Abstract
Low levels of vitamin D have been linked with increased adiposity and diminished muscle strength. Whether it is also related to fat deposition in muscle tissues is not studied well. This study explored the associations between circulating 25-hydroxyvitamin D (25(OH)D) and fat deposition in muscle tissues of adult Arab males. A total 465 adult Saudi males were included in this cross-sectional study. Anthropometrics, body composition and muscle strength were assessed. Serum 25(OH)D was determined and quantified enzymatically. They were grouped according to vitamin D status: deficient (25(OH)D < 50 nmol/l) N = 325 (69.9%) and sufficient (25(OH)D > 50 nmol/l)140 (30.1%). Mean level of lean/height2, lean-arm-legs and lean-arms-legs/height2 were significantly higher in 25(OH)D deficient participants (p-values 0.03; 0.05 and 0.01 respectively). Thigh strength was significantly higher in 25(OH)D sufficient participants than their deficient counterparts (p = 0.02). In all participants, a significant correlation between 25(OH)D was observed with age and thigh-strength (p-values < 0.05), while a significant inverse correlation between 25(OH)D and lean/height2, lean-arms-legs, lean-arms-legs/height2, fat (%) region, fat arms, fat legs, fat trunk, lean legs were noted. In conclusion, low circulating 25(OH)D is associated with enhanced fat infiltration in muscle tissues of adult Arab males.
Keywords: Vitamin D deficiency, Muscle fats, Obesity, Vitamin D sufficiency
1. Introduction
Vitamin D (secosteroid) is one of the lipid soluble vitamins mainly present in various food products such as oily fish, cod liver oil, chicken eggs and mushrooms, respectively, as well as direct sun light sources. Different types of vitamin D such as cholecalciferol (animal source) and ergocalciferol (plant source) are biologically inactive and it can be effectively charged by list of enzymatic degradation activities. In general, the inactive vitamin D moieties were enzymatically treated in the liver cells and produced the bi products such as calcidiol and further absorbed in the cells various hormones namely hypophosphatemia and growth hormones, respectively. The deficiency of vitamin D in the human causes rickets in infants and osteomalacia in old people. The deficiency vitamin D is associated with the amount of calcium exists in the human bone and cells. world Health organization suggested that the consumption of 200 mg/day (0–6 months), 260 mg/day (6–12 months), 300 mg/day (1–3 years), 700 mg/day (4–8 years), 1000 mg/day (4–8 years), 1300 mg/day (9–18 years), 1000 mg/day (9–18 years), 1000 mg/day (19–51 years), 1000 mg/day (51–70 years males), 1200 mg/day (51–71 years females), 1200 mg/day (>70 years) and 1300 mg/day (pregnant/lactating) respectively (Pilz et al., 2019).
Vitamin D plays a major in the regulation of bone metabolism and is associated with muscles strength (Ward et al., 2009). Vitamin D deficiency may cause bone disorders as well as muscles weakness (Crocombe et al., 2004), especially proximal muscles (Ladhani et al., 2004). In the elderly, the vitamin D deficiency has been associated with muscle weakness, decrease body control, increased body sway and higher risk for fall and fracture, all of which can be modestly reversed by vitamin D and calcium supplementation (Al-Said et al., 2009, Bischoff et al., 2003). Vitamin D status has also been associated with muscle strength in healthy girls, suggesting that vitamin D is necessary for efficient muscle contractility (Ward et al., 2009). How vitamin D affects muscle strength is not fully understood but could be due to its direct influence in calcium homeostasis, which greatly influences muscle strength. Furthermore, vitamin D receptors are widely available in various tissues including skeletal muscles (Bischoff et al., 2001). The higher fat content in muscles are associated with lower levels of muscle strength and physical activity, independent of muscle mass (Manini et al., 2007). In the present cross-sectional study, we aim to assess whether fat deposition in muscles are associated with levels of 25-hydroxyvitamin D (25(OH)D) independent of muscle mass in a cohort of Saudi adult males.
