Abstract
Tumor organoids inherit the genomic and molecular characteristics of the donor tumor, which not only bridge the gap between genome and phenotype but also circumvent the disadvantages such as genetic information change by using 2D cell lines and the mouse-specific tumor evolution in patient-derived xenograft (PDX). So, cancer organoid has been widely applied to preclinical drug evaluation, biomarker identification, biological research, and individualized therapy. Besides, cancer organoid can be preserved, resuscitated, passed infinitely, and mechanically cultured on a chip for drug screening; it has become one of the partial models for low/high-throughput drug screening in the preclinical trial in vitro. Therefore, this review presents the recent developments of tumor organoids for drug screening, which will introduce from four aspects, including the stability/credibility, types, application, deficiency and prospect of the tumor organoids model for drug screening.
Keywords: Cancer organoid, Drug screening, Precision therapy
Graphical abstract
Highlights
-
•
Stability and accuracy of tumor organoids as a drug screening model.
-
•
Methods of tumor organoids for drugs screening.
-
•
Application of tumor organoids in drug screening.
-
•
Shortcomings and prospects of tumor organoids as a drug screening model.
Introduction
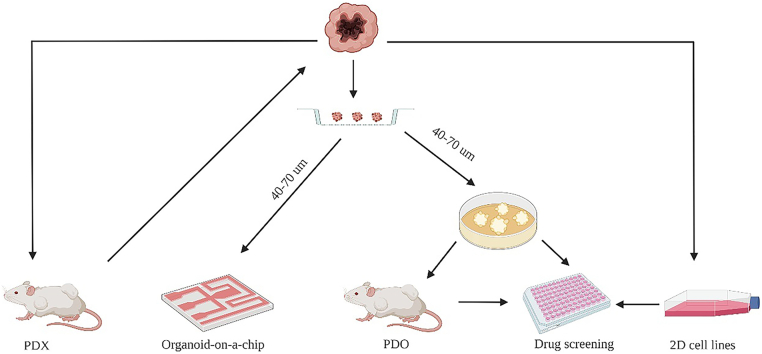
Richard K. Harrison et al. found that most drug candidate attrition in Phase II and Phase III for the period 2013–2015 was due to the lack of effectiveness (52%) and safety (24%) [1]. Thus, it was of considerable significance to test the efficacy and safety of drugs in vitro, which not only improved the success rate of tumor drug screening in clinical trials but also benefited for finding the most effective treatment for cancer patients. So, it was necessary and urgent to establish effective and stable preclinical tumor drug screening models, which could faithfully recapitulate the morphological and molecular characteristics of various human tumors. In the era of precision oncology, scientists and oncologists were committed to finding drug screening models that would be more effective, realistic, time-saving and labor-saving to study the response of tumor patients to drugs [2,3]. Recently, more and more preclinical drug screening models had been emerging, Such as commercial 2D cell lines, patient-derived xenograft (PDX), primary patient-derived 2D cell lines, and organoid etc. [4].
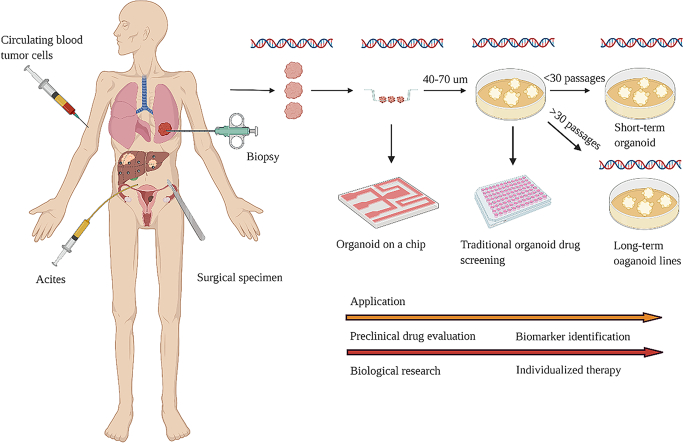
On the one hand, commercial 2D cell lines originated from the establishment of the Hela cell line in the 1950s [5]. As it was easy to operate and culture, 2D cell lines had gradually become the basic research model for tumor research and drug screening [[6], [7], [8]]. However, 2D cell lines grew in plastic bottles and accumulated plenty of gene mutations in the process of passage, so 2D cell lines could not conclusively reveal the drug response of clinical patients (Table 1). On the other hand, PDX had also served as the workhorse model in cancer research for many years [[9], [10], [11]]. It reported that the PDX model could retain the molecular and morphological characteristics of the primary tumor [[12], [13], [14]] and reveal the heterogeneity of the primary tumor [15]. However, PDX models lacked the immune system, and mouse-specific tumor evolution occurred in PDX (Table 1). Thus, a tumor model that was more similar to the original tumor issue was required urgently. Subsequently, the appearance of cancer organoid technology satisfied such requirements [16,17]. Cancer organoid facilitated personalized therapy and provided an alternative for drug screening researches besides 2D cell lines and PDX [[18], [19], [20]] (Fig. 1). Tumor spheres cultured in BME, which was surrounded by a medium containing Respond-1 EGF Noggin, could maintain the cell composition and self-renewal ability of the original cancer tissue [21,22]. For example, eleven malignant ascites-derived organoids (MADOs) established from gastric cancer patients were cultured for long-term expansion without any change in morphology and genome [23]. Many studies had shown that organoids were superior to 2D cell lines and PDX in terms of stability and fidelity as a drug screening model [24,25]. However, due to the expensive and elaborate culture conditions of tumor organoids, not all types of tumors were suitable for organoids' establishment (Table 1). Therefore, in pre-clinical drug screening experiments, the rational combination of preclinical drug screening models was essential to improve the success rate of clinical drug screening tests [9]. This review will summarize the recent progress of tumor organoids for drug screening from its stability and fidelity, applications, types, deficiencies and prospects.
Table 1.
Pros and cons of cancer drug screening models.
| Model | Cost | Time | Manipulate | Stability | Condition | Source |
|---|---|---|---|---|---|---|
| Commercial 2D cell lines | + | + | + | + | + | + |
| PDX | +++ | +++ | +++ | ++ | ++ | +++ |
| Organoid | +++ | ++ | ++ | +++ | +++ | +++ |
+: Cost low; timesaving; easy to manipulate; poor stability; easy to culture; easy to get.
