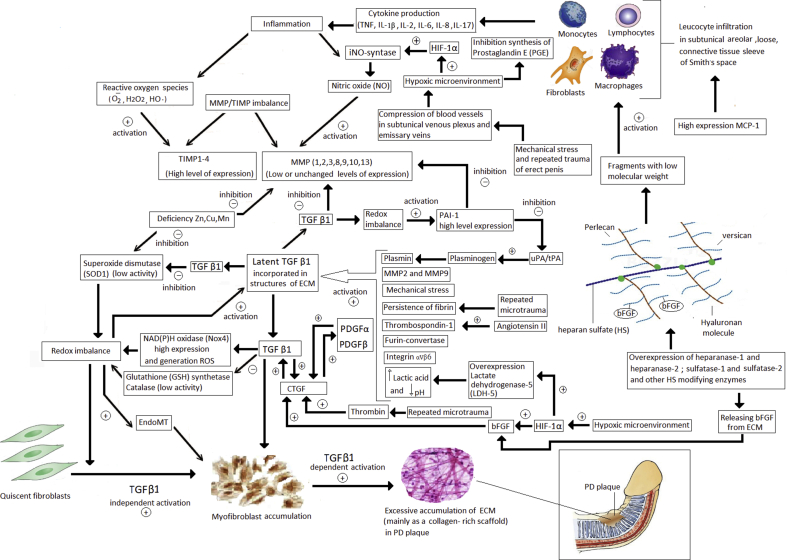
Fig. 1.
Proposed pathogenetic mechanisms, alterations of ECM, and influences of growth factors and cytokines involved in the maintenance and development of PD. Leukocyte infiltration occurs during acute tissue damage, then pro-inflammatory cytokine production begins, which triggers an inflammatory reaction. This leads to formation of a large number of reactive oxygen species and nitric oxide synthesis. During repeated microtraumas and TA mechanical stress, blood vessels become compressed in subshell venous plexus and emissary veins, which lead to a hypoxic microenvironment and increased expression of HIF-1α that activates NO synthase and suppresses prostaglandin E synthesis. Under influence of many factors at low pH, activities LDH, furin convertase, thrombospondin, fibrin persistence, and other hemostatic disorders increase, against a background of ECM reorganization, release of latent TGF β1, followed TGF β1. TGF-β1 signaling cascade is activated by CTGF, which can be activated by PDGF α and PDGF β. During PD, against background of increased expression of heparanase 1 and 2 and other HS-modifying enzymes, bFGF is cleaved from the ECM, which activates CTGF, and low-molecular fragments form that lead to leukocyte infiltration along with an increased MCP-1 expression. At high levels of TGF-β, redox becomes imbalanced by suppressing antioxidant systems (Glutathione synthase and catalase) against a background of increased expression of NAD(P)H oxidase Nox 4 and a deficiencies in Cu and Mn. Redox imbalance results in increased PAI-1 expression, which disrupts fibrinolysis with subsequent fibrin persistence. With increased TGF-β1 expression, MMP synthesis is suppressed and TIMP level increases against the background of redox imbalance and deficiency of Zn ions. Against the backgrounds of all these factors, EndoMT and myofibroblasts accumulation occurs with the subsequent formation of PD plaque consisting of desorbed ECM enriched mainly with collagen. EMC: extracel-lular matrix; PD: Peyronie's disease; TA: tunica albuginea; HIF-1α: hypoxia-inducible factor-1 alpha; NO: nitric oxide; LDH: lactate dehydrogenase; TGF: transforming growth factor; CTGF: connective tissue growth factor; PDGF: platelet-derived growth factor; bFGF: basic fibroblast growth factor; MCP-1: monocyte chemotactic protein-1; PAI: plasminogen activator inhibitor; MMP: matrixins; TIMP: matrix metalloproteinases; EndoMT: endothelial to mesenchymal transition.
Changes in the levels of cytokines and growth factors, and the role of myofibroblastic differentiation in pathogenesis of PD and other fibrotic conditions
The central profibrotic cytokine TGF-β1 is found in all fibrotic processes, including PD. Many cell types, including macrophages secrete inactive (latent) TGF-β that is linked to the polypeptides, latent TGF-β-binding protein (LTBP) and (LAP).24,62 In a paradigm of impaired wound healing, we consider changes in levels not only of TGF-β1, but also of other cytokines and growth factors, as well as the role of oxidative stress in maintaining the fibrotic state in PD, taking into account the unique anatomical localization of the pathological process. An inflammatory cascade is evidently activated during the acute phase of PD and multiple pro-inflammatory cytokines are released accompanied by oxidative stress in response to damage. The exact etiology of the PD remains unknown. A prevalent hypothesis is that PD is caused by repeated traumatic microvascular injury of the TA with further inflammation, elastic fiber disruption, and plasma extravasation with further fibrin deposition.66 Other viewpoints have been presented regarding the etiology of PD with respect to endothelial dysfunction in patients without risk factors for hypertension, atherosclerosis, and diabetes mellitus. Agrawal et al67 showed that endothelium-dependent flow-mediated dilation (FMD) is impaired in patients with PD who do not have risk factors such as atherosclerosis. Atar et al68 showed that patients with early PD have more abundant IL-6 and pentraxin 3, and that this does not correlate with disease duration, degree of curvature, age, and level of C-reactive protein. These cytokines are released by endothelial cells, fibroblasts, monocytes as a result of traumatic events.
A study of 91 patients with stable PD showed elevated serum levels of TGF-β1 and interferon-γ, decreased levels of TNF-α, and undetectable IL-6.69 Myostatin (GDF-8) is overexpressed in PD plaques, it stimulates myofibroblast generation and collagen expression in healthy TA cells and potentiates the effects of TGF-β1.70 Many studies of fibrosis have shown that several essential factors play significant roles during impaired healing.
These factors include myofibroblast transformation from fibroblasts, fibrocytes of different origin, smooth muscle cells, MSC, and avoidance of apoptotic mechanisms.19,25 Gabbiani et al originally described in 1971, that fibroblasts acquire smooth muscle cell features with the expression of desmin, caldesmon, and actin isoforms that aid progress of fibrotic events after injury, or during the development of granulation tissue.71 Myofibroblasts generally participate in physiological wound healing by acting in tissue contraction and ECM secretion. Given the specific localization of the pathological process in PD, the origins of myofibroblasts might be tissue-resident fibroblasts and MSC in the Smith space, circulating bone marrow-derived fibrocytes and MSC, smooth muscle cells and endothelial cells from erectile tissue covering cavernosal spaces and emissary veins passing through TA (Fig. 2). In addition to TGF-β1, several other factors are involved in maintaining the α-SMA positive phenotype and activating myofibroblasts. These factors include platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), reactive oxygen species (ROS), mechanical tension, pro-inflammatory cytokines (TNF-α, IL-1β, IL-2, IL-6, IL-8, IL-17), angiotensin II, monocyte chemotactic protein-1 (MCP-1), and others. Several studies of fibrosis have shown that PDGF have mitogenic and anti-apoptotic effects on ECM. They are secreted by endothelial cells, macrophages and released from platelets upon degranulation. The expression of PDGF α and β receptors increases during fibrosis in many organs. Lucattelli et al72 showed significantly increased levels of mRNA expression of PDGF α, PDGF β, and their receptors PDGFR α and PDGFR β in rat models of PD. That study also found high levels of hypoxia-inducible factor-1 (HIF-1) and inducible NO-synthase (iNOS) expression in rats with PD-like lesions.73,74 Another important factor that contributes to the maintenance of the fibrotic process is CTGF. This cysteine-rich matricellular protein of the CCN family is involved in the control of many biological processes, such as cell proliferation, adhesion, differentiation, and angiogenesis.75 The main profibrotic role of CTGF is to promote TGF-β1 activity by decreasing Smad7 and increasing Smad2 when they act together and consequently result a sustained fibrotic response.76 A pilot study has shown that administering a prostacyclin I2 analog decreased CTGF expression in fibroblasts, and in 29% of patients with PD in whom penile curvature was improved.77 The redox imbalance plays an important role in the development of PD and other fibrotic processes. Several sources lead to a redox imbalance; NAD(P)H oxidase of the Nox family, and particularly Nox4, play pivotal roles in ROS generation.
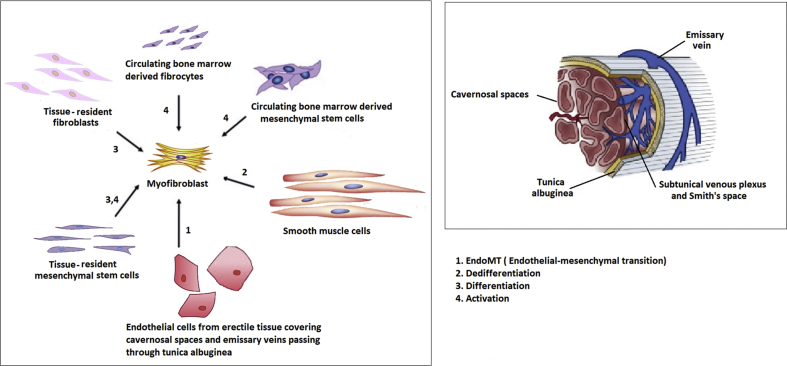
Fig. 2.
