Abstract
Green synthesis of silver nanoparticles has gained great interest among scientists. In view of this data, we conducted this study to identify the ameliorative effect of green synthesis of silver nanoparticles using Nigella sativa extract in diabetic neuropathy induced experimentally. In this study, 50 adult male albino rats were used and they were randomly divided into five groups; the first group was the healthy control group, the second group were the diabetic neuropathy diabetic neuropathy induced, Groups (3-6) diabetic neuropathy induced group and treated with silver nanoparticles, Nigella sativa extract and green synthesized silver nanoparticles using Nigella sativa extract respectively. Biochemical parameters including diabetic, inflammatory and antioxidant biomarkers were evaluated. Brain histopathology was also performed. Results revealed substantial rise in glucose, AGE, aldose reductase with insulin reduction in diabetic neuropathy induced group as compared to healthy control. Also, inflammatory markers increased significantly in diabetic neuropathy induced group. A remarkable change in oxidative status was observed in the same group. Furthermore, significant decline in nitrotyrosin level was observed. Regarding gene expression, we found significant down regulation in brain TKr A accompanied by upregulation of nerve growth factor in diabetic neuropathy group comparing with healthy control. Several treatments for diabetic neuropathy remarkably ameliorate all the investigated biomarkers. Histological findings are greatly relied on for the results achieved in this study. Therefore, it can be established that green synthesis of silver nanoparticles in combination with Nigella sativa extract could be a newly neuroprotective agents against inflammation and oxidative stress characterizing diabetic neuropathy through their antidiabetic, anti-inflammatory and anti-oxidants effects.
Keywords: Diabetic neuropathy, Green synthesis, Silver nanoparticles, Nigella sativa
1. Introduction
Being the seventh leading cause of death globally, diabetes mellitus, resulted in 1.4 million deaths in the year 2011 (approximately 2.6 percent of entire worldwide deaths for the same year) (Zhang et al., 2012). Neuropathy is one of the most common risks associated with diabetes, this condition affects 20 percent of adults with diabetes currently, and in the long run, it will affect about 50 percent of all adults with diabetes. There are different forms of diabetic neuropathy that can be classified further through focal or diffuse neuropathy (Callaghan et al., 2012).
Having sensory neuropathy being the most frequently experienced, diabetic neuropathy comprises of autonomic, motor and sensory nerve lesions. The DN’s main clinical features include progressive loss of movement and sensation within the distal limbs, showing symptoms such as intense numbness of limbs, weakness and muscles atrophy, severe pain, and weakening or total loss of tendon reflexes, eventually resulting to foot ulcers, infection and in other cases, amputation (Li, 2013). The symptoms lead to a several disease burden in relation to disability and exhaustion of resources for health care (Alleman et al., 2015).
Since ancient times, human diseases are believed to be treated using medicinal plants (Benhaddou et al., 2011). Use of medicinal plants has been on significant rise in the recent years in comparison with chemical drugs due to various factors, such as low-cost, easy access without prescription, considering the natural products have less side effects, as well as no need to refer to medical advice. (Awad et al., 2005).
Nigella sativa NS is a flowering from the family Ranunculaceae. It is a therapeutic plant that grows in different places all over the world especially Saudi Arabia, Syria, Pakistan and Turkey (Ahmad et al., 2013). It is considered as enriched source of nutritionally vital constituents and its oil contains polyunsaturated fatty acids (PUFA), as well as other several phytochemicals that exhibit strong antioxidant properties and cause lowering effect on glucose (Farooqui et al., 2016).
In the food industry, silver nanocomposites and nanoparticles remain among the most commonly used nanomaterials. They are used as antimicrobials. Silver nanoparticles can be used as a source of Ag ions, that bind to membrane proteins, and catalyze the generation of ROS in bacterial cells. This causes cell death due to oxidative stress (Kumaran, 2018). However, a number of research studies done in the recent years indicated that silver nanocomposites are safe and can be used for food packaging, with insignificant levels or no detectable level of silver nanoparticles produced and moved from containers into actual samples of food (Bieberstein et al., 2013).
In KSA, a diagnosis was carried on out on 552 patients with diabetes mellitus where they were found to have diabetic neuropathy (Wang et al., 2016). In a study carried out in KSA, of 91 patients with foot ulcer, the incidences of lower extremity amputation were 29.7 percent, and there was a rise of amputation risk in patients with peripheral arterial disease and diabetic neuropathy, especially those patients with a more advanced infection and ulceration stage (Ahmed et al., 2015). In a cross-sectional study of three hundred and fifty diabetic patients who were present at a primary care in KSA, the occurrence of peripheral vascular and peripheral neuropathy disease was 15% and 47.5% respectively. In a specialist diabetes center in Riyadh, a cross-sectional study involving 351 patients with diabetes was carried out where diabetic neuropathy, was found in 22.8% of the patients (Madanat et al., 2015). A research carried out by Li and Huang (2008) demonstrated that the speed through which the cells up took the nanoparticles, might be the typical reason for their capability to enhance the antioxidant capacity inside the brain cells
Several synthetic techniques have been employed for the synthesis of silver-based nanoparticles in relation to biochemical, physical and chemical methods. (Huang et al., 2007). Due to the use of noxious stabilizing and/or reducing agents such as N, N-dimethylformamide, toxic agents and sodium borohydride, chemical-based methods are not recommended (Amin et al., 2012). With the increasing attention on green chemistry, natural compounds such as soluble starch, glucose, chitosan and certain microorganisms (Shahverdi et al., 2007), have become a focal point for researches as low toxic alternatives, stabilizing and reducing agents to the silver nanosphere’s synthesize. The use of biochemical routes to synthesize nanoparticles through plant extracts as capping and reducing agents, has been given a lot of special attention among others conferring the silver nanoparticles with extra properties of pharmacology (Prathna et al., 2011). As a result, medicinal plants are being largely utilized for the shape and size- controlled synthesis of silver nanoparticles since these plants have proven to be significant in therapeutic uses (Amin et al., 2012).
In the present study, we focused on the antidiabetic, anti-inflammatory together with antioxidant effect of silver nanoparticles prepared by using Nigella sativa extract in experimental model. Furthermore, we would like to explore the beneficial effect of this combination in ameliorating neurodegeneration accompanied diabetes.
2. Materials and methods
2.1. Animals and chemicals
50 male albino rats (200 ± 10 g) were divided into 5 groups (10 rats in each). Rats received water ad libitum in addition to Standard laboratory diet.
2.2. Preparing the plant extract
Briefly, according to Jalali et al. (2014), 100 g of powdered seeds Nigella Sativa was mixed in a Soxhlet extractor with 70% ethanol. The final extract yielded 32% was then concentrated under low pressure and stored at −20 °C until being used.
2.3. Preparing Silver nanoparticles
Chemical reduction method has been used in the preparation of Silver nanoparticles as reported by Lee and Meisel (1982).