++: Degree between + and +++.
+++: Cost high; time-consuming; hard to manipulate; good stability; hard to culture; hard to get.
PDX (patient-derived xenograft).
Fig. 1.
Cancer models for drug screening in vitro.
Stability and fidelity of cancer organoid drug screening model
The stability and fidelity of the cancer organoid model for drug screening could be classified into four aspects. Firstly, the drug screening results of different generations of tumor organoids were almost consistent. A study of colorectal cancer found that different generations of colorectal cancer organoids behaved nearly the same reaction to plocabulin [26]. Besides, drug responses showed little difference when authors compared 5FU and cisplatin reactions of different generations of organoids in a study of gastric cancer [27].
Secondly, the drug screening results of the tumor organoids were similar to the clinical ones of patients [28,29]. In a clinical follow-up of radiotherapy and chemotherapy for rectal cancer, when the organoids were sensitive to at least one kind of treatment, the prognosis of the corresponding patient was good. In contrast, when the organoids were resistant to any kind of treatment, the patient progressed quickly [30]. Similarly, Hervé Tiriac et al. found that five-sixths of the organoids derived from patients of good prognosis were sensitive to at least one chemotherapeutic drug. While two-thirds of the PDAC organoids from patients of poor prognosis were resistant to all chemotherapeutic drugs. As for the other cases with inconsistent results, it found that the purity of tumor cells in the initial tumor was too low [31]. Also, the half-inhibitor-concentration (IC50) of patient-derived organoids (PDOs) obtained from paclitaxel responsive patients were reduced by approximately 4-fold compared to PDOs of the patients in progression. Interestingly, these resistant PDOs showed similar paclitaxel dose-response to the patient of refractory paclitaxel [19].
Thirdly, drug screening of the same organoid cell line were tested repeatedly for three times, and drug screening results of the three repeated times matched with each other [32]. In a study of gastric cancer, the results with the semblable tendency were also obtained when we carried out the biological replicates of tumor organoids in drug screening tests. Furthermore, the sensitivity of three patients to 5FU and cisplatin was almost consistent with the response of corresponding organoids [27]. In a study related to colorectal cancer, authors provided a method to establish the living biobank of colorectal cancer organoids, which retained genetic information in colorectal cancer patients such as mutation information and copy number changes. It demonstrated that the results of three different technical replicates drug screening were nearly consistent in high-throughput drug screening tests [33].
Finally, the results of organoids drug screening were almost consistent with previously reported ones, which referred to the association of reported genes and phenotypes. For example, Crizotinib targeted organoids with EGFR-mutant/MET-amplified and BRCA2-mutant Lung cancer organoids were sensitive to olaparib, while Erlotinib was useful to EGFR-mutant organoids [34].
Types of cancer organoid model used for drug screening
Traditional cancer organoid model for drug screening
In 2009, since Hans Clever et al. discovered that Lgr5+ intestinal stem cells could grow and passage in BME with medium containing EGF, Noggin, and R-spondin1 cytokines, the traditional culture methods of the tumor organoids model for drug screening have blossomed for nearly ten years [35], also, some improvements were developed for different tumor organoids' growth characteristics [36,37]. The protocol of traditional tumor organoids for drug screening was described as followed [38]. Tumor organoids were planted in the 384-well plate or 96-well plate and treated with drugs for 3–6 days and then, CellTiter-Glo® (Promega) organoid activity was tested based on the manufacturer's instructions [39]. The traditional tumor organoids activity delivered the results of drug screening, which had been utilized by many researchers [40,41]. It reported that breast cancer organoids grew well under the traditional organoid culture conditions. The genetic and molecular characteristics of breast cancer organoids were consistent with those of the initial tumors. Furthermore, its drug screening results also matched with PDX and clinical drug tests [42]. Additionally, it exhibited that the organoids with BRCA mutation were sensitive to PARP inhibitors, which was consistent with the previous studies [43]. Parallelly, five colorectal cancer organoid cell lines derived from KRAS and BRAF mutant patients were cross-resistance to Olaparib and Oxaliplatin [44]. Therefore, traditional cancer organoid model served as a useful tool for drug screening.
Cancer organoid on a chip for drug screening
On the one hand, as a cultural tool of cancer organoids, the chip could be permitted to imitate the physiological environment of tumor cells as much as possible. Organoid-on-a-chip produced the components of vascularized tissue, lymphatic vessels, and immune system. It also simulated the characteristics of microcirculation that a fluid outflow brought nutrients and drugs for tumor cells at the end of the capillary artery with net absorption of liquid at the venous end [45]. A micro-physiological system (MPS) provided a 3D microfluidic culture device similar to the sophisticated living environment of lung cancer, which could complete drug screening in one step. The organoid cultured in MPS maintained better stemness than the traditional organoid grown in the matrix. Another device simulated the microenvironment of the lung parenchyma by creating a thin alveolar barrier. Hence, lung-on-a-chip became the most similar candidate in terms of representing human lung [46]. Similarly, under the conditions of flow and circulatory deformation, small intestinal epithelial cells on the porous membrane formed a multi-lineage differentiation similar to the small intestinal villi structure in the microfluidic device [47]. Thus, though the organoid-on-a-chip was expensive, it was one of the most emulating devices of tumor microenvironment at present.
On the other hand, it could mechanize the cultivation of tumor organoids and complete the drug screening of tumor organoids on a chip, which could save the human resources and reduce human operational error [48]. Without tedious manual procedures, human brain organoids on a micropillar array expanded its application in developmental biology, drug testing, and disease modeling [49]. Besides, if a patient had multiple tumors or metastasis tumors, the chip could complete the culture of multi-site tumor organoids. As a result, the results of organoid drug screening in a chip was closer to the clinical tests than previous tumor models. In a lung cancer organoid chip, lung cancer cells invaded vascular channels and migrated to the distant liver, bone, or brain chip; metastatic diffusion could be detected, and the growth of metastatic lesions could be identified and studied [50,51]. Analogously, Sunghee Estelle Park et al. proposed that organoid-on-a-chip could imitate the microenvironment of tumor, carry out drug screening mechanically on a large scale, and realize the interaction of multiple organs and tissues [52]. Therefore, organoid-on-a-chip had a perspective application for the frontier of biomedical research and shortens the distance to precision therapy.
