Abstract
Background
Tuberculosis (TB) of the middle ear cleft (MEC) is a rare extra-pulmonary manifestation. Signs and symptoms of tuberculous otitis media are indistinguishable from that of non-tuberculous otitis media making early diagnosis difficult.
Objectives
To study the clinical presentations, complications and effective diagnostic modalities in tuberculosis of middle ear cleft.
Methods
We retrospectively studied 10 patients diagnosed with chronic otitis media, unresponsive to 2 months conventional treatment. Pure tone audiogram, High resolution computed tomography (HRCT) of temporal bone, and AFB staining of ear discharge were done. All patients underwent mastoid surgery. AFB staining and histopathological examination of granulation tissue removed from the middle ear and mastoid were also done.
Results
Clinical findings were mastoid swelling, facial palsy and post-aural fistula 3,4 & 2 patients respectively. All patients had persistent ear discharge and three had vertigo. Hearing loss was of moderate conductive type in five, sensorineural type in three and mixed type in two. HRCT of temporal bone revealed soft tissue density in MEC in 9 and evidence of bone destruction in 6 cases. Diagnosis of TB was confirmed either by (a) demonstration of AFB in ear discharge (4 patients)/tissue removed during surgery (4 patients) or (b) by demonstration of tuberculous granulomas with necrosis on histopathological examination of tissue from MEC (8 patients).
Conclusion
Tuberculosis should be suspected in all cases of chronic otitis media unresponsive to conventional treatment particularly in endemic areas. Histopathological examination and AFB staining of tissue removed during mastoid surgery are reliable diagnostic methods.
Keywords: Tuberculosis, Otitis media, Mastoiditis, Histopathological diagnosis
1. Introduction
Tuberculosis (TB) is a major health problem in the developing world and India accounts for ¼th of the global TB burden (2.79 million) (C Carvalho et al., 2018; TB India report 2017). Tuberculous otitis media (TOM) is a relatively rare disease and accounts for 4% of head and neck TB (S Jesic et el 2009; Saltzman SJ et al., 1971) and is characterized by indolent and heterogeneous presentations (Bhatkar D et al., 2017). It often presents as otitis media not responding to conventional management (Saltzman SJ et al., 1971). Lack of suspicion of tuberculosis leads to long delay in diagnosis and resultant complications.
In this study, we assess the clinical features, complications and effective.
Diagnostic modalities in ten patients with tuberculous otitis media (TOM).
These patients presented rather late in the course of the disease after prolonged and unsuccessful medical treatment for chronic middle ear infection. In this group of patients, the diagnosis was made following demonstration of Acid Fast Bacilli (AFB) in ear discharge/granulations and histopathological examination of tissues taken during mastoid surgery (Verma R et al., 2017).
2. Material and methods
2.1. Study subjects
This is a retrospective observational study carried out at the Department of ENT, St. Stephen’s Hospital, Delhi, India, from 2008 to 2018. Institutional ethics and scientific committee clearance were obtained and identifying information of patients are not documented. 10 patients were diagnosed with tuberculous otitis media during this period.
2.2. Assessment and evaluation methods
We have reviewed the medical records and clinical presentation of all patients. Pure tone audiogram, High resolution computed tomography of temporal bone, and AFB staining of discharge from ear were done in all cases. All patients underwent surgery in the form of a mastoid exploration. AFB staining and histopathological examination of granulation tissue removed from the middle ear and mastoid were also done in all cases. TB Polymerase Chain Reaction (PCR) of discharge & granulation tissue was done only in five cases.
2.3. Medical treatment
In all proven cases of tuberculosis, the chemotherapy regime using a combination of drugs was given for a minimum period of six months. Chemotherapy included Rifampicin, Isoniazid, Ethambutol and Pyrazinamide for two months followed by Rifampicin and Isoniazid for 4 months.
3. Results
3.1. Clinical presentation (Table 1)
Table 1.