2.4. Green synthesis of silver nanoparticles using nigella sativa
5 ml of aqueous extract of (NS) was placed and paced on the stirrer that is magnetic with hot plate. Then 50 ml of 1 Mm silver nitrate solution was added to this in drops and stirred continuously 120 rpm at 100 °C. The way the color of the solution changed was checked from time to time. The changes from yellow to brown color of the solution shows the formation of AgNPs (Kokate et al., 2009).
2.5. Characterization of silver nanoparticles
Scanning Electron Microscopy (SEM) and change in color and UV–Visible were used to characterize synthesized AgNPs. Through visual observation, a change of color was observed in the flask which has AgNO3 solution with extracts.
2.6. UV–Visible spectroscopy
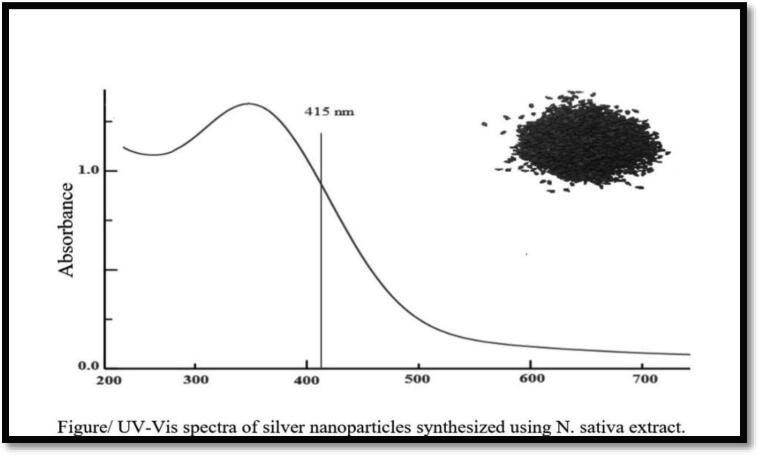
UV–Visible spectrophotometer was used to confirm the formation of AG-NPs. The sample’s UV-V absorption spectrum was carried out in Perkin Elmer Spectrophotometer, at diverse time 5, 10, 20 min after extracting step, AgNO3 solution was added to obtain the UV–Visible spectra of the sample Fig. A.
Fig. A.
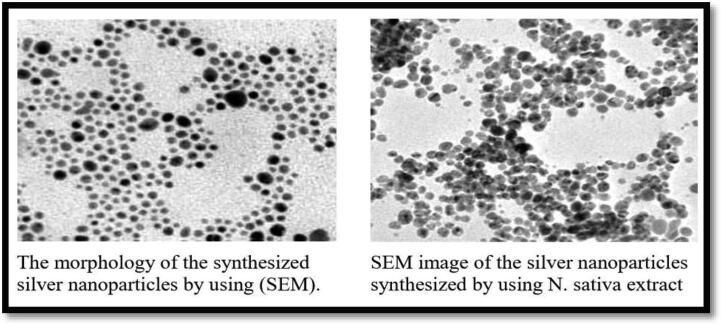
2.7. Scanning electron microscopy
Scanning Electron Microscopy (SEM) was applied to detect AgNPs’ surface morphology. The Ag NPs acquired with Nigella sativa was predominantly spherical with 25.2 nm (Fig. B).
Fig. B.
Table/Absorbance of the Ag-NPs of extracts
| Wavelength | Size | Absorbance | |
|---|---|---|---|
| Nigella sativa + A(combination) | 415 nm | 25.2 nm | 1.2 |
2.8. Experimental induction of diabetic neuropathy
Stimulation of diabetes was done through the single intraperitoneal injection of streptozotocin (STZ) (50 mg/kg b.w. in 0.1 M citrate buffer (pH = 4.5) that was freshly prepared to rats after overnight fasting. After three weeks rats with high glucose level (more than 250 mg/dl) having glycosuria and hyperglaycemia, were considered as diabetic neuropathy rats and used for the experiment.
2.9. Experimental design
Group (1): Healthy control rats treated by saline daily Group (2): Diabetic Neuropathy (DN) group (Streptozotocin intraperitoneally injected (50 mg/kg) to induce diabetic neuropathy Group (3): Diabetic Neuropathy group treated with AgNPs in oral dose of (0.25 mg/kg bw) daily for 21 days. (4): Diabetic Neuropathy group treated with Nigellia Sativa extract in oral dose of (200 mg/kg bw. ip) daily for 21 days. Group (5): Diabetic Neuropathy group treated with AgNPs prepared by Nigellia Sativa extract in oral dose of (200 mg/kg bw. ip) daily for 21 days.
Animals were anaesthetized by sodium thiopental injection, blood samples were obtained by retro-orbital bleeding at the end of experimental period. Then, sera were separated for subsequent use of different biochemical measurements. Rats were then decapitated, and their brain cautiously dissected and extraneous tissues were removed, and apart of brain tissue was directly moved to 10% formalin for histopathological assessments. For gene expression, the second part was preserved at −80 °C, homogenization was done on the other part using 0.1 M phosphate buffer saline at pH7.4, in order to get ultimate concentration of 10% w/v and centrifuged at 3000xg for fifteen minutes at −4 °C. The resulting supernatant was utilized for subsequent biochemical assessments.
3. Biochemical analysis
Glucose level was measured using peripheral blood from the tail by (one touch sure step meter life scan, CA). Serum insulin and advanced glycation end product (AGE) were measured using Eliza Kit (Cayman chem., Ann Arbor, MI, USA; Cat. No: 589501) according to manufacture instruction. TNF-α, Nuclear factor Kappa B (NFκB), S-100B were measured using sandwich enzyme-connected immunosorbent assay (ELISA) method (provided by WKEA Med Supplies Corp., Changchun, China) and (Human S100B, BioVendor, No: RD192090100R) respectively. Serum Aldose reductase, was also measured. Brain Antioxidant Status Includes malondialdehyde (MDA), nitric oxide (NO) levels, glutathione (GSH) were also measured according to manufacture instruction. Moreover, nitrotyrosine was measured using Eliza immunoassay (HyCult Biotechnology, Holland). Histopathological analysis was performed on brain tissue according to Yoo and Lee (2016).
4. Quantitative real-time polymerase chain reaction (qPCR)
Using Sepasol RNA Super G, Total RNA was obtained from the brain tissue (Nacalai Tesque, Kyoto, Japan) and transcribed in reverse using ReverTra Ace- PCR RT Master Mix with genomic DNA Remover (Toyobo Co., Osaka, Japan) in relation to protocol of the manufacturer.