Short-term cancer organoid for drug screening
The cancer organoid model for drug screening was named short-term drug screening model when tumor organoids were passed on within thirty generations or three months [53]. It had found that short-term small cell lung cancer (SCLC) organoid complemented reliably drug sensitivity predicting, when combined genes and phenotypes interaction [54]. The sensitivity of colorectal cancer tissue-originated spheroids (CTOSs) to 5FU was measured within several generations, which provided a new platform for the study of tumor biology and individualized therapy [55]; the short-term head and neck cancer organoid showed the same function [56]. The genomic and morphological characteristics of short-term cancer organoid culture were more similar to primary tumors, which provides more accurate drug screening results than long-term ones [57]. However, due to the limited generation and culture time, it was impossible to complete the high-throughput drug screening like long-term culture; thus, it was currently used for screening several drugs. The results of gene analysis and short-term screening drugs of ovarian cancer organoids implied that homologous recombination (HR) deficient organoids were sensitive to PARP inhibitors. In contrast, organoids' functional defects in replication fork protection were sensitive to DNA damage drugs such as CHK inhibitor and ATR inhibitor [58]. Another short-term culture of ovarian cancer organoids screened a drug which could convert mutated p53 into active p53, leading to apoptosis and cell cycle arrest of ovarian cancer [59]. In summary, the short-term organoid drug screening model was mainly applied to low-throughput drug screening, and it was suitable for identifying the biomarker of drug and exploring the mechanism of drug resistance.
Long-term cancer organoid for drug screening
Tumor organoids were long-term organoid cell lines after cultured for more than three months or more than thirty generations [60]. The long-term tumor organoids model for drug screening still imprinted the genomic features of the primary tumor after conservation, resuscitation and passage. Hence, the authors established cancer organoid cell lines, which revealed disease-associated traits and cancer-linked mutations of each patient [61]. Besides, long-term cancer organoid cell lines could achieve not only high-throughput drug screening but also accomplish individual treatment [62,63]. In 2019, Jumpei Kondo et al. utilized machine-processed drugs in organoid systems to screen 2427 drugs, and the results manifested that colorectal cancer organoid lines had different sensitivity to hit compounds. It indicated that this system could be used for personalized medicine [64]. In the same year, Oded Kopper et al. established 56 ovarian cancer organoid cell lines from 32 patients. These ovarian cancer organoid cell lines were utilized to screen patients who were sensitive or resistant to standard chemotherapy regimens. Then the ovarian cancer organoid lines were subcutaneously implanted into NCG mice to complete drug screening of assay in vivo [65]. Similarly, Matteo Boretto et al. established the organoids library of endometrial carcinoma from patients with a broad spectrum of endometrial pathologies. The organoid cell lines that had been passaged for more than eight months still replicated the mutational landscape, maintained the pathological features of endometrial carcinoma, and showed patient-specific drug responses [66]. Another organoid study of endometrial cancer exhibited the same results [67]. Since it was insufficient to use genetic information for predicting patients' response to drugs, Chantal Pauli et al. performed high-throughput drug screening of four organoid cell lines and corresponding PDXs. Then, the authors integrated genomic information and the results of cancer organoid cell lines as well as PDX drug screening to identify effective drugs and drug combination therapy for patients [68]. Moritz Schutte et al. also integrated the genomic information of colorectal cancer, and the drug screening results of PDX/PDO to identify the biomarker of EGFR inhibitors [69]. Additionally, the living biobank of bladder cancer organoids from patients not only retained tumor histopathology and gene mutations but also proceeded the tumor evolution in the process of passage [70]. Therefore, long-term cancer organoids were appropriate for high-throughput drug screening and individualized therapy [71].
Application of cancer organoid model for screening drugs
Firstly, the drug screening of normal organoids and tumor organoids was extended to detect the drug toxicity and predict the prognosis of tumor patients [72]. For example, the organoids derived from the central nervous system were employed to detect the neurotoxicity of small molecular inhibitors [73,74]. After integrating the transcriptome data of organoid derived from Biliary Tract Carcinoma (BTC) and clinical data of BTC patients, Yoshimasa Saito et al. discovered that SOX2, KLK6, and CPB2 were associated with BTC patients' prognosis [75].
Secondly, several patient-derived organoids (PDO) and patient-derived xenograft organoids (PDXO) were applied to the fundamental investigations of drug combination and the reversal of drug resistance [76,77]. The transgenic mouse model that had spontaneous breast cancer was utilized to explore the mechanism of resistance of PARP inhibitors [78]. Also, it reported that PDXO and PDO of castrate-resistant prostate cancer (CRPC) were combined to detect the sensitivity of PARP inhibitors and carboplatin [79] (Fig. 1).
Thirdly, cancer organoid was used for drug screening to find the most effective drug for the cancer patient and the biomarker of a drug for specific cancer patients [80,81]. For instance, the organoid cell lines of colorectal cancer exhibited different responses to EZH2 inhibitors, because ATRX and PAX2 mutation organoids were sensitive to EZH2 inhibitors, while the p53 mutant organoid lines were resistant to EZH2 inhibitors [82]. Anti-EGFR strongly inhibited colorectal cancer organoids with Microsatellite stable (MSS), parallelly, microsatellite instable (MSI) ones were resistant to anti-EGFR [83]. Another study inferred that one of the mechanisms of colorectal cancer resistance to the chemotherapeutic drug 5FU was the maintenance of stemness [84]. In a non-small cell lung cancer (NSCLC) survey, organoids treated with MEK inhibitors and anti-PD-L1 could kill tumor cells by activating immunity [85].
Fourthly, the cancer organoid model could achieve individualized therapy through high-throughput drug screening. Yuki Kita et al. screened 2098 compounds in bladder cancer organoid cell lines. They discovered that Disulfiram (DSF), an anti-alcoholism drug, and cisplatin had a cooperative effect [86]. After screening 484 compounds in six Cholangiocarcinoma's (CCA) organoid cell lines, Lampis A et al. presented that the sensitivity of HSP90 inhibitors was related to the mutation of MIR21 gene [87].
