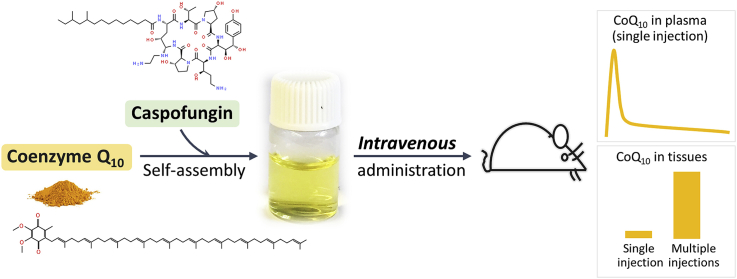
Abstract
Coenzyme Q10 (CoQ10; also known as ubiquinone) is a vital, redox-active membrane component that functions as obligate electron transporter in the mitochondrial respiratory chain, as cofactor in other enzymatic processes and as antioxidant. CoQ10 supplementation has been widely investigated for treating a variety of acute and chronic conditions in which mitochondrial function or oxidative stress play a role. In addition, it is used as replacement therapy in patients with CoQ deficiency including inborn primary CoQ10 deficiency due to mutations in CoQ10-biosynthetic genes as well as secondary CoQ10 deficiency, which is frequently observed in patients with mitochondrial disease syndrome and in other conditions. However, despite many tests and some promising results, whether CoQ10 treatment is beneficial in any indication has remained inconclusive. Because CoQ10 is highly insoluble, it is only available in oral formulations, despite its very poor oral bioavailability. Using a novel model of CoQ-deficient cells, we screened a library of FDA-approved drugs for an activity that could increase the uptake of exogenous CoQ10 by the cell. We identified the fungicide caspofungin as capable of increasing the aqueous solubility of CoQ10 by several orders of magnitude. Caspofungin is a mild surfactant that solubilizes CoQ10 by forming nano-micelles with unique properties favoring stability and cellular uptake. Intravenous administration of the formulation in mice achieves unprecedented increases in CoQ10 plasma levels and in tissue uptake, with no observable toxicity. As it contains only two safe components (caspofungin and CoQ10), this injectable formulation presents a high potential for clinical safety and efficacy.
Keywords: Coenzyme Q, Ubiquinone, Mitochondrial diseases, Micelle, Caspofungin
Graphical abstract
Highlights
-
•
Coenzyme Q10 (CoQ10) can be solubilized by the antifungal drug caspofungin (CF).
-
•
CF is a mild surfactant and solubilizes CoQ10 in water by forming micellar structures with a high CoQ10 content.
-
•
CF/CoQ10 micelles have unique properties favoring rapid and efficient uptake into cells and mitochondria.
-
•
CF/CoQ10 micelles can be intravenously administrated without signs of toxicity.
-
•
Intravenous administration of CF/CoQ10 in mice achieves unprecedented elevation of CoQ10 plasma levels and tissue uptake.
1. Introduction
Coenzyme Q (CoQ), also called ubiquinone (UQ), is a redox-active, lipid-soluble component of biological membranes. It is an obligate mobile electron carrier in the mitochondrial electron transport chain (ETC) [1,2]. In addition, CoQ has antioxidant properties that are believed to protect membrane lipids from oxidative damage [3,4]. Several other redox functions have also been described for CoQ, including in sulfide oxidation, in regulating the mitochondrial permeability transition pore, and in plasma membrane electron transport and proton translocation across lysosomal membranes [[4], [5], [6]].
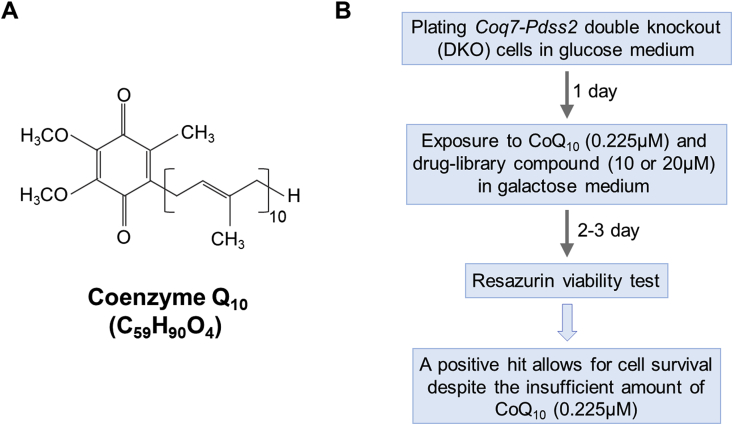
CoQ is comprised of a redox-active benzoquinone ring linked to an isoprenoid side-chain (Fig. 1A). The length of the side-chain, indicated by a subscript, is species-specific. Humans have CoQ10 and mice mostly CoQ9, with some CoQ10. The final steps of CoQ biosynthesis in the inner mitochondrial membrane begin with the assembly of the polyisoprenoid side-chain and its attachment to the benzoquinone ring, which then undergoes a series of modifications (decarboxylation, hydroxylations and methylations) to obtain the final product [[7], [8], [9], [10], [11], [12], [13]]. In addition to the actual enzymes, several other proteins are necessary for CoQ biosynthesis [[14], [15], [16], [17], [18]].
Fig. 1.
Screen design for compounds that enhance cellular uptake of exogenous CoQ10. (A) Structure of CoQ10. (B) We screened for the ability of compounds to rescue the survival of DKO cells in galactose medium supplemented with an amount of CoQ10 (0.225 μM) that is insufficient to allow for survival by itself.
Patients with mutations in genes involved in the biosynthesis of CoQ suffer from inborn primary CoQ deficiency (MIM 607426). So far, pathogenic mutations for 9 of the 11 genes currently known to participate in human CoQ biosynthesis have been identified, with an estimated 125,000 individuals affected worldwide [7,[19], [20], [21]]. CoQ deficiency is associated with numerous symptoms, including nephrotic syndrome, infantile encephalomyopathy, ataxia and cerebellar atrophy [7,[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]]. Like mitochondrial disease syndrome (MDS), primary CoQ deficiency is often a multi-systems disorder with heterogeneous clinical manifestations. In addition, patients with MDS and other diseases frequently suffer from secondary CoQ10 deficiency [16,[48], [49], [50], [51]]. A decline of CoQ10 levels is also observed during aging and in some age-related diseases (e.g., Parkinson disease) [[52], [53], [54]]. The mechanisms responsible for secondary deficiency remain unknown.
In addition to the possibility of treating CoQ10 deficiency, supplementing CoQ10 has long been put forward as a potential treatment for many conditions that have components of mitochondrial dysfunction or oxidative stress, and numerous clinical trials have been conducted with CoQ10 for a variety of disorders. Examples include inborn mitochondrial disorders, heart disease, diabetes and neurodegenerative diseases [49,[55], [56], [57], [58], [59], [60]]. However, only oral CoQ10 is currently available and, despite the sensible rationale and some positive reports, it appears to be only minimally effective [14,37,[42], [43], [44], [45],61]. In fact, although numerous oral CoQ10 products are available, CoQ10 supplementation is not yet FDA-approved to treat any medical condition.
One reason for the therapeutic failure of CoQ10 is its poor oral bioavailability and its extreme hydrophobicity which prevents parenteral delivery. Here we report a water-soluble CoQ10 formulation made by using the FDA-approved drug caspofungin (brand name Cancidas). We show that this formulation can be safely administered intravenously to animals, which raises CoQ10 levels by unprecedented amounts in all tissues tested. Given that the only component of the formulation besides CoQ10 is safe and approved, there should be few obstacles to its clinical development and approval for the treatment of CoQ deficiency and other diseases.
2. Results
2.1. Identification of caspofungin (CF) in a screen for drugs that enhance uptake of exogenous CoQ
We obtained mouse cells entirely devoid of CoQ and CoQ biosynthetic intermediates by viral delivery of Cre-recombinase into mouse embryonic fibroblasts (MEFs) carrying floxed alleles of two CoQ-biosynthetic genes, Pdss2 and Coq7. Pdss2 (prenyldiphosphate synthase subunit 2) is one of two subunits of the prenyl diphosphate synthase that is responsible for the first committed step of CoQ biosynthesis, the assembly and elongation of the polyisoprenoid side chain [13]. The Coq7 hydroxylase catalyzes the penultimate step which is the C6-hydroxylation of 5-demethoxyubiquinone (DMQ). Coq7 null mutants, in addition to losing the ability to make CoQ, accumulate DMQ [10,11,62,63]. As detailed in Fig. S1A and B, the expression of both genes was lost in the Pdss2f/f/;Coq7fl/fl MEFs after infection with the Cre-bearing retrovirus, with CoQ and DMQ becoming undetectable, as expected. Below we refer to the cells with the excised genes as double knockout (DKO) cells, with the Pdss2fl/fl;Coq7fl/fl MEFs infected with an empty viral vector serving as controls. DKO cells show no growth impairment under standard growth conditions in a medium containing high levels of glucose. However, they display a severely impaired oxygen consumption rate (OCR) and a virtually complete loss of spare respiratory capacity (Fig. S1C). These cells die within 2 days after transfer from a glucose-rich medium to a glucose free, galactose-containing, medium. The replacement of glucose with galactose forces cells to rely more heavily on aerobic respiration for energy production. A dose-dependent rescue of survival in galactose medium was observed when the medium was supplemented with CoQ10 (Fig. S1D).