The sequences of the primers utilized for NGF and TrkA are listed below: NGF primers, forward, 50-ACCTCTTCGGACACTCTGG-30, and backward, 50-CGTGGCTGTGGTCTTATCTC-30; trkA primers, forward, 50-CCATGCTACAGCACCAACAC-30, and backward, 50-AAGGACCAGGAGCCACATC-30; qPCR was achieved through the use of a Bio- Rad iCycler - qPCR system as well as SYBR- Ex Premix taq™ (Takara Bio Inc., Shiga, Japan). All the primer pairs had cycling conditions as shown below: 5 s at 95 °C and 30 s at 60 °C, as per the protocol of the manufacturer. The ratios of NGF and trkA mRNA expression to the β actin level in the different samples were taken into consideration based on the different levels of mRNA and are expressed based on the mRNA levels of the control group (Obata et al., 2006). Data are indicated as mean ± SE.
4.1. Statistical analysis
The results of the current study were carried as Mean + SE. Data analyzation was done through ONE WAY analysis of variance (ANOVA) utilizing the Statistical Package for the Social Sciences (SPSS) program. Variance was recognized to be significant when P value was <0.05.
5. Results
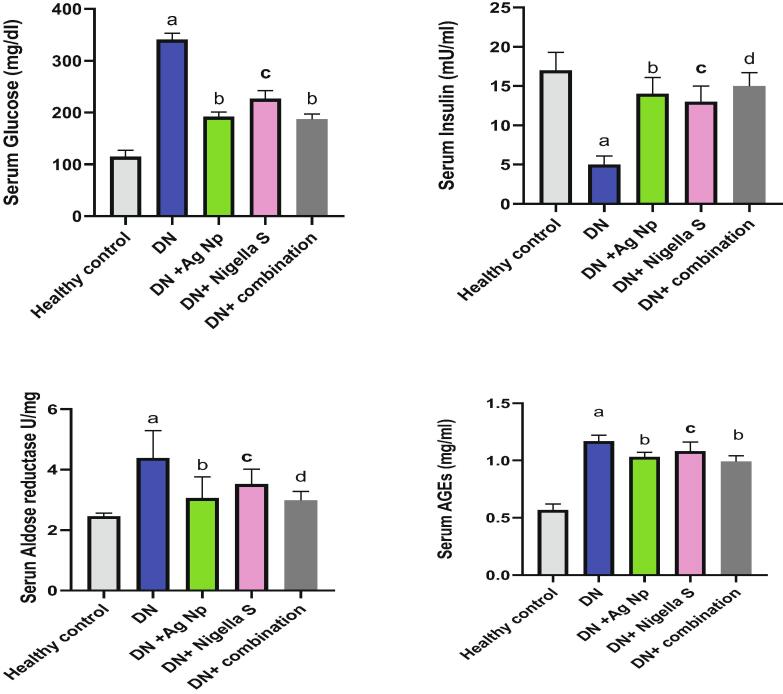
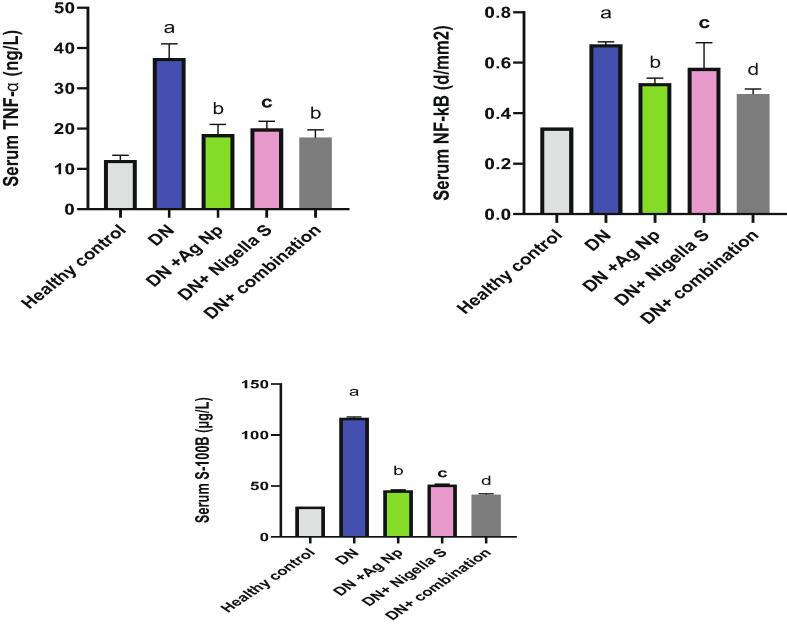
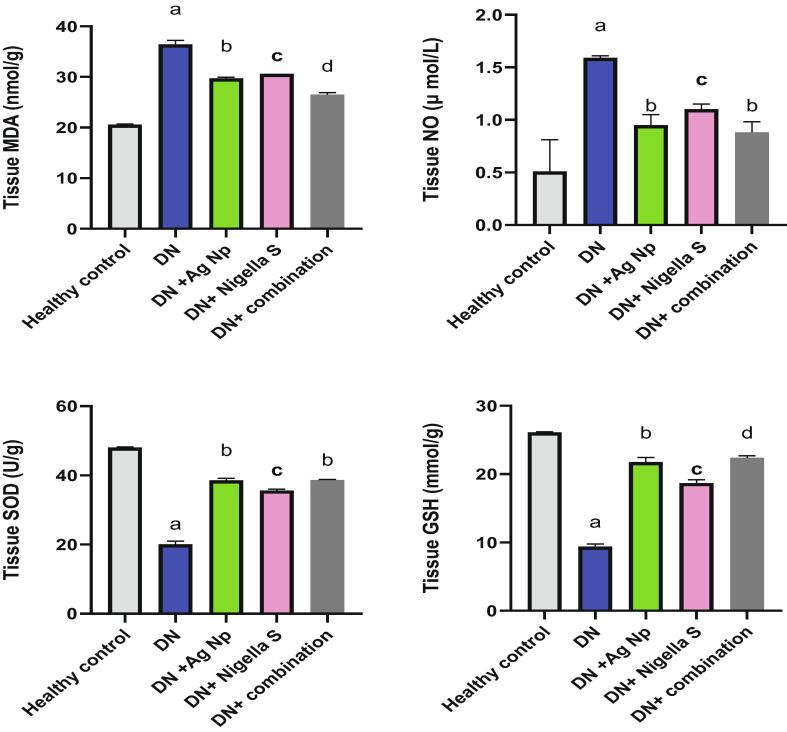
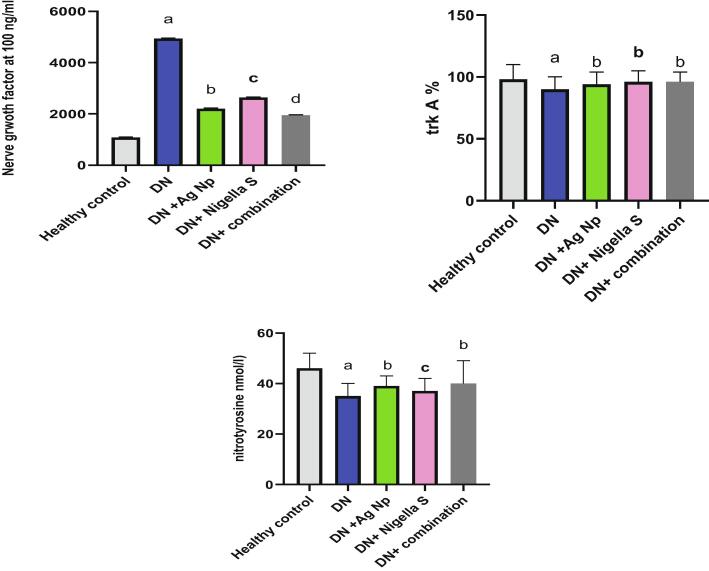
Fig. 1 illustrates the effect of AgNPs, nigella sativa and their combination on serum level of glucoses, insulin, aldose reductase and AGE in all groups. It is clear from this figure that, there was significant increase (P < 0.05) in blood glucose, AGE and aldose reductase levels in Diabetic neuropathy induced group accompanied by a significant decline (P < 0.05) in insulin concentration as compared to control group. Groups treated with AgNPs, Nigella sativa and their combinations, showed significant decrease (P < 0.05) in glucose and AGE and aldose reductase level comparing with DN induced group, on the other hand, insulin level increased significantly (P < 0.05) in treated groups as compared to DN induced group. Our results from Fig. 2 revealed significant elevation in inflammatory biomarkers (TNF-α, NFκB and S100B) in Diabetic neuropathy induced groups as compared to healthy control. While treatments with AgNPs, Nigella sativa and their combination remarkably ameliorate inflammatory markers comparing to Diabetic neuropathy induced group. Nano silver prepared using Nigella sativa extract (green synthesis) showed the best effect as compared to other treatments. Regarding the oxidative stress status, It is clear from the present data (Fig. 3) that there was significant elevation (P < 0.05) in brain MDA, NO content in Diabetic neuropathy induced