2. Material and methods
2.1. Subjects
In this cross-sectional study, 465 healthy males from Saudi Arabian native (22–45 years) were recruited from the Department of Exercise Physiology, College of Sport Sciences and Physical Activity, King Saud University (KSU), Riyadh, Saudi Arabia. Subjects taking medications or having severe clinical conditions such as renal, hepatic diseases and cardiovascular diseases as well as neurological issues excluded. All Saudi males were questioned to complete a general questionnaire containing questions on demographics and past medical history and the role of the participatory males were orally described thereby informed written consents were obtained from all individuals. The study protocol was approved by the ethics committee of the College of Medicine, KSU, Riyadh, Kingdom of Saudi Arabia (IRB No. E-16-1785).
Anthropometrics were also recorded as described previously (Al-Daghri et al., 2015) and included height (nearest 0.5 cm), weight (nearest 0.1 kg), waist and hip circumference (nearest 0.5 cm), and mean blood pressure (mmHg). Body mass index (BMI) was computed accordingly (kg/m2) and subjects with BMI ≥ 25.00 kg/m2 and/or ≥30.00 kg/m2 were noted as the extreme body weight and denoted as obese, respectively.
2.2. Sample collection
About 10CC of fasting blood donor samples were carefully obtained from all subjects and transferred to the Chair for Biomarkers of Chronic Diseases in KSU for serum separation while the remaining was treated with freshly prepared EDTA tubes for routine laboratory analysis. It is carefully noted that blood samples collected from the individuals and serum samples obtained from the individuals were immediately stored at −80 °C for routine laboratory analysis.
2.3. Biochemical analyses
The automated analyzer was used to measure serum 25(OH) D (nmol/l) (Cobas e411, Roche Diagnostics, Mannheim, Germany). The inter- and intra-assay variations (CV) were 8.0 and 5.6%, respectively, with a lower detection limit (LOD) of 50 nmol/l. Vitamin D status was based on the national and regional recommendations, which considers 25(OH)D level <50 nmol/l as deficient (Al-Daghri et al., 2017, Al-Saleh et al., 2020).
2.4. Muscle and fat measurements
Body composition was determined and included total and appendicular muscle mass, fat mass and bone mineral density (BMD) using DXA (Lunar iDXA, GE Healthcare, General Electric Company, USA). Appendicular lean mass (ALM) was calculated by dividing appendicular skeletal muscle mass (kg) to height (m2). Subjects with −1SD and −2SD were determined following the gender-specific means for Saudi adults. Fat mass index (FMI) was recorded as the quotient of total body fat mass (kg) to height (m2). Handgrip dynamometer was used to measure grip strength while standing, while rotational dynamometer was used to measure thigh strength. The average of two trials were recorded. Paper version of long IPAQ was completed by all subjects under the supervision of a trained research assistant who was on standby for queries.
2.5. Statistical analysis
All statistical analysis was done using SPSS (version 21.0, IBM). The obtained results were represented as mean ± standard deviation (SD) for Gaussian variables and median (1st and 3rd) percentiles for non-Gaussian variables. Categorical variables were denoted as frequencies (%). All the continuous samples variables were assessed using Kolmogorov-Smirnov testfor normality. Non Gaussian variables were normalized before analysis. Pearson’s correlation analysis was used to determine correlations between variables of interest. Student T-test was used to match variations in mean. Area under the curve (AUC) was done using MedCalc software. P value <0.05 was effectively considered as statistically significant data.