Clinical, Investgation And operative findings.
| No | Age | Sex | Clinical Presentation | Radiology | Audiology | Intra-operative findings |
|---|---|---|---|---|---|---|
| 1 | 52 | M | (R)ear discharge, Hearing loss, Facial palsy -GrIV, Post -aural abscess | Soft tissue density in MEC, Destruction of ossicles, facial canal erosion, mastoid cortex |
Conductive hearing loss | Granulations, bone erosion ossicular detruction, facial canal erosion |
| 2 | 60 | F | (R) ear discharge, post-aural abscess, facial palsy-Gr III, tinnitus vertigo, headache, neck stiffness | Erosion of mastoid cortex, soft tissue density in MEC | SN hearing loss | Extensive destruction and sequestra of mastoid bone, labrynth, facial canal.Pale granulations |
| 3 | 19 | M | Bilateral ear discharge, Hearing loss, (R) ear-large perforation of pars tensa, (L) ear double perforation. | Soft tissue density in MEC, ossicular destruction | Conductive hearing loss- Bilateral | Ossicular necrosis, Extensve, pale granulations in mesotympanum & mastoid |
| 4 | 36 | F | Bilateral ear discharge, postaural fistula,(R), perforation of pars tensa, both sides | Erosion of mastoid cortex, soft tissue density in mastoid | Conductive hearing loss-Bilateral | Post- aural fistula fistula leading to mastoid cavty, bone erosion & granulations |
| 5 | 21 | F | (L) Ear discharge, Hearing loss, white flakes in postero-superior part | Soft tissue density in MEC | Conductive hearing loss | Thick caseous pus in mastoid and middle ear, erosion of facial canal |
| 6 | 53 | M | (R) ear discharge, postaural abscess, facial palsy Gr- IV | Soft tissue density & fluid levels in MEC, erosion of facial canal | SN hearing loss | Pus & granulations in the mastoid cavity, facial canal erosion |
| 7 | 50 | M | (R) ear discharge, decreased hearing, otalgia, polyp in external cana,l Facial palsy Gr-III | Soft tissue density in MEC, erosion if postr wall of external canal | Mixed hearing loss | Polyp occupying the mastoid & external canal, erosion postr canal wall, facial canal, granulations. |
| 8 | 17 | M | (R) ear discharge, decrease hearing, post-aural fistula, granulations in pars flaccida, history of ear surgery, tinnitus | Soft tissue density in MEC, erosion of ossicles & mastoid cortex | Mixed loss | Post aural fistula leading to mastoid cavity, granulations and bone destruction in and sequestra formation |
| 9 | 11 | F | Recurrent ear discharge from both ears, | Soft tissue density in MEC, | Conductive hearing loss | Granulation tissue in MEC, ossicular chain disruption |
| 10 | 25 | F | (L) ear decreased hearing, intact ear drumwith gromet, tinnitus, headache, vertigo otalgia, history of myringotomy | Soft tissue density & fluid levels in MEC | SN hearing loss | Abundant granulations with extensive bone destruction and sequestra formation mastoid, pus in the middle ear & mastoid |
This retrospective analysis includes 10 patents (6 males; 4 females) and age ranged from 11 years to 60 years (Mean age = 34.4). Duration of symptoms varied widely: in five patients it was less than one year, in one patient it was one year, in another patient it was 3 years and in two patients it was more than 10 years. But all of them had an exacerbation of symptoms at the time of presentation. All patients had mucopurulent ear discharge for more than 2 months, not responding to medical treatment. Two patients had history of previous surgical treatment for chronic otitis media. Seven had unilateral ear discharge while three had bilateral discharge. Otalgia was present only in three patients.
Three patients presented with post aural abscess, and 2 had post aural fistula. Facial palsy was present in four patients (House- Brackmann grade II, III & IV). 3 patients reported tinnitus and two had experienced vertigo.