group accompanied by significant decline (P < 0.05) in SOD and GSH as compared to healthy control group. All the other treatment remarkably attenuates oxidative status in DN induced rats. Fig. 4 illustrates the level of nerve growth factor and nitrotyrosine and TKr A in all treated groups. Results revealed a significant elevation (P < 0.05) in expression of nerve growth factor associated by decrease (P < 0.05) in TKr A level in DN induced group when compared to healthy control group, while expression of nitrotyrosine significantly reduced (P < 0.05). Groups treated with either AgNPs, nigella sativa or their combinations showed significant (P < 0.05) improvement of these biomarkers.
Fig. 1.
Effect of different treatments on serum levels of Glucose, Insulin and AGEs a: Significantly different from (normal control) group value at p < 0.05. b: Significantly different from (Diabetic Neuropathy) group value at p < 0.05. c: Significantly different from (Diabetic Neuropathy + AgNP) group value at p < 0.05. d: c: Significantly different from (Diabetic Neuropathy + Nigella Sativa) group value at p < 0.05.
Fig. 2.
Effect of different treatments on serum levels of TNF-α, NF-kB and S-100B, a: Significantly different from (normal control) group value at p < 0.05. b: Significantly different from (Diabetic Neuropathy) group value at p < 0.05. c: Significantly different from (Diabetic Neuropathy + AgNP) group value at p < 0.05. d: c: Significantly different from (Diabetic Neuropathy + Nigella Sativa) group value at p < 0.05.
Fig. 3.
Effect of different treatments on serum levels of MDA, NO, SOD and GSH, a: Significantly different from (normal control) group value at p < 0.05. b: Significantly different from (Diabetic Neuropathy) group value at p < 0.05. c: Significantly different from (Diabetic Neuropathy + AgNP) group value at p < 0.05. d: c: Significantly different from (Diabetic Neuropathy + Nigella Sativa) group value at p < 0.05.
Fig. 4.
Effect of different treatments on expression of Nerve Growth Factor, trKA, and Nitrotyrosine. a: Significantly different from (normal control) group value at p < 0.05. b: Significantly different from (Diabetic Neuropathy) group value at p < 0.05. c: Significantly different from (Diabetic Neuropathy + AgNP) group value at p < 0.05. d: c: Significantly different from (Diabetic Neuropathy + Nigella Sativa) group value at p < 0.05.
Interestingly, group treated with green synthesis of nano silver prepared by nigella sativa extract showed the best effect between all other treatment.
5.1. Histological findings
See Fig. 5.
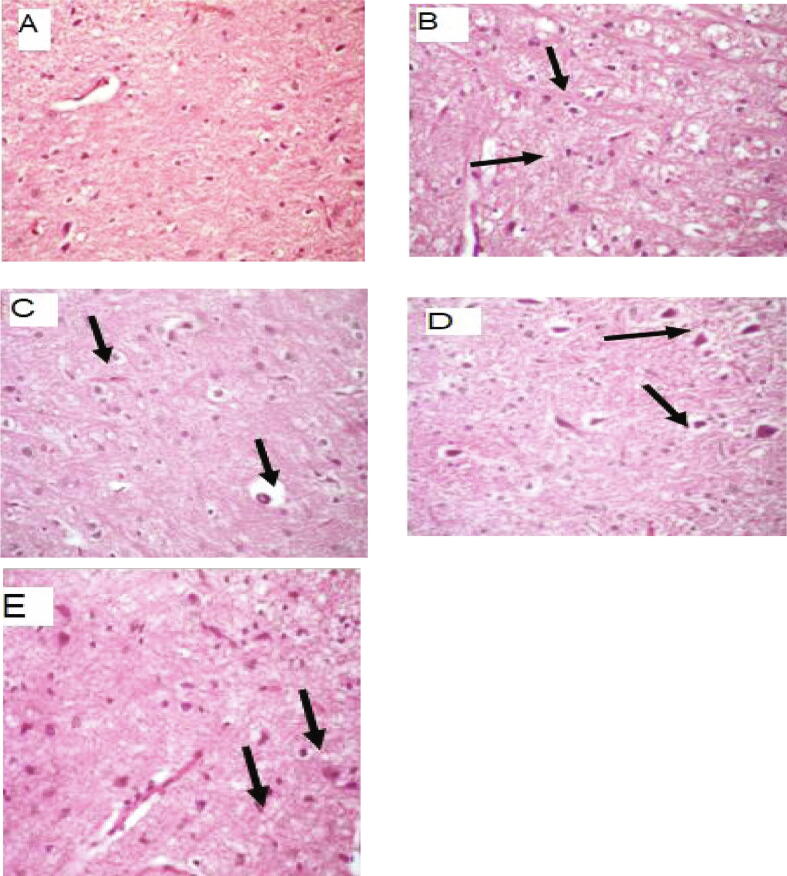
Fig. 5.