Besides, this model was also used to the lack of tumor models in vitro. Due to the lack of a drug screening model of mitigate CRPC in vitro, the authors established seven organoid cell lines that summarized the phenotypic diversity of CRPC, including TMPRSS2-ERG fusion, SPOP mutation, SPINK1 overexpression, and CHD1 loss [88]. Xiaodun Li et al. established the Esophageal adenocarcinoma (EAC) organoid cell lines They found the potential capacity of targeting receptor tyrosine kinases and downstream mediators in EAC after medium-throughout drug screening [89]. Except for these, Else Driehuis et al. found that the expression of EGFR in head and neck malignant organoids was higher than that in normal head and neck organoids. Therefore the organoid model was used to investigate the deadly effect of EGFR-targeted photodynamic therapy (PDT) on tumor and its side effects on normal tissues in vitro [90]. Bernhard W. Renz et al. found that when b2 receptor blockers were injected into the medium, the pancreatic tumor grew slower [91]; such a drug screening model could be employed to explore the interaction between tumor and nerve in vitro. Therefore, the drug screening model of tumor organoids was mainly applied to preclinical drug evaluation, biomarker identification, biological research, and individualized therapy.
The deficiency and prospect of cancer organoid model for drug screening
So far, there were still many defects in the preclinical drug screening model of tumor organoids. Firstly, because the cancer organoid establishment was highly tissue specific, not all types of tumors were suitable for organoid culture. Also, the non-tumor components were not suitable for tumor organoids. Consequently, at present, tumor organoids were mainly applied in the following types of tumors (Table 2). Besides, some tumors had a low culture success rate, such as breast cancer. Furthermore, although tumor organoids could be cryopreserved, the recovery rate was not as high as 2D cell lines. Thus, the development of the organoid model was limited to a certain extent.
Table 2.
Various types of cancer organoid models for drug screening.
| Cancer type | Culture method | Culture method | Application | Reference |
|---|---|---|---|---|
| Glioblastoma | Traditional organoid | Long-term | Biological research | [18] |
| HNSCC | Traditional organoid | Long-term | Personalized therapy | [35] |
| Lung cancer | Traditional organoid | Short-term | LTS | [34] |
| Lung cancer | Organoid-on-a-chip | Short-term | LTS | [51] |
| Esophageal adenocarcinoma | Traditional organoid | Long-term | Personalized therapy | [89] |
| Gastrointestinal cancer | Traditional organoid | Long-term | Preclinical drug evaluation | [19] |
| Pancreatic cancer | Traditional organoid | Long-term | Biological research | [40] |
| Liver cancer | Traditional organoid | Long-term | Personalized therapy | [61] |
| Biliary tract carcinoma | Traditional organoid | Long-term | Biomarker identification | [75] |
| Colorectal cancer | Traditional organoid | Long-term | HTS | [33] |
| Rectal cancer | Traditional organoid | Long-term | Preclinical drug evaluation | [29] |
| Breast cancer | Traditional organoid | Long-term | HTS | [42] |
| Ovarian cancer | Traditional organoid | Short-term | Biological research | [58] |
| Ovarian cancer | Traditional organoid | Long-term | Personalized therapy | [65] |
| Endometrial cancer | Traditional organoid | Long-term | Biological research | [66] |
| Prostate cancer | Traditional organoid | Long-term | Biological research | [88] |
| Bladder cancer | Traditional organoid | Long-term | Biological research | [70] |
HTS: high-throughput drug screening.
LTS: low-throughput drug screening.
HNSCC: head and neck squamous carcinoma.
Secondly, although tumor organoids retained the genetic characteristics of tumor tissue during long-term passages, clonal drift inevitably occurred. Except for that, the culture condition of cancer organoid was elaborate and expensive. Although 2D cell lines were widely used to produce growth factors instead of commercial cytokines to reduce the cost, cytokines may have an impact on the drug screening results of tumor organoids [92]. Helen H.N. Yan et al. removed L-wnt3A as well as Sachs N et al. removed Y-27632 and N-Acetylcysteine from organoids' growth medium when they used cancer organoid for drug screening [27,42].
Thirdly, the source of cancer tissues for non-operative tumor patients was difficult to obtain. Although the development of tumor organoids derived from biopsy specimens and circulating tumor cell-derived organoids enriched the models of drug screening in vitro for non-operative tumor patients [93,94], still, some kinds of tumor organoids could not be established from circulating tumor cell or biopsy specimens as reported, which also badly affected the application of tumor organoids in personal therapy. The liver cancer organoid cell lines constructed from the biopsy specimens of patients regained the mutation, number variation, and heterogeneity of the original tumor [95]. Bee Luan Khoo et al. used a 3D microfluidic device to culture circulating tumor cells, in which drug screening could be carried out to achieve individualized treatment [96].
Except for the mentioned shortcomings, cancer organoid model for drug screening had also been continuously breaking through in recent years.
On the one hand, co-culturing tumor organoids with non-tumor cells or human induced pluripotent stem cells (iPSC)-induced tumor organoids compensated for the lack of other non-tumor cellular components in tumor organoids [97,98]. Chiara M. Cattaneo et al. provided a protocol to co-cultured tumor cells and T cells to detect T cell-based immunotherapy in vitro [99]. Yotam E. Bar-Ephraim et al.co-cultured tumor organoids and immune cells to study the interaction between tumor cells and immune cells [100]. Furthermore, iPSC-derived organoids were a 3D, self-organized spherical structure of multiple cell types, which was more faithful to tumor tissue in terms of structure [97,101]. Also, iPSC-derived organoids could retain tumor cell and non-tumor cell components, which more veritably revealed the sensitivity of tumor patients to drugs [102].
On the other hand, as the tumor organoids lost their original arrangement style, it was necessary to develop new technology such as scaffolds, 3D printing technology, and air-liquid interface, which facilitated tumor organoids form similar structures to primary tumor tissues. 3D printing technology had been used to construct an engineered organ, which enabled the organ to complete the spatial arrangement of cells, the composition of ECM, and the multicellular activity [103,104]. In 3D scaffolds, cancer cells and endothelial cells were co-cultured, a vascular structure could be formed by using microfluidic technology [105]. In the future, with the rapid development of cancer organoid technology, tumor organoids will be first expected to predict the effectiveness and accuracy of drug responses in vitro [106].
