Abstract
Aedes mosquitoes are the most important group of vectors that transmit pathogens, including arboviruses, and cause human diseases such as dengue fever, yellow fever, Zika virus, and Chikungunya. Biosynthesis and the use of green silver nanoparticles (AgNPs) is a vital step to identify reliable and eco-friendly controls for these vectors. In this study, Aedes (Ae.) aegypti larvae (2nd and 3rd instar) were exposed to leaf extracts of Ricinus communis (Castor) and AgNPs synthesized from the extract to evaluate their larvicidal potential. Synthesized AgNPs were characterized by UV–Vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), and energy-dispersive X-ray spectroscopy (XRD). Ae. aegypti larvae were treated with different concentrations (50–250 ppm) of the leaf extract and synthesized AgNPs. There were five replicates per treatment, in addition to a positive (temephos) and negative control (dechlorinated water). Mortality was recorded after 12, 24, 36, and 48 h and the data were subjected to Probit analysis. The nanoparticles were more toxic (LC50 = 46.22 ppm and LC90 = 85.30 ppm) than the plant extract (106.24 and 175.73 ppm, respectively). The leaf extracts of Ricinus communis were subjected to HPLC analysis to identify their chemical constituents. This study suggests that plant extracts and synthesized nanoparticles are excellent alternatives to hazardous chemical pesticides used to control vector mosquitoes. This is a potentially useful technique that can reduce aquatic toxicity from insecticide use.
Keywords: Larvicidal, Ricinus communis, Mosquito larvae, AgNPs, Yellow fever mosquito
1. Introduction
Mosquito-borne diseases such as malaria, dengue fever, chikungunya, Zika virus, Japanese encephalitis, and leishmaniasis are serious threats to public health (WHO, 2006). These diseases occur worldwide and cause millions of deaths annually (Ravikumar and Rahuman, 2011). Pakistan, in particular, is at a high risk for vector-borne diseases, such as dengue fever, due to its packed cities, insufficient sanitation, and poor vaccination rates. In Pakistan, cases of dengue fever are reported throughout the year, but the highest occurrence rate is in the post-monsoon period (Jahan, 2011). The only way to mitigate such disease outbreaks is to manage the mosquito population. Mosquito management is often chemical-based, which causes serious health issues, environmental pollution, and vector resistance. Therefore, alternate methods are being used (Isman, 2006).
Medicinal plants have played a key role in human health. These plants are a good source of bioactive insecticidal phytochemicals (e.g., saponins, isoflavonoids, tannins, terpenes, steroids) that can kill mosquito larvae with high mortality rates (Mdoe et al., 2014). The chemicals work by inducing changes in the development, midgut epithelium, mutating the DNA, and producing reactive oxygen species in the larvae (Arjunan et al., 2012). Moreover, these phytochemicals are highly specific, rapidly biodegradable, eco-friendly, and less toxic to human health (Ghosh et al., 2012).
Green silver nanoparticles (AgNPs), synthesized from plant extracts, are more toxic larvicides than phytochemicals (Bilal and Hassan, 2012). AgNPs are efficient larvicidal agents because their small size (1–100 nm) and large surface area make them effective at very low concentrations (Borase et al., 2013). Ricinus communis (castor) is a flowering plant in the Euphorbiaceae family, that is used as laxative, fungicide, anti-oxidant, antiasthmatic, antiulcer, wound healer, and an insecticide/larvicidal agent due to presence of glycosides, alkaloids, flavonoids, steroids, and saponins, etc. (Palanivelu et al., 2015). This study aimed to evaluate the larvicidal potential of the liquid extract and AgNPs synthesized from R. communis (Castor) against the 2nd and 3rd larval instar of Ae. aegypti under laboratory conditions.
2. Materials and methods
2.1. Preparation of leaf extract
Leaves of the R. communis (Castor) plant were collected from the University of Agriculture Faisalabad, Pakistan (31°26′2.18″N, 73°3′53.6″E). After identification by a Botanist, the leaves were washed with distilled water, shade dried, and ground in an electric grinder (Anex, Germany). Extractions were performed with a Soxhlet apparatus using 250 ml acetone and 50 g leaf powder for 8 to 15 h. The collected extract was stored at 4 °C until further analyses (Vogel, 1978).
2.2. Preparation of green AgNPs
Silver nitrate (AgNO3) was purchased from Sigma Aldrich, UK and a 1 mM solution was prepared in a 250 ml Erlenmeyer flask in darkness. The acetone plant extract (10 ml) was added to a 250 ml conical flask with 90 ml of the 1 mM silver nitrate solution. Two to three drops of 1% NaOH were added to adjust the pH to 8 while being mixed continuously by a magnetic stirrer. This mixture was kept at 40 °C for 1 h under clear sky conditions for irradiation. The formation of AgNPs was indicated by a solution color change to reddish-brown (Satyavani et al., 2011).