3. Results
A total of 465 Saudi male adults were included in this cross-sectional vitamin D study, 325 (69.9%) of whom were vitamin D deficient (25(OH)D < 50 nmol/l) and 140 (30.1%) were vitamin D sufficient (25(OH)D > 50 nmol/l). Mean BMI was comparatively better in the vitamin D deficient group before and after adjusting for age (p-values 0.02 and 0.005, respectively). Waist to Hip ratio (WHR) adjusted for age was also significantly more in the vitamin D depleted subjects (p < 0.01). Mean value of lean/height2, lean-arm-legs and lean-arms-legs/height2 were significantly more in vitamin D depleted samples (p-values 0.034, 0.046 and 0.012, respectively). Thigh strength was significantly higher in 25(OH)D sufficient participants than their 25(OH)D deficient counterparts (p = 0.017) (Fig. 1). Mean value of fat arms, fat legs, fat trunk, fat android, lean legs and fat gynoid were effectively higher in the 25(OH)D deficient group before and after adjusting for age, while fat region, fat/height2,fat-trunk/total, visceral-volume(cm3) and visceral mass (g) were significantly higher only after adjusting for age (P < 0.05) (Table 1).
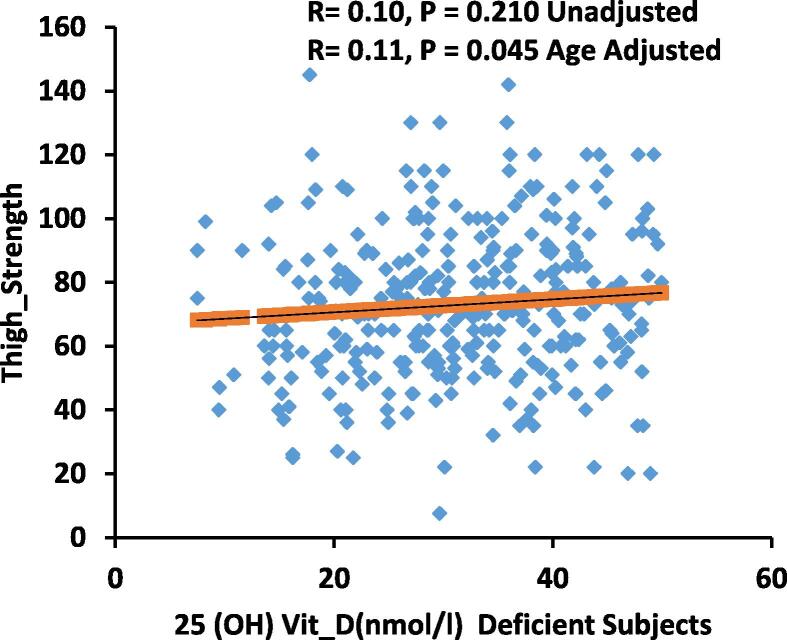
Fig. 1.
Correlation of 25(OH)D(nmol/l) Deficient Subjects and Thigh Strength.
Table 1.
General characteristics of subjects according to vitamin D status.
| Parameters | All | Vitamin D Deficient | Vitamin D Sufficient | P-Value | Age Adjusted P-Value |
|---|---|---|---|---|---|
| N | 465 | 325 (69.9) | 140 (30.1) | ||
| Age | 32.6 ± 11.0 | 31.1 ± 10.4 | 34.9 ± 11.0 | <0.001 | |
| BMI | 28.1 ± 5.3 | 28.5 ± 5.5 | 27.2 ± 4.8 | 0.02 | 0.005 |
| WHR | 0.89 ± 0.07 | 0.89 ± 0.07 | 0.88 ± 0.07 | 0.35 | 0.01 |
| 25(OH)D (nmol/l) | 38.4 (27.4–54.3) | 31.0 (23.2–39.6) | 64.1 (56.1–84.6) | <0.001 | <0.001 |