Conclusion
In summary, tumor organoids can be passed on infinitely and do not lose or change genetic information in the process of passage. It can also capture the intra-/interpatient heterogeneity. The traditional cancer organoid model for drug screening saves money, while organoid-on-a-chip frees human resources. Short-term drug screening takes little time. However, long-term drug screening can be used for high-throughput drug screening. So far, the cancer organoid model for low-/high-throughput drug screening has been widely used in preclinical drug evaluation, biomarker identification, biological research, and individualized therapy. Although tumor organoids have many shortcomings in the application of drug screening, the cancer organoid model for drug screening is also continuously developing and breaking through. Besides, the drug screening results of tumor organoids are very stable and fidelity. However, 2D cell lines are easy to operate and cheap, and the drug screening results of PDX are not affected by cytokines. Therefore, we should rationally use a combination of pre-clinical drug screening models according to the characteristics of cancer types.
Funding
National Natural Science Foundation of China under Grant numbers NFSC 81974408.
Author contributions statement
Chen Liu, Tianyu Qin: Conceptualization, Methodology, Software.
Chen Liu, Tianyu Qin: Data curation, Writing- Original draft preparation.
Yuhan Huang: Visualization, Investigation.
Gang Chen: Supervision.
Yuan Li: Software, Validation.
Chaoyang Sun: Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Harrison R.K. Phase II and phase III failures: 2013-2015. Nat. Rev. Drug Discov. 2016;15:817–818. doi: 10.1038/nrd.2016.184. [DOI] [PubMed] [Google Scholar]
- 2.Tuveson D., Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364:952–955. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 3.Kalamara A., Tobalina L., Saez-Rodriguez J. How to find the right drug for each patient? Advances and challenges in pharmacogenomics. Curr Opin Syst Biol. 2018;10:53–62. doi: 10.1016/j.coisb.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava P., Kumar M., Nayak P. 2016. Role of Patient Derived Cell Lines and Xenograft in Cancer Research. The Pharmstudent; p. 27. [Google Scholar]
- 5.Scherer W.F., Syverton J.T., Gey G.O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu Z., Li H., Zhang Z., Zhu Z., He S., Wang X. A pharmacogenomic landscape in human liver cancers. Cancer Cell. 2019;36:179–193. doi: 10.1016/j.ccell.2019.07.001. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S.V., Haber D.A., Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer. 2010;10:241–253. doi: 10.1038/nrc2820. [DOI] [PubMed] [Google Scholar]
- 9.Bruna A., Rueda O.M., Greenwood W., Batra A.S., Callari M., Batra R.N. A biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anticancer compounds. Cell. 2016;167:260–274. doi: 10.1016/j.cell.2016.08.041. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J.F., Palakurthi S., Zeng Q., Zhou S., Ivanova E., Huang W. Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for preclinical evaluation of novel therapeutics. Clin. Cancer Res. 2017;23:1263–1273. doi: 10.1158/1078-0432.CCR-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmar K., Kochupurakkal B.S., Lazaro J.B., Wang Z.C., Palakurthi S., Kirschmeier P.T. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin. Cancer Res. 2019;25:6127–6140. doi: 10.1158/1078-0432.CCR-19-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drapkin B.J., George J., Christensen C.L., Mino-Kenudson M., Dries R., Sundaresan T. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov. 2018;8:600–615. doi: 10.1158/2159-8290.CD-17-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeRose Y.S., Wang G., Lin Y.C., Bernard P.S., Buys S.S., Ebbert M.T. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Fu S., Zhao J., Zhao W., Shen Z., Wang D. Transbronchoscopic patient biopsy-derived xenografts as a preclinical model to explore chemorefractory-associated pathways and biomarkers for small-cell lung cancer. Cancer Lett. 2019;440-441:180–188. doi: 10.1016/j.canlet.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Kondrashova O., Topp M., Nesic K., Lieschke E., Ho G.Y., Harrell M.I. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat. Commun. 2018;9:3970. doi: 10.1038/s41467-018-05564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia X., Li F., He J., Aji R., Gao D. Organoid technology in cancer precision medicine. Cancer Lett. 2019;457:20–27. doi: 10.1016/j.canlet.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Ben-David U., Ha G., Tseng Y.Y., Greenwald N.F., Oh C., Shih J. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 2017;49:1567–1575. doi: 10.1038/ng.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob F., Salinas R.D., Zhang D.Y., Nguyen P.T.T., Schnoll J.G., Wong S.Z.H. A patient-derived Glioblastoma Organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180:188–204. doi: 10.1016/j.cell.2019.11.036. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlachogiannis G., Hedayat S., Vatsiou A., Jamin Y., Fernandez-Mateos J., Khan K. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ooft S.N., Weeber F., Dijkstra K.K., McLean C.M., Kaing S., van Werkhoven E. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. 2019;11(513) doi: 10.1126/scitranslmed.aay2574. eaay2574. [DOI] [PubMed] [Google Scholar]
- 21.Fujii M., Shimokawa M., Date S., Takano A., Matano M., Nanki K. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18:827–838. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Usui T., Sakurai M., Umata K., Elbadawy M., Ohama T., Yamawaki H. Hedgehog signals mediate anti-cancer drug resistance in three-dimensional primary colorectal cancer organoid culture. Int. J. Mol. Sci. 2018;19(4):1098. doi: 10.3390/ijms19041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J., Xu H., Zhang L., Song L., Feng D., Peng X. Malignant ascites-derived organoid (MADO) cultures for gastric cancer in vitro modelling and drug screening. J. Cancer Res. Clin. Oncol. 2019;145:2637–2647. doi: 10.1007/s00432-019-03004-z. [DOI] [PubMed] [Google Scholar]