2.3. Characterization of AgNPs
The biosynthesized silver nanoparticles were characterized by UV–Vis spectroscopy, powdered X-ray Diffraction (PXRD), and Fourier transform infrared (FTIR) spectroscopy, with assistance from the Hi-Tech central laboratory of the Government College University Faisalabad.
2.4. UV–Vis absorbance spectroscopy
To monitor the formation of the green AgNPs, the absorption spectra of the samples were measured using a Cary 60 double beam UV–Vis spectrophotometer (Spectramax M3 molecular devices) operating at the resolution of 1 nm. UV–Vis spectra were recorded after 15 and 30 min (Rajesh et al., 2009).
2.5. Powdered X-ray diffraction
The structure and size of the silver nanoparticles were investigated by recording the diffracted intensities at 40 kV and 30 mA, with a scan range of 0–80° 2θ using CuKα radiation (Rigaku, Ultima IV, and X-ray diffractometer system).
2.6. Fourier transform infrared spectroscopy
After the reaction, 100 ml of the residue solution was centrifuged at 5 000 rpm for 10 min to remove the free biomass residue. The supernatant was again centrifuged at 10 000 rpm for 60 min to pellet the silver nanoparticles (Vivek et al., 2011). Fresh samples with a volume of 1–2 ml in aqueous form were sent for FTIR analysis to the Hi-Tech Lab, Government College University Faisalabad.
2.7. Scanning electron microscopy (SEM)
A carbon-coated copper grid was used to prepare thin films of the Ag nanoparticles from a very small amount of the sample; the extra solution was removed with blotting paper. The films were dried under a mercury lamp for 5 min (Santoro et al., 2017).
2.8. Phytochemical analysis
The plant extract was further subjected to qualitative phytochemical analysis. This analysis was performed to detect alkaloids, terpenoids, tannins, cardiac glycosides, steroids, saponins, and phenols (Bargah, 2015).
2.9. High performance liquid chromatography (HPLC)
The HPLC analysis of the leaf extracts of R. communis (Castor) was conducted on a Chromera HPLC system (Perkin Elmer, USA) attached to a Flexer Binary LC pump, and a UV/Vis LC Detector (Shelton CT, 06,484 USA) controlled by software V 4.2. (Ghramh et al., 2019). Solvent A (acetonitrile and methanol, 70:30) and solvent B (double distilled water with 0.5% glacial acetic acid) was used as the mobile phase to separate the phenolic acids as follows: 10–15% A for 0 to 5 min, 15–20% A for 5 to 18 min, and 20–40% A for 18 to 40 min. The UV spectra were recorded at 275 nm. The identification of the different compounds was completed by matching the retention times and spiking samples to standards.
2.10. Collection and rearing of mosquitoes
Larvae and pupae were collected from indoor breeding sites with a dipper from the Faisalabad district, Punjab. The specimens were transported to the Zoology Lab in the Department of Zoology, Government College University Faisalabad, inside beakers closed with a muslin cloth. After identification (Qasim et al., 2014), the larvae and pupae were reared to the adult stage in 1000 ml beakers with water and a fish diet under lab conditions (27 ± 3 °C and 80 ± 3% RH). The adults were further reared in glass cages. Male adults were fed with a 10% sugar solution, and females were fed the blood of live white rats (for egg-laying). The larvae that emerged from the eggs were reared on a fish diet in batches of 300 in 1 200 ml deionized water in stainless steel trays (35 × 30 × 5 cm) for the bioassays (Ahmad et al., 2017).
2.11. Bioassay
Groups of 20 actively swimming 2nd and 3rd instar larvae were released in a 250 ml beaker containing 200 ml distilled water. Five concentrations (50, 100, 150, 200, and 250 ppm) of the larvicidal solutions from the R. communis extract and green AgNPs were prepared separately using distilled water. The bioassays were conducted at 27 ± 3 °C, 80 ± 3% relative humidity (RH), with five replications per treatment, including a positive (temephos) and negative controls (dechlorinated water). The mortality rates were calculated using the World Health Organization (WHO, 2006) bioassay protocol, with slight modifications. The percentage data were corrected with Abbott’s formula (Abbott, 1925).
| (1) |
| (2) |
2.12. Data analysis
The average mortality data of the larvae were subjected to Probit analysis using Minitab 17 statistical software (2017). For each treatment, we calculated the lethal concentrations (LC50 and LC90) and the dose and time mortality regression lines (Cheng et al., 2009).
3. Results
3.1. Synthesis of silver nanoparticles
AgNPs were formed through the reduction of Ag+ with the extract of R. communis and the color of the mixture (plant extract + AgNO3 solution) turned reddish-brown (Fig. 1) in 1 h at 40 °C.
Fig. 1.
UV–Vis spectra of the silver nanoparticles synthesized by treating R. communis leaf extracts with 1 mM AgNO3 solution.