Origin of myofibroblasts and localization of sources in PD. Myofibroblasts are crucial elements in all fibrotic process, including PD, and they might have various origins. They can arise as a result of smooth myocyte dedifferentiation, via endothelial–mesenchymal transition of endothelial structures, as well as activation and differentiation of MSC, fibrocytes, fibroblasts, both tissue and bone marrow. PD: Peyronie's disease; MSC: mesenchymal stem cells.
A redox imbalance also participates in the maintenance of TGF-β1-mediated fibrosis, myofibroblast differentiation, fibrotic/profibrotic gene expression, and the endothelial–mesenchymal transition.78, 79, 80 A redox imbalance is also supported by TGF-β1, which inhibits antioxidant enzymes such as catalase, glutathione peroxidase, and glutathione (GSH) synthetase.81 The extent of fibrosis in many tissues depends on collagen synthesis rates and its degradation by cellular proteolytic activities via the uPA/tPA/plasmin/MMP system. Plasminogen activator inhibitor (PAI)-1 is the most potent inhibitor of fibrinolysis, as it controls activities of the uPA/tPA/plasmin/MMPs system and participates in the maintenance of tissue homeostasis.82 It is a critical element of the TGF-β network along with the redox imbalance and the growth factors described above, and is mainly deposited in platelets and secreted into the bloodstream. Aside from platelets, PAI-1 is also synthesized in endothelial cells, megakaryocytes, fibroblasts, macrophages, and adipocytes.83 Davila et al showed using immunohistochemistry and real-time PCR that PAI-1 is intensely immunostained in human PD plaque that PAI-1 mRNA levels are increased 16-fold compared with normal TA tissue.84 Abundant basic fibroblast growth factor (bFGF) is expressed during the early stages of tissue injury because activated chemotactic cytokines are released from the ECM by heparanase. The main function of bFGF is to stimulate angiogenesis and fibroblast proliferation during the development of granulation tissue. It fills up a wound space early in the wound-healing process by binding with heparan sulfate proteoglycans in the ECM and exerts local action in a paracrine mode.85,86 Cell lines derived from PD plaque tissue express more abundant basic FGF than normal TA.87 Adenosine might also play an important role in the progression of fibrotic changes in damaged tissues. High levels of adenosine expression promote the pathogenesis of penile fibrosis through ADORA2B signaling. Mateus et al showed that among the four knowns adenosine receptors, ADORA1 and ADORA2B are significantly more prevalent in cells derived from PD plaque than non-PD TA tissues.88,89
Angiotensin II also mediates penile fibrosis by increasing the expression of thrombospondin-1, a major activator of latent TGF-beta. A previous study confirmed that losartan administered to a rat model reduces penile fibrosis after bilateral cavernous nerve injury.90,91 Along with various degrees of buckling stress within the TA during intercourse, the number of layers of the tunica might also play a role in the development of PD. Shafik et al92 clearly found the following morphological variations of the TA in 28 cadaveric specimens: a two-layered (inner circular and an outer longitudinal) TA in 71.4% of men, and a three-layered (inner circular, intermediate longitudinal and outer circular) and single-layered (longitudinal) TA in 21.4% and 7.2% of the specimens, respectively.
The single-layer TA is likely to be more disturbed under the influence of mechanical stress during sexual intercourse than the three-layer structure of TA in patients with PD.92 The resistance of the collagen network in TA to mechanical stress is also affected by the overexpression of aquaporin 1 (AQP1) as a result of a localized maladaptive response to repeated microtrauma of the connective tissues of TA in patients with PD compared with normal TA.93 Insulin-like growth factor 1 (IGF 1) also plays an important role in tissue homeostasis and the level of its expression can change to influence wound healing. Thomas et al94 showed that the expression of all IGF1 isoforms (Ea, Eb, and Ec) was significantly suppressed in PD plaque compared with normal TA. When tissue is damaged, the expression of MCP 1 increases and it is involved in the recruitment of dendritic cells and monocytes, then followed by a cascade of inflammatory reactions. Significantly more MCP-1 was expressed in cells from PD plaque than normal TA cells, while the level of CTGF between non-PD and PD-fibroblasts remained unchanged. Szardening-Kirchner et al also confirmed significant up-regulation of MCP-1 gene expression in fibroblasts from PD and non-PD tissue after prolonged incubation with TGF-β1.95 Kottmann et al96,97 found that the metabolite lactic acid is an important mediator of myofibroblast differentiation via a pH-dependent activation of TGF-β and overexpression of lactate dehydrogenase 5 (LDH5), and that HIF-1α induces myofibroblast differentiation in cultured human lung fibroblasts from patients with idiopathic pulmonary fibrosis. The role of apoptosis of myofibroblasts in wound healing has been emphasized. Myofibroblast apoptosis is inhibited during wound healing, and the excessive accumulation of ECM is a subsequent hallmark of fibrotic pathologic conditions, including PD.98,99 In this review, we discussed some factors that might be implicated in the inhibition of myofibroblast apoptosis during the development of fibrosis and PD. Apoptosis is a crucial fundamental process aimed at the maintenance of tissue homeostasis and it is involved in metamorphosis, and normal tissue turnover during wound healing. A disturbance of its regulation is involved in the development of fibrosis and several other diseases. It can proceed either through extrinsic (death receptor-mediated) or intrinsic (mitochondrial) pathways. Pro-apoptotic and anti-apoptotic proteins such as Bax, Bad, and Bcl-2 are often imbalanced during the development of fibrotic changes. Safaeian et al reported that imbalanced Bax/Bcl-2 defines the susceptibility of a cell to apoptosis. They showed that high Bcl-2 expression contributes to myofibroblast resistance to apoptosis with further fibroproliferative changes in the lung.100 The roles of caspase 3 and 9, Bax/Bcl-2, and Fas-ligand have been examined in patients with PD.
Using immunohistochemistry and western blotting analysis, Loreto et al101 showed the overexpression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and its death receptor (DR5), indicating activation of apoptosis through an extrinsic pathway in PD tissue compared with normal TA. Another study using immunohistochemical staining showed Bax overexpression in myofibroblasts of PD plaque and metaplastic bone tissue, whereas the level of Bcl-2 was minimal. In addition, PD tissues are intensely and moderately immunostained for caspases-3 and -9, respectively. Therefore, plaque apoptotic events are partly induced by the intrinsic (mitochondrial) and extrinsic (CD95 or Fas ligand-mediated) pathways in stabilized PD.102 In contrast, Zorba et al.19 found high levels of Bcl-2, expression and decreased levels of caspase 3 and 8 expression using PCR. Despite these differences in data, myofibroblast apoptosis is activated at least partially, and inhibited by many factors. For instance, heat shock proteins (HSP) can inhibit apoptosis by interacting with proteins involved in programmed cell death, such as apoptotic protease activating factor 1 (Apaf-1), cytochrome c, and caspases. Although HSP 70 is involved in TGF-β receptor degradation and limits Smad phosphorylation, it also participates in the inhibition of apoptosis by inactivating Apaf-1 and AIF (apoptosis-inducing factor). At the mitochondrial level, HSP 70 blocks the translocation of Bax to the mitochondria and mitochondrial protein release to increase Bcl-2 stability. Both HSP 27 and HSP 90 inhibit the function of cytochrome C and Apaf-1, respectively.103 The anti-apoptotic activity of HSP 70 might also be enhanced by its mitochondrial chaperone, mortalin, which inhibits p53 translocation to the nucleus. Lee et al104 found more abundant mortalin expression in keloid fibroblasts than in adjacent normal tissues. Ajayi et al105 showed that Fas-resistant lung fibroblasts express abundant X-linked inhibitor of apoptosis protein (XIAP) in patients with idiopathic pulmonary fibrosis, and that its antagonists increased the susceptibility of lung fibroblasts to Fas ligand. On the other hand, using immunohistochemistry and western blotting, Liu et al106 showed that the expression of mitochondrial protein binding inhibitors of apoptosis, Smac/DIABLO, was lower in fibroblasts of hypertrophic scars than that in normal tissue.
High levels of Smac/DIABLO expression increase caspase-3 and -9 activities and inhibit the mRNA expression of types I and III pro-collagen in fibroblasts of hypertrophic scar tissue.106 All these factors and others that inhibit myofibroblast apoptosis are potentially involved in PD (Fig. 3).