- 24.Kondo J., Inoue M. Application of cancer organoid model for drug screening and personalized therapy. Cells. 2019;8(5):470. doi: 10.3390/cells8050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L., Yang S., Li X., Li B., Li Y., Zhang X. Tumor organoids: From inception to future in cancer research. Cancer Lett. 2019;454:120–133. doi: 10.1016/j.canlet.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Costales-Carrera A., Fernandez-Barral A., Bustamante-Madrid P., Guerra L., Cantero R., Barbachano A. Plocabulin displays strong cytotoxic activity in a personalized colon cancer patient-derived 3D organoid assay. Mar Drugs. 2019;17(11):648. doi: 10.3390/md17110648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan H.H.N., Siu H.C., Law S., Ho S.L., Yue S.S.K., Tsui W.Y. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–897. doi: 10.1016/j.stem.2018.09.016. e11. [DOI] [PubMed] [Google Scholar]
- 28.Buzzelli J.N., Ouaret D., Brown G., Allen P.D., Muschel R.J. Colorectal cancer liver metastases organoids retain characteristics of original tumor and acquire chemotherapy resistance. Stem Cell Res. 2018;27:109–120. doi: 10.1016/j.scr.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganesh K., Wu C., O'Rourke K.P., Szeglin B.C., Zheng Y., Sauve C.G. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 2019;25:1607–1614. doi: 10.1038/s41591-019-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Y., Xu X., Yang L., Zhu J., Wan J., Shen L. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26:17–26. doi: 10.1016/j.stem.2019.10.010. e6. [DOI] [PubMed] [Google Scholar]
- 31.Tiriac H., Belleau P., Engle D.D., Plenker D., Deschenes A., Somerville T.D.D. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8:1112–1129. doi: 10.1158/2159-8290.CD-18-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson L., Tighe A., Golder A., Littler S., Bakker B., Moralli D. A living biobank of ovarian cancer ex vivo models reveals profound mitotic heterogeneity. Nat. Commun. 2020;11:822. doi: 10.1038/s41467-020-14551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Wetering M., Francies H.E., Francis J.M., Bounova G., Iorio F., Pronk A. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim M., Mun H., Sung C.O., Cho E.J., Jeon H.J., Chun S.M. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019;10:3991. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Driehuis E., Kolders S., Spelier S., Lõhmussaar K., Willems S.M., Devriese L.A. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discovery. 2019;9:852–871. doi: 10.1158/2159-8290.CD-18-1522. [DOI] [PubMed] [Google Scholar]
- 36.Drost J., Karthaus W.R., Gao D., Driehuis E., Sawyers C.L., Chen Y. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 2016;11:347–358. doi: 10.1038/nprot.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 38.Aberle M.R., Burkhart R.A., Tiriac H., Olde Damink S.W.M., Dejong C.H.C., Tuveson D.A. Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br. J. Surg. 2018;105:e48–e60. doi: 10.1002/bjs.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francies H.E., Barthorpe A., McLaren-Douglas A., Barendt W.J., Garnett M.J. Drug sensitivity assays of human cancer organoid cultures. Methods Mol. Biol. 2019;1576:339–351. doi: 10.1007/7651_2016_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boj S.F., Hwang C.I., Baker L.A., Chio I.I., Engle D.D., Corbo V. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dekkers J.F., Wiegerinck C.L., de Jonge H.R., Bronsveld I., Janssens H.M., de Winter-de Groot K.M. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 42.Sachs N., de Ligt J., Kopper O., Gogola E., Bounova G., Weeber F. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–386. doi: 10.1016/j.cell.2017.11.010. e10. [DOI] [PubMed] [Google Scholar]
- 43.Robson M., Im S.A., Senkus E., Xu B., Domchek S.M., Masuda N. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 44.Arena S., Corti G., Durinikova E., Montone M., Reilly N.M., Russo M. A subset of colorectal cancers with cross-sensitivity to olaparib and oxaliplatin. Clin. Cancer Res. 2020;26:1372–1384. doi: 10.1158/1078-0432.CCR-19-2409. [DOI] [PubMed] [Google Scholar]
- 45.Shirure V.S., Bi Y., Curtis M.B., Lezia A., Goedegebuure M.M., Goedegebuure S.P. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip. 2018;18:3687–3702. doi: 10.1039/c8lc00596f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kizilkurtlu A.A., Polat T., Aydin G.B., Akpek A. Lung on a chip for drug screening and design. Curr. Pharm. Des. 2018;24:5386–5396. doi: 10.2174/1381612825666190208122204. [DOI] [PubMed] [Google Scholar]
- 47.Kasendra M., Tovaglieri A., Sontheimer-Phelps A., Jalili-Firoozinezhad S., Bein A., Chalkiadaki A. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 2018;8:2871. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jabs J., Zickgraf F.M., Park J., Wagner S., Jiang X., Jechow K. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol. Syst. Biol. 2017;13:955. doi: 10.15252/msb.20177697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y., Wang L., Yu H., Yin F., Wang Y., Liu H. In situ generation of human brain organoids on a micropillar array. Lab Chip. 2017;17:2941–2950. doi: 10.1039/c7lc00682a. [DOI] [PubMed] [Google Scholar]
- 50.Sontheimer-Phelps A., Hassell B.A., Ingber D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer. 2019;19:65–81. doi: 10.1038/s41568-018-0104-6. [DOI] [PubMed] [Google Scholar]
- 51.Jung D.J., Shin T.H., Kim M., Sung C.O., Jang S.J., Jeong G.S. A one-stop microfluidic-based lung cancer organoid culture platform for testing drug sensitivity. Lab Chip. 2019;19:2854–2865. doi: 10.1039/c9lc00496c. [DOI] [PubMed] [Google Scholar]
- 52.Park S.E., Georgescu A., Huh D. Organoids-on-a-chip. Science. 2019;364:960–965. doi: 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi R., Radulovich N., Ng C., Liu N., Notsuda H., Cabanero M. Organoid cultures as preclinical models of non-small cell lung cancer. Clin. Cancer Res. 2020;26:1162–1174. doi: 10.1158/1078-0432.CCR-19-1376. [DOI] [PubMed] [Google Scholar]