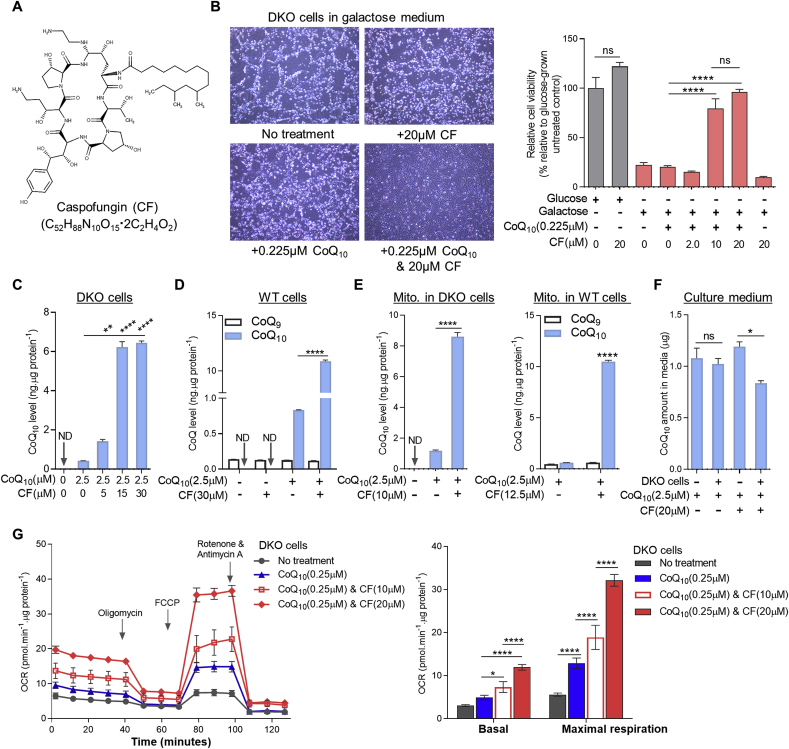
We used the DKO cells to screen a library of FDA-approved drugs (Selleckchem) for compounds that can boost the cellular uptake of exogenous CoQ. The screen design is shown in Fig. 1B. Briefly, we screened for compounds that can rescue the death of DKO cells in a glucose free, but galactose-containing medium (referred to as galactose medium below) supplemented with a very small amount of CoQ10 (an amount that is insufficient by itself to restore the viability of DKO cells in this medium). The screen could therefore identify compounds that can boost the uptake or the functional efficiency of the minimal amount of added CoQ10. We tested the 1018 compounds of the library at two concentrations (10 and 20 μM). Only one compound, caspofungin acetate (CF) (Fig. 2A), was able to rescue DKO cell viability in presence of the minimal amount of CoQ10 (Fig. 2B). CF is an antifungal drug that inhibits fungal cell wall synthesis and has no known target in mammalian cells [64]. Addition of CoQ10 at 0.225 μM or CF alone did not affect survival of DKO cells in galactose medium, but inclusion of both completely rescued viability (Fig. 2B and Fig. S2).
Fig. 2.
Caspofungin (CF) increases cellular uptake of exogenous CoQ. (A) Chemical structure of CF, a semi-synthetic lipopeptide composed of a cyclic hexapeptide N-linked to an acyl fatty acid side-chain. (B) Viability of DKO cells after 2 days of treatment under various conditions. Cell viability was measured by the resazurin viability assay (n = 8). See Fig. S2 for cell viability data measured by crystal violet staining. (C-D) Changes of CoQ10 levels in DKO or WT cells treated with CoQ10 and/or CF for 3 days (n = 3). (E) Mitochondrial CoQ concentrations in DKO or WT cells after 3-day treatment with either CoQ10 and/or CF (n = 3). Mito. means mitochondria. (F) Total CoQ10 amount in culture medium (10 ml per 10 cm dish) after 2 days of incubation under different conditions (n = 3). In b-f, data shown are mean ± SEM. One-way ANOVA followed by Tukey's post hoc test or Student's t-test was used to compare CoQ9 or CoQ10 levels between groups. (G) Comparison of the effects of CoQ10 treatment only and co-treatment of CoQ10 and CF on mitochondrial respiration of DKO cells. Representative oxygen consumption rate (OCR) traces are shown on the left. Bar graph shows summary data. Error bars represent standard deviations (SD) (n = 5). The data were subjected to two-way ANOVA followed by Tukey's multiple comparison test. ND: not detectable. ns: not significant. *p < 0.05, **p < 0.05, and ****p < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To examine the effect of CF on CoQ uptake into cells, we used higher concentrations of CoQ10 (2.5 μM) than to examine viability. As shown in Fig. 2C and D, in comparison to treatment with CoQ10 alone, there was a much greater, dose-dependent, increase of total cellular CoQ10 in both DKO and Pdss2fl/fl;Coq7fl/fl MEFs (referred to as WT cells below) when CF was added in combination with CoQ10. In WT cells the presence of CF increased CoQ10 uptake >13-fold, resulting in >78-fold more CoQ10 than endogenous CoQ9 (Fig. 2D). As part of its biological function, CoQ can exist in both reduced and oxidized states. However, reduced CoQ is inherently unstable and readily converts to oxidized CoQ when exposed to air and light during the extraction process [65]. Therefore, the amount of absorbance at 275 nm at which we measure CoQ, and which corresponds to oxidized CoQ, measures the total CoQ concentration (See Materials and Methods).
We further measured CoQ levels in mitochondria and observed that there was >7–17 times more CoQ10 uptake in cells co-treated with CF and CoQ10 relative to cells treated with the same amount of CoQ10 alone (Fig. 2E). Consistent with this, we found that after incubation of DKO cells with CF and CoQ10, the amount of CoQ10 in the culture media was decreased, further pointing to a dramatic and active CoQ10 uptake by the cells (Fig. 2F). Of note, no treatment had any effect on the level of endogenous CoQ (CoQ9 in mouse cells) (Fig. 2D and E). In order to confirm the intra-mitochondrial functionality of the exogenous CoQ10 after uptake, we measured effects on mitochondrial respiration. After 2 days of treatment with 0.25 μM of CoQ10, DKO cells showed no change of basal respiration rate, but a small increase of mitochondrial respiratory capacity (assessed by addition of the uncoupler FCCP). However, a much larger dose-dependent increase was observed for both basal and maximal respiration after 2 days of treatment with CF (10 or 20 μM) and CoQ10 (0.25 μM) simultaneously (Fig. 2G). CF by itself had no effect, as shown in Fig. S3.
2.2. Caspofungin increases the water solubility of CoQ10
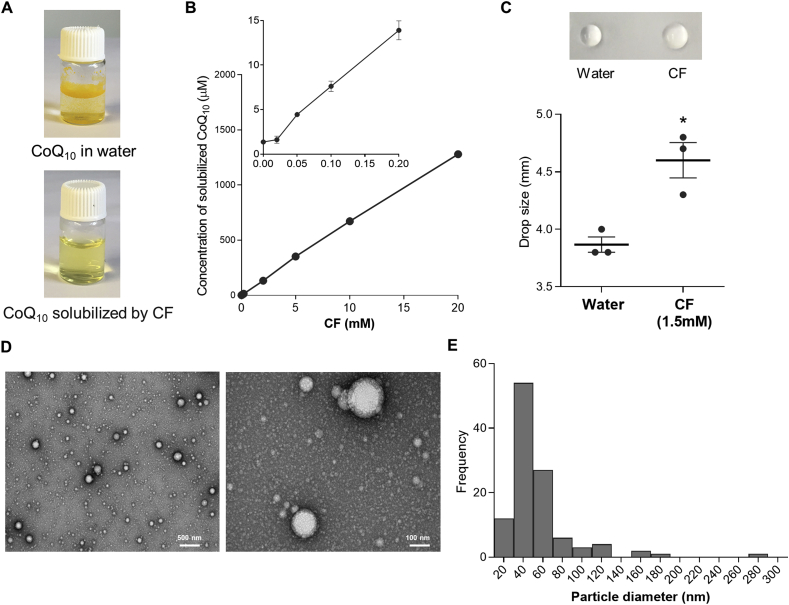
The solubility of CoQ10 in water is extremely low. Fig. 3A shows that the yellow powder that is pure CoQ10 cannot dissolve in water. As CF is water-soluble, we added CoQ10 powder into a CF water solution and mixed by sonicating for ~2 min. The mixture was then filtered through a 0.22 μm sterile filter to remove undissolved material and to sterilize the solution. This resulted in a clear yellow solution with 2–5 mM of CoQ10 (Fig. S4). The aqueous solubility of CoQ10 by itself is reported to be around 0.7 ng/ml [66]. Therefore, a CoQ10 concentration of >2 mM indicates an >2.4 × 106 fold increase of solubility. Fig. 3B shows that the solubilization of CoQ10 by CF follows a linear dose-dependency. In the drop-collapse test, which is commonly used for rapid determination of wetting properties of a surfactant [67], we observed that CF is surface-active (Fig. 3C). Transmission electron microscopy (TEM) of the CF/CoQ10 mix revealed sharp-contrast particles with spherical morphology that have a wide size distribution with a mode of 40 nm (Fig. 3D and E). In contrast, CF by itself produces very few and smaller particles (Fig. S5). Taken together these observations indicate that CF acts as a surfactant that solubilizes CoQ10 by micellization.