Otoscopic examination revealed a single perforation in eight patients, double perforations in one and intact tympanic membrane in yet another patient. Polyps/granulations had partially replaced the tympanic membrane in three cases. Moderate conductive hearing loss was present in five patients, sensorineural hearing loss in three and two had mixed hearing loss.
Two patients were already on anti-tuberculous treatment for pulmonary tuberculosis. None of the patients Ihad cervical lymphadenopathy.
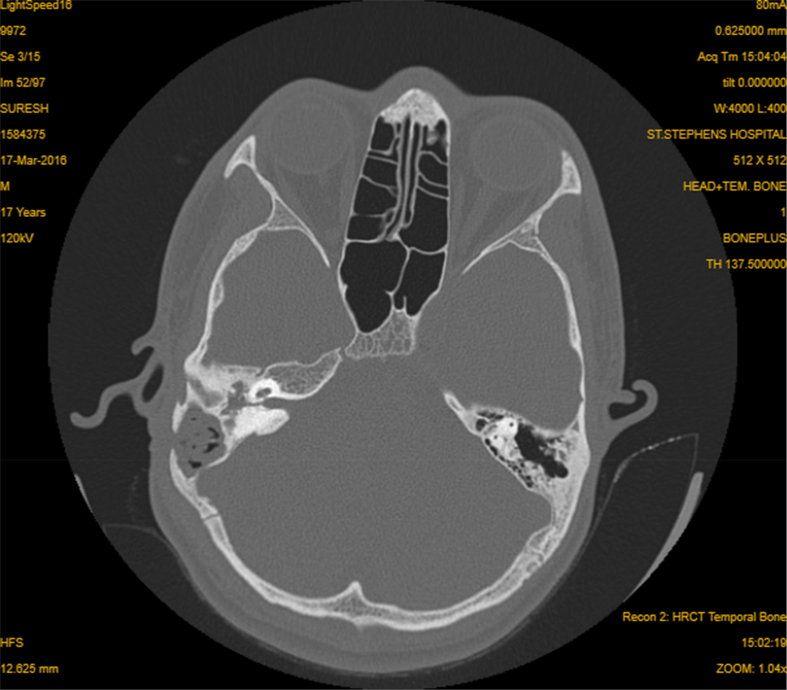
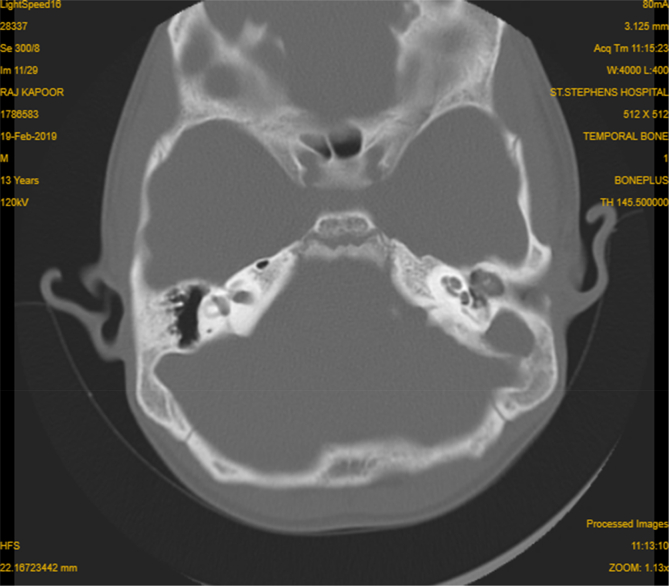
HRCT imaging of the temporal bone revealed multifocal destructive lesions of the temporal bone in 6 cases with destruction of outer cortex of the mastoid bone in five ears (Fig. 1). Soft tissue attenuation was present in the Middle Ear Cleft (MEC) in the scans of 9 patients (Fig. 2).
Fig. 1.