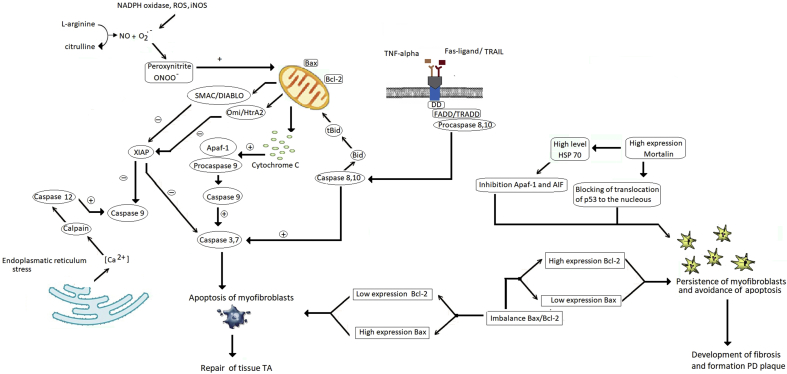
Fig. 3.
Factors and mechanisms that inhibit and activate myofibroblast apoptosis in Peyronie's disease (PD). Myofibroblast apoptosis is violated during all fibrotic process, because myofibroblasts would undergo apoptosis during normal wound healing. This contributes to various factors. Imbalanced expression of B-cell lymphoma-2 protein (Bcl-2) and Bcl-2 associated X protein (bax) in which levels of Bcl-2 increase and those of bax decrease, leads to persistent myofibroblasts and excessive ECM production. Heat shock proteins that interact with caspases, apoptotic protease activating factor-1(Apaf-1), and cytochrome C might function in inhibiting apoptosis. Heat shock protein (HSP) 70, HSP 27 and HSP 90 inhibit functions of Apaf-1 and cytochrome C. Heat shock protein 70 blocks Bax translocation to mitochondria and mitochondrial protein release and increases Bcl-2 stability. Anti-apoptotic activities of HSP 70 can be enhanced by mitochondrial chaperone mortalin, which inhibits p53 translocation into nucleus. Despite the expression of Fas and TNF-related apoptosis inducing ligand (TRAIL) and partial activation of external and internal activation pathways, other factors inhibit apoptosis. During fibrosis, amounts of X-linked inhibitor of apoptosis protein(XIAP) expression are increased against a background of low levels of mitochondrial proteins, second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO) and Omi-stress regulated endoprotease/high temperature requirement factor A2(Omi/HtrA2), which are regulators of apoptosis inhibitors.
Interactions among various signal pathways in cells participating in development of PD and possible treatment targets based on data from studies of other fibrotic conditions
One of the conditions for the viability of a complex multicellular organism is a persistent, yet flexible, system of inter- and intracellular communications. Cell interactions with multiple external and internal stimuli constitute a whole intracellular cascade of signaling molecules and pathways of biochemical transformations. Critical target molecules and crosstalk between signal cell pathways, which have potential implications in the development and maintenance of fibrosis in PD, are reviewed. In the previous sections, we discussed the functional roles of the main elements of TGFβ signaling. Here, we reviewed other main cell signal transduction pathways that might be implicated in the excessive proliferation of myofibroblasts and their resistance to apoptosis, as well as in the chronic inflammation and fibrosis associated with PD. These include the Wnt/β-catenin, Notch, Hedgehog, Hippo, Rho/ROCK, PI3K/Akt/mTOR, p38 MAPK/ERK, JAK-STAT, and NF-κB signal pathways. As noted above, TGFβ signaling starts from the activation of latent TGFβ from LAP/LTBP under the influence of various cytokines and mechanical factors. Thereafter, it binds to receptor complexes on the membranes of fibroblasts, macrophages, and other cells. The synthesis of TGF-β1 is controlled by paracrine and autocrine regulation. Released TGF-β1 ligand exerts biological effects by binding to specific type I and II TGF-β1 receptors with serine–threonine kinase activity and further assembly into complexes, within which TGFβRII phosphorylates and activates TGFβRI. After receptor complexes are assembled, the TGFβ ligand mediates its signal through Smad proteins, and hyperactivated Smad2/3 leads to fibrosis, while Smad6 and 7 prevent it. Jang et al showed that inhibiting the TGF-β type I receptor, activin receptor-like kinase 5 (ALK5) using the synthetic inhibitor IN-1130 suppresses the subsequent TGF-β1-induced phosphorylation of Smad2 and Smad3 and nuclear accumulation of Smad proteins in human fibroblasts of PD-plaque. The ALK5 inhibitor, SKI2162 not only blocks TGF-β1-induced phosphorylation and nuclear translocation of Smad2 and Smad3 but also inhibits the production of extracellular matrix in human PD tissue.107,108 Haag et al109 demonstrated rapid nuclear translocation of Smad2/3, Smad3 and Smad4 overexpression in TGF-β1-stimulated fibroblasts from PD-plaque compared with control normal TA, using PCR, western blotting, and immunofluorescence staining. The activation of Smad via transcriptional regulation promotes the expression of fibrogenic intermediate effectors, such as PDGF or CTGF, which are coactivators of TGF-β.
The crucial downstream mediators of TGFβ along with PDGF are epidermal growth factors, and the activation of their receptors (PDGFR/EGFR/ErbB) also contributes to the profibrotic effect of TGFβ. Andrianifahanana et al showed that inhibition of TGFβ/PDGF and ErbB pathways not only prevented myofibroblast differentiation, but also improved organ function in a mouse model of bleomycin-induced pulmonary fibrosis.110,111 The effects of TGFβ can be exerted in a Smad-independent manner through the Wnt/β-catenin signal pathway. Akhmetshina et al showed that TGF-β activates canonical Wnt/β-catenin signaling by decreasing the expression of the Wnt antagonist, Dickkopf-1 (DKK1), and the overexpression of Wnt-1 and Wnt-10b ligands with increased nuclear accumulation of β-catenin in fibroblasts from fibrotic skin.112 Overexpression of DKK1 prevents fibrosis through the activation of myofibroblast differentiation from other sources such as bone marrow-derived mesenchymal stem cells and epithelial tissue. This fact has been confirmed in many studies, but the role of Wnt/β-catenin signaling has not been studied in the context of PD pathogenesis.113,114 Crosstalk between Wnt/β-catenin and TGF-β signal pathways are involved in several fibroproliferative disorders such as Dupuytren disease and pulmonary fibrosis. Here, TGF-β inhibits the expression of DKK1 and up-regulates profibrotic genes by interaction with Wnt ligands. The other morphogenic Hedgehog and Notch pathways tightly interact with Wnt/β-catenin signaling, which is aberrantly activated under many fibrotic states, including PD.115,116 Studies of skin, lung, liver, and kidney fibrosis have shown that the Notch signaling pathway is involved in the regulation of myofibroblast differentiation in the fibrotic area and that this is accompanied by the overexpression of Notch-1,2,3,4 and Jagged 1,2 ligands with increased nuclear accumulation of NICD (Notch intracellular domain) and further activation profibrotic genes. Crosstalk between TGFβ and Notch signaling has also been identified in various human fibrotic diseases and experimental animal models. The expression of Jagged-1 and notch-1 is induced by TGFβ, and inhibition of the γ-secretase complex leads to the amelioration of fibrosis by inhibiting activation of the TGF/Smad2/3 signaling pathway.117,118 Deregulation of the transcriptional factor, yes-associated protein (YAP), and of transcriptional coactivators with a PDZ-binding motif (TAZ), a terminal effector of the Hippo pathway, are associated with pathological states such as PD, pulmonary, renal and liver fibrosis. Normal tissue regeneration occurs with hypomorphic (partial) activation of YAP/TAZ but under pathological conditions including fibrosis, it is enhanced due to the convergence of differently activated signal cascades such as Rho/ROCK, Wnt/β-catenin, TGF-β, and mechanosignaling. Therefore, the increased nuclear accumulation of YAP/TAZ as transcription coactivators promotes the expression of the central mediator of tissue fibrosis-CTGF by binding with the TEAD complex in the nucleus.119, 120, 121
The overexpression of CTGF, which contributes to fibrosis, can also occur through excessive activation of ROCK signaling and the phosphorylation of Rho protein upon binding to the ligand-bound TGF-β receptor II/Alk-1 complex.122 Matrix stiffness-dependent fibroblast activation is partially triggered through TGF-β-independent expression of plasminogen activator inhibitor (PAI)-1, which promotes persistent YAP/TAZ nuclear localization by attenuating pericellular plasmin activity and this leads to constant cellular activation and fibrogenesis.123 Mechanistically, TGF-β1 signaling in PD plaque and fibroblasts derived from TA occurs via Rho/ROCK-mediated YAP/TAZ nuclear translocation. The ROCK inhibitor, Y-27632, and simvastatin prevent fibrotic changes in TA by inhibiting YAP/TAZ nuclear translocation and downregulating CTGF mRNA expression.124 Fasudil, a RhoA/Rho kinase (ROCK) inhibitor significantly induces the apoptosis of human fibroblasts derived from urethral scar tissues and decreases their proliferative activity in the absence or presence of TGF-β1 stimulation in a dose and time-dependent manner. Fasudil also suppresses actin polymerization and collagen synthesis, and induces apoptosis in human urethral scar fibroblasts stimulated with or without TGF-β1 via the Rho/ROCK signaling pathway.125,126 Hedgehog (Hh) signaling plays an important role in tissue repair after injury, and its hyperactivation promotes the development of fibrosis in combination with other signaling pathways. For instance, Hedgehog signaling upregulates Wnt-2b and Wnt-5a and the Notch ligand Jaggad-2, and such interaction between several signal pathways can accelerate the progression of tissue fibrosis.127 The Hh ligands Indian, Desert, and Sonic Hedgehog (Ihh, Dhh, Shh) are upregulated during all fibrotic process, including PD along with overexpression of their transmembrane receptors: 12-path transmembrane protein patched (Ptch) and 7-transmembrane protein smoothened (Smo). Their activation leads to the dissociation of SUFU-Gli complexes in myofibroblasts along with further release of Gli proteins, which translocate into the nucleus and activate a target gene, including Smo, Ptch and transcriptional activators of the canonical Hh signal pathway Gli-1 and Gli-2 proteins. The Hedgehog signal pathway might be driven by a Gli protein-independent mechanism through interaction with TGFb, PDGF, and EGFR.128,129 One of the universal signaling pathways characteristic of most human cells is PI3K/Akt/mTOR, which is responsible for growth, cell proliferation, metabolism, and avoiding apoptosis. This signal pathway is hyperactivated in fibroblasts from fibrotic tissue. Lin et al showed that supplementation with rapamycin improved penile fibrosis by up-regulation of eNOS, nNOS, and cGMP in a rat model of streptozotocin-induced type 1 diabetes.130 However, this is insufficient to reverse the fibrotic process. The mTOR inhibitor, rapamycin, does not inhibit dermal fibrosis due to increasing the upregulation of phosphorylated Akt on Ser473 in a rat model of systemic sclerosis.