- 54.Kodack D.P., Farago A.F., Dastur A., Held M.A., Dardaei L., Friboulet L. Primary patient-derived cancer cells and their potential for personalized cancer patient care. Cell Rep. 2017;21:3298–3309. doi: 10.1016/j.celrep.2017.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondo J., Endo H., Okuyama H., Ishikawa O., Iishi H., Tsujii M. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6235–6240. doi: 10.1073/pnas.1015938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka N., Osman A.A., Takahashi Y., Lindemann A., Patel A.A., Zhao M. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol. 2018;87:49–57. doi: 10.1016/j.oraloncology.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarro L.M., Bradshaw C.R. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill S.J., Decker B., Roberts E.A., Horowitz N.S., Muto M.G., Worley M.J., Jr. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8:1404–1421. doi: 10.1158/2159-8290.CD-18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soragni A., Janzen D.M., Johnson L.M., Lindgren A.G., Thai-Quynh Nguyen A., Tiourin E. A designed inhibitor of p53 aggregation rescues p53 tumor suppression in ovarian carcinomas. Cancer Cell. 2016;29:90–103. doi: 10.1016/j.ccell.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Krawczyk E., Suprynowicz F.A., Palechor-Ceron N., Yuan H., Dakic A. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 2017;12:439–451. doi: 10.1038/nprot.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu H., Gehart H., Artegiani B., LO-I C., Dekkers F., Basak O. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175:1591–1606. doi: 10.1016/j.cell.2018.11.013. e19. [DOI] [PubMed] [Google Scholar]
- 62.Sachs N., Papaspyropoulos A., Zomer-van Ommen D.D., Heo I., Bottinger L., Klay D. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;34:1906–1910. doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saito Y. Establishment of an organoid bank of biliary tract and pancreatic cancers and its application for personalized therapy and future treatment. J. Gastroenterol. Hepatol. 2019;34:1906–1910. doi: 10.1111/jgh.14773. [DOI] [PubMed] [Google Scholar]
- 64.Kondo J., Ekawa T., Endo H., Yamazaki K., Tanaka N., Kukita Y. High-throughput screening in colorectal cancer tissue-originated spheroids. Cancer Sci. 2019;110:345–355. doi: 10.1111/cas.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kopper O., de Witte C.J., Lohmussaar K., Valle-Inclan J.E., Hami N., Kester L. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019;25:838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 66.Boretto M., Maenhoudt N., Luo X., Hennes A., Boeckx B., Bui B. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019;21:1041–1051. doi: 10.1038/s41556-019-0360-z. [DOI] [PubMed] [Google Scholar]
- 67.Kiyohara Y., Yoshino K., Kubota S., Okuyama H., Endo H., Ueda Y. Drug screening and grouping by sensitivity with a panel of primary cultured cancer spheroids derived from endometrial cancer. Cancer Sci. 2016;107:452–460. doi: 10.1111/cas.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pauli C., Hopkins B.D., Prandi D., Shaw R., Fedrizzi T., Sboner A. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7:462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schutte M., Risch T., Abdavi-Azar N., Boehnke K., Schumacher D., Keil M. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat. Commun. 2017;8:14262. doi: 10.1038/ncomms14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee S.H., Hu W., Matulay J.T., Silva M.V., Owczarek T.B., Kim K. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173:515–528. doi: 10.1016/j.cell.2018.03.017. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed M., Jinks N., Babaei-Jadidi R., Kashfi H., Castellanos-Uribe M., May S.T. Repurposing antibacterial AM404 as a potential anticancer drug for targeting colorectal cancer stem-like cells. Cancers (Basel) 2019;12(1):106. doi: 10.3390/cancers12010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prestwich G.D. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc. Chem. Res. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 73.Chhibber T., Bagchi S., Lahooti B., Verma A., Al-Ahmad A., Paul M.K. CNS organoids: an innovative tool for neurological disease modeling and drug neurotoxicity screening. Drug Discov. Today. 2020;25:456–465. doi: 10.1016/j.drudis.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pamies D., Block K., Lau P., Gribaldo L., Pardo C.A., Barreras P. Rotenone exerts developmental neurotoxicity in a human brain spheroid model. Toxicol. Appl. Pharmacol. 2018;354:101–114. doi: 10.1016/j.taap.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saito Y., Muramatsu T., Kanai Y., Ojima H., Sukeda A., Hiraoka N. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27:1265–1276. doi: 10.1016/j.celrep.2019.03.088. e4. [DOI] [PubMed] [Google Scholar]
- 76.Hai J., Zhang H., Zhou J., Wu Z., Chen T., Papadopoulos E. Generation of genetically engineered mouse lung organoid models for squamous cell lung cancers allows for the study of combinatorial immunotherapy. Clin. Cancer Res. 2020;26:3431–3442. doi: 10.1158/1078-0432.CCR-19-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakagawa H., Kasagi Y., Karakasheva T.A., Hara T., Aaron B., Shimonosono M. Modeling epithelial homeostasis and reactive epithelial changes in human and murine three-dimensional esophageal organoids. Curr Protoc Stem Cell Biol. 2020;52 doi: 10.1002/cpsc.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duarte A.A., Gogola E., Sachs N., Barazas M., Annunziato S., RdR J. BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat. Methods. 2018;15:134–140. doi: 10.1038/nmeth.4535. [DOI] [PubMed] [Google Scholar]
- 79.Beshiri M.L., Tice C.M., Tran C., Nguyen H.M., Sowalsky A.G., Agarwal S. A PDX/Organoid biobank of advanced prostate cancers captures genomic and phenotypic heterogeneity for disease modeling and therapeutic screening. Clin. Cancer Res. 2018;24:4332–4345. doi: 10.1158/1078-0432.CCR-18-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sudhan D.R., Guerrero-Zotano A., Won H., Gonzalez Ericsson P., Servetto A., Huerta-Rosario M. Hyperactivation of TORC1 drives resistance to the pan-HER tyrosine kinase inhibitor neratinib in HER2-mutant cancers. Cancer Cell. 2020;37:183–199. doi: 10.1016/j.ccell.2019.12.013. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu J., Qin B., Moyer A.M., Nowsheen S., Liu T., Qin S. DNA methyltransferase expression in triple-negative breast cancer predicts sensitivity to decitabine. J. Clin. Invest. 2018;128:2376–2388. doi: 10.1172/JCI97924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koppens M.A., Bounova G., Cornelissen-Steijger P., de Vries N., Sansom O.J., Wessels L.F. Large variety in a panel of human colon cancer organoids in response to EZH2 inhibition. Oncotarget. 2016;7:69816–69828. doi: 10.18632/oncotarget.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Angelis M.L., Bruselles A., Francescangeli F., Pucilli F., Vitale S., Zeuner A. Colorectal cancer spheroid biobanks: multi-level approaches to drug sensitivity studies. Cell Biol. Toxicol. 2018;34:459–469. doi: 10.1007/s10565-018-9423-3. [DOI] [PubMed] [Google Scholar]