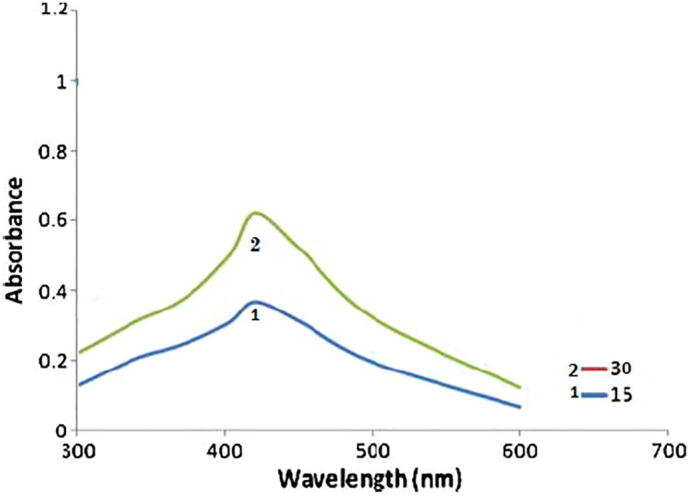
3.2. UV–Vis spectrum of AgNPs
The synthesis of AgNPs was confirmed by UV–Visible absorption spectroscopy (Fig. 2). The UV–visible spectra of the AgNPs at different reaction times (15 vs. 30 min) was used to monitor the reaction between Ag+ and the leaf extract in the aqueous solution (Fig. 2). The localized surface plasmon resonance band showed maximum absorbance at 430 nm after 30 min. Ahmed et al. (2010) also reported the same result.
Fig. 2.
PXRD spectrum of the silver nanoparticles.
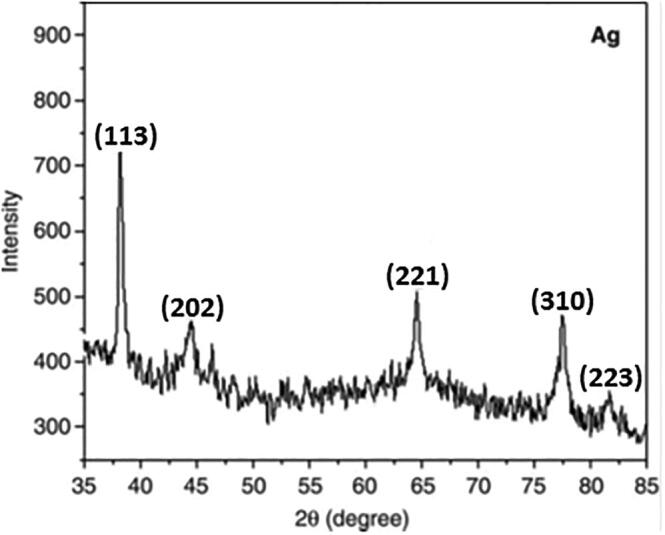
3.3. Powdered X-ray diffraction studies
Diffraction peaks were observed at 38.1, 44.5, 64.5, 77.5, and 81.6° with facets 113, 202, 221, 310, and 223 of the face-centered cubic crystal structure (Fig. 3). Satyavani et al. (2011) also documented peaks at 44.5, 52.2, and 76.7° with facets 111, 200, and 222. Our findings are also consistent with those of Nirmala et al. (2010).
Fig. 3.
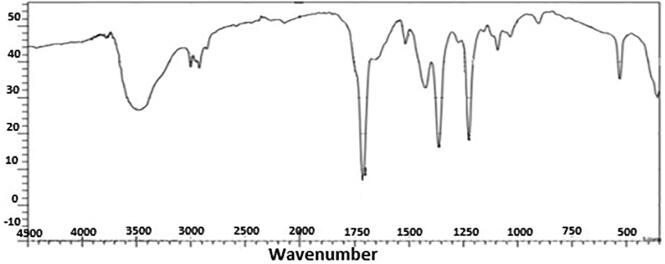
FTIR spectra of the AgNPs synthesized from leaf extracts of R. communis (Castor).
3.4. Fourier transform infrared spectroscopy analysis
The FTIR spectra of aqueous AgNPs (Fig. 4) showed transmittance peaks at 1263.2, 978.6, 849.1, 710.5, 662.8, 502.7, and 435.6 nm. These peaks indicate that the carbonyl group formed amino acid residues that capped the silver nanoparticles. These residues prevented the agglomeration of AgNPs and made the medium stable. The FTIR results highlight the role of the proteins and other compounds in the leaf extracts in the formation and stabilization of AgNPs.
Fig. 4.
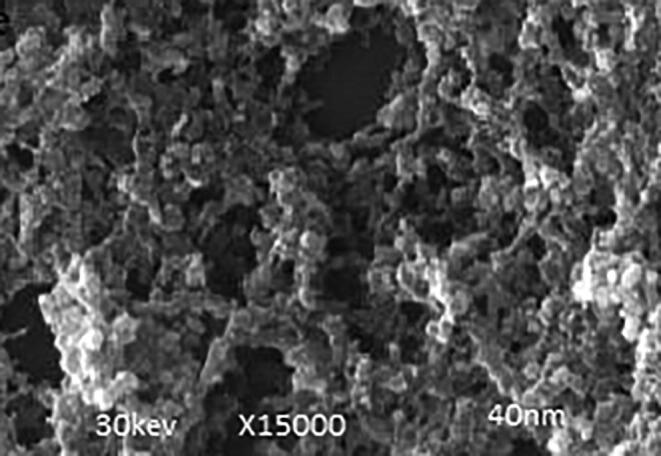
SEM image of the AgNPs synthesized from Ricinus communis.