Fig. 3.
CF increases the water solubility of CoQ10. (A) Pictures of CoQ10 in aqueous solution alone or solubilized by CF. The final concentration of CF and CoQ10 in the clear yellow solution is 4 mM and 2.2 mg/mL (2.5 mM), respectively. (B) Concentration of solubilized CoQ10 in aqueous CF solutions of various concentrations. The smaller graph on the upper left zooms over the region where CF concentrations are lower than 0.2 mM. Each point represents the average and SEM of 2 samples (some error bars are not visible due to their small size). In A, the CF/CoQ10 water solution was prepared by sonication-aided mixing followed by filtration with 0.22 μm filters to remove undissolved material. In B, to better control the mixing force and time, CF and CoQ10 were mixed by overnight shaking at 220 rpm and insoluble particles were removed by centrifugation prior to quantification of CoQ10 concentrations. (C) Drop collapse test result. Photos of typical samples are shown, and the graph below presents the average drop size of 3 independent measures. *p < 0.05, unpaired student's t-test. (D) Negative-staining TEM images of CF/CoQ10 nanoparticles at two different magnifications. (E), Particle size distribution histogram determined from TEM images of a clear mixture of CF and CoQ10. See Fig. S5 for TEM images of nanoparticles in a CF-only solution. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. In vitro cellular uptake of CF/CoQ10 micelles
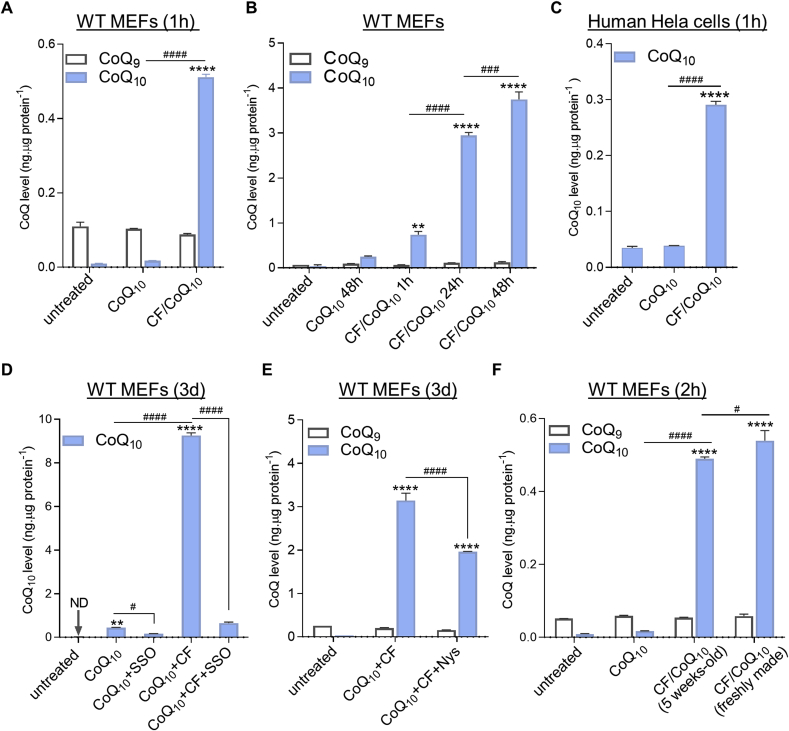
We tested the capacity of CF/CoQ10 micelles to deliver CoQ10 into cells and mitochondria. The liquid formulations of CF/CoQ10 was prepared as described above. To treat cells with CoQ10 alone, we used a 0.5 mM CoQ10 stock in ethanol. Fig. 4A shows that 1-h after addition of CF/CoQ10, there was already a dramatic increase of CoQ10 in WT cells, while there was essentially no uptake of free CoQ10 at the same dose. Furthermore, the total CoQ10 loading increased with time (Fig. 4B). Quick uptake was also observed in human HeLa cells (Fig. 4C).
Fig. 4.
Rapid cellular uptake of CF/CoQ10micelles and CoQ accumulation over time. (A) CoQ levels in WT MEFs after 1-h treatment with free CoQ10 or micellar CF/CoQ10 (n = 2). (B) Quantification of time-dependent cellular uptake of CF/CoQ10 micelles (n = 3). (C) Changes of CoQ10 levels in human HeLa cells after 1-h treatment with micellar CF/CoQ10 or free CoQ10 (n = 2). (D-E) Effects of sulfosuccinimidyl oleate (SSO) or nystatin (Nys) on uptake of CF/CoQ10 micelles. The dosage for SSO and Nys was 75 μM and 40 μg/mL (43.2 μM), respectively. CoQ10 levels at the end of 3-day treatment are shown. n = 3. (F) Comparison of CoQ-delivery efficacy between 5-weeks old and freshly made CF/CoQ10 micelles. WT MEFs were used, and CoQ extraction was carried out after 2-h treatment. For all micelle treatment groups, the micellar solutions of CF/CoQ10 used to treat cells were prepared by sonication-aided mixing (see Materials and Methods) of 4 mM of CF and excess CoQ10 powder. CoQ10 concentration in the solutions varied between 2 mM and 5 mM because they were prepared at different times and sometimes made with different batches of materials. However, the final concentration of CoQ10 in the treatment media was always 6 μM. Data are mean ± SEM, and one-way ANOVA followed by Tukey's multi-comparisons was used to compare CoQ9 or CoQ10 levels between groups. ND: not detected. **p < 0.01 and ****p < 0.0001 (vs. untreated cells). #p < 0.05, ###p < 0.001, and ####p < 0.0001 (comparison of CoQ10 levels between treatment groups). No difference in CoQ9 levels was found between any groups.
We sought to establish the nature of the molecular mechanism that allows for uptake of CoQ10 via CF/CoQ10 micelles. The class B scavenger receptor CD36 is a ubiquitous membrane glycoprotein that has been implicated in CoQ uptake by brown adipose tissue in mice [68]. We tested the effect of sulfosuccinimidyl oleate (SSO) which binds irreversibly and specifically to the hydrophobic cavity of CD36 [69]. We observed an inhibitory effect of SSO on the small amount of uptake of free CoQ10 and a very dramatic effect on the large uptake of CF/CoQ10 micelles (Fig. 4D). Although the inhibition of CoQ10 uptake by SSO is dramatic, it is not complete (Fig. 4D). We therefore also tested nystatin, which disrupts caveolae-dependent endocytosis [70]. The moderate effect that was observed could imply a small role for caveolae but could also result from toxicity or an indirect effect on CD36 (Fig. 4E). Finally, the storage stability of CF/CoQ10 micelles was studied by comparing the efficiency of CoQ10 delivery by micellar CF/CoQ10 stored under different conditions (Fig. 4F and Supplementary Results). The observation of efficient CoQ10 delivery by CF/CoQ10 after storage under various conditions, suggesting high physicochemical stability of the micelles.
2.4. In vivo administration of CF/CoQ10 micelles by intravenous (IV) injection
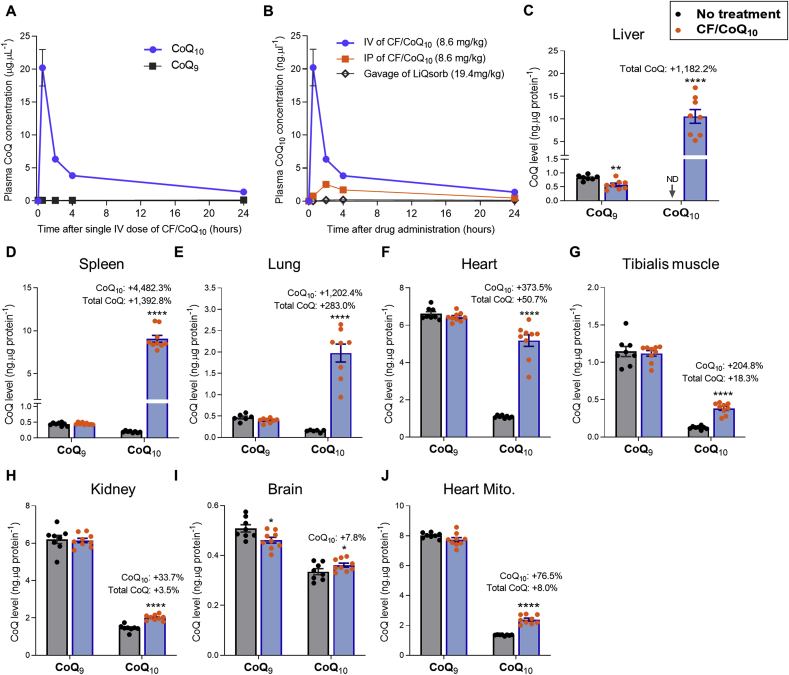
We injected wild-type mice through the tail vein with micellar CF/CoQ10 and followed the plasma CoQ concentrations after a single dose of 8.6 mg/kg body weight (BW) of CoQ10 (Fig. 5A and Fig. S10). The treatment created a dramatic supraphysiological peak of CoQ10 concentration, followed by fast clearance. At the dose used, the CoQ10 plasma concentration reached 20.23 ± 6.22 μg/ml after 30 min, which is > 160 times higher than that of CoQ9, the major endogenous CoQ species in mice. A rapid decline in concentration, representing a distribution phase, was observed for the first 4 h after dosing. However, notably, 24 h after dosing, the plasma concentration of CoQ10 was 1.37 ± 0.29 μg/mL, that is, still about 10 times higher than that CoQ9 (0.14 ± 0.01 μg/mL). We also administered CF/CoQ10 by intraperitoneal (IP) injection for comparison and observed a much lower plasma peak level of CoQ10 (Fig. 5B). For a direct comparison of CF/CoQ10 treatments with an available oral CoQ product, we administered LiQsorb (Tishcon) by oral gavage. LiQsorb is a liposomal formulation of CoQ10 that we administered at 19.4 mg/kg BW, which is approximately equivalent to the highest safe daily dose for humans (1200 mg/day) [71], and about 1.6 times higher than the IV and IP dose of CF/CoQ10. Compared to IV CF/CoQ10, the plasma CoQ10 level increase after the one-time gavage of LiQsorb was excessively small (Fig. 5B).