| Lean/Height2 (FFMI) | 18.2 ± 2.1 | 18.3 ± 2.1 | 17.8 ± 2.0 | 0.03 | 0.02 |
| Lean Arms & Legs | 26.2 ± 4.5 | 26.4 ± 4.5 | 25.6 ± 4.3 | 0.05 | 0.11 |
| Lean Arms & Legs/BMI | 0.94 ± 0.14 | 0.94 ± 0.14 | 0.95 ± 0.15 | 0.71 | 0.12 |
| Lean Arms & Legs/Height2 | 8.8 ± 1.3 | 8.9 ± 1.3 | 8.6 ± 1.2 | 0.01 | 0.02 |
| Handgrip strength | 42.6 ± 7.7 | 42.6 ± 7.7 | 42.6 ± 7.7 | 0.97 | 0.71 |
| Thigh Strength | 74.7 ± 24.0 | 72.7 ± 24.0 | 78.5 ± 23.7 | 0.02 | 0.001 |
| BMD (g/cm2) | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.12 | 0.56 | 0.65 |
| %Fat Region | 31.4 ± 8.0 | 31.8 ± 8.3 | 30.5 ± 7.8 | 0.10 | 0.02 |
| Lean (g) | 53.6 ± 8.0 | 53.9 ± 8.2 | 52.8 ± 7.5 | 0.18 | 0.21 |
| Fat Arms | 2.7 ± 1.1 | 2.8 ± 1.2 | 2.5 ± 0.9 | 0.006 | 0.002 |
| Fat Legs | 9.1 ± 3.8 | 9.5 ± 4.0 | 8.5 ± 3.3 | 0.02 | 0.03 |
| Fat Trunk | 14.2 ± 6.9 | 14.7 ± 7.3 | 13.2 ± 6.2 | 0.03 | 0.003 |
| Fat Android | 2.5 ± 1.4 | 2.6 ± 1.5 | 2.2 ± 1.3 | 0.04 | 0.004 |
| Fat Gynoid | 4.3 ± 1.9 | 4.5 ± 2.0 | 4.0 ± 1.7 | 0.02 | 0.019 |
| Lean Arms | 6.5 ± 1.2 | 6.6 ± 1.2 | 6.4 ± 1.2 | 0.36 | 0.49 |
| Lean Legs | 19.6 ± 3.6 | 19.9 ± 3.6 | 19.1 ± 3.3 | 0.04 | 0.09 |
| Fat/Height2 (FMI) | 10.7 ± 2.8 | 10.8 ± 2.8 | 10.4 ± 2.9 | 0.13 | 0.018 |
| Fat Trunk/total | 0.51 ± 0.07 | 0.51 ± 0.07 | 0.49 ± 0.07 | 0.55 | 0.006 |
| Fat Legs/total | 0.34 ± 0.05 | 0.35 ± 0.05 | 0.36 ± 0.05 | 0.68 | 0.006 |
| Fat Arms & Legs/trunk | 0.91 ± 0.24 | 0.91 ± 0.24 | 0.92 ± 0.25 | 0.57 | 0.007 |
| Visceral volume(cm3)# | 900 (429–1521) | 936 (432–1533) | 839 (400–1479) | 0.30 | 0.005 |
| Visceral mass(g)# | 850 (405–1429) | 882 (407–1447) | 806 (381–1367) | 0.40 | 0.006 |
Note: Data Presented as Mean ± SD and Median (1st−3rd) percentiles for Gaussian and Non Gaussian variables. P-value and adjusted p-value significant at 0.05 and 0.01 level.