3.5. Electron microscopy
Fig. 5 represents the SEM image of the AgNPs synthesized from Ricinus communis showing the spherical shape 40 nm in size.
Fig. 5.
Chromatogram profile of R. communis leaf extract.
3.6. Larvicidal activity of the leaf extracts and synthesized silver nanoparticles
The larvicidal activity of the leaf extracts of Ricinus communis (Castor) and the synthesized AgNPs of varying concentrations (50–250 ppm) on the 2nd and 3rd instar larvae of Ae. aegypti after 12, 24, 36, and 48 h exposure are presented in Table 1, Table 2. Dose- and time-dependent toxic effects were observed in all treatments except the control (no mortality was observed in the control groups). The AgNPs at 250 ppm caused 100% mortality for all of the larvae within 36 h (Table 1). For the 2nd instar larvae, LC50 = 46.22 ppm and LC90 = 85.30 ppm. The 95% lower and upper confidence limits were (36.05–53.29 ppm) and (77.30–97.89 ppm), respectively, and the regression equation was Y = −1.515 + 0.3279x. For the 3rd instar larvae, the same values were: 81.25 ppm, 157.09 ppm, (70.74–90.31 ppm), (145.25–172.76 ppm), and Y = −1.372 + 0.1709x, respectively.
Table 1.
Larvicidal activity of the AgNPs synthesized from Ricinus communis against Aedes aegypti larvae.
| Time | Larval instars | Lethal concentration | LFL | UFL | Chi-square | P value | Regression equation | |
|---|---|---|---|---|---|---|---|---|
| 12 h | 2nd | LC50 | 339.35 | 289.442 | 441.420 | 0.86 | 0.835 | Y = −1.86 + 0.0055x |
| LC90 | 571.93 | 462.395 | 805.604 | 0.86 | 0.835 | Y = −1.86 + 0.0055x | ||
| 3rd | LC50 | 513.08 | 380.197 | 1034.82 | 1.34 | 0.712 | Y = −1.96 + 0.0038x | |
| LC90 | 847.98 | 591.325 | 1873.27 | 1.34 | 0.712 | Y = −1.96 + 0.0038x | ||
| 24 h | 2nd | LC50 | 231.83 | 211.541 | 260.779 | 5.843 | 0.109 | Y = −1.69 + 0.0073x |
| LC90 | 407.01 | 356.895 | 488.235 | 5.843 | 0.109 | Y = −1.69 + 0.0073x | ||
| 3rd | LC50 | 455.96 | 351.970 | 786.414 | 0.174 | 0.918 | Y = −1.83 + 0.0040x | |
| LC90 | 774.01 | 561.843 | 1463.36 | 0.174 | 0.918 | Y = −1.83 + 0.0040x | ||
| 36 h | 2nd | LC50 | 104.30 | 93.4270 | 114.113 | 3.778 | 0.001 | Y = −1.14 + 0.0136x |
| LC90 | 198.05 | 183.979 | 216.453 | 3.778 | 0.001 | Y = −1.14 + 0.0136x | ||
| 3rd | LC50 | 200.83 | 185.533 | 219.929 | 0.146 | 0.983 | Y = −1.66 + 0.0082x | |
| LC90 | 355.25 | 318.485 | 410.968 | 0.146 | 0.983 | Y = −1.66 + 0.0082x | ||
| 48 h | 2nd | LC50 | 46.227 | 36.0508 | 53.2955 | 0.0367 | 0.005 | Y = −1.51 + 0.327x |
| LC90 | 85.306 | 77.3013 | 97.8951 | 0.0367 | 0.005 | Y = −1.51 + 0.327x | ||
| 3rd | LC50 | 81.252 | 70.7438 | 90.3100 | 4.252 | 0.058 | Y = −1.37 + 0.170x | |
| LC90 | 157.09 | 145.255 | 172.765 | 4.252 | 0.058 | Y = −1.37 + 0.170x | ||
LFL: Lower Fiducial Limit, UFL: Upper Fiducial Limit.
Table 2.