Fig. 5.
Plasma and tissue CoQ levels after intravenous administration of CF/CoQ10. (A) Plasma CoQ concentrations versus time after a single tail vein injection of CF/CoQ10 (8.6 mg of CoQ10 per kg BW). (B) Plasma CoQ10 concentrations after a single administration of CF/CoQ10 (via the tail vein or by IP injection) in comparison to one-time gavage with LiQsorb. n = 4–6 mice per group. (C-J) CoQ concentrations in various tissues or isolated heart mitochondria 2 h after 10 daily injection of CF/CoQ10 (at a dosage of 12.0 mg of CoQ10 per kg BW/day). Data are mean ± SEM (n = 7–10/group). When not shown, error bars are smaller than the symbol size. *p < 0.05 and ****p < 0.0001 (Student's t-test). The labels above CoQ10 bars indicate the percentage increase of CoQ10 and total CoQ levels (CoQ9+CoQ10) in the treated mice compared to no-treatment control. 10-wk-old female C57BL/6J mice were used in the experiment. ND: not detectable.
CoQ10 tissue uptake was studied by performing single daily injections of CF/CoQ10 for 10 consecutive days and scoring CoQ 2 h after the last injection. Fig. 5, C-J present the findings in female C57BL/6J mice at a dosage of 12.0 mg/kg BW/day of CoQ10. The most dramatic increase of CoQ10 concentrations was seen in the liver, spleen and lung, with a 3.8- to 14.9-fold increase of total CoQ (CoQ9+CoQ10) (Fig. 5, C-E). The heart showed a >4.7-fold increase of CoQ10 and a ~50.7% increase in total CoQ (Fig. 5F). The CoQ10 increase in skeletal muscle was ~3.0-fold which resulted in ~18.3% higher total CoQ (Fig. 5G). The kidney also showed significant uptake but to a lesser degree: ~33.7% increase of CoQ10 and ~3.5% increase in total CoQ (Fig. 5H). The smallest (but significant) elevation of CoQ10 level (~7.8%) was observed in the brain, where the level of endogenous CoQ9 was lowered and therefore total CoQ levels remained unchanged (Fig. 5I). The low uptake of CoQ10 might be due to the blood-brain barrier. The low CoQ9 levels, for which we have no explanation, were not seen with a different mouse strain (see below). For the heart, we extended our analysis to purified mitochondria and observed a ~76.5% increase in the level of CoQ10 and a ~8.0% increase in total CoQ (Fig. 5J).
To demonstrate reproducibility and possible effects of genetic background, we repeated the 10 injections experiments in a mixed C57BL/6J × 129/Sv background. Our findings were essentially indistinguishable from those made with the pure C57BL/6J background (Fig. S7 A-H). In this background we also scored CoQ in tissues and in heart mitochondria after a single dose injection (8.6 mg/kg body weight of CoQ10), and observed elevated CoQ10 levels in the liver, spleen, lung, heart, and heart mitochondria, but not in other tissues (Fig. S7, I–O). In the tissues in which we observed CoQ10 uptake after a single injection, the elevation of CoQ10 was much lower than the elevation in the same tissues after 10 injections. This indicates that repeated injections allow for tissue accumulation of CoQ10. On the other hand, the plasma concentrations achieved after 1 or 10 daily injections were very similar (Fig. S8A). This suggests that there is rapid clearance of CF/CoQ10 micelles from circulation and distribution into tissues. All animal experiments described above were with females. We therefore also tested male C57BL/6J mice with the 10 injections protocol but examined only blood, heart, liver and heart mitochondria for CoQ levels. As with females there was a dramatic elevation of plasma CoQ10 concentration, a ~3.3-fold increase of CoQ10 level in the heart, and a ~9.7-fold increase of total CoQ in the liver (Figs. S9A–C). In the heart mitochondria, we observed a ~63.2% increase of CoQ10 level and a ~8.3% higher level of total CoQ (Fig. S9D).
Intriguingly, in experiments with both females and males, and in both genetic backgrounds, we found that the dramatic rise of CoQ10 concentration in the plasma resulting from CF/CoQ10 injection was accompanied by a reduction of plasma CoQ9 levels (Figs. S8, S9A, and S10). In addition, we observed a small decrease of CoQ9 in the liver, but in no other tissues, after 10 injections of CF/CoQ10 in C57BL/6J mice (both male and female) (Fig. 5C and Fig. S9C), but not in the mixed C57BL/6J × 129/Sv background (Fig. S7A).
For a direct comparison with 10 daily injections of CF/CoQ10, we administrated LiQsorb by oral gavage daily for 10 days. As shown above, we observed a much smaller increase of plasma CoQ10 level after one-time gavage of LiQsorb compared to one-time IV injection of CF/CoQ10. In fact, we observed a CoQ10 peak concentration of 0.20 ± 0.04 ng μl−1, 4 h after LiQsorb feeding (Fig. S11A). This is about 100 time less than that detected after a single dose of intravenous CF/CoQ10 (20.22 ± 6.22 ng μl−1, 0.5 h after dosing). At tissue level, we found a small uptake only in the liver and spleen after oral feeding of LiQsorb for 10 days (fig. S11, B and C), and this is a >100-fold lesser uptake compared to IV dosing of CF/CoQ10 in these organs (Fig. 5C and D). Other tissues examined (heart, kidney, muscle, lung and brain) showed no detectable increase in CoQ10 after LiQsorb gavage (fig. S11, D-H). As with CF/CoQ10 IV treatment, LiQsorb feeding also resulted in a decrease in the level of CoQ9 in the plasma and liver with no effect in other tissues (Fig. S11, B–I). In the blood, CoQ is thought to be carried in liver-derived lipoproteins which may serve to deliver dietary CoQ into other tissues [72]. Thus, low liver CoQ9 could result in low serum CoQ9. However, how CoQ10 dosing results in low liver CoQ9 remains to be investigated.
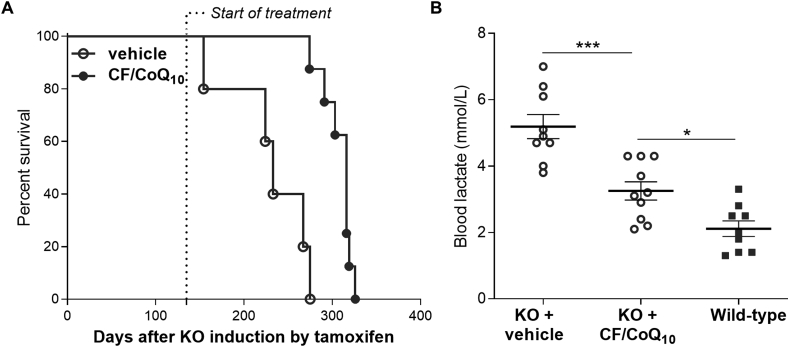
We sought to demonstrate phenotypic rescue of CoQ deficiency in mice by parenteral delivery of CF/CoQ10. We previously showed that complete loss of Coq7 by induced conditional knockout (KO) in young adults, led to progressive but rapid loss of CoQ, severe ETC dysfunction, and death after ~9 months [73]. Oral CoQ10 supplementation had no meaningful effect on the mutant phenotypes [73]. Unfortunately, the KO animals become rapidly too sick to allow for reliable IV treatment. We therefore turned to intraperitoneal (IP) injection to determine if parenteral treatment with micellar CF/CoQ10 could alleviate their phenotype. We started the IP CF/CoQ10 treatment (~8.6 mg/kg BW/day) at 4 months following the induction of the Coq7 KO. At that time, the condition of the animals had already deteriorated significantly but with no mortality [73]. As shown in Fig. 6A, we found that the treated Coq7 KO mice lived significantly longer than saline-treated KO mice. Blood lactate is a systemic measure of mitochondrial dysfunction. In a separate experiment we therefore scored blood lactate after IP treatment starting ~2 weeks after KO induction. We measured blood lactate after 2 months of treatment and observed significantly lower levels in the treated animals (Fig. 6B). As shown above, single CF/CoQ10 IP injections are sufficient to increase plasma CoQ10 level, but to a much lesser degree than by IV. Furthermore, although IP injections resulted in significant CoQ10 uptake in the liver there was no measurable uptake in the heart after 5 daily injections (see Supplementary Results). Taken together, the positive effects of IP treatment on lactate levels and survival, despite comparatively poor effect on plasma and organ CoQ10 levels, suggest that profound phenotypic rescue could be obtained by IV CF/CoQ10.