Photomicrograph of brain tissue (A) healthy control group showing normal brain parenchyma with normal nerve cells and neurons, (score lesion: 0), (H&E X 400); (B) Diabetic neuropathy group showing severe intracellular neuronal edema (arrow) with marked demyelination of nerve fibers (nerve arrow), (lesion score: +++), (H&E X 400); (C) Diabetic neuropathy + AgNPs group, showing low frequent neuronal edema (arrow), with slight nerve fibers demylenation (arrow head), (score lesion: +), (H&E X 400); (D) Diabetic neuropathy + Nigella Sativa group showing massive areas of neuronal degeneration (arrows) with severely degenerated nerve fibers (arrow head), (score lesion: +++), (H&E X 400);(E) Diabetic neuropathy + Combination group showing multifocal areas of neuronal degenerations (arrows) together with vacuolation of nerve fibers (arrow head), (score lesion: ++), (H&E X 400).
6. Discussion
Diabetic neuropathy is a pathology that grow on fast rate and should have a global concern. In most cases, diabetic neuropathy starts as a fiber neuropathy that is small and influenced by serum glucose levels. Factors that play significant roles in this pathophysiological process include low blood flow and increased serum glucose levels (Akbarzadeh et al., 2007). Therefore, it is important to discover new compounds and treatment techniques to avoid diabetes risks (Suryavanshi and Kulkarni, 2017). In this section, the ameliorative potential of green synthesis of AgNPs is discussed through the use of Nigella sativa extract on diabetic neuropathy in the model of a rat of Streptozotocin -induced diabetes. Streptozotocin STZ destroys pancreas cells that produce insulin and is usually used as an experimental model for diabetes (Furman et al., 2015)
6.1. Effect of different treatments on serum levels of glucose, aldose reductase and AGEs
Results showed that, Diabetic neuropathy rat group had an elevation in serum levels of glucose, aldose reductase and AGEs accompanied by reduction in insulin level. High levels of blood glucose, results in several health risks including neuropathy, renal failure, blindness etc. In addition, elevated levels of glucose in blood encouraged auto-oxidative glycosylation, generation of a product of glycation, stimulation of kinase-C protein and nuclear transcription factor (NF-kB) (Kumar et al., 2007). For a mammalian brain, glucose is the major source of energy, according to (Howarth et al., 2012), neuron is the part that require the most energy in the brain of an adult, demanding uninterrupted delivery of glucose from blood. Our findings agreed with Yang et al. (2014) who demonstrated that diabetic neuropathy induces metabolic imbalance and induces hyperglycemia. Similar results have been demonstrated in both type 1 and 2 diabetic rodent models (Zhang et al., 2015). Hyperglycemia in patients suffering from diabetes is concerned with, oxidative and glycosylation damage to proteins, fats and nucleic acids leading to structural and functional damage of nerve fibers, with diabetic neuropathy development (Albers and Pop-Busui, 2014).
Advanced glycation end product AGEs have been considered as possible biomarkers for diagnosing diabetes complications. AGEs are generated through a non-enzymatic reaction of glucose using amino groups on amino acids from proteins during diabetes (Xing et al., 2016). The increased levels of AGEs were due the effect of diabetes on increasing receptors of AGEs, induction of inflammatory cascade and generation of RNS and ROS, leading to cellular damage and dysfunction (Yagihashi et al., 2011). Additionally, the AGEs encourage vascular and neuronal alteration of protein, leading to vasoconstriction of the neuronal blood vessel and neuronal dysfunction, following deprivation of neuronal cells from its source of blood (Hosseini and Abdollahi, 2013).
It was noticed in the present study that, treatment with either Nigella sativa or AgNPs, modified these level parameters, Nigella sativa have conveyed activities that lower glucose in the earlier literature in different mechanisms of action and considered as pancreas protective herb (Kaatabi et al., 2015). As stated by Balbaa et al. (2016), Nigella sativa’s ethanolic extract has the property of proliferation and regeneration of beta cells in the pancreas. It also has the capability of improving the glucose uptake, as well as inducing insulin secretion from beta cells in pancreas. Furthermore, Halimi et al (2008) considered Nigella sativa as a herb that used in treating diabetes and several other diseases. A study by Abel-Salam (2012) exposed that, volatile fractions and N. sativa lipid holds insulinotropic properties and retains the integrity of β-cells that improve the production of the insulin which is vital in facilitating diabetes mellitus. These results strongly support our findings.
Regarding antidiabetic effect of AgNPs, it has established to make an increase in the bone’s mineral density making it easy for the β-cells of the pancreas to release insulin through a related pathway, of the sulfonylureas that are presently utilized in the therapy for anti-diabetic. This due to their ability to induce endogenous insulin secretions, as well as to enhance the uptake of glucose and its usage inside the muscle and plays the role of free radical scavenger reducing oxidative stress in rat models that are diabetic (Alkaladi et al., 2014).
6.2. Effect of different treatments on TNF-α, NF- ĸB and S-100B
In our study, results obtained from diabetic neuropathy group showed an increase in serum levels of TNF-α, NF- ĸB and S-100B as compared to control rats.
During diabetes, inflammation can be triggered through an increase in reactive oxygen species ROS (Behzad et al., 2017). NFκB, which is a transcription aspect that controls the expression of proinflammatory genes, can be activated by ROS (Nadeem et al., 2013).
Glucose auto-oxidation and excessive production of ROS is strongly induced by hyperglycemia. Protein kinase C is activated by excess ROS and then NFκB, resulting to injury. Being a redox-sensitive protein complexes, NFκB has a major function in inflammation (Khanra et al., 2015). Activated NFκB encourages the transcription, as well as, the release of inflammatory mediators, for instance, tumor necrosis factor-α TNF-α and thus, provoking inflammation (Saisho, 2014). Previous study by Wang et al. (2013) reveal that, enhancement in serum levels of proinflammatory cytokines like TNF-a represents the state of insulin resistance.
The S100 proteins, a family of calcium-binding cytosolic proteins, have a broad range of intracellular and extracellular functions through regulating calcium balance, cell apoptosis, migration, proliferation, differentiation, energy metabolism, and inflammation (Crisan et al., 2018). An increased S100B expression has been recorded inside the plasma and adipose tissue of obese animals (Buckman et al., 2014). A close connection between increased levels of S100B and insulin resistance has been indicated. The secretion of pro-inflammatory cytokines in the adipose tissue such as TNF-α is usually controlled by adipocyte derived S100B (Zhang et al., 2017).