Larvicidal activity of the leaf extracts of Ricinus communis against Aedes aegypti larvae.
| Time | Larval instars | Lethal concentration | LFL | UFL | Chi-square | P value | Regression equation | |
|---|---|---|---|---|---|---|---|---|
| 12 h | 2nd | LC50 | 410.812 | 333.837 | 607.800 | 0.888 | 0.828 | Y = −2.15 + 0.005x |
| LC90 | 655.074 | 504.179 | 1051.09 | 0.888 | 0.828 | Y = −2.06 + 0.005x | ||
| 3rd | LC50 | 612.106 | 416.283 | 2047.60 | 1.225 | 0.738 | Y = −2.98 + 0.003x | |
| LC90 | 1007.14 | 642.127 | 3716.84 | 1.225 | 0.738 | Y = −2.98 + 0.003x | ||
| 24 h | 2nd | LC50 | 362.911 | 303.314 | 495.486 | 0.141 | 0.986 | Y = −1.84 + 0.0050x |
| LC90 | 614.370 | 485.639 | 911.276 | 0.141 | 0.986 | Y = −1.84 + 0.005x | ||
| 3rd | LC50 | 410.824 | 324.773 | 656.965 | 0.88 | 0.821 | Y = −1.62 + 0.004x | |
| LC90 | 734.236 | 543.609 | 1295.86 | 0.88 | 0.821 | Y = −1.62 + 0.004x | ||
| 36 h | 2nd | LC50 | 188.196 | 172.390 | 207.370 | 0.778 | 0.809 | Y = −1.42 + 0.007x |
| LC90 | 357.114 | 317.509 | 418.588 | 0.778 | 0.809 | Y = −1.42 + 0.007x | ||
| 3rd | LC50 | 242.616 | 220.363 | 275.703 | 4.170 | 0.253 | Y = −1.719 + 0.007x | |
| LC90 | 423.457 | 368.578 | 514.795 | 4.170 | 0.253 | Y = −1.719 + 0.007x | ||
| 48 h | 2nd | LC50 | 136.243 | 116.3101 | 155.477 | 6.778 | 0.011 | Y = −1.721 + 0.159x |
| LC90 | 245.731 | 218.209 | 293.903 | 6.778 | 0.011 | Y = −1.721 + 0.159x | ||
| 3rd | LC50 | 136.981 | 116.7967 | 156.306 | 2.270 | 0.059 | Y = −1.558 + 0.418x | |
| LC90 | 251.956 | 211.792 | 271.888 | 2.270 | 0.059 | Y = −1.558 + 0.418x | ||
LFL: Lower Fiducial Limit, UFL: Upper Fiducial Limit.
The leaf extracts at 250 ppm resulted in a mortality rate of 100% for the 2nd instar larvae and 98% for 3rd instar larvae (Table 2). The values of LC50 and LC90 were 136.24 and 245.73 ppm for the 2nd instar larvae and 136.98 and 251.95 ppm for the 3rd instar larvae, respectively. The 95% lower and upper confidence limits for the 2nd instar larvae were (116.31–155.47 ppm) and (218.20–293.90 ppm), respectively, and regression equation was Y = −1.721 + 0.1594x. For the 3rd instar larvae, the 95% lower and upper confidence limits were (116.79–156.30 ppm) and (211.79–271.88 ppm), respectively, with a regression equation of Y = −1.558 + 0.4179x.
The activity of the positive control group also indicated higher LC50 values in the 3rd instar larvae than in the 2nd instar larvae (Table 3). About 100% mortality was observed after 36 h in both larval instars.
Table 3.
Larvicidal activity of temephos (positive control) against Aedes aegypti larvae.
| Time | Larval instars | Lethal concentration | LFL | UFL | Chi-square | P value | Regression equation | |
|---|---|---|---|---|---|---|---|---|
| 12 h | 2nd | LC50 | 1.17 | 0.92 | 1.39 | 2.69 | 0.00 | Y = −0.420 + 1.351x |
| LC90 | 1.45 | 1.15 | 1.67 | 2.69 | 0.00 | Y = −0.420 + 1.351x | ||
| 3rd | LC50 | 1.78 | 1.39 | 1.98 | 3.34 | 0.00 | Y = −0.492 + 1.274x | |
| LC90 | 1.99 | 1.86 | 2.16 | 3.34 | 0.00 | Y = −0.492 + 1.274x | ||
| 24 h | 2nd | LC50 | 0.95 | 0.88 | 1.07 | 13.40 | 0.004 | Y = −0.064 + 3.000x |
| LC90 | 1.14 | 0.97 | 1.56 | 13.40 | 0.004 | Y = −0.064 + 3.000x | ||
| 3rd | LC50 | 1.26 | 0.99 | 1.43 | 13.69 | 0.003 | Y = −0.051 + 1.94x | |
| LC90 | 1.68 | 1.47 | 1.84 | 13.69 | 0.003 | Y = −0.051 + 1.94x | ||
| 36 h | 2nd | LC50 | 0.79 | 0.59 | 0.96 | 8.12 | 0.000 | Y = 0.723 + 2.42x |
| LC90 | 0.98 | 0.86 | 1.15 | 8.12 | 0.000 | Y = 0.723 + 2.42x | ||
| 3rd | LC50 | 1.07 | 0.91 | 1.23 | 0.40 | 0.022 | Y = 0.604 + 2.45x | |
| LC90 | 1.27 | 1.12 | 1.40 | 0.40 | 0.022 | Y = 0.604 + 2.45x | ||
| 48 h | 2nd | LC50 | 0.63 | 0.41 | 0.87 | 0.023 | 0.999 | Y = 0.96 + 10.65x |
| LC90 | 0.82 | 0.56 | 1.05 | 0.023 | 0.999 | Y = 0.96 + 10.65x | ||
| 3rd | LC50 | 0.88 | 0.65 | 1.03 | 0.005 | 1.0 | Y = 0.78 + 9.74x | |
| LC90 | 1.02 | 0.81 | 1.62 | 0.005 | 1.0 | Y = 0.78 + 9.74x | ||
LFL: Lower Fiducial Limit, UFL: Upper Fiducial Limit.