HRCT temporal bone, Axial cut-showing soft tissue density occupying the middle ear, mastoid cells And external canal with erosion of the mastoid cortex and sinus plate.
Fig. 2.
HRCT temporal bone Axial cut-howing soft tissue density occupying middle ear and mastoid cavity with bone destruction.
All the patients underwent mastoid surgery for non-healing otitis media. Intra-operative findings showed abundant pale granulations in 8 patients and bone destruction of fallopian canal in five. Extensive bone destruction and sequestra formation with involvement of the labryrinth was encountered in three cases.
3.2. Laboratory investigations (Table 2)
Table 2.
| No | AFB Ear Discharge | AFB Tissue | Histopathology |
|---|---|---|---|
| 1 | + | _ | Epitheloid granulomas with multinucleate giant cells & necrosis |
| 2 | _ | _ | Granulomas of epitheloid cells with multinucleate giant cells & palisading histiocytes |
| 3 | _ | + | Granulomas of epitheloid cells, mononuclear infitrates & necrosis. |
| 4 | _ | _ | Granulomas of epitheloid cells multinucleate giant, plasmacells & necrosis cells |
| 5 | _ | + | Granulomas of epitheloid cells, histiocytes and multinucleate giant cells |
| 6 | _ | + | Keratinized squamous epithelium with inflammatory cells & dense granulations |
| 7 | + | + | Otic polyp & epitheloid granulomas with multinucleate giant cells |
| 8 | + | _ | Non-specific granulations with abscess formation. No epitheloid granuloma |
| 9 | + | _ | Epitheloid granuloma with focal necrosis |
| 10 | _ | _ | Nectrotising granulomatous inflammation with epitheloid granulomas and multinucleate giant cells |
Histopathological examination of the granulations removed from middle ear and mastoid showed evidence of necrotising granulomatous inflammation in 8 cases. Ziehl–Neelson staining of the granulations was positive for AFB in four cases. Positive AFB staining of ear discharge was present in four patients. TB Polymerase Chain Reaction (PCR) was done in five cases only out of which 2 were positive.
At the end of six months of ATT, nine patients had good disease control and dry ears. Four patients with conductive hearing loss preoperatively had hearing improvement following surgical and medical treatment. One patient expired following stroke during the course of treatment.
3.3. Follow-up
Three patients were under follow up for a period of 2 years, two had completed one year and one patient had completed only 10 month’s follow up. None of them showed any evidence of recurrence of tuberculosis. Four patients were lost for follow up after completion of 6 months of chemotherapy.
4. Discussion
Tuberculosis of the middle ear cleft and temporal bone are not common. It accounts for only 0.05–0.9% of all cases of all chronic otitis media (D Djeric et al., 2013). It is usually secondary to infection in the lungs, larynx, pharynx or nose (Windle-Taylor PC et al., 1980). The routes of entry of tubercular bacilli into the temporal bone can be via aspiration through Eustachian tube, haematogenous spread from distant sites, or direct implantation through the external auditory canal and tympanic membrane perforation (N T Hoca et al., 2008). In our series, two patients were already diagnosed with pulmonary TB. Rest of the patients did not have any other focus of TB and nine patients had tympanic membrane perforations. Hence direct implantation through the external canal and tympanic membrane may be considered as an important route of spread for TOM (Cho Y S et al., 2006).
Total duration of the symptoms varied widely but did not correlate with the extent of disease and the presence of complications, at the time presentation to the hospital.
Tuberculous otitis media remains a diagnostic challenge because of its non-specific clinical features and the difficulty in confirming diagnosis by microbiological tests of ear discharge. Classical features of TOM like painless otorrhoea and multiple tympanic membrane perforations are not constant features. The appearance of tympanic membrane can be varied. (Yaniv E et al., 1987; Adhikari P 2008). It can have single or multiple perforations or may even be intact. Only one patient had multiple perforations of the tympanic membrane in this series. All patients had history of prolonged ear discharge that did not respond to local or parenteral antibiotic therapy and it was painless in nine out of ten patients.