Administering a combination of Nigella sativa extract and Silver NPs modulated the inflammatory biomarkers. The anti-inflammatory effect of Nigella sativa has been previously demonstrated in several inflammation-based models (Kokku et al., 2014). These results agreed with Vance et al. (2015) who stated that AgNPs possesses anti-inflammatory activities. Interestingly, several Ag-NPs’ studies have illustrated cytoprotective activities, both in vitro and in vivo (Tian et al., 2007). In addition, in vitro experimental data assigned AgNPs anti-inflammatory impact and the role it plays in the process of healing wounds through lowering the levels of TNF-α, interferons and interleukin 1, as well as, inhibition of COX-2 and MMP-3 expressions (Frankova et al., 2016). As it was also discovered, silver NPs has the potential of hindering the activities of TNF-α which are involved in inflammations (Shin et al., 2007).
6.3. Effect of different treatments on oxidative stress biomarkers
Results revealed that Diabetic neuropathy could affect oxidative parameters as it increases serum levels of MDA and NO with a parallel reduction in SOD and GSH. These enzymes act as a major factor in the removal of free radicals from the tissues and they get protected from the impact of oxidative stress through their high expression and activities levels in brain cells (May et al., 2014). The cell’s components such as, DNA, protein and lipids are severely damaged by oxidative stress (El-Hussein et al., 2012). In diabetic neuropathy, endothelial function is severely affected by insulin resistance resulting to reduced synthesis of nitric oxide NO. Superoxide and nitric oxide combine rapidly in solution which produce a potent protein-oxidizing agent called peroxynitrite. Through the detection of nitrotyrosine residues in the protein, the peroxynitrite concentrations can be monitored indirectly, showing the amount of damage caused by oxidative stress (Safinowski et al., 2009). It is a major factor in the neuronal degeneration since it activates the extracellular modulation, inflammatory cascade and autoimmune apoptosis. The inflammatory mediators such as TNF-α, NF-κβ are activated by the production of ROS in neuronal cells.
Results indicated that the oxidative stability was impaired by diabetic neuropathy (Mittal et al., 2014). Among the several mechanisms that lead to induction of oxidative stress in diabetes is the disruption in the mitochondrial electron transport sequence, caused by excessive levels of glucose, that lead to a superoxide anions’ overproduction (Nishikawa et al., 2000). Previous studies by Saribeyoglu et al., 2011, Weidinger and Kozlov, 2015 indicated increased serum MDA concentration in diabetic patients as well as a reduction in plasma total antioxidant capacity TAC which confirmed our findings.
After treatment with either Nigella sativa or AgNPs, or combination of them, the antioxidant status is restored significantly in comparison to the diabetic neuropathic group. Our results agreed with Kanter et al. (2004) who stated that administering Nigella sativa in STZ-induced diabetic rats, may protect pancreatic β-cells, and reduce the concentration of pancreatic MDA and serum levels of nitric oxide. Nigella sativa has protective activities by induction of endogenous anti-oxidative enzymes, scavenging superoxide, and inhibition of NF-κB (Shabana et al., 2013). Additionally, it indicated the efficacy of Nigella sativa antioxidant property in preventing environmental toxic agent-induced oxidative stress.
6.4. Effect of different treatments on expression of nerve growth factor, trKA, and nitrotyrosine
A diabetic state encourages delay in the generation of nerves during diabetic neuropathy (Malysz et al., 2010). Yasuda et al. (2003) reviewed different factors relating to nerve regeneration factor NGF that are recorded to cause a down-regulation during experimental neuropathy in diabetic rodents. Additionally, through the stimulation of a receptor tropomyosin kinase receptor A, the mature NGF can apply its biological action.
According to the in vitro and in vivo results, NGF promotes differentiation, role and survival of sympathetic nerve and peripheral sensory cells. These results suggest that an NGF that is purified might be of great importance in the prevention and/or protection of peripheral nerve degeneration, for instance, those that occur in diabetes, surgical traumas and leprosy (Aloe et al., 2012). Our result was in line with Manni et al. (2011) which covered an experimental model of diabetic neuropathy illustrating how the early increase of peripheral and spinal NGF, was linked to the development of thermal hyperglycaemia and hyperalgesia. The results achieved in this study were in line with Ceriello et al. (2002) which stated that there was a significant increase of fasting nitrotyrosine in diabetic patients.
Several studies revealed that Nigella sativa has pharmacological properties among them are the neuroprotective improvement of neurodegenerative illnesses, as well as memory enhancement (Imam et al., 2018). A previous study by Yuste et al. (2015) evaluated the Nigella sativa protective effect against Chlopyrifos which has been linked to the pathophysiology of many neurodegenerative illnesses. On the other hand, concomitant administration of ethanolic extract of Nigella sativa, suppressed the increased hippocampal NO levels in the rats that were exposed, and as a result inferred to lower neuro-inflammation (Kunjiappan et al. 2015)
6.5. Histological findings
Histological changes were extremely noticed in tissues inside the brain of Diabetic neuropathic rats. The brain cells can be affected directly by diabetes, through the production of an oxidative stress resulting to apoptosis. The main tool is demonstrated a rise in glucose metabolism because of hyperglycemia that stimulates the respiration of mitochondrial, leading to the release of superoxide and other reactive nitrogen or oxygen species into the cytoplasm (Zhang et al., 2015).
7. Conclusion
We provide the evidence that, green synthesis of AgNPs using nigella sativa extract has a promising antidiabetic effect with special reference to one of the most complications of diabetes (diabetic neuropathy) via their ability to mitigate hyperglycemia, oxidative stress, inflammation, and apoptosis. Further studies are required to find other mechanisms involved in this action.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This project was funded by the Deanship of Scientific Research - at King Abdulaziz University, Jeddah, Saudi Arabia, under grant number (G:315-363-1439). The authors thank Deanship of Scientific Research at King Abdulaziz University for providing the funding for this research and for technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abel-salam B. Immunomodulatory effect of black seed and garlic on alloxan induced diabetes in Albino rats. Allergol. Immunopathol. (Madr.) 2012;40:336–340. doi: 10.1016/j.aller.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Damanhouri Z.A., Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pacific J. Trop. Biomed. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.A., Algamdi S.A., Alzahrani A.M. Surveillance of risk factors for diabetic foot ulceration with particular concern to local practice. Diab. Metabolic Syndrome: Clin. Res. Rev. 2015;9(4):310–315. doi: 10.1016/j.dsx.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh A., Norouzian D., Mehrabi M.R., Jamshidi S.H., Farhangi A., Verdi A.A., Mofidian S.M.A., Rad B.L. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007;22(2):60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers J.W., Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr. Neurol. Neurosci. Rep. 2014;14(8):473. doi: 10.1007/s11910-014-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkaladi A., Abdelazim A.M., Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014;15(2):2015–2023. doi: 10.3390/ijms15022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleman C.J., Westerhout K.Y., Hensen M., Chambers C., Stoker M., Long S., van Nooten F.E. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res. Clin. Pract. 2015;109(2):215–225. doi: 10.1016/j.diabres.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Aloe L., Rocco M.L., Bianchi P., Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J. Translat. Med. 2012;10(1):239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M., Anwar F., Janjua M.R.S.A., Iqbal M.A., Rashid U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012;13(8):9923–9941. doi: 10.3390/ijms13089923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad E.M., Binder B.R. In vitro induction of endothelial cell fibrinolytic alterations by Nigella sativa. Phytomedicine. 2005;12(3):194–202. doi: 10.1016/j.phymed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Balbaa M., El-Zeftawy M., Ghareeb D., Taha N., Mandour A.W. Nigella sativa relieves the altered insulin receptor signaling in streptozotocin-induced diabetic rats fed with a high-fat diet. Oxidat. Med. Cell. Longevity. 2016;2016 doi: 10.1155/2016/2492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzad, S., Sureda, A., Barreca, D., Nabavi, S.F., Rastrelli, L., Nabavi, S.M., Health effects of phloretin 2017 from chemistry to medicine, Phytochem. Rev., pp. 1–7.