In contrast, dual administration with BEZ235, an inhibitor of PI3K/Akt/mTOR signaling significantly reduced fibrosis by blocking Akt and GSK-3.131 Song et al132 showed that naringin inhibits the phosphorylation of Akt and downstream proteins of Akt and suppresses the proliferation of fibroblasts in hypertrophic scar tissue. Levels of mRNA and TGF-β1, PI3K, Akt, mTOR, and 70S6K proteins are higher in hypertrophic scar tissue than in normal skin.133 Jung et al134 showed that HS-173 (a derivate of imidazo[1,2-a] pyridine) an inhibitor of PI3K, decreased both fibroblast proliferation and differentiation into myofibroblasts in PD plaque by inhibiting PI3K/Akt/mTOR signaling. The activation of p38 mitogen-activated protein kinase (MAPK) and ERK1/2 intracellular signal pathways is potentially implicated in the Smad-independent production of proinflammatory and profibrotic mediators in PD. Tamoxifen inhibits TGF-b-mediated fibroblast activation in cultured primary human fibroblasts by blocking ERK 1/2 signaling.135,136 However, the simultaneous inhibition of several hyperactivated signaling pathways results in more regression of fibrous tissue than the suppression of any single signaling cascade. For instance, Wang et al showed that the multikinase inhibitor sorafenib, which inhibits TGF-β/Smad and MAPK/ERK signaling pathways, increased the ratio of MMP-2/TIMP-1 and MMP-13/TIMP-1 and decreased the expression of CTGF, IL-6, and IL-8 in primary keloid fibroblasts.137 These effects have also been found in a few models of renal fibrosis after the creation of unilateral ureteral obstruction. The pharmacological inhibition of p38 MAPK-ERK1/2 and PI3K-Akt signaling leads to fibrotic regression and the decreased expression of profibrotic factors.138,139 Since PD initially proceeds from the acute phase with repeated exposure to a damaging factor followed by plaque formation, the chronicity of the inflammatory and fibrotic proceeds on a background of altered ECM. This might be associated with constant activation of the JAK-STAT and NF-κB signal cell pathways. The pharmacological inhibition of JAK and STAT proteins suppresses the production of collagen IV, fibronectin, and other components of fibrotic tissue by decreasing the expression of pro-fibrogenic factors.140 Tao et al141 found increased proliferation and differentiation in human hypertrophic scar fibroblasts treated with CTGF, and blocking the JAK/STAT pathway with STAT1 ASODN was obviously inhibited, whereas α-SMA expression was not significantly altered.
The role of the JAK/STAT and NF-κB pathways in fibrosis has been shown in various models of obstructive uropathy. Pang et al142 confirmed the functional role of STAT3 activation in a rat model of unilateral ureteral obstruction, and a STAT3 inhibitor abolished the expression of ɑ-smooth muscle actin and fibronectin, which are markers of myofibroblasts.
Wang et al143 showed that α-keto acid supplements alleviate ischemia-reperfusion (IR)-induced inflammation and apoptosis in animal models of IR-induced renal injury by inhibiting activation of the NF-κB and MAPK pathways. Chuang et al144 showed that the expression of NF-κB significantly correlated with levels of IL-6 and TNF-α in a rat model of ureteral obstruction, and the number of apoptotic cells and their pharmacological inhibition attenuated the tissue damage and inflammatory process. Based on these data, we constructed a scheme of proposed cell signal pathways that might be involved in the pathogenesis of PD (Fig. 4). Despite improved understanding of the roles of the above signaling pathways in fibrogenesis, study results are considerably heterogeneous, and most studies have included animal models and primary cultures of human fibroblasts.
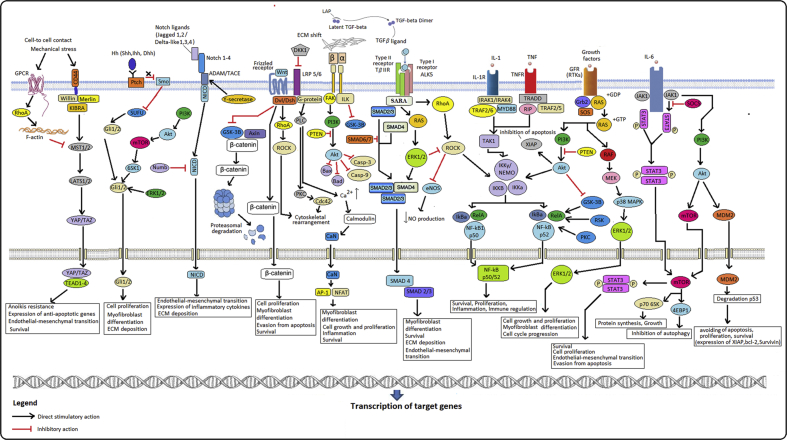
Fig. 4.
Proposed cell signal pathways involved in PD. Several signaling cell cascades that can interact and are hyperactivated can participate in maintaining fibrotic processes. Akt: Protein kinase B; AP-1: activator protein 1; DDK-1: Dickkopf-1; Dsh/Dvl: dishevelled protein; ERK1/2: extracellular regulated kinases; FAK: focal adhesion kinase; Gli1/2, Zinc finger proteins; GF-β1, transforming growth factor β1; Ihh, Dhh, Shh Hedgehog ligands: Indian, Desert, or Sonic Hedgehog; IRAK: interleukin-1 receptor-associated kinase; IKK: inhibitor of nuclear factor kappa-B kinase; JAK: Janus kinase; MEK/p38 MAPK: mitogen-activated protein kinase; mTOR: mechanistic target of rapamycin; NICD: Notch intracellular domain; PI3K: p70S6K1, 70 kDa ribosomal protein S6 kinase 1; Phosphoinositide 3-kinase; PKC: protein kinase C; Ptch: 12-path transmembrane protein patched; PDK: phosphoinositide-dependent protein kinase; PTEN: Phosphatase and tensin homolog; RhoA: Ras homolog gene family, member A; ROCK: Rho-associated protein kinase; SOCS: suppressor of cytokine signaling; Smo: transmembrane protein smoothened; STAT: signal transducer and activator of transcription; SUFU: suppressor of fused homolog; TAK: transforming growth factor beta-activated kinase; TNF: tumor necrosis factor; YAP: Yes-associated protein; SARA: SMAD anchor for receptor activation; SMAD: Mothers Against Decapentaplegic Homolog; 4E-BP1: 4E-binding protein 1; SOS: son of sevenless; TEAD: transcriptional enhanced associate domain; GSK-3B: Glycogen synthase kinase 3 beta; CaN: Calcineurin; NFAT-1: Nuclear factor of activated T-cells 1; Mdm2: E3 ubiquitin-protein ligase; Cdc42: Cell division control protein 42; NEMO: NF-κB essential modulator; TRAF: tumor necrosis factor receptor (TNF-R)-associated factor; MyD88: myeloid differentiation primary response 88; TRADD: TNFR1-associated death domain protein; RIP: receptor-interacting protein.