Fig. 6.
Effects of intraperitoneal CF/CoQ10treatment on Coq7 KO mice. (A) Kaplan-Meier survival curves comparing CF/CoQ10-treated Coq7 KO mice to vehicle (saline)-treated mutant mice. Administration of CF/CoQ10 treatment was conducted by intraperitoneal (IP) injection and started at ~ 4 months after induction of the Coq7 KO (n = 5 for vehicle treatment and n = 8 for CF/CoQ10 treatment). p < 0.001 by log-rank test. (B) IP administration of CF/CoQ10 decreases blood lactate concentration in Coq7 KO mice. IP injection of micellar CF/CoQ10 started ~2 weeks after completion of induction of Coq7 KO (by IP injections of tamoxifen) and was performed every day until the mice died. The blood lactate level was measured after 2 months of CF/CoQ10 treatment. Means ± SEM are shown (n = 9–10). *p < 0.05 and ****p < 0.0001 (one-way ANOVA with a Tukey's multi-comparison test).
2.5. Effects of caspofungin analogs on CoQ10 solubilization and uptake
CF is the first echinocandin antifungal agent to gain FDA-approval for clinical use. It is a semisynthetic water-soluble lipopeptide composed of a cyclic hexapeptide, of which all six amino acids are hydroxylated, and a branched C14 fatty acid side-chain (Fig. 2A) [74,75]. There are two other semi-synthetic echinocandin derivatives, anidulafungin (AFG) and micafungin (MFG), which are also approved anti-fungal agents for candidiasis. Structurally, they differ from CF in a number of ways but particularly at the side chains: MFG has an aromatic side-chain (3.5-diphenyl-substituted isoxazole ring side chain) and the side chain of AFG is an alkoxytriphenyl (terphenyl) side-chain [[74], [75], [76]] (Fig. S14). AFG is practically insoluble in water whereas MFG is water soluble. We found that both compounds did not produce drop collapse (Fig. S15A). However, mixing CoQ10 with MFG by sonication resulted in an increase of CoQ10 aqueous solubility, while it was ineffective with AFG (Fig. S15B). Both showed no effect on uptake of CoQ10 from the medium (Fig. S15C), and consistently failed to enhance the rescue of DKO cells in galactose medium supplemented with the minimal, ineffective, amount of CoQ10 (0.225 μM) (Fig. S15D).
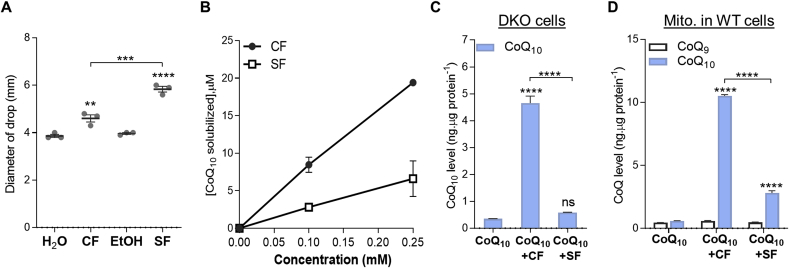
We also tested two other commercially available lipopeptides, daptomycin and surfactin. Daptomycin, which is highly soluble in water and contains a 10-carbon carboxylic acid chain (Fig. S14), showed only a small effect on CoQ10 uptake (Supplementary Results). Interestingly, although surfactin is a much stronger surfactant than CF (Fig. 7A), it is substantially poorer at solubilizing CoQ10 (Fig. 7B). We speculate that this is attributable to its very poor aqueous solubility. Although surfactin could enhance CoQ10 uptake, the effect was much smaller for both cells and mitochondria compared to CF (Fig. 7, C and D). Thus, it appears that CF possesses a unique set of properties by which the micelles it forms with CoQ10 are stable, have a high CoQ10 content, can be efficiently taken up by cells, and can release their CoQ10 once in the cells. We also tested the ability of a dozen other well-known surfactants to solubilize and deliver CoQ10, including block copolymers. None compares advantageously with CF. The findings are presented in Supplementary Results.
Fig. 7.
Effects of surfactin on CoQ10solubilization and uptake. (A) Drop collapse test of surfactin (SF). CF and SF were tested at the same concentration of 1.5 mM **p < 0.01, ****p < 0.0001 vs water or ethanol (EtOH) control for CF and SF, respectively; ***p < 0.001 comparing CF and SF (one-way ANOVA and Tukey's multi-comparison test). (B) Aqueous CoQ10 solubility as a function of CF or SF concentrations. (C-D) CoQ10 levels in DKO cells or mitochondrial CoQ levels in WT cells after 3 days of co-treatment of CoQ10 and CF or SF. The final concentrations of the test compounds in cell medium were: CoQ10: 2.5 μM, CF: 12.5 μM, SF: 12.5 μM. Statistical analysis was by one-way ANOVA followed by Tukey's post-hoc test. ns: not significant. ****p < 0.0001. All data are presented as mean ± SEM (n = 3). Some error bars are concealed by the symbols.
3. Discussion
Despite only moderate surfactant activity (Fig. 3C), CF solubilizes CoQ10 by forming micellar nanoparticles (Fig. 3D). The CF/CoQ10 micelles we observed were spherical and mostly smaller than 200 nm in diameter, with a mode of 40 nm (Fig. 3E). A nanoparticle size range of 20–200 nm is preferred for IV delivery, as it allows for long circulation times and sufficient extravasation and tissue uptake [[77], [78], [79]]. A critical micelle concentration (CMC) in the low millimolar range is considered desirable in a drug carrier as it indicates a good stability following dilution in the blood after IV administration [80]. The CMC of CF/CoQ10 micelles is close to 50 μM (Fig. 3B). Therefore, CF/CoQ10 are unlikely to dissociate rapidly due to high dilution upon IV injection.
In fact, our CF/CoQ10 formulation can be safely administered to mice by IV injection and CoQ10 reaches all the tissues examined. Following intravenous CF/CoQ10 at 8.6 mg/kg BW of CoQ10, the highest plasma CoQ10 concentration detected was >160 times higher than the normal level of endogenous CoQ9 (CoQ10 was undetectable in our measurements of plasma CoQ from untreated mice) (Fig. 5A and Fig. S10). To the best of our knowledge, the greatest increase in COQ10 plasma level reported upon long-term oral administration was never more than 10 times that of CoQ9 [[81], [82], [83], [84]]. Note that although CoQ9 levels were slightly reduced by administration of CF/CoQ10 (figs. S8, S9A and S10), the effect on CoQ9 levels were negligible with respect to the total elevation of CoQ (CoQ10+CoQ9).
In all the tissues examined, including the liver, kidney, heart, skeletal muscle, spleen, lung and brain, we observed a significant increase of CoQ10 levels after 10 daily IV doses of 8.6–12 mg of CoQ10/kg of BW (Fig. 5, C–I and fig. S7, A-G). Uptake in all these organs was cumulative (10 injections produce much more uptake than one-time injection). In the liver, spleen, lung, heart and skeletal muscle, from 18.3% to 1392% higher levels of total CoQ were observed. Further studies are needed to address the mechanism behind the differences in uptake between tissues. Remarkably, an increase in the levels of CoQ10 and total CoQ was found in heart mitochondria after only a single IV dosing (Fig. 5G, Fig. S7, H and O, and Fig. S9D). In addition to patient studies suggesting general secondary CoQ deficiency in mitochondrial disorders [48,49,85], a recent animal study demonstrated that mitochondrial dysfunction in the heart leads to low CoQ biosynthesis [51]. Furthermore, cardiac manifestations are prevalent in mitochondrial disease patients [86]. The quick uptake of exogenous CoQ10 in heart mitochondria provided by CF/CoQ10 treatment is thus particularly encouraging.
Both CF and CoQ10 are already used in the clinic and have very favorable safety profiles [53,87]. We did not observe any adverse effects on the mice during the 10-day treatments at ~12.0 mg/kg and ~19.4 mg/kg for CoQ10 and CF, respectively (Fig. S18). Previous studies with mice showed that oral CoQ10 supplementation in wild-type animals can result in some uptake in the liver, ovaries and brown adipose tissue but perhaps not in other tissues despite sporadic positive reports [68,73,83,[88], [89], [90]]. In the present study, we directly compared oral feeding with LiQsorb at a ~1.6 times higher dose than that used with IV CF/CoQ10 and observed only low plasma concentrations (Fig. 5B and Fig. S11A). At tissue level, LiQsorb gavage only increased CoQ10 in the liver and spleen with a much weaker effect than with IV CF/CoQ10 even in these organs (Fig. 5C and D, and fig. S11, B and C). Taken together, our results demonstrate a promise of high delivery and excellent tolerance for intravenous CF/CoQ10.