In the literature, the incidence of facial paralysis is reported to range from 1% to 3.5% in patients in non-tuberculous chronic otitis media and is frequently associated with cholesteatoma (J W Choi et al., 2015). In TOM, facial palsy can occur in approximately 20% of cases and it seems to occur at the acute stage of the disease (Cho YS et al., 2006). The incidence of facial palsy was much higher in our series (40%).
Moderate to severe hearing loss which is out of proportion to the apparent involvement of the ear is often decribed in TOM(Adhikari P.2008). In the present series also all patients had hearing impairment where 5 patients had moderate conductive hearing loss.
Radiological features of TB mastoiditis are also not diagnostic, but it is valuable in assessing the extent of involvement of various parts of the temporal bone. CT findings can vary from non-specific clouding of the mastoid to extensive soft tissue densities with fluid levels in the middle ear, mastoid and petrous air cells and multifocal bone erosions.(Hoshino T et al., 1994). In this study, five cases had erosion of the outer cortex of mastoid process which is not a frequent site of bone erosion in non-tuberculous chronic otitis media (Cho YS et al., 2006).
Certain intraoperative findings like pale granulations and sequestra can give a clue to the diagnosis. Extensive granulations in the middle ear and mastoid are frequent intraoperative findings. Temporal bone destruction can be marked in advanced cases with sequestration or fistulas.(Saltzman SJ et al., 1971). In our study, three patients had extensive sequestration of temporal bone with involvement of labryrinth and five had fallopian canal erosion.
In extrapulmonary tuberculosis, the mycobacterial counts are low. Hence Ziehl-Neelsen staining of the ear discharge gives a poor yield. Same is true for AFB cultures. Microbiological confirmation can be further delayed because of the presence of secondary infection (Yaniv E et al., 1986). In the present study, AFB staining of tissue removed during mastoid surgery was positive in four cases (40%).
Histopathology of the tissue reveals more definitive features of tuberculosis. Well-formed epitheloid granulomas with, Langerhans giant cells, histiocytes and epitheloid cells and focal areas of necrosis are suggestive of tuberculous etiology (Verma R et al., 2017; Windle-Taylor. Maheshwari A et al., 2017).. In our study eight patients had definite histopathological features of tuberculosis.(80%). Histopathology along with AFB staining of tissue removed from the middle ear cleft confirmed the diagnosis of TB in 9 cases (90%). Hence it is important to collect granulation tissue during middle ear and mastoid surgery for histopathological examination and AFB staining, in regions where TB is endemic (L Bruschini et al., 2016). Surgical debridement along with ATT gave good disease control in our patients. None of patients in this series who were followed up for a period ranging from 10 months to 2 years had recurrence of the disease (60%).
5. Conclusion
This study highlights the importance of suspecting tuberculosis in all cases of chronic otitis media that do not respond to conventional antibacterial therapy particularly in endemic areas. Severe conductive hearing loss and an increased incidence of otogenic complications are often present. As mixed infections are often present microbiological diagnosis is difficult. But AFB staining and histopathological examination of tissue removed from middle ear cleft are reliable diagnostic modalities. Treatment with ATT combined with surgery give good disease control even in advanced cases.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Susan K. Sebastian, Email: drsusanks@gmail.com.
Aditya Singhal, Email: adirocker@gmail.com.
Ankur Sharma, Email: ankuracms@gmail.com.
Pankajkumar Doloi, Email: Doloi.pankaj@gmail.com.