- Benhaddou-Andaloussi A., Martineau L., Vuong T., Meddah B., Madiraju P., Settaf A., Haddad P.S. The in vivo antidiabetic activity of Nigella sativa is mediated through activation of the AMPK pathway and increased muscle Glut4 content. Evidence Compl. Alternat. Med. 2011;2011 doi: 10.1155/2011/538671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberstein A., Roosen J., Marette S., Blanchemanche S., Vandermoere F. Consumer choices for nano-food and nano-packaging in France and Germany. Eur Rev. Agric. Econ. 2013;40(1):73–94. [Google Scholar]
- Buckman L.B., Anderson-Baucum E.K., Hasty A.H., Ellacott K.L. Regulation of S100B in white adipose tissue by obesity in mice. Adipocyte. 2014;3(3):215–220. doi: 10.4161/adip.28730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.C., Cheng H.T., Stables C.L., Smith A.L., Feldman E.L. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A., Quagliaro L., Catone B., Pascon R., Piazzola M., Bais B., Marra G., Tonutti L., Taboga C., Motz E. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25(8):1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- Crisan D., Scharffetter-Kochanek K., Crisan M., Schatz S., Hainzl A., Olenic L., Filip A., Schneider L.A., Sindrilaru A. Topical silver and gold nanoparticles complexed with Cornus mas suppress inflammation in human psoriasis plaques by inhibiting NF-κB activity. Exp. Dermatol. 2018;27(10):1166–1169. doi: 10.1111/exd.13707. [DOI] [PubMed] [Google Scholar]
- El-Hussein A., Harith M., Abrahamse H. Assessment of DNA damage after photodynamic therapy using a metallophthalocyanine photosensitizer. Int. J. Photoenergy. 2012;2012:1–10. doi: 10.1155/2012/281068. [DOI] [Google Scholar]
- Farooqui Z., Afsar M., Rizwan S., Khan A.A., Khan F. Oral administration of Nigella sativa oil ameliorates the effect of cisplatin on membrane enzymes, carbohydrate metabolism and oxidative damage in rat liver. Toxicol. Rep. 2016;3:328–335. doi: 10.1016/j.toxrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franková J., Pivodová V., Vágnerová H., Juráňová J., Ulrichová J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016;14(2):137–142. doi: 10.5301/jabfm.5000268. [DOI] [PubMed] [Google Scholar]
- Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protocols Pharmacol. 2015;70(1):5–47. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- Halimi S., Schweizer A., Minic B., Foley J., Dejager S. Combination treatment in the management of type 2 diabetes: focus on vildagliptin and metformin as a single tablet. Vascular Health Risk Manage. 2008;4(3):481. doi: 10.2147/vhrm.s2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A., Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid. Med. Cell. Longevity. 2013;2013:1–15. doi: 10.1155/2013/168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C., Gleeson P., Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012;32(7):1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Li Q., Sun D., Lu Y., Su Y., Yang X., Wang H., Wang Y., Shao W., He N., Hong J. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18(10):105–1104. [Google Scholar]
- Imam A., Lawal A., Oyewole L.A., Ajibola M.I., Williams V., Chengetanai S., Shittu T.S.T., Ajao M.S. Nigella sativa conserved hippocampal oxidative and neurogenic activities to salvage neuro-cognitive integrities in chlorpyrifos insult. Scientific African. 2018;1:e00008. [Google Scholar]
- Jalali M., Tehranipour M., Mahdavi Shahri N. Effect of alcoholic extract of Nigella sativa seed on alpha motor neurons density of spinal cord following sciatic nerve compression in rats. J. Gorgan Univ. Med. Sci. 2014;15(4) [Google Scholar]
- Kaatabi H., Bamosa A.O., Badar A., Al-Elq A., Abou-Hozaifa B., Lebda F., Al-Khadra A., Al-Almaie S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS ONE. 2015;10(2) doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter M., Coskun O., Korkmaz A., Oter S. Effects of Nigella sativa on oxidative stress and β-cell damage in streptozotocin-induced diabetic rats. Anatom. Rec. Part A: Disc. Mole. Cell. Evolut. Biol.: Off. Publ. Am. Assoc. Anatomists. 2004;279(1):685–691. doi: 10.1002/ar.a.20056. [DOI] [PubMed] [Google Scholar]
- Khanra R., Dewanjee S., Dua T.K., Sahu R., Gangopadhyay M., De Feo V., Zia-Ul-Haq M. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J. Translat. Med. 2015;13(1):6. doi: 10.1186/s12967-014-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate C.K., Purohit A.P., Gokhale S.B. Pharmacognostical and Physicochemical Standardization of Leaves of Spathodea Campanulata P. Beauv Pharmacognosy. 2009;1:1–6. [Google Scholar]
- Kokku S.B., Mahapatra B., Tucker S., Saggurti N., Prabhakar P. Effect of public-private partnership in treatment of sexually transmitted infections among female sex workers in Andhra Pradesh, India. Indian J. Med. Res. 2014;139(2):M285–M293. [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kaundal R.K., Iyer S., Sharma S.S. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007;80(13):1236–1244. doi: 10.1016/j.lfs.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Kumaran N.S. In vitro anti-inflammatory activity of silver nanoparticle synthesized Avicennia marina (Forssk.) Vierh.: a green synthetic approach. Int. J. Green Pharm. (IJGP) 2018;2(03):6. [Google Scholar]
- Kunjiappan S., Bhattacharjee C., Chowdhury R. In vitro antioxidant and hepatoprotective potential of Azolla microphylla phytochemically synthesized gold nanoparticles on acetaminophen–induced hepatocyte damage in Cyprinus carpio L. Vitro Cell. Dev. Biol.-Animal. 2015;51(6):630–643. doi: 10.1007/s11626-014-9841-3. [DOI] [PubMed] [Google Scholar]
- Lee P., Meisel D. Adsorption and surface – enhanced raman of dyes on silver and gold sols. J. Phys. Chem. 1982;86:3391–3395. [Google Scholar]
- Li H.J. Progress of diabetic peripheral nerve lesions. Cap Med. 2013;20:12–14. [Google Scholar]