The potential range of uses of CF/CoQ10 to treat CoQ deficiencies and other diseases remains to be established. How each tissue in an organism lacking sufficient CoQ will react to effective CoQ supplementation is not clear. Some tissue might be refractory to correction of the damage sustained through the deficiency, while in others CoQ might show a disproportional beneficial effect, as has been repeatedly suggested for the kidney [73,91,92]. It is encouraging that treatment of Coq7 KO mice with CF/CoQ10 led to increased survival despite the need to use the much less efficient IP route (Fig. 6A).
For use in patients, the CF/CoQ10 formulation can very likely be further developed and improved. Methods exist to improve micelle drug loading capacity and optimal micelle shapes and sizes can be engineered. Finally, structure-function relationship studies could lead to discovery of CF analogs with further enhanced CoQ10 solubilization and delivery properties. However, the potential toxicity of a new chemical entity is necessarily unknown, in contrast to CF which is known to be safe.
4. Materials and Methods
4.1. Cell culture and compound screening
Pdss2fl/fl;Coq7fl/fl MEFs were isolated from E12.5 embryos and cultured in standard DMEM supplemented with 10% (v/v) FBS and 1% (v/v) antibiotic-antimycotic (referred to as glucose medium). To excise the floxed genes, Pdss2fl/fl;Coq7fl/fl MEFs were infected with a pBabe-Puro-Cre retrovirus as previously described [63]. The resulting cells lacking the expression of both Pdss2 and Coq7 genes were referred to as DKO cells. Pdss2fl/fl;Coq7fl/fl MEFs infected with empty pBabe-puro retrovirus were used as wild-type control. For use in compound screening, DKO cells were seeded in 96-well plates in glucose medium for 12–24 h. After washing once with phosphate-buffered saline (PBS), medium was switched to DMEM deprived of glucose but supplemented with 10 mM galactose, 10% dialyzed FBS, and 1% antibiotic-antimycotic (referred to as galactose medium). The compounds of the Selleckchem FDA-approved drug library were added at 2 different concentrations (10 and 20 μM) along with or without 0.225 μM CoQ10. Cell viability was determined after 2–3 days of culture in galactose medium by resazurin assay.
4.2. Preparation of CF/CoQ10 micelle solution for cell treatments and animal tests
An excess of CoQ10 powder were added to 4 mM of CF solution in water followed by mixing by sonication for 2–3 min (QSonica XL-2000 sonicator at the power setting of 8–9). The sonication mixture was then passed through a 0.22-μm-pore-size sterile filter to remove undissolved material and to sterilize it for use in cell treatments or animal tests. Filtrate concentration of CoQ10 was determined from absorbance at 275 nm using a molar extinction coefficient of 14.2 mM−1cm−1. The filtrate (CF/CoQ10) was kept at 4 °C before use.
4.3. Electron microscopy
A droplet of CF or CF/CoQ10 solution was directly deposited onto an electron microscope grid (carbon-coated copper grid) and the samples were allowed to dry for 2 min under ambient conditions. Then, a droplet of 1% uranyl acetate was added on to grids and left standing for 45 s. Observations were performed on a Tecnai 12 BioTwin Transmission Electron Microscope (FEI Electron Optics), operated at 120 kV, using a 40-μm objective aperture. Images were digitized with the use of an AMT XR80C (8 megapixel) charge-coupled device camera and Image Capture Engine Software (version 601).
4.4. Mice
C57BL/6 mice were obtained from Charles River Laboratories. Pdss2fl/f mice were kindly provided by Dr. David L. Gasser (University of Pennsylvania) [13]. All procedures were approved by McGill University's Animal Care Committee and were in accordance with the ethical guidelines of the Canadian Council on Animal Care. All intravenous injections of CF/CoQ10 solution via the tail vein were made in a volume of 0.1 ml, which corresponds to 8.6–12.0 mg/kg body weight (BW) per dose. LiQsorb (Tishcon) treatment was given by oral gavage at dose of 19.4 mg/kg BW daily for 10 days.
4.5. CoQ detection by high performance liquid chromatography (HPLC)
CoQ quantification was carried out by HPLC, as previously described with some modifications [25,63,93]. An Agilent 1260 Infinity LC system equipped with a quaternary pump (G7111A) and a variable wavelength detector (G7114A) was used. Cells and mouse tissues were thoroughly homogenized in a radioimmunoprecipitation buffer (20 mM Tris-HCl, pH 7.5, 1% NP-40, 0.5% deoxycholate, 10 mM EDTA, 150 mM NaCl) before CoQ extraction. Culture medium (500 μL) and mouse plasma (100 μL) samples were directly used for CoQ extraction with ethanol and hexane (v/v 2/5). Chromatography was carried out on a reverse-phase C18 column (2.1 × 50 mm, 1.8 μm, Agilent) with 70% methanol and 30% ethanol as the mobile phase at a flow rate of 0.3 mL/min. The detector was set at 275 nm CoQ9 and CoQ10 peaks in extracts were identified and quantified using pure CoQ standards. CoQ concentrations in cells or mouse tissues were normalized to the amount of protein measured with BCA assay (Thermo Scientific). The whole procedure was carried out under normal conditions with exposure to air and light. Given that CoQ in the reduced state is highly unstable and is readily oxidized in the presence of oxygen and light, the detection at 275 nm (maximal absorbance of oxidized CoQ), should measure total CoQ.
4.6. Statistical analysis
Quantitative data were analyzed by GraphPad Prism Version 8.4.2 (GraphPad Software, Inc.). Comparisons between untreated and treated mice were performed using an unpaired two-tailed Student's t-test unless otherwise specified. For multiple comparisons, one-way ANOVA followed by an appropriate post-hoc comparison test was performed. Kaplan-Meier survival data was analyzed by the log rank test. For all analyses, the level of statistical significance is set at p < 0.05.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
We wish to acknowledge the excellent technical assistance of Ms. Angeline de Bruyns for compound screening. We thank the personnel at the Facility for Electron Microscopy Research (FEMR) of McGill University for technical assistance with TEM imaging. We are also thankful to Dr. Tom Barnes for helpful discussions. Research in the laboratory of SH is funded by a Foundation grant from the Canadian Institutes of Health Research: FDN-159916.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101680.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Crane F.L. Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion. 2007;7(Suppl):S2–S7. doi: 10.1016/j.mito.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Burger N., Logan A., Prime T.A., Mottahedin A., Caldwell S.T., Krieg T. A sensitive mass spectrometric assay for mitochondrial CoQ pool redox state in vivo. Free Radic. Biol. Med. 2020;147:37–47. doi: 10.1016/j.freeradbiomed.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Hekimi S. Understanding ubiquinone. Trends Cell Biol. 2016;26(5):367–378. doi: 10.1016/j.tcb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta. 2004;1660(1–2):171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Quinzii C.M., Luna-Sanchez M., Ziosi M., Hidalgo-Gutierrez A., Kleiner G., Lopez L.C. The role of sulfide oxidation impairment in the pathogenesis of primary CoQ deficiency. Front. Physiol. 2017;8:525. doi: 10.3389/fphys.2017.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echtay K.S., Winkler E., Klingenberg M. Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature. 2000;408(6812):609–613. doi: 10.1038/35046114. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Hekimi S. Molecular genetics of ubiquinone biosynthesis in animals. Crit. Rev. Biochem. Mol. Biol. 2013;48(1):69–88. doi: 10.3109/10409238.2012.741564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsui H.S., Clarke C.F. Ubiquinone biosynthetic complexes in prokaryotes and eukaryotes. Cell Chem. Biol. 2019;26(4):465–467. doi: 10.1016/j.chembiol.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyadera H., Amino H., Hiraishi A., Taka H., Murayama K., Miyoshi H. Altered quinone biosynthesis in the long-lived clk-1 mutants of Caenorhabditis elegans. J. Biol. Chem. 2001;276(11):7713–7716. doi: 10.1074/jbc.C000889200. [DOI] [PubMed] [Google Scholar]
- 10.Levavasseur F., Miyadera H., Sirois J., Tremblay M.L., Kita K., Shoubridge E. Ubiquinone is necessary for mouse embryonic development but is not essential for mitochondrial respiration. J. Biol. Chem. 2001;276(49):46160–46164. doi: 10.1074/jbc.M108980200. [DOI] [PubMed] [Google Scholar]