Nevertheless, we constructed a model of the pathogenesis of PD, based on most ubiquitous signaling pathways involved in fibrogenesis in various tissue structures. Notably, the relatively small numbers of qualitative translational studies of the role of signaling cell pathways have been directly conducted in cultured PD plaque, which has significant limitations. To superimpose knowledge gained about various fibrotic diseases onto PD is difficult due to the heterogeneity of fibroblasts and the intercellular matrix in specific organs and tissues. Given all of the above, translational studies of PD need to be expanded in terms of investigating more intracellular signaling cascades and changes in the intercellular matrix that occur during the development of this disease. Ultimately, the emergence of new agents that will more effectively eliminate fibrous changes in PD plaque, in contrast to the present conservative treatment recommended by EAU with conflicting results, will depend on such investigations. Surely more research will promote the development of multikinase agents or effective combinations that will block several signaling cell pathways involved in the fibrotic process.
Conclusions
The pathogenesis of PD is still not fully understood. Nevertheless, this pathology should be considered as a uniquely localized type of impaired wound processes within the TA and the Smith space. The key to understanding the cause of PA lies in an accurate interpretation of the histological and anatomical features of the PA plaque and the nature of the pathological stimulus that leads to its formation. Many factors influence the imbalance of MMP/TIMP, including a deficiency of specific cofactors, and their replenishment might prevent the pathogenesis and/or improve the course of PD.
Despite the nature of the pathological stimulus, constant hyperactivation of intracellular signaling pathways plays essential roles in the development of the fibrotic process in PD. Eventually, this leads to disruption of myofibroblast apoptosis, circulation of the matrix, and persistent inflammation. The development of multikinase drugs as well as various combinations that can affect intermediate downstream mediators that are common to many signaling cell cascades can significantly block the development of fibrosis rather than blocking mediators of individual intracellular cascades. Further translational studies are needed to determine changes in ubiquitous intracellular signaling pathways directly in models of TA and other structures involved in PD. This will facilitate the construction of a more accurate scheme of pathogenesis, considering various changes in the ECM and cytokine deregulation.
Conflicts of interest
None.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Garaffa G., Trost L.W., Serefoglu E.C., Ralph D., Hellstrom W.J. Understanding the course of Peyronie's disease. Int J Clin Pract. 2013;67:781–788. doi: 10.1111/ijcp.12129. [DOI] [PubMed] [Google Scholar]
- 2.Aliperti L.A., Mehta A. Peyronie's disease: intralesional therapy and surgical intervention. Curr Urol Rep. 2016;17:60. doi: 10.1007/s11934-016-0622-2. [DOI] [PubMed] [Google Scholar]
- 3.De Young L.X., Bella A.J., O'Gorman D.B., Gan B.S., Lim K.B., Brock G.B. Protein biomarker analysis of primary Peyronie's disease cells. J Sex Med. 2010;7:99–106. doi: 10.1111/j.1743-6109.2009.01556.x. [DOI] [PubMed] [Google Scholar]
- 4.Ilg M.M., Cellek S. Unwinding fibrosis in peyronie's disease. J Sex Med. 2020;17:838–840. doi: 10.1016/j.jsxm.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Levine L.A., Larsen S.M. Surgical correction of persistent Peyronie's disease following collagenase clostridium histolyticum treatment. J Sex Med. 2015;12:259–264. doi: 10.1111/jsm.12721. [DOI] [PubMed] [Google Scholar]
- 6.Levine L., Larsen S. Surgery for Peyronie's disease. Asian J Androl. 2012;15:27–34. doi: 10.1038/aja.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twidwell J., Levine L. Topical treatment for acute phase Peyronie's disease utilizing a new gel, H-100: a randomized, prospective, placebo-controlled pilot study. Int J Impot Res. 2016;28:41–45. doi: 10.1038/ijir.2015.22. [DOI] [PubMed] [Google Scholar]
- 8.Chung E., Ralph D., Kagioglu A. Evidence-based management guidelines on peyronie's disease. J Sex Med. 2016;13:905–923. doi: 10.1016/j.jsxm.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 9.Carson C.C., Levine L.A. Outcomes of surgical treatment of Peyronie's disease. BJU Int. 2014;113:704–713. doi: 10.1111/bju.12565. [DOI] [PubMed] [Google Scholar]
- 10.Nehra A., Alterowitz R., Culkin D.J. Peyronie's disease: AUA guideline. J Urol. 2015;194:745–753. doi: 10.1016/j.juro.2015.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herati A.S., Pastuszak A.W. The genetic basis of Peyronie disease: a review. Sex Med Rev. 2016;4:85–94. doi: 10.1016/j.sxmr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somers K.D., Winters B.A., Dawson D.M. Chromosome abnormalities in Peyronie's disease. J Urol. 1987;137:672–675. doi: 10.1016/s0022-5347(17)44170-x. [DOI] [PubMed] [Google Scholar]
- 13.Salonia A., Bettocchi C., Carvalho J. 2020. Sexual and Reproductive Health. Uroweb.https://uroweb.org/guideline/sexual-and-reproductive-health at: [Google Scholar]
- 14.Mulhall J.P., Nicholson B., Pierpaoli S., Lubrano T., Shankey T.V. Chromosomal instability is demonstrated by fibroblasts derived from the tunica of men with Peyronie's disease. Int J Impot Res. 2004;16:288–293. doi: 10.1038/sj.ijir.3901170. [DOI] [PubMed] [Google Scholar]
- 15.Mulhall J.P., Branch J., Lubrano T., Shankey T.V. Perturbation of cell cycle regulators in Peyronie's disease. Int J Impot Res. 2001;13:S21–S28. doi: 10.1038/sj.ijir.3900771. [DOI] [PubMed] [Google Scholar]
- 16.Hauck E.W., Hauptmann A., Schmelz H.U., Bein G., Weidner W., Hackstein H. Prospective analysis of single nucleotide polymorphisms of the transforming growth factor beta-1 gene in Peyronie's disease. J Urol. 2003;169:369–372. doi: 10.1016/S0022-5347(05)64129-8. [DOI] [PubMed] [Google Scholar]
- 17.Dolmans G.H., Werker P.M., de Jong I.J. WNT2 locus is involved in genetic susceptibility of Peyronie's disease. J Sex Med. 2012;9:1430–1434. doi: 10.1111/j.1743-6109.2012.02704.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma K.L., Alom M., Trost L. The etiology of peyronie's disease: pathogenesis and genetic contributions. Sex Med Rev. 2020;8:314–323. doi: 10.1016/j.sxmr.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Zorba O.U., Sirma S., Ozgon G., Salabas E., Ozbek U., Kadioglu A. Comparison of apoptotic gene expression profiles between Peyronie's disease plaque and tunica albuginea. Adv Clin Exp Med. 2012;21:607–614. [PubMed] [Google Scholar]
- 20.Wynes M.W., Edelman B.L., Kostyk A.G. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 21.Dodi A.E., Ajayi I.O., Chang C. Regulation of fibroblast Fas expression by soluble and mechanical pro-fibrotic stimuli. Respir Res. 2018;19:91. doi: 10.1186/s12931-018-0801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon K.D., Choi M.J., Park J.M. Silencing histone deacetylase 2 using small hairpin RNA induces regression of fibrotic plaque in a rat model of Peyronie's disease. BJU Int. 2014;114:926–936. doi: 10.1111/bju.12812. [DOI] [PubMed] [Google Scholar]
- 23.Korfei M., Skwarna S., Henneke I. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax. 2015;70:1022–1032. doi: 10.1136/thoraxjnl-2014-206411. [DOI] [PubMed] [Google Scholar]
- 24.Ryu J.K., Kim W.J., Choi M.J. Inhibition of histone deacetylase 2 mitigates profibrotic TGF-β1 responses in fibroblasts derived from Peyronie's plaque. Asian J Androl. 2013;15:640–645. doi: 10.1038/aja.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang D.H., Yin G.N., Choi M.J. Silencing histone deacetylase 7 alleviates transforming growth factor-β1-induced profibrotic responses in fibroblasts derived from peyronie's plaque. World J Mens Health. 2018;36:139–146. doi: 10.5534/wjmh.170005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramzy M.M., Abdelghany H.M., Zenhom N.M., El-Tahawy N.F. Effect of histone deacetylase inhibitor on epithelial-mesenchymal transition of liver fibrosis. IUBMB Life. 2018;70:511–518. doi: 10.1002/iub.1742. [DOI] [PubMed] [Google Scholar]
- 27.Hu S.W., Ni W., Chen C.F. Comparison between the effects of valproic acid and trichostatin A on in vitro development of sheep somatic cell nuclear transfer embryos. J Anim Vet Adv. 2012;11:1868–1872. [Google Scholar]