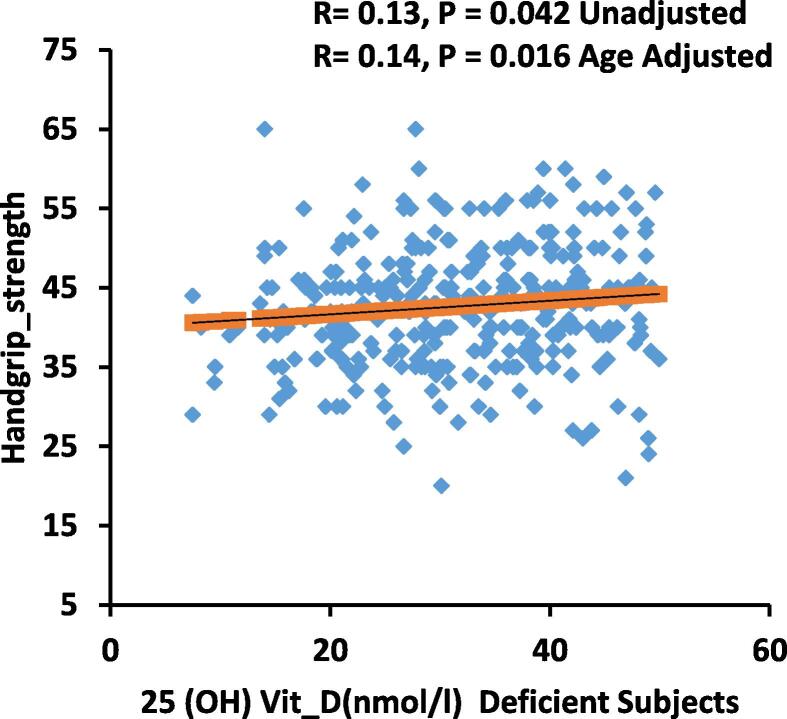
The bivariate relationships of 25(OH)D with various anthropometric, biochemical, muscle strength and muscle fats in all subjects as well as in deficient and sufficient vitamin D level are shown in Table 2. A significant direct correlation between vitamin D were observed with age, thigh- strength (P < 0.05 for all) for all subjects, while a significant inverse correlation between vitamin D and lean/height2, lean-arms-legs, lean-arms-legs/height2, fat (%) region, fat arms, fat legs, fat trunk, lean legs were observed. In vitamin D deficient subjects, a significant correlation between 25(OH)D, lipids (total and LDL-cholesterol) and handgrip-strength (Fig. 2) were observed (P < 0.05).
Table 2.
Significant 25(OH)D associations with measured parameters.
| Parameters | All |
Vitamin D Deficient |
Vitamin D Sufficient |
|||
|---|---|---|---|---|---|---|
| N | 465 |
325 (69.9) |
140 (30.1) |
|||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Age | 0.21** | – | 0.13* | – | – | – |
| BMI | −0.16** | −0.16** | −0.18** | −0.16** | – | – |
| Lean/Height2 (FFMI) | −0.11* | −0.11* | – | – | – | – |
| Lean Arms&Legs | −0.12* | −0.10* | –– | – | – | – |
| Lean Arms&Legs/Height2 | −0.13** | −0.12* | – | – | – | – |
| Handgrip strength | 0.13* | – | – | –– | ||
| Thigh Strength | 0.12* | 0.16** | – | 0.17** | – | – |
| %Fat Region | −0.11* | −0.11* | −0.13* | −0.12* | – | – |
| Fat Arms | −0.16** | −0.14** | −0.15** | −0.14* | – | – |
| Fat Legs | −0.19** | −0.16** | −0.23** | −0.20** | – | – |
| Fat Trunk | −0.14** | −0.17** | −0.15** | −0.16** | – | – |
| Fat Android | −0.14** | −0.16** | −0.15** | −0.16** | – | – |
| Fat Gynoid | −0.18** | −0.16** | −0.22** | −0.18** | – | – |
| Lean Legs | −0.14** | −0.12* | −0.13* | – | – | |
| Visceral volume (cm3)# | – | −0.14** | – | −0.12* | – | – |
| Visceral mass (g)# | – | −0.13* | – | −0.13* | – | – |
Note: Only parameters with significant correlations were presented. Data presented as coefficient (R).
Denotes significance at 0.05 level.
Denotes significance at 0.01 level also adjusted for age.
Fig. 2.
Correlation of 25(OH)D(nmol/l) Deficient Subjects and Handgrip strength.
Table 3 shows the significant predictors of 25(OH)D in all participants. Using 25(OH)D as dependent variable and the various body composition measures as well as the hand and grip strength as independent variables, it was found that age is the single most significant predictor for 25(OH)D in adult Arab males, predicting 22% of the variance in 25(OH)D levels (p < 0.001) (model 1). Age and thigh strength on the other hand predicted a combined 25% of the variance perceived in 25(OH)D levels, with thigh strength alone contributing as much as 15% (p < 0.001) (model 2). Lean arms and legs/height2, while inversely associated with 25(OH)D, cumulatively predicts 26% of the variance in 25(OH)D levels together with thigh strength and age (p < 0.001). Furthermore, AUC revealed that a thigh strength 63 kg and above corresponds to 25(OH)D levels ≥ 50 nmol (AUC 0.566 (P = 0.02). The sensitivity, specificity, negative predictive and positive predictive values were 75.36%, 36.65%, 0.67 and 1.19, respectively.