3.7. Phytochemical analysis
Phytochemical analysis of the leaf extract revealed the presence of alkaloids, terpenoids, tannins, saponins, and other components (Table 4).
Table 4.
Phytochemical analysis of Ricinus communis leaves.
| Plant | Alkaloids | Terpenoids | Tannins | Cardiac glycosides | Steroids | Saponins | Phenols |
|---|---|---|---|---|---|---|---|
| R. communis | + | + | + | + | + | ++ | + |
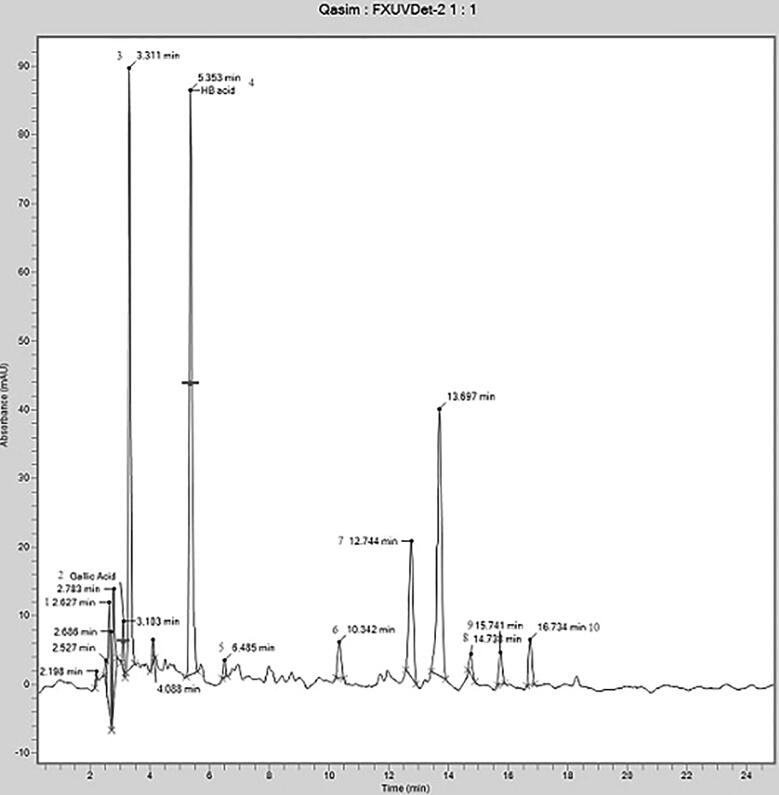
3.8. HPLC analysis
The phenolic compounds were separated and identified by matching their retention time to standards, as shown in Table 5 and Fig. 5.
Table 5.
Phenolic compounds identified in the leaf extracts of Ricinus communis.
| Sr. No. | Retention Time (minutes) | Name of compound |
|---|---|---|
| 1 | 2.627 | Anisole |
| 2 | 2.783 | Gallic acid |
| 3 | 3.311 | Propyl acetate |
| 4 | 5.353 | 3-hydroxybutanoic acid |
| 5 | 6.485 | Pterin-6-carboxylic acid |
| 6 | 10.342 | Thymol |
| 7 | 12.744 | 12-methyl-E,E-2,13-octadecadien-1-ol |
| 8 | 14.738 | 13-Heptadecyn-1-ol |
| 9 | 15.741 | Behenic alcohol |
| 10 | 16.734 | Phenol,2,2′-methylene bis[6-(1,1-dimethyl ethyl)-4-methyl- |
4. Discussion
Our study revealed that the leaf extract of R. communis contained constituents that were toxic to mosquito larvae. Moreover, our results showed that this toxicity increased when the extracts were combined with AgNPs. This larvicidal activity is due to the presence of phytochemicals such as alkaloids, tannins, lignin, saponins, gallic acid, flavones, and kaempferols that were detected in a prior study (Naz and Bano, 2012). The insecticidal properties of the plant extract depend not only on the plant parts, mosquito species, and solvent but also on the method of extraction used (Zarai et al., 2012).