- 28.Choi H.S., Song J.H., Kim I.J. Histone deacetylase inhibitor, CG200745 attenuates renal fibrosis in obstructive kidney disease. Sci Rep. 2018;8:11546. doi: 10.1038/s41598-018-30008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi M.J., Song K.M., Park J.M. Effect of SMAD7 gene overexpression on TGF-β1-induced profibrotic responses in fibroblasts derived from Peyronie's plaque. Asian J Androl. 2015;17:487–492. doi: 10.4103/1008-682X.142130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S.P., Yang Z., Li F.R., Liu X.D., Chen H.T., Su D.N. Smad7-overexpressing rat BMSCs inhibit the fibrosis of hepatic stellate cells by regulating the TGF-β1/Smad signaling pathway. Exp Ther Med. 2017;14:2568–2576. doi: 10.3892/etm.2017.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Chen L., Cao C. The long non-coding RNA LncRNA8975-1 is upregulated in hypertrophic scar fibroblasts and controls collagen expression. Cell Physiol Biochem. 2016;40:326–334. doi: 10.1159/000452548. [DOI] [PubMed] [Google Scholar]
- 32.Chen L., Li J., Li Q. Non-coding RNAs: the new insight on hypertrophic scar. J Cell Biochem. 2017;118:1965–1968. doi: 10.1002/jcb.25873. [DOI] [PubMed] [Google Scholar]
- 33.Miscianinov V., Martello A., Rose L. MicroRNA-148b targets the TGF-β pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther. 2018;26:1996–2007. doi: 10.1016/j.ymthe.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 35.Naba A., Pearce O., Del Rosario A. Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J Proteome Res. 2017;16:3083–3091. doi: 10.1021/acs.jproteome.7b00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naba A., Clauser K.R., Ding H., Whittaker C.A., Carr S.A., Hynes R.O. The extracellular matrix: tools and insights for the "omics" era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randles M., Lennon R. Applying proteomics to investigate extracellular matrix in health and disease. Curr Top Membr. 2015;76:171–196. doi: 10.1016/bs.ctm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Giannandrea M., Parks W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apte S.S., Parks W.C. Metalloproteinases: a parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015;44–46:1–6. doi: 10.1016/j.matbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Gunes M., Aslan R., Eryılmaz R., Demir H., Taken K. Levels of serum trace elements in patients with Peyronie. Aging Male. 2018:1–4. doi: 10.1080/13685538.2018.1474195. [DOI] [PubMed] [Google Scholar]
- 41.Sakiyama H., Fujiwara N., Yoneoka Y., Yoshihara D., Eguchi H., Suzuki K. Cu,Zn-SOD deficiency induces the accumulation of hepatic collagen. Free Radic Res. 2016;50:666–677. doi: 10.3109/10715762.2016.1164856. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X., Liang D., Lian X. Effect of zinc deficiency on mouse renal interstitial fibrosis in diabetic nephropathy. Mol Med Rep. 2016;14:5245–5252. doi: 10.3892/mmr.2016.5870. [DOI] [PubMed] [Google Scholar]
- 43.Del Carlo M., Cole A.A., Levine L.A. Differential calcium independent regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by interleukin-1beta and transforming growth factor-beta in Peyronie's plaque fibroblasts. J Urol. 2008;179:2447–2455. doi: 10.1016/j.juro.2008.01.093. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe M.S., Theodoro T.R., Coelho N.L. Extracellular matrix alterations in the Peyronie's disease. J Adv Res. 2017;8:455–461. doi: 10.1016/j.jare.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodall K.J., Poon I.K., Phipps S., Hulett M.D. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PloS One. 2014;9 doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yue X., Lu J., Auduong L., Sides M.D., Lasky J.A. Overexpression of Sulf2 in idiopathic pulmonary fibrosis. Glycobiology. 2013;23:709–719. doi: 10.1093/glycob/cwt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parish C.R., Freeman C., Ziolkowski A.F. Unexpected new roles for heparanase in Type 1 diabetes and immune gene regulation. Matrix Biol. 2013;32:228–233. doi: 10.1016/j.matbio.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., Khalil R.A. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog Mol Biol Transl Sci. 2017;148:355–420. doi: 10.1016/bs.pmbts.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batra J., Robinson J., Soares A.S., Fields A.P., Radisky D.C., Radisky E.S. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: binding studies and crystal structure. J Biol Chem. 2012;287:15935–15946. doi: 10.1074/jbc.M112.341156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobsen J.A., Major Jourden J.L., Miller M.T., Cohen S.M. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta. 2010;1803:72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Akman T., Tefekli A., Armagan A. Decorin as a new treatment alternative in Peyronie's disease: preliminary results in the rat model. Andrologia. 2013;45:101–106. doi: 10.1111/j.1439-0272.2012.01318.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z., Garron T.M., Li X.J. Recombinant human decorin inhibits TGF-beta1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35:527–537. doi: 10.1016/j.burns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Jiang N., Zhang Q., Chau M.K. Anti-fibrotic effect of decorin in peritoneal dialysis and PD-associated peritonitis. EBioMedicine. 2020;52:102661. doi: 10.1016/j.ebiom.2020.102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kantola A.K., Ryynänen M.J., Lhota F., Keski-Oja J., Koli K. Independent regulation of short and long forms of latent TGF-beta binding protein (LTBP)-4 in cultured fibroblasts and human tissues. J Cell Physiol. 2010;223:727–736. doi: 10.1002/jcp.22082. [DOI] [PubMed] [Google Scholar]
- 55.Van de Water L. Mechanisms by which fibrin and fibronectin appear in healing wounds: implications for Peyronie's disease. J Urol. 1997;157:306–310. doi: 10.1097/00005392-199701000-00103. [DOI] [PubMed] [Google Scholar]
- 56.Davila H.H., Ferrini M.G., Rajfer J., Gonzalez-Cadavid N.F. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie's disease. BJU Int. 2003;91:830–838. doi: 10.1046/j.1464-410x.2003.04224.x. [DOI] [PubMed] [Google Scholar]
- 57.Graziottin T.M. The pathophysiology of Peyronie's disease: beyond the Smith's space. Int Braz J Urol. 2015;41:1040–1042. doi: 10.1590/S1677-5538.IBJU.2015.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernau K., Torr E.E., Evans M.D., Aoki J.K., Ngam C.R., Sandbo N. Tensin 1 is essential for myofibroblast differentiation and extracellular matrix formation. Am J Respir Cell Mol Biol. 2017;56:465–476. doi: 10.1165/rcmb.2016-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wight T.N. Provisional matrix: a role for versican and hyaluronan. Matrix Biol. 2017;60–61:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gefen A., Elad D., Chen J. Biomechanical aspects of Peyronie's disease in development stages and following reconstructive surgeries. Int J Impot Res. 2002;14:389–396. doi: 10.1038/sj.ijir.3900866. [DOI] [PubMed] [Google Scholar]
- 61.Herwig R., Bayerl M. Superficial tunica albuginea rupture as initial starting point of Peyronie's disease: a topic for interdisciplinary consideration. BioMed Res Int. 2015;2015:751372. doi: 10.1155/2015/751372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong X., Zhao B., Iacob R.E. Force interacts with macromolecular structure in activation of TGF-β. Nature. 2017;542:55–59. doi: 10.1038/nature21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu W., Hutcheon A., Sriram S., Tran J.A., Zieske J.D. Initiation of fibrosis in the integrin Αvβ6 knockout mice. Exp Eye Res. 2019;180:23–28. doi: 10.1016/j.exer.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koivisto L., Bi J., Häkkinen L., Larjava H. Integrin αvβ6: structure, function and role in health and disease. Int J Biochem Cell Biol. 2018;99:186–196. doi: 10.1016/j.biocel.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Lord M.S., Tang F., Rnjak-Kovacina J., Smith J., Melrose J., Whitelock J.M. The multifaceted roles of perlecan in fibrosis. Matrix Biol. 2018;68–69:150–166. doi: 10.1016/j.matbio.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Hussein A.A., Alwaal A., Lue T.F. All about Peyronie's disease. Asian J Urol. 2015;2:70–78. doi: 10.1016/j.ajur.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agrawal V., Ellins E., Donald A., Minhas S., Halcox J., Ralph D.J. Systemic vascular endothelial dysfunction in Peyronie's disease. J Sex Med. 2008;5:2688–2693. doi: 10.1111/j.1743-6109.2008.00947.x. [DOI] [PubMed] [Google Scholar]