Table 3.
Significant Predictors of 25(OH) D status.
| Model | Unstandardized Coefficients |
Standardized Coefficients |
P-Value | ||
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| Model 1 | (Constant) | 27.17 | 3.80 | <0.001 | |
| Age | 0.51 | 0.11 | 0.22 | <0.001 | |
| Model 2 | (Constant) | 13.22 | 5.99 | 0.028 | |
| Age | 0.58 | 0.12 | 0.25 | <0.001 | |
| Thigh Strength | 0.15 | 0.05 | 0.15 | 0.003 | |
| Model 3 | (Constant) | 38.41 | 9.71 | <0.001 | |
| Age | 0.60 | 0.11 | 0.26 | <0.001 | |
| Thigh Strength | 0.20 | 0.05 | 0.19 | <0.001 | |
| Lean Arms & Legs/Height2 | −3.27 | 1.00 | −0.16 | 0.001 | |
4. Discussion
In this study, associations between muscle mass, strength and muscle adiposity with vitamin D status in apparently healthy Saudi adult males were determined. Data revealed that serum 25(OH)D was significantly associated with age and thigh strength for all subjects, while a significant inverse correlation was found between 25(OH)D and lean/height2, lean-arms-legs, lean-arms-legs/height2, fat arms, fat legs and fat trunk even after adjusting for age. It is well known that muscle strength is strongly influenced by the amount of fat present in muscle tissues, and it has been proven that reduced physical activity and less exercise increase intramuscular fats (Berggren et al., 2008, Goodpaster et al., 2008, Manini et al., 2007). It has been documented that fats in skeletal muscles can be oxidized through extensive workout and exercise (Berggren et al., 2008, Durheim et al., 2008). In the present study, we found that a thigh strength corresponding to 63 kg was sensitive enough to detect 25(OH)D deficiency at 50 nmol/l. Consequently, in our recent study, we observed that adult Arab men who had pre-sarcopenia also had mean thigh strength of 63 kg (Yakout et al., 2019). Previous reports demonstrated that 25(OH)D status is positively related to muscular strength, force, velocity, and jump height (Al-Eisa et al., 2016, Ward et al., 2009). Most individuals in the current study were overweight, so we could say that vitamin D also correlated with overall body adiposity and level of muscle strength. Vitamin D supplementation is helpful to gain muscular strength without any apparent changes in muscle mass (Verhaar et al., 2000). Muscular mass and strength are correlated but the association is modest (Goodpaster et al., 2006), and strength of the muscles can significantly increase without having a significant change in muscle mass (Goodpaster et al., 2008). Unfortunately, we did not study the effect of activity level on muscular strength and mass. It is apparent that due to the hot environment, daylight outdoor activity is very less, partially explaining why obesity level in Saudi population is very high. Due to less exposure to sunlight, many individuals have vitamin D deficiency (Farhat et al., 2019). In the current study, vitamin D deficient subjects, 25(OH) D was inversely correlated with BMI, fat arms, fat legs and fat trunk. It has been reported that inactivity positively correlated with weight, vitamin D status, sluggishness and spending time on TV or computer etc., and were not associated with muscular adiposity (Gilsanz et al., 2010). A comprehensive in-vivo or in-vitro studies are required to explain the underlying mechanisms of vitamin D deficiency and its association with muscular fats. It has been studied that vitamin D mediates the protein synthesis at acellular level and also accumulation of the cellular ATPs, elevate the troponin C, and the up-regulation of the actin and sarcoplasmic proteins in striated muscles (de Boland et al., 1983). It is unknown that these cellular pathways induce the accumulation of muscular fats. Muscular fats are very essential for the various biological mechanisms like; lipids regulate myocytes, infiltration of the muscle fat weakens muscle mitochondrial function and also alters oxidative phosphorylation (Morino et al., 2006). High triglyceride level reduces the insulin depend glucose uptake in muscles; therefore, muscular fats were closely related with the insulin secretion and diabetes (Albu et al., 2005, Hulver and Dohm, 2004, Miljkovic-Gacic et al., 2008). Low circulating vitamin D level have also been interlinked with glucose level in the blood serum (Hypponen and Power, 2006) suggesting cross-talk between glucose homeostasis, vitamin D status and muscle fat content.