References
- Adhikari P. Tuberculous otitis media: a review of literature. Internet J. Otorhinolaryngol. 2008;9(1):10. [Google Scholar]
- Bhatkar D., Utpat K., Desai U., Joshi J.M. Bilateral tuberculous otitis media: an unique presentation. Indian J Tuberc ulosis. 2017;64(4):334–336. doi: 10.1016/j.ijtb.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Bruschini Luca, Ciabotti Annalisa, Berrettini Stefano. Chronic tuberculous otomastoiditis: a case report. J. Int. Adv. Otol. 2016;12(2):219–221. doi: 10.5152/iao.2016.2097. [DOI] [PubMed] [Google Scholar]
- Carvalho Cassandra, Velankar Haritosh, Pusalkar A.G. Tuberculous otitis media with facial paralysis-case report and review of literature. Otolaryngol. Open Acc. J. 2018;3(2) 000171. [Google Scholar]
- Cho Y.S., Lee H.S., Kim S.W., Chung K.H., Lee D.K., Koh W.J., Kim M.G. Tuberculous otitis media: a clinical and radiologic analysis of 52 patients. The Laryngoscope. 2006;116(6):921–927. doi: 10.1097/01.mlg.0000214861.87933.00. [DOI] [PubMed] [Google Scholar]
- Choi Jin Woong, Park Yong-Ho. vol. 8. 2015 Sep. pp. 218–223. (Facial Nerve Paralysis in Patients with Chronic Ear Infections: Surgical Outcomes and Radiologic Analysis Clinical Exp Otorhinolaryngology). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeric Dragoslava, Tomanovic Nada, Boricic Ivan. Tuberculous otitis media- diagnosis and treatment of four cases. J. Int. Adv. Otol. 2013;9(2):255–259. [Google Scholar]
- Hoca Nevin Taci, Ogretensoy Mihriban, Dayioglu Didem. Tuberculousotitis media–A Case report. Turkish Resp. J. 2008;9(1):48–50. [Google Scholar]
- Hoshino T., Miyashita H., Asai Y. CT temporal bone in Tuberculous otitis media. J. Laryngol. Otol. 1994;108:702–705. doi: 10.1017/s0022215100127896. [DOI] [PubMed] [Google Scholar]
- India T.B. Annual Status Report Central TB Division; New Delhi: 2017. Revised National Tuberculosis Control Programme. [Google Scholar]
- Jesic Snezana, Stosic Svetlana, Milenkovic Branislava, Nesic Vladimir, Dudvarski Zoran, Jotic Ana. Nikola slijepcevic:middle ear tuberculosis: diagnostic criteria. Srp. Arh. Celok. Lek. 2009;137(81):346–350. doi: 10.2298/sarh0908346j. [DOI] [PubMed] [Google Scholar]
- Maheshwari A., Panigrahi R. vol. 3. 2017. pp. 82–87. (Tubercular Otitis Media : an under Diagnosed Entity). 1. [Google Scholar]
- Saltzman S.J., Feigin R.D. Tuberculous otitis media and mastoiditis. J. Pediatr. 1971;79(6):1004–1006. doi: 10.1016/s0022-3476(71)80199-3. [DOI] [PubMed] [Google Scholar]
- Verma R. Tuberculous otomastoiditis: a therapeutic and diagnostic challenge. Indian J. Otology. 2017;23:260–263. [Google Scholar]
- Windle-Taylor P.C., Bailey C.M. Tuberculous otitis media: a series of 22 patients. The Laryngoscope. 1980;90(6):1039–1044. doi: 10.1002/lary.1980.90.6.1039. [DOI] [PubMed] [Google Scholar]
- Yaniv E. Tuberculous otitis: an underdiagnosed disease. Am. J. Otolaryngol. 1987;8(6):356–360. doi: 10.1016/s0196-0709(87)80020-0. 2018. [DOI] [PubMed] [Google Scholar]
- Yaniv E., Traub P., Conradie R. Middle ear tuberculosis — a series of 24 patients. Int. J. Pediatr. Otorhinolaryngol. 1986;12(1):59–63. doi: 10.1016/s0165-5876(86)80058-1. [DOI] [PubMed] [Google Scholar]