- 68.Atar A., Kural A., Yenice G., Comez I., Tugcu V. Role of interleukin-6 and pentraxin 3 as an early marker in Peyronie's disease. Kaohsiung J Med Sci. 2017;33:195–200. doi: 10.1016/j.kjms.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Zimmermann R.P., Feil G., Bock C., Hoeltl L., Stenzl A. Significant alterations of serum cytokine levels in patients with Peyronie's disease. Int Braz J Urol. 2008;34:457–466. doi: 10.1590/s1677-55382008000400008. [DOI] [PubMed] [Google Scholar]
- 70.Cantini L.P., Ferrini M.G., Vernet D. Profibrotic role of myostatin in Peyronie's disease. J Sex Med. 2008;5:1607–1622. doi: 10.1111/j.1743-6109.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 71.Ribatti D., Tamma R. Giulio Gabbiani and the discovery of myofibroblasts. Inflamm Res. 2019;68:241–245. doi: 10.1007/s00011-018-01211-x. [DOI] [PubMed] [Google Scholar]
- 72.Lucattelli M., Lunghi B., Fineschi S. A new mouse model of Peyronie's disease: an increased expression of hypoxia-inducible factor-1 target genes during the development of penile changes. Int J Biochem Cell Biol. 2008;40:2638–2648. doi: 10.1016/j.biocel.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 73.Papadopoulos N., Lennartsson J. The PDGF/PDGFR pathway as a drug target. Mol Aspect Med. 2018;62:75–88. doi: 10.1016/j.mam.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Klinkhammer B.M., Floege J., Boor P. PDGF in organ fibrosis. Mol Aspect Med. 2018;62:44–62. doi: 10.1016/j.mam.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Ramazani Y., Knops N., Elmonem M.A. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018;68–69:44–66. doi: 10.1016/j.matbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Lipson K.E., Wong C., Teng Y., Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pavone C., Napoli G., Caruana G., Alonge V., Usala M., Abbadessa D. Safety and tolerability of local treatment with iloprost, a prostacyclin analogue, in patients with Peyronie's disease: a phase I study. BJU Int. 2012;110:117–121. doi: 10.1111/j.1464-410X.2011.10733.x. [DOI] [PubMed] [Google Scholar]
- 78.Barnes J.L., Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sampson N., Berger P., Zenzmaier C. Redox signaling as a therapeutic target to inhibit myofibroblast activation in degenerative fibrotic disease. BioMed Res Int. 2014;2014:1–14. doi: 10.1155/2014/131737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan E.C., Peshavariya H.M., Liu G.S., Jiang F., Lim S.Y., Dusting G.J. Nox4 modulates collagen production stimulated by transforming growth factor β1 in vivo and in vitro. Biochem Biophys Res Commun. 2013;430:918–925. doi: 10.1016/j.bbrc.2012.11.138. [DOI] [PubMed] [Google Scholar]
- 81.Liu R.M., Desai L.P. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang W.T., Akhter H., Jiang C. Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis. Exp Gerontol. 2015;61:62–75. doi: 10.1016/j.exger.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rabieian R., Boshtam M., Zareei M., Kouhpayeh S., Masoudifar A., Mirzaei H. Plasminogen activator inhibitor type-1 as a regulator of fibrosis. J Cell Biochem. 2018;119:17–27. doi: 10.1002/jcb.26146. [DOI] [PubMed] [Google Scholar]
- 84.Davila H.H., Magee T.R., Zuniga F.I., Rajfer J., Gonzalez-Cadavid N.F. Peyronie's disease associated with increase in plasminogen activator inhibitor in fibrotic plaque. Urology. 2005;65:645–648. doi: 10.1016/j.urology.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 85.Bishen K.A., Radhakrishnan R., Satyamoorthy K. The role of basic fibroblast growth factor in oral submucous fibrosis pathogenesis. J Oral Pathol Med. 2008;37:402–411. doi: 10.1111/j.1600-0714.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 86.Barrientos S., Stojadinovic O., Golinko M., Brem H., Tomic-Canic M. Perspective article: growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 87.Mulhall J., Thom J., Lubrano T., Shankey T. Basic fibroblasts growth factor expression in peyronie's' disease. J Urol. 2001;165:419–423. doi: 10.1097/00005392-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 88.Mateus M., Ilg M.M., Stebbeds W.J. Understanding the role of adenosine receptors in the myofibroblast transformation in peyronie's disease. J Sex Med. 2018;15:947–957. doi: 10.1016/j.jsxm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 89.Wen J., Jiang X., Dai Y. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. Faseb J. 2010;24:740–749. doi: 10.1096/fj.09-144147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gur S., Kadowitz P.J., Hellstrom W.J. Drugs of the future for Peyronie's disease. Med Hypotheses. 2012;78:305–311. doi: 10.1016/j.mehy.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Canguven O., Lagoda G., Sezen S.F., Burnett A.L. Losartan preserves erectile function after bilateral cavernous nerve injury via antifibrotic mechanisms in male rats. J Urol. 2009;181:2816–2822. doi: 10.1016/j.juro.2009.01.097. [DOI] [PubMed] [Google Scholar]
- 92.Shafik A., El-Sharkawy A., Khamis A., Zaghloul S., Abdel Gawad M., Elwy D. Histologic study of the tunica albuginea of the penis and mode of cavernosus muscles' insertion in it. Arch Androl. 2006;52:1–8. doi: 10.1080/01485010500203667. [DOI] [PubMed] [Google Scholar]
- 93.Castorina A., Loreto C., Vespasiani G. Increased aquaporin 1 expression in the tunica albuginea of Peyronie's disease patients: an in vivo pilot study. Histol Histopathol. 2016;31:1241–1249. doi: 10.14670/HH-11-755. [DOI] [PubMed] [Google Scholar]
- 94.Thomas C.G., Psarros C., Gekas A., Vandoros G.P., Philippou A., Koutsilieris M. Alternative splicing of IGF1 gene as a potential factor in the pathogenesis of peyronie's disease. In Vivo. 2016;30:251–256. [PubMed] [Google Scholar]
- 95.Szardening-Kirchner C., Konrad L., Hauck E.W., Haag S.M., Eickelberg O., Weidner W. Upregulation of mRNA expression of MCP-1 by TGF-beta1 in fibroblast cells from Peyronie's disease. World J Urol. 2009;27:123–130. doi: 10.1007/s00345-008-0320-x. [DOI] [PubMed] [Google Scholar]
- 96.Kottmann R.M., Kulkarni A.A., Smolnycki K.A. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med. 2012;186:740–751. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kottmann R.M., Trawick E., Judge J.L. Pharmacologic inhibition of lactate production prevents myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol. 2015;309:1305–1312. doi: 10.1152/ajplung.00058.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darby I.A., Zakuan N., Billet F., Desmoulière A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci. 2016;73:1145–1157. doi: 10.1007/s00018-015-2110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gelfand R.A., Vernet D., Kovanecz I., Rajfer J., Gonzalez-Cadavid N.F. The transcriptional signatures of cells from the human Peyronie's disease plaque and the ability of these cells to generate a plaque in a rat model suggest potential therapeutic targets. J Sex Med. 2015;12:313–327. doi: 10.1111/jsm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Safaeian L., Abed A., Vaseghi G. The role of Bcl-2 family proteins in pulmonary fibrosis. Eur J Pharmacol. 2014;741:281–289. doi: 10.1016/j.ejphar.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 101.Loreto C., Barbagli G., Djinovic R. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and its death receptor (DR5) in Peyronie's disease. A biomolecular study of apoptosis activation. J Sex Med. 2011;8:109–115. doi: 10.1111/j.1743-6109.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- 102.Loreto C., La Rocca G., Anzalone R. The role of intrinsic pathway in apoptosis activation and progression in Peyronie's disease. BioMed Res Int. 2014;2014:1–10. doi: 10.1155/2014/616149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bellaye P.S., Burgy O., Causse S., Garrido C., Bonniaud P. Heat shock proteins in fibrosis and wound healing: good or evil. Pharmacol Ther. 2014;143:119–132. doi: 10.1016/j.pharmthera.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 104.Lee W.J., Ahn H.M., Na Y., Wadhwa R., Hong J., Yun C.O. Mortalin deficiency suppresses fibrosis and induces apoptosis in keloid spheroids. Sci Rep. 2017;7:12957. doi: 10.1038/s41598-017-13485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ajayi I.O., Sisson T.H., Higgins P.D. X-linked inhibitor of apoptosis regulates lung fibroblast resistance to Fas-mediated apoptosis. Am J Respir Cell Mol Biol. 2013;49:86–95. doi: 10.1165/rcmb.2012-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu B.H., Chen L., Li S.R., Wang Z.X., Cheng W.G. Smac/DIABLO regulates the apoptosis of hypertrophic scar fibroblasts. Int J Mol Med. 2013;32:615–622. doi: 10.3892/ijmm.2013.1442. [DOI] [PubMed] [Google Scholar]