About 70% of the current Saudi male adults are vitamin D deficient and this is consistent with other studies conducted in the warmest regions of the world such as South Asia, Middle East, South America and Australia, showing that 30–50% of individuals have 25(OH)D levels less than 20 ng/ml (Holick, 2007, Peters et al., 2009).
The author acknowledges some caveats. The cross-sectional design of the study limits its findings as it is unable to determine causality. Interventional studies are needed to see whether vitamin D correction can influence muscle fat mass as well as strength. Since vitamin D receptors are present in skeletal muscle tissues, causation is possible and functional studies may further shed light in the inferential findings of the present study.
5. Conclusion
In conclusion, vitamin D status is inversely associated with levels of fat deposition in muscle tissues independent of body mass and is modestly but significantly associated with thigh muscle strength among Saudi male adults. Functional studies are needed to illustrate the physiology and cross-talk between muscle fat and vitamin D.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful for the Researchers Supporting Group (RSP-2019/22) in King Saud University, Riyadh, Saudi Arabia for their support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Daghri N.M., Al-Attas O.S., Wani K., Alnaami A.M., Sabico S., Al-Ajlan A., Chrousos G.P., Alokail M.S. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc. Diabetol. 2015;14:101. doi: 10.1186/s12933-015-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daghri N.M., Al-Saleh Y., Aljohani N., Sulimani R., Al-Othman A.M., Alfawaz H., Fouda M., Al-Amri F., Shahrani A., Alharbi M., Alshahrani F., Tamimi W., Sabico S., Rizzoli R., Reginster J.Y. Vitamin D status correction in Saudi Arabia: an experts’ consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Muskuloskeletal Diseases (ESCEO) Arch. Osteoporos. 2017;12(1):1. doi: 10.1007/s11657-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eisa E.S., Alghadir A.H., Gabr S.A. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin. Interv. Aging. 2016;11:513–522. doi: 10.2147/CIA.S102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Said Y.A., Al-Rached H.S., Al-Qahtani H.A., Jan M.M. Severe proximal myopathy with remarkable recovery after vitamin D treatment. Can. J. Neurol. Sci. Le journal canadien des sciences neurologiques. 2009;36(3):336–339. doi: 10.1017/s0317167100007083. [DOI] [PubMed] [Google Scholar]
- Albu J.B., Kovera A.J., Allen L., Wainwright M., Berk E., Raja-Khan N., Janumala I., Burkey B., Heshka S., Gallagher D. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am. J. Clin. Nutrit. 2005;82(6):1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saleh Y., Beshyah S.A., Hussein W., Almadani A., Hassoun A., Al Mamari A., Ba-Essa E., Al-Dhafiri E., Hassanein M., Fouda M.A., Al Ali N., Aljohani N., Al-Sayed N., Gittoes N., Elhadd T., Al-Baker W., Sabico S., Al-Daghri N. Diagnosis and management of vitamin D deficiency in the Gulf Cooperative Council (GCC) countries: an expert consensus summary statement from the GCC vitamin D advisory board. Arch. Osteoporos. 2020;15(1):35. doi: 10.1007/s11657-020-0709-8. [DOI] [PubMed] [Google Scholar]
- Berggren J.R., Boyle K.E., Chapman W.H., Houmard J.A. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am. J. Physiol. Endocrinol. Metabol. 2008;294(4):E726–732. doi: 10.1152/ajpendo.00354.2007. [DOI] [PubMed] [Google Scholar]