- 84.Engel R.M., Chan W.H., Nickless D., Hlavca S., Richards E., Kerr G. Patient-derived colorectal cancer organoids upregulate revival stem cell marker genes following chemotherapeutic treatment. J. Clin. Med. 2020;9(1):128. doi: 10.3390/jcm9010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Della Corte C.M., Barra G., Ciaramella V., Di Liello R., Vicidomini G., Zappavigna S. Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J. Exp. Clin. Cancer Res. 2019;38:253. doi: 10.1186/s13046-019-1257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kita Y., Hamada A., Saito R., Teramoto Y., Tanaka R., Takano K. Systematic chemical screening identifies disulfiram as a repurposed drug that enhances sensitivity to cisplatin in bladder cancer: a summary of preclinical studies. Br. J. Cancer. 2019;121:1027–1038. doi: 10.1038/s41416-019-0609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lampis A., Carotenuto P., Vlachogiannis G., Cascione L., Hedayat S., Burke R. MIR21 drives resistance to heat shock protein 90 inhibition in cholangiocarcinoma. Gastroenterology. 2018;154:1066–1079. doi: 10.1053/j.gastro.2017.10.043. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao D., Vela I., Sboner A., Iaquinta P.J., Karthaus W.R., Gopalan A. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X., Francies H.E., Secrier M., Perner J., Miremadi A., Galeano-Dalmau N. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun. 2018;9:2983. doi: 10.1038/s41467-018-05190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Driehuis E., Spelier S., Beltran Hernandez I., de Bree R., S M.W., Clevers H. Patient-derived head and neck cancer organoids recapitulate EGFR expression levels of respective tissues and are responsive to EGFR-targeted photodynamic therapy. J. Clin. Med. 2019;8(11):1880. doi: 10.3390/jcm8111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Renz B.W., Takahashi R., Tanaka T., Macchini M., Hayakawa Y., Dantes Z. beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33:75–90. doi: 10.1016/j.ccell.2017.11.007. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Broutier L., Andersson-Rolf A., Hindley C.J., Boj S.F., Clevers H., Koo B.K. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016;11:1724–1743. doi: 10.1038/nprot.2016.097. [DOI] [PubMed] [Google Scholar]
- 93.Gao M., Lin M., Rao M., Thompson H., Hirai K., Choi M. Development of patient-derived gastric cancer organoids from endoscopic biopsies and surgical tissues. Ann. Surg. Oncol. 2018;25:2767–2775. doi: 10.1245/s10434-018-6662-8. [DOI] [PubMed] [Google Scholar]
- 94.Yang C., Xia B.R., Jin W.L., Lou G. Circulating tumor cells in precision oncology: clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int. 2019;19:341. doi: 10.1186/s12935-019-1067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nuciforo S., Fofana I., Matter M.S., Blumer T., Calabrese D., Boldanova T. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018;24:1363–1376. doi: 10.1016/j.celrep.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khoo B.L., Grenci G., Lim Y.B., Lee S.C., Han J., Lim C.T. Expansion of patient-derived circulating tumor cells from liquid biopsies using a CTC microfluidic culture device. Nat. Protoc. 2018;13:34–58. doi: 10.1038/nprot.2017.125. [DOI] [PubMed] [Google Scholar]
- 97.Duttenhoefer F., Lara de Freitas R., Meury T., Loibl M., Benneker L.M., Richards R.G. 3D scaffolds co-seeded with human endothelial progenitor and mesenchymal stem cells: Evidence of prevascularisation within 7 days. Eur Cell Mater. 2013;26:49–64. doi: 10.22203/ecm.v026a04. [DOI] [PubMed] [Google Scholar]
- 98.Ye W., Luo C., Li C., Huang J., Liu F. Organoids to study immune functions, immunological diseases and immunotherapy. Cancer Lett. 2020;477:31–40. doi: 10.1016/j.canlet.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 99.Cattaneo C.M., Dijkstra K.K., Fanchi L.F., Kelderman S., Kaing S., van Rooij N. Tumor organoid-T-cell coculture systems. Nat. Protoc. 2020;15:15–39. doi: 10.1038/s41596-019-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bar-Ephraim Y.E., Kretzschmar K., Clevers H. Organoids in immunological research. Nat Rev Immunol. 2020;20:279–293. doi: 10.1038/s41577-019-0248-y. [DOI] [PubMed] [Google Scholar]
- 101.Mills R.J., Parker B.L., Quaife-Ryan G.A., Voges H.K., Needham E.J., Bornot A. Drug screening in human PSC-cardiac organoids identifies pro-proliferative compounds acting via the mevalonate pathway. Cell Stem Cell. 2019;24:895–907. doi: 10.1016/j.stem.2019.03.009. e6. [DOI] [PubMed] [Google Scholar]
- 102.Sharma A., Sances S., Workman M.J., Svendsen C.N. Multi-lineage human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell. 2020;26:309–329. doi: 10.1016/j.stem.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park J., Wetzel I., Dreau D., Cho H. 3D miniaturization of human organs for drug discovery. Adv Healthc Mater. 2018;7(2) doi: 10.1002/adhm.201700551. [DOI] [PubMed] [Google Scholar]
- 104.Yoo S.S. 3D-printed biological organs: Medical potential and patenting opportunity. Expert Opin Ther Pat. 2015;25:507–511. doi: 10.1517/13543776.2015.1019466. [DOI] [PubMed] [Google Scholar]
- 105.Gholobova D., Terrie L., Gerard M., Declercq H., Thorrez L. Vascularization of tissue-engineered skeletal muscle constructs. Biomaterials. 2020;235:119708. doi: 10.1016/j.biomaterials.2019.119708. [DOI] [PubMed] [Google Scholar]
- 106.Rossi G., Manfrin A., Lutolf M.P. Progress and potential in organoid research. Nat Rev Genet. 2018;19:671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]