In our study, R. communis AgNPs showed 100% mortality at 250 ppm for the Ae. aegypti larvae after 48 h with LC50 and LC90 values of 46.22 and 85.30 ppm, respectively. The LC50 and LC90 values of the leaf extract of R. communis were 136.24 and 245.73 ppm, respectively. This suggests that the R. communis AgNPs were more toxic (3 times more) than the leaf extracts. Several prior studies have similarly reported the larvicidal potential of plants belonging to the Euphorbiaceae family. For example, crude methanol extracts of Euphorbia hirta leaves with concentrations of 50 to 250 ppm showed LC50 values of 121.51, 145.40, 169.11, and 197.40 ppm and LC90 values of 236.44, 293.75, 331.42, and 371.34 ppm for An. stephensi 1st to 4th instar larvae, respectively (Karthikeyan et al., 2012). Asaad and Basheer (2014) reported 96% mortality after 24 h with an LC50 of 0.390 mg/l and 100% mortality after 48 h with an LC50 of 0.284 mg/l for An. arabiensis 3rd instar larvae. R. communis seed extracts exhibited 100% larval mortality at different concentrations (32–64 μg/mL), with an LC50 value of 16.84 μg/mL for Ae. albopictus (Mandal, 2010). Lata et al. (2009) reported LC50 values of 144.11 and 92.44 ppm and LC90 values of 432.42 and 352.89 ppm after 24 and 48 h, respectively, for Culex quinquefasciatus. Prior results are close to the results we present here but vary due to differences in the plant and mosquito species, larval stage, and solvent for plant extraction. Karthikeyan et al. (2012) also studied the toxic effects of silver nanoparticles synthesized from Euphorbia hirta leaf extracts for Anopheles stephensi 1st to 4th instar larvae. They reported LC50 values of 10.1, 16.8, 21.5, and 27.9 ppm and LC90 values of 31.9, 50.4, 60.1, 69.9 ppm for the 1st to 4th larval instars, respectively. LC50 values of 3.5 to 7.0 ppm and 4.4 to 8.7 ppm were reported for the 2nd and 4th instar larvae of Ae. aegypti, respectively, after 24 h exposure to AgNPs from Euphorbia tirucalli (Hemant et al., 2013). Namita and Ramesh (2017) also investigated the toxicity of R. communis synthesized silver nanoparticles on An. stephensi and Ae. aegypti third instar larvae and found similar results to our study. Prior work has shown that the positive control (temephos) also resulted in 100% mortality at lower concentrations and in less exposure time, but the potentially harmful residual effects remained for 2–3 months (SEARO, 2011). Plant extracts and green nanoparticles, on the other hand, easily biodegrade and are safer for other aquatic fauna (Zarai et al., 2012).
5. Conclusions
In conclusion, our results show that the leaf extract and synthesized silver nanoparticles of R. communis have excellent potential to control mosquito larvae. Their application on the breeding areas will likely decrease the population size of vector mosquitoes, control many harmful diseases, and prevent environmental pollution. Field applications of these nanoparticles should be conducted to test their efficacy and the side effects on other aquatic fauna under natural conditions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
“The authors (SM and KAAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. RG-1435-012”. “The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their providing facility for language editing service”.
Author contributions
MW and SN designed, SA, MA and BA performed the experiment, NAK, BH, NM and SM tabulated and analysed the data, MW and SN prepared initial draft of the manuscript, KAG and FAM contributed the final draft of the manuscript
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbott W.S. A method of computing the effectiveness of insecticide. J. Eco. Ent. 1925;18:265–267. [PubMed] [Google Scholar]
- Ahmad T., Nasir S., Nasir I., Nawaz T., Rafiq A., Yousaf I. Response surface modeling for West Nile viral encephalitis mosquito control experiments. Pak. Vet. J. 2017;37:465–469. [Google Scholar]
- Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2010;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjunan N., Murugan K., Rejeeth C., Madhiyazhagan P., Barnard D. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vec. Bor. Zo. Dis. 2012;12:262–268. doi: 10.1089/vbz.2011.0661. [DOI] [PubMed] [Google Scholar]
- Asaad G., Basheer M. Ricinus communis (CASTOR) as Larvicide on Anopheles arabiensis Patton. Int. J. adv. Pharm. Biol. Chem. 2014;3:319–328. [Google Scholar]
- Bilal H., Hassan S.A. Plants secondary metabolites for mosquito control. Asian. Pac. J. Trop. Dis. 2012;2 168 168. [Google Scholar]
- Bargah R.K. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. J. Pharmacogn. Phytochem. 2015;4(1):7–9. [Google Scholar]
- Borase H., Patil C., Patil R. Phyto-synthesized silver nanoparticles: a potent mosquito biolarvicidal agent. J. Nanomed. Biotherap. Discov. 2013;3:1. [Google Scholar]