- 11.Jonassen T., Larsen P.L., Clarke C.F. A dietary source of coenzyme Q is essential for growth of long-lived Caenorhabditis elegans clk-1 mutants. Proc. Natl. Acad. Sci. U.S.A. 2001;98(2):421–426. doi: 10.1073/pnas.021337498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozeir M., Pelosi L., Ismail A., Mellot-Draznieks C., Fontecave M., Pierrel F. Coq6 is responsible for the C4-deamination reaction in coenzyme Q biosynthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2015;290(40):24140–24151. doi: 10.1074/jbc.M115.675744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng M., Falk M.J., Haase V.H., King R., Polyak E., Selak M. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4(4) doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefely J.A., Pagliarini D.J. Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem. Sci. 2017;42(10):824–843. doi: 10.1016/j.tibs.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asquith C.R.M., Murray N.H., Pagliarini D.J. ADCK3/COQ8A: the choice target of the UbiB protein kinase-like family. Nat. Rev. Drug Discov. 2019;18(11):815. doi: 10.1038/d41573-019-00158-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Hekimi S. The complexity of making ubiquinone. Trends Endocrinol. Metabol. 2019;30(12):929–943. doi: 10.1016/j.tem.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Bradley M.C., Yang K., Fernandez-Del-Rio L., Ngo J., Ayer A., Tsui H.S. COQ11 deletion mitigates respiratory deficiency caused by mutations in the gene encoding the coenzyme Q chaperone protein Coq10. J. Biol. Chem. 2020;295(18):6023–6042. doi: 10.1074/jbc.RA119.012420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian K., Jochem A., Le Vasseur M., Lewis S., Paulson B.R., Reddy T.R. Coenzyme Q biosynthetic proteins assemble in a substrate-dependent manner into domains at ER-mitochondria contacts. J. Cell Biol. 2019;218(4):1353–1369. doi: 10.1083/jcb.201808044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes B.G., Harrison P.M., Hekimi S. Estimating the occurrence of primary ubiquinone deficiency by analysis of large-scale sequencing data. Sci. Rep. 2017;7(1):17744. doi: 10.1038/s41598-017-17564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinzii C.M., Hirano M. Primary and secondary CoQ(10) deficiencies in humans. Biofactors. 2011;37(5):361–365. doi: 10.1002/biof.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doimo M., Desbats M.A., Cerqua C., Cassina M., Trevisson E., Salviati L. Genetics of coenzyme q10 deficiency. Mol. Syndromol. 2014;5(3–4):156–162. doi: 10.1159/000362826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desbats M.A., Lunardi G., Doimo M., Trevisson E., Salviati L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J. Inherit. Metab. Dis. 2015;38(1):145–156. doi: 10.1007/s10545-014-9749-9. [DOI] [PubMed] [Google Scholar]
- 23.Malicdan M.C.V., Vilboux T., Ben-Zeev B., Guo J., Eliyahu A., Pode-Shakked B. A novel inborn error of the coenzyme Q10 biosynthesis pathway: cerebellar ataxia and static encephalomyopathy due to COQ5 C-methyltransferase deficiency. Hum. Mutat. 2018;39(1):69–79. doi: 10.1002/humu.23345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanyi B., Racz G.Z., Gal P., Brinyiczki K., Bodi I., Kalmar T. Diffuse mesangial sclerosis in a PDSS2 mutation-induced coenzyme Q10 deficiency. Pediatr. Nephrol. 2018;33(3):439–446. doi: 10.1007/s00467-017-3814-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Smith C., Parboosingh J.S., Khan A., Innes M., Hekimi S. Pathogenicity of two COQ7 mutations and responses to 2,4-dihydroxybenzoate bypass treatment. J. Cell Mol. Med. 2017;21(10):2329–2343. doi: 10.1111/jcmm.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sondheimer N., Hewson S., Cameron J.M., Somers G.R., Broadbent J.D., Ziosi M. Novel recessive mutations in COQ4 cause severe infantile cardiomyopathy and encephalopathy associated with CoQ10 deficiency. Mol. Genet. Metab. Rep. 2017;12:23–27. doi: 10.1016/j.ymgmr.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danhauser K., Herebian D., Haack T.B., Rodenburg R.J., Strom T.M., Meitinger T. Fatal neonatal encephalopathy and lactic acidosis caused by a homozygous loss-of-function variant in COQ9. Eur. J. Hum. Genet. 2016;24(3):450–454. doi: 10.1038/ejhg.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barca E., Musumeci O., Montagnese F., Marino S., Granata F., Nunnari D. Cerebellar ataxia and severe muscle CoQ deficiency in a patient with a novel mutation in ADCK3. Clin. Genet. 2016;90(2):156–160. doi: 10.1111/cge.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freyer C., Stranneheim H., Naess K., Mourier A., Felser A., Maffezzini C. Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid. J. Med. Genet. 2015;52(11):779–783. doi: 10.1136/jmedgenet-2015-102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brea-Calvo G., Haack T.B., Karall D., Ohtake A., Invernizzi F., Carrozzo R. COQ4 mutations cause a broad spectrum of mitochondrial disorders associated with CoQ10 deficiency. Am. J. Hum. Genet. 2015;96(2):309–317. doi: 10.1016/j.ajhg.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y.T., Hersheson J., Plagnol V., Fawcett K., Duberley K.E., Preza E. Autosomal-recessive cerebellar ataxia caused by a novel ADCK3 mutation that elongates the protein: clinical, genetic and biochemical characterisation. J. Neurol. Neurosurg. Psychiatry. 2014;85(5):493–498. doi: 10.1136/jnnp-2013-306483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumkin L., Leshinsky-Silver E., Zerem A., Yosovich K., Lerman-Sagie T., Lev D. Heterozygous mutations in the ADCK3 gene in siblings with cerebellar atrophy and extreme phenotypic variability. JIMD Rep. 2014;12:103–107. doi: 10.1007/8904_2013_251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scalais E., Chafai R., Van Coster R., Bindl L., Nuttin C., Panagiotaraki C. Early myoclonic epilepsy, hypertrophic cardiomyopathy and subsequently a nephrotic syndrome in a patient with CoQ10 deficiency caused by mutations in para-hydroxybenzoate-polyprenyl transferase (COQ2) Eur. J. Paediatr. Neurol. 2013;17(6):625–630. doi: 10.1016/j.ejpn.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Multiple-System Atrophy Research C. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N. Engl. J. Med. 2013;369(3):233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- 35.Jakobs B.S., van den Heuvel L.P., Smeets R.J., de Vries M.C., Hien S., Schaible T. A novel mutation in COQ2 leading to fatal infantile multisystem disease. J. Neurol. Sci. 2013;326(1–2):24–28. doi: 10.1016/j.jns.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Ashraf S., Gee H.Y., Woerner S., Xie L.X., Vega-Warner V., Lovric S. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Invest. 2013;123(12):5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salviati L., Trevisson E., Rodriguez Hernandez M.A., Casarin A., Pertegato V., Doimo M. Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J. Med. Genet. 2012;49(3):187–191. doi: 10.1136/jmedgenet-2011-100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horvath R., Czermin B., Gulati S., Demuth S., Houge G., Pyle A. Adult-onset cerebellar ataxia due to mutations in CABC1/ADCK3. J. Neurol. Neurosurg. Psychiatry. 2012;83(2):174–178. doi: 10.1136/jnnp-2011-301258. [DOI] [PubMed] [Google Scholar]
- 39.Emmanuele V., Lopez L.C., Berardo A., Naini A., Tadesse S., Wen B. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch. Neurol. 2012;69(8):978–983. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heeringa S.F., Chernin G., Chaki M., Zhou W., Sloan A.J., Ji Z. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 2011;121(5):2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duncan A.J., Bitner-Glindzicz M., Meunier B., Costello H., Hargreaves I.P., Lopez L.C. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am. J. Hum. Genet. 2009;84(5):558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mollet J., Delahodde A., Serre V., Chretien D., Schlemmer D., Lombes A. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am. J. Hum. Genet. 2008;82(3):623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagier-Tourenne C., Tazir M., Lopez L.C., Quinzii C.M., Assoum M., Drouot N. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am. J. Hum. Genet. 2008;82(3):661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mollet J., Giurgea I., Schlemmer D., Dallner G., Chretien D., Delahodde A. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J. Clin. Invest. 2007;117(3):765–772. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diomedi-Camassei F., Di Giandomenico S., Santorelli F.M., Caridi G., Piemonte F., Montini G. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 2007;18(10):2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 46.Quinzii C., Naini A., Salviati L., Trevisson E., Navas P., Dimauro S. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am. J. Hum. Genet. 2006;78(2):345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez L.C., Schuelke M., Quinzii C.M., Kanki T., Rodenburg R.J., Naini A. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 2006;79(6):1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montero R., Grazina M., Lopez-Gallardo E., Montoya J., Briones P., Navarro-Sastre A. Coenzyme Q(1)(0) deficiency in mitochondrial DNA depletion syndromes. Mitochondrion. 2013;13(4):337–341. doi: 10.1016/j.mito.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Yubero D., Montero R., Martin M.A., Montoya J., Ribes A., Grazina M. Secondary coenzyme Q10 deficiencies in oxidative phosphorylation (OXPHOS) and non-OXPHOS disorders. Mitochondrion. 2016;30:51–58. doi: 10.1016/j.mito.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Sacconi S., Trevisson E., Salviati L., Ayme S., Rigal O., Redondo A.G. Coenzyme Q10 is frequently reduced in muscle of patients with mitochondrial myopathy. Neuromuscul. Disord. 2010;20(1):44–48. doi: 10.1016/j.nmd.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 51.Kuhl I., Miranda M., Atanassov I., Kuznetsova I., Hinze Y., Mourier A. Transcriptomic and proteomic landscape of mitochondrial dysfunction reveals secondary coenzyme Q deficiency in mammals. Elife. 2017;6 doi: 10.7554/eLife.30952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mischley L.K., Allen J., Bradley R. Coenzyme Q10 deficiency in patients with Parkinson's disease. J. Neurol. Sci. 2012;318(1–2):72–75. doi: 10.1016/j.jns.