- Li S.D., Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- Madanat A., Sheshah E., Badawy E.B., Abbas A., Anas A.B. Utilizing the Ipswich Touch Test to simplify screening methods for identifying the risk of foot ulceration among diabetics: The Saudi experience. Primary Care Diab. 2015;9(4):304–306. doi: 10.1016/j.pcd.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Malysz T., Ilha J., Nascimento P.S.D., Angelis K.D., Schaan B.D.A., Achaval M. Beneficial effects of treadmill training in experimental diabetic nerve regeneration. Clinics. 2010;65(12):1329–1337. doi: 10.1590/S1807-59322010001200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni L., Florenzano F., Aloe L. Electroacupuncture counteracts the development of thermal hyperalgesia and the alteration of nerve growth factor and sensory neuromodulators induced by streptozotocin in adult rats. Diabetologia. 2011;54:1900–1908. doi: 10.1007/s00125-011-2117-5. [DOI] [PubMed] [Google Scholar]
- May J.M., Jayagopal A., Qu Z.C., Parker W.H. Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes. Biochem. Biophys. Res. Commun. 2014;452(1):112–117. doi: 10.1016/j.bbrc.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A., Naveed A.K., Hussain M.M., Raza S.I. Correlation of inflammatory markers with type 2 Diabetes Mellitus in Pakistani patients. J. Postgraduate Med. Inst. (Peshawar-Pakistan) 2013;27(3) [Google Scholar]
- Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Prathna T.C., Chandrasekaran N.A., Raichur M., Mukherjee A. Kinetic evolution study of silver nanoparticles in bio-based green synthesis process. Colloids Surf. A Physicochem. Eng. Aspects. 2011;377:212–216. [Google Scholar]
- Safinowski M., Wilhelm B., Reimer T., Weise A., Thomé N., Hänel H., Forst T., Pfützner A. Determination of nitrotyrosine concentrations in plasma samples of diabetes mellitus patients by four different immunoassays leads to contradictive results and disqualifies the majority of the tests. Clin. Chem. Lab. Med. 2009;47(4):483–488. doi: 10.1515/CCLM.2009.095. [DOI] [PubMed] [Google Scholar]
- Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int. J. Mol. Sci. 2014;15(10):18381–18406. doi: 10.3390/ijms151018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saribeyoglu K., Aytac E., Pekmezci S., Saygili S., Uzun H., Ozbay G., Aydin S., Seymen H.O. Effects of clinoptilolite treatment on oxidative stress after partial hepatectomy in rats. Asian J. Surg. 2011;34(4):153–157. doi: 10.1016/j.asjsur.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Shabana A., El-Menyar A., Asim M., Al-Azzeh H., Al Thani H. Cardiovascular benefits of black cumin (Nigella sativa) Cardiovasc. Toxicol. 2013;13(1):9–21. doi: 10.1007/s12012-012-9181-z. [DOI] [PubMed] [Google Scholar]
- Shahverdi A.R., Minaeian S., Shahverdi H.R., Jamalifar H., Nohi A.A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem. 2007;42(5):919–923. [Google Scholar]
- Shin S.H., Ye M.K., Kim H.S., Kang H.S. The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int. Immunopharmacol. 2007;7(13):1813–1818. doi: 10.1016/j.intimp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Suryavanshi S.V., Kulkarni Y.A. NF-κβ: a potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017;8:798. doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Wong K.K., Ho C.M., Lok C.N., Yu W.Y., Che C.M., Chiu J.F., Tam P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem: Chem. Enabl. Drug Disc. 2007;2(1):129–136. doi: 10.1002/cmdc.200600171. [DOI] [PubMed] [Google Scholar]
- Vance M.E., Kuiken T., Vejerano E.P., McGinnis S.P., Hochella M.F., Jr, Rejeski D., Hull M.S. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015;6(1):1769–1780. doi: 10.3762/bjnano.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.D., Jamjoom R.A., Alzahrani A.H., Hu F.B., Alzahrani H.A. Prevalence and correlates of lower-extremity amputation in patients with diabetic foot ulcer in Jeddah, Saudi Arabia. Int. J. Lower Extremity Wounds. 2016;15(1):26–33. doi: 10.1177/1534734615601542. [DOI] [PubMed] [Google Scholar]
- Wang J., Chen Y., Zhang W., Zheng G., Meng S., Che H., Ke T., Yang J., Chen J., Luo W. Akt activation protects liver cells from apoptosis in rats during acute cold exposure. Int. J. Biol. Sci. 2013;9(5):509. doi: 10.7150/ijbs.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger A., Kozlov A.V. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5(2):472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L.V., Gao-Hong L.V., Guo-Ying D.A.I., Hong-Mei S.U.N., Hui-Qin X.U. Food-advanced glycation end products aggravate the diabetic vascular complications via modulating the AGEs/RAGE pathway. Chinese J. Natl. Med. 2016;14(11):844–855. doi: 10.1016/S1875-5364(16)30101-7. [DOI] [PubMed] [Google Scholar]
- Yagihashi S., Mizukami H., Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J. Diabetes Investig. 2011;2:18–32. doi: 10.1111/j.2040-1124.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Xia C., Li S., Du L., Zhang L., Hu Y. Mitochondrial dysfunction driven by the LRRK2-mediated pathway is associated with loss of Purkinje cells and motor coordination deficits in diabetic rat model. Cell Death Dis. 2014;5:e1217. doi: 10.1038/cddis.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H., Terada M., Maeda K., Kogawa S., Sanada M., Haneda M., Kashiwagi A., Kikkawa R. Diabetic neuropathy and nerve regeneration. Prog. Neurobiol. 2003;69(4):229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Yuste J.E., Tarragon E., Campuzano C.M., Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015;9:322. doi: 10.3389/fncel.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Liang X.C., Sun Q., Yin D.H., Jiang N., Han S.M. Study on the characteristics of syndrome of traditional Chinese medicine for diabetic peripheral neuropathy. China J. Tradit. Chinese Med. Pharm. 2012:6. [Google Scholar]
- Zhang Y., Reichel J.M., Han C., Zuniga-Hertz J.P., Cai D. Astrocytic process plasticity and IKKβ/NF-κB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 2017;25(5):1091–1102. doi: 10.1016/j.cmet.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Xu H., Yu X., Wang Y., Sun F., Sui D. Simvastatin ameliorates low-dose streptozotocin-induced type 2 diabetic nephropathy in an experimental rat model. Int. J. Clin. Exp. Med. 2015;8(4):6388. [PMC free article] [PubMed] [Google Scholar]