- Bischoff H.A., Borchers M., Gudat F., Duermueller U., Theiler R., Stahelin H.B., Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001;33(1):19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- Bischoff H.A., Stahelin H.B., Dick W., Akos R., Knecht M., Salis C., Nebiker M., Theiler R., Pfeifer M., Begerow B., Lew R.A., Conzelmann M. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 2003;18(2):343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- Crocombe S., Mughal M.Z., Berry J.L. Symptomatic vitamin D deficiency among non-Caucasian adolescents living in the United Kingdom. Arch. Dis. Child. 2004;89(2):197–199. doi: 10.1136/adc.2003.026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boland A.R., Albornoz L.E., Boland R. The effect of cholecalciferol in vivo on proteins and lipids of skeletal muscle from rachitic chicks. Calcif. Tissue Int. 1983;35(6):798–805. doi: 10.1007/BF02405126. [DOI] [PubMed] [Google Scholar]
- Durheim M.T., Slentz C.A., Bateman L.A., Mabe S.K., Kraus W.E. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am. J. Physiol. Endocrinol. Metabol. 2008;295(2):E407–412. doi: 10.1152/ajpendo.90397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat K.H., Arafa M.A., Rabah D.M., Amin H.S., Ibrahim N.K. Vitamin D status and its correlates in Saudi male population. BMC Public Health. 2019;19(1):211. doi: 10.1186/s12889-019-6527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz V., Kremer A., Mo A.O., Wren T.A., Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J. Clin. Endocrinol. Metabol. 2010;95(4):1595–1601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster B.H., Chomentowski P., Ward B.K., Rossi A., Glynn N.W., Delmonico M.J., Kritchevsky S.B., Pahor M., Newman A.B. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J. Appl. Physiol. 2008;105(5):1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V., Simonsick E.M., Tylavsky F.A., Visser M., Newman A.B. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hulver M.W., Dohm G.L. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc. Nutr. Soc. 2004;63(2):375–380. doi: 10.1079/pns2004351. [DOI] [PubMed] [Google Scholar]
- Hypponen E., Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabet. Care. 2006;29(10):2244–2246. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- Ladhani S., Srinivasan L., Buchanan C., Allgrove J. Presentation of vitamin D deficiency. Arch. Dis. Child. 2004;89(8):781–784. doi: 10.1136/adc.2003.031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini T.M., Clark B.C., Nalls M.A., Goodpaster B.H., Ploutz-Snyder L.L., Harris T.B. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am. J. Clin. Nutrit. 2007;85(2):377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- Miljkovic-Gacic I., Gordon C.L., Goodpaster B.H., Bunker C.H., Patrick A.L., Kuller L.H., Wheeler V.W., Evans R.W., Zmuda J.M. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am. J. Clin. Nutrit. 2008;87(6):1590–1595. doi: 10.1093/ajcn/87.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K., Petersen K.F., Shulman G.I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B.S., dos Santos L.C., Fisberg M., Wood R.J., Martini L.A. Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann. Nutr. Metab. 2009;54(1):15–21. doi: 10.1159/000199454. [DOI] [PubMed] [Google Scholar]
- Pilz S., Zittermann A., Trummer C. Vitamin D testing and treatment: a narrative review of current evidence. Endocr Connect. 2019;8(2):R27–R43. doi: 10.1530/EC-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaar H.J., Samson M.M., Jansen P.A., de Vreede P.L., Manten J.W., Duursma S.A. Muscle strength, functional mobility and vitamin D in older women. Aging. 2000;12(6):455–460. doi: 10.1007/BF03339877. [DOI] [PubMed] [Google Scholar]
- Ward K.A., Das G., Berry J.L., Roberts S.A., Rawer R., Adams J.E., Mughal Z. Vitamin D status and muscle function in post-menarchal adolescent girls. J. Clin. Endocrinol. Metabol. 2009