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez-Camacho J.D., Bernier M., Lopez-Lluch G., Navas P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 2018;9:44. doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varela-Lopez A., Giampieri F., Battino M., Quiles J.L. Coenzyme Q and its role in the dietary therapy against aging. Molecules. 2016;21(3):373. doi: 10.3390/molecules21030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hargreaves I.P. Coenzyme Q10 as a therapy for mitochondrial disease. Int. J. Biochem. Cell Biol. 2014;49:105–111. doi: 10.1016/j.biocel.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Yoritaka A., Kawajiri S., Yamamoto Y., Nakahara T., Ando M., Hashimoto K. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Park. Relat. Disord. 2015;21(8):911–916. doi: 10.1016/j.parkreldis.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 57.Fotino A.D., Thompson-Paul A.M., Bazzano L.A. Effect of coenzyme Q(1)(0) supplementation on heart failure: a meta-analysis. Am. J. Clin. Nutr. 2013;97(2):268–275. doi: 10.3945/ajcn.112.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang S.Y., Yang K.L., Zeng L.T., Wu X.H., Huang H.Y. Effectiveness of coenzyme Q10 supplementation for type 2 diabetes mellitus: a systematic review and meta-analysis. Internet J. Endocrinol. 2018;2018:6484839. doi: 10.1155/2018/6484839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parikh S., Saneto R., Falk M.J., Anselm I., Cohen B.H., Haas R. A modern approach to the treatment of mitochondrial disease. Curr. Treat. Options Neurol. 2009;11(6):414–430. doi: 10.1007/s11940-009-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaturvedi R.K., Beal M.F. Mitochondria targeted therapeutic approaches in Parkinson's and Huntington's diseases. Mol. Cell. Neurosci. 2013;55:101–114. doi: 10.1016/j.mcn.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Lopez L.C., Luna-Sanchez M., Garcia-Corzo L., Quinzii C.M., Hirano M. Pathomechanisms in coenzyme q10-deficient human fibroblasts. Mol. Syndromol. 2014;5(3–4):163–169. doi: 10.1159/000360494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stepanyan Z., Hughes B., Cliche D.O., Camp D., Hekimi S. Genetic and molecular characterization of CLK-1/mCLK1, a conserved determinant of the rate of aging. Exp. Gerontol. 2006;41(10):940–951. doi: 10.1016/j.exger.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Hekimi S. Mitochondrial respiration without ubiquinone biosynthesis. Hum. Mol. Genet. 2013;22(23):4768–4783. doi: 10.1093/hmg/ddt330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deresinski S.C., Stevens D.A. Caspofungin. Clin. Infect. Dis. 2003;36(11):1445–1457. doi: 10.1086/375080. [DOI] [PubMed] [Google Scholar]
- 65.Turkowicz M.J., Karpinska J. Analytical problems with the determination of coenzyme Q10 in biological samples. Biofactors. 2013;39(2):176–185. doi: 10.1002/biof.1058. [DOI] [PubMed] [Google Scholar]
- 66.Celik B., Sagiroglu A.A., Ozdemir S. Design, optimization and characterization of coenzyme Q10- and D-panthenyl triacetate-loaded liposomes. Int. J. Nanomed. 2017;12:4869–4878. doi: 10.2147/IJN.S140835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walter V., Syldatk C., Hausmann R. Screening concepts for the isolation of biosurfactant producing microorganisms. Adv. Exp. Med. Biol. 2010;672:1–13. doi: 10.1007/978-1-4419-5979-9_1. [DOI] [PubMed] [Google Scholar]
- 68.Anderson C.M., Kazantzis M., Wang J., Venkatraman S., Goncalves R.L., Quinlan C.L. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep. 2015;10(4):505–515. doi: 10.1016/j.celrep.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuda O., Pietka T.A., Demianova Z., Kudova E., Cvacka J., Kopecky J. Sulfo-N-succinimidyl oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164: SSO also inhibits oxidized low density lipoprotein uptake by macrophages. J. Biol. Chem. 2013;288(22):15547–15555. doi: 10.1074/jbc.M113.473298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutta D., Donaldson J.G. Search for inhibitors of endocytosis: intended specificity and unintended consequences. Cell. Logist. 2012;2(4):203–208. doi: 10.4161/cl.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hathcock J.N., Shao A. Risk assessment for coenzyme Q10 (Ubiquinone) Regul. Toxicol. Pharmacol. 2006;45(3):282–288. doi: 10.1016/j.yrtph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Miles M.V. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7(Suppl):S72–S77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Oxer D., Hekimi S. Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat. Commun. 2015;6:6393. doi: 10.1038/ncomms7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denning D.W. Echinocandin antifungal drugs. Lancet. 2003;362(9390):1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 75.Patil A., Majumdar S. Echinocandins in antifungal pharmacotherapy. J. Pharm. Pharmacol. 2017;69(12):1635–1660. doi: 10.1111/jphp.12780. [DOI] [PubMed] [Google Scholar]
- 76.Kofla G., Ruhnke M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature. Eur. J. Med. Res. 2011;16(4):159–166. doi: 10.1186/2047-783X-16-4-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pepic I., Lovric J., Hafner A., Filipovic-Grcic J. Powder form and stability of Pluronic mixed micelle dispersions for drug delivery applications. Drug Dev. Ind. Pharm. 2014;40(7):944–951. doi: 10.3109/03639045.2013.791831. [DOI] [PubMed] [Google Scholar]
- 78.Garnett M.C., Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occup. Med. (Lond). 2006;56(5):307–311. doi: 10.1093/occmed/kql052. [DOI] [PubMed] [Google Scholar]
- 79.Rabanel J.M., Aoun V., Elkin I., Mokhtar M., Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012;19(19):3070–3102. doi: 10.2174/092986712800784702. [DOI] [PubMed] [Google Scholar]
- 80.Rangel-Yagui C.O., Pessoa A., Jr., Tavares L.C. Micellar solubilization of drugs. J. Pharm. Pharmaceut. Sci. 2005;8(2):147–165. [PubMed] [Google Scholar]
- 81.Ashida Y., Yamanishi H., Terada T., Oota N., Sekine K., Watabe K. CoQ10 supplementation elevates the epidermal CoQ10 level in adult hairless mice. Biofactors. 2005;25(1–4):175–178. doi: 10.1002/biof.5520250120. [DOI] [PubMed] [Google Scholar]
- 82.Thomas S.R., Leichtweis S.B., Pettersson K., Croft K.D., Mori T.A., Brown A.J. Dietary cosupplementation with vitamin E and coenzyme Q(10) inhibits atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler. Thromb. Vasc. Biol. 2001;21(4):585–593. doi: 10.1161/01.atv.21.4.585. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Corzo L., Luna-Sanchez M., Doerrier C., Ortiz F., Escames G., Acuna-Castroviejo D. Ubiquinol-10 ameliorates mitochondrial encephalopathy associated with CoQ deficiency. Biochim. Biophys. Acta. 2014;1842(7):893–901. doi: 10.1016/j.bbadis.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Kamzalov S., Sumien N., Forster M.J., Sohal R.S. Coenzyme Q intake elevates the mitochondrial and tissue levels of Coenzyme Q and alpha-tocopherol in young mice. J. Nutr. 2003;133(10):3175–3180. doi: 10.1093/jn/133.10.3175. [DOI] [PubMed] [Google Scholar]
- 85.Zierz S., Jahns G., Jerusalem F. Coenzyme Q in serum and muscle of 5 patients with Kearns-Sayre syndrome and 12 patients with ophthalmoplegia plus. J. Neurol. 1989;236(2):97–101. doi: 10.1007/BF00314404. [DOI] [PubMed] [Google Scholar]
- 86.Duran J., Martinez A., Adler E. Cardiovascular manifestations of mitochondrial disease. Biology. 2019;8(2) doi: 10.3390/biology8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maertens J., Boogaerts M. Caspofungin in the treatment of candidosis and aspergillosis. Int. J. Infect. Dis. 2003;7(2):94–101. doi: 10.1016/s1201-9712(03)90003-8. [DOI] [PubMed] [Google Scholar]
- 88.Ben-Meir A., Burstein E., Borrego-Alvarez A., Chong J., Wong E., Yavorska T. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vazquez-Fonseca L., Schaefer J., Navas-Enamorado I., Santos-Ocana C., Hernandez-Camacho J.D., Guerra I. ADCK2 haploinsufficiency reduces mitochondrial lipid oxidation and causes myopathy associated with CoQ deficiency. J. Clin. Med. 2019;8(9) doi: 10.3390/jcm8091374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sikorska M., Lanthier P., Miller H., Beyers M., Sodja C., Zurakowski B. Nanomicellar formulation of coenzyme Q10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model: potential use as an adjuvant treatment in Parkinson's disease. Neurobiol. Aging. 2014;35(10):2329–2346. doi: 10.1016/j.neurobiolaging.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saiki R., Lunceford A.L., Shi Y., Marbois B., King R., Pachuski J. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am. J. Physiol. Ren. Physiol. 2008;295(5):F1535–F1544. doi: 10.1152/ajprenal.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kleiner G., Barca E., Ziosi M., Emmanuele V., Xu Y., Hidalgo-Gutierrez A. CoQ10 supplementation rescues nephrotic syndrome through normalization of H2S oxidation pathway. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2018;1864(11):3708–3722. doi: 10.1016/j.bbadis.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lapointe J., Wang Y., Bigras E., Hekimi S. The submitochondrial distribution of ubiquinone affects respiration in long-lived Mclk1+/- mice. J. Cell Biol. 2012;199(2):215–224. doi: 10.1083/jcb.201203090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.