- 107.Jang J.H., Ryu J.K., Suh J.K. Activin receptor-like kinase 5 inhibitor attenuates fibrosis in fibroblasts derived from peyronie's plaque. Korean J Urol. 2012;53:44–49. doi: 10.4111/kju.2012.53.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Piao S., Choi M.J., Tumurbaatar M. Transforming growth factor (TGF)-β type I receptor kinase (ALK5) inhibitor alleviates profibrotic TGF-β1 responses in fibroblasts derived from Peyronie's plaque. J Sex Med. 2010;7:3385–3395. doi: 10.1111/j.1743-6109.2010.01753.x. [DOI] [PubMed] [Google Scholar]
- 109.Haag S.M., Hauck E.W., Szardening-Kirchner C. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie's disease. Eur Urol. 2007;51:255–261. doi: 10.1016/j.eururo.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 110.El-Sakka A.I., Yassin A.A. Amelioration of penile fibrosis: myth or reality. J Androl. 2010;31:324–335. doi: 10.2164/jandrol.109.008730. [DOI] [PubMed] [Google Scholar]
- 111.Andrianifahanana M., Wilkes M.C., Gupta S.K. Profibrotic TGFβ responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. Faseb J. 2013;27:4444–4454. doi: 10.1096/fj.12-224907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Akhmetshina A., Palumbo K., Dees C. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guo Y., Sun L., Xiao L. Aberrant wnt/beta-catenin pathway activation in dialysate-induced peritoneal fibrosis. Front Pharmacol. 2017;8:774. doi: 10.3389/fphar.2017.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y., Qiu S.S., Shao Y. Dickkopf-1 has an inhibitory effect on mesenchymal stem cells to fibroblast differentiation. Chin Med J (Engl). 2016;129:1200–1207. doi: 10.4103/0366-6999.181974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Enzo M.V., Rastrelli M., Rossi C.R., Hladnik U., Segat D. The Wnt/β-catenin pathway in human fibrotic-like diseases and its eligibility as a therapeutic target. Mol Cell Ther. 2015;3:1. doi: 10.1186/s40591-015-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dees C., Distler J.H. Canonical Wnt signalling as a key regulator of fibrogenesis - implications for targeted therapies. Exp Dermatol. 2013;22:710–713. doi: 10.1111/exd.12255. [DOI] [PubMed] [Google Scholar]
- 117.Dees C., Zerr P., Tomcik M. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011;63:1396–1404. doi: 10.1002/art.30254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu B., Phan S.H. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol Res. 2016;108:57–64. doi: 10.1016/j.phrs.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moya I.M., Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20:211–226. doi: 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- 120.Jin H., Lian N., Zhang F. Inhibition of YAP signaling contributes to senescence of hepatic stellate cells induced by tetramethylpyrazine. Eur J Pharmaceut Sci. 2017;96:323–333. doi: 10.1016/j.ejps.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Piersma B., Bank R.A., Boersema M. Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med (Lausanne) 2015;2:59. doi: 10.3389/fmed.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patel D.P., Christensen M.B., Hotaling J.M., Pastuszak A.W. A review of inflammation and fibrosis: implications for the pathogenesis of Peyronie's disease. World J Urol. 2020;38:253–261. doi: 10.1007/s00345-019-02815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu F., Lagares D., Choi K.M. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Milenkovic U., Ilg M.M., Zuccato C., Ramazani Y., De Ridder D., Albersen M. Simvastatin and the Rho-kinase inhibitor Y-27632 prevent myofibroblast transformation in Peyronie's disease-derived fibroblasts via inhibition of YAP/TAZ nuclear translocation. BJU Int. 2019;123:703–715. doi: 10.1111/bju.14638. [DOI] [PubMed] [Google Scholar]
- 125.Xu N., Chen S.H., Qu G.Y. Fasudil inhibits proliferation and collagen synthesis and induces apoptosis of human fibroblasts derived from urethral scar via the Rho/ROCK signaling pathway. Am J Transl Res. 2017;9:1317–1325. [PMC free article] [PubMed] [Google Scholar]
- 126.Li X.D., Wu Y.P., Chen S.H. Fasudil inhibits actin polymerization and collagen synthesis and induces apoptosis in human urethral scar fibroblasts via the Rho/ROCK pathway. Drug Des Dev Ther. 2018;12:2707–2713. doi: 10.2147/DDDT.S156095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu L., Lin X., Lu H., Chen B., Bai Y. An overview of hedgehog signaling in fibrosis. Mol Pharmacol. 2015;87:174–182. doi: 10.1124/mol.114.095141. [DOI] [PubMed] [Google Scholar]
- 128.Briscoe J., Thérond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 129.Kramann R. Hedgehog Gli signalling in kidney fibrosis. Nephrol Dial Transplant. 2016;31:1989–1995. doi: 10.1093/ndt/gfw102. [DOI] [PubMed] [Google Scholar]
- 130.Lin H., Wang T., Ruan Y. Rapamycin supplementation may ameliorate erectile function in rats with streptozotocin-induced type 1 diabetes by inducing autophagy and inhibiting apoptosis, endothelial dysfunction, and corporal fibrosis. J Sex Med. 2018;15:1246–1259. doi: 10.1016/j.jsxm.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 131.Liang M., Lv J., Chu H. Vertical inhibition of PI3K/Akt/mTOR signaling demonstrates in vitro and in vivo anti-fibrotic activity. J Dermatol Sci. 2014;76:104–111. doi: 10.1016/j.jdermsci.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Song Y., Guo B., Ma S., Chang P., Tao K. Naringin suppresses the growth and motility of hypertrophic scar fibroblasts by inhibiting the kinase activity of Akt. Biomed Pharmacother. 2018;105:1291–1298. doi: 10.1016/j.biopha.2018.06.103. [DOI] [PubMed] [Google Scholar]
- 133.Zhai X.X., Tang Z.M., Ding J.C., Lu X.L. Expression of TGF-β1/mTOR signaling pathway in pathological scar fibroblasts. Mol Med Rep. 2017;15:3467–3472. doi: 10.3892/mmr.2017.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jung K.H., Ryu Y.L., Lee H.S. A novel PI3K inhibitor alleviates fibrotic responses in fibroblasts derived from Peyronie's plaques. Int J Oncol. 2013;42:2001–2008. doi: 10.3892/ijo.2013.1905. [DOI] [PubMed] [Google Scholar]
- 135.Lee J., An J.N., Hwang J.H., Lee H., Lee J.P., Kim S.G. p38 MAPK activity is associated with the histological degree of interstitial fibrosis in IgA nephropathy patients. PloS One. 2019;14 doi: 10.1371/journal.pone.0213981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carthy J.M., Sundqvist A., Heldin A. Tamoxifen inhibits TGF-β-mediated activation of myofibroblasts by blocking non-smad signaling through ERK1/2. J Cell Physiol. 2015;230:3084–3092. doi: 10.1002/jcp.25049. [DOI] [PubMed] [Google Scholar]
- 137.Wang W., Qu M., Xu L. Sorafenib exerts an anti-keloid activity by antagonizing TGF-β/Smad and MAPK/ERK signaling pathways. J Mol Med (Berl) 2016;94:1181–1194. doi: 10.1007/s00109-016-1430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodríguez-Peña A.B., Grande M.T., Eleno N. Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int. 2008;74:196–209. doi: 10.1038/ki.2008.160. [DOI] [PubMed] [Google Scholar]
- 139.Gao F., Wang Y., Li S., Wang Z., Liu C., Sun D. Inhibition of p38 mitogen-activated protein kinases attenuates renal interstitial fibrosis in a murine unilateral ureteral occlusion model. Life Sci. 2016;167:78–84. doi: 10.1016/j.lfs.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 140.Pedroza M., Le T.T., Lewis K. STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation. Faseb J. 2016;30:129–140. doi: 10.1096/fj.15-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tao L., Liu J., Li Z., Dai X., Li S. Role of the JAK-STAT pathway in proliferation and differentiation of human hypertrophic scar fibroblasts induced by connective tissue growth factor. Mol Med Rep. 2010;3:941–945. doi: 10.3892/mmr.2010.349. [DOI] [PubMed] [Google Scholar]
- 142.Pang M., Ma L., Gong R. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010;78:257–268. doi: 10.1038/ki.2010.154. [DOI] [PubMed] [Google Scholar]
- 143.Wang M., Xu H., Chong Lee Shin O.L. Compound α-keto acid tablet supplementation alleviates chronic kidney disease progression via inhibition of the NF-kB and MAPK pathways. J Transl Med. 2019;17:122. doi: 10.1186/s12967-019-1856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chuang Y.H., Chuang W.L., Huang S.P., Liu C.K., Huang C.H. Inhibition of nuclear factor-kappa B (NF-kappaB) activation attenuates ureteric damage in obstructive uropathy. Pharmacol Res. 2009;60:347–357. doi: 10.1016/j.phrs.2009.05.004. [DOI] [PubMed] [Google Scholar]