- Cheng S.S., Huang C.G., Chen Y.J., Yu J.J., Chen W.J., Chang S.T. Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species. Bioresour. Technol. 2009;100:452–456. doi: 10.1016/j.biortech.2008.02.038. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Chowdhury N., Chandra G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012;13:581–598. [PMC free article] [PubMed] [Google Scholar]
- Ghramh H.A., Khan K.A., Ibrahim E.H., Setzer W.N. Synthesis of gold nanoparticles (AuNPs) using Ricinus communis leaf ethanol extract, their characterization, and biological applications. Nanomaterials. 2019;9(5):765. doi: 10.3390/nano9050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemant P.B., Chandrashekhar D.P., Rahul B.S. Phyto-synthesized silver nanoparticles: a potent mosquito biolarvicidal agent. J. Nanomed. Biotherap. Discov. 2013;3:1. [Google Scholar]
- Isman B.M. Botanical insecticides deterrents and repellents in modern agriculture and an increasingly regulated world. An. Rev. Ent. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Jahan F. Dengue fever (DF) in Pakistan. Asia Pac. Fam. Med. 2011;10:1. doi: 10.1186/1447-056X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan A.P., Kadarkarai M., Chellasamy P. Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae) Parasitol. Res. 2012;11:997–1006. doi: 10.1007/s00436-012-2924-8. [DOI] [PubMed] [Google Scholar]
- Lata B., Sharma P., Mohan L., Maurya P., Srivastava C.N. Relative toxicity of neem fruit, bitter gourd, and castor seed extracts against the larvae of filaria vector, Culex quinquefasciatus (Say) Parasitol. Res. J. 2009:1205–1210. doi: 10.1007/s00436-009-1541-7. [DOI] [PubMed] [Google Scholar]
- Mandal S. Exploration of larvicidal and adult emergence inhibition activities of Ricinus communis seed extract against three potential mosquito vectors in Kolkata, India. Asian Pac. J. Trop. Med. 2010:605–609. [Google Scholar]
- Mdoe F.P., Cheng S.S., Msangi S., Nkwengulila G., Chang S.T., Kweka E.J. Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae. Par. Vec. 2014;7:209. doi: 10.1186/1756-3305-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namita S., Ramesh C.D. Phytochemical, anti-oxidant, larvicidal, and antimicrobial activities of castor (Ricinus communis) synthesized silver nanoparticles. Chin. Herb. Med. 2017;9:289–294. [Google Scholar]
- Naz R., Bano A. Antimicrobial potential of Ricinus communis leaf extracts in different solvents against pathogenic bacterial and fungal strains. Asian Pac. J. Trop. Biomed. 2012;2:944–947. doi: 10.1016/S2221-1691(13)60004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmala R., Faheem A.S., Kanjwal M.A., Lee J.H. Synthesis and characterization of bovine femur bone hydroxyapatite containing silver nanoparticles for the biomedical applications. J. Nano Res. 2010;10:9944–9950. [Google Scholar]
- Palanivelu J., Kunjumon M.M., Suresh A., Nair A., Ramalingam C. Green synthesis of silver nanoparticles from Dracaena mahatma leaf extract and its antimicrobial activity. J. Pharm. Sci. Res. 2015;7:690–695. [Google Scholar]
- Qasim M., Naeem M., Bodlah I. Mosquito (Diptera: Culicidae) of Murree Hills, Punjab, Pakistan. Pak. J. Zool. 2014;46:523–529. [Google Scholar]
- Rajesh W.R., Jaya R.L., Niranjan S.K., Vijay D.M., Sahelebrao B.K. Phytosynthesis of silver nanoparticles using Gliricidia sepium (Jaeq) Cur. Nanosci. 2009;5:117–122. [Google Scholar]
- Ravikumar G., Rahuman A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 2011;118:196–203. doi: 10.1016/j.actatropica.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Santoro C., Kodali M., Kabir S., Soavi B., Serov A. Three-dimensional grapheme nanosheets as cathode catalysts in standard and super capacitive microbial fuel cell. J. Power Sources. 2017;356:371–380. doi: 10.1016/j.jpowsour.2017.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyavani K., Ramanathan T., Gurudeeban S. Plant mediated synthesis of biomedical silver nanoparticles by leaf extract of Citrullus colosynthis. Res. J. NanoTech. 2011;1:95–101. [Google Scholar]
- SEARO . SEARO; New Delhi: 2011. Prevention and Control of Dengue and Dengue Haemorrhagic Fever: Comprehensive Guidelines. [Google Scholar]
- Vivek M., Kumar P.S., Steffi S., Sudha S. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. J. Med. Biotech. 2011;3:143–148. [PMC free article] [PubMed] [Google Scholar]
- Vogel A.I. The English Language Book Society and Longman; London: 1978. Text Book of Practical Organic Chemistry. [Google Scholar]
- WHO, 2006. Pesticides and their application for the control of vectors and pests of public health importance. Geneva, Switzerland.
- Zarai Z., Ben Chobba I., Ben Mansour R., Békir A., Gharsallah N., Kadri A. Essential oil of the leaves of Ricinus communis L.: in vitro cytotoxicity and antimicrobial properties. Lipids Health Dis. 2012;11:102. doi: 10.1186/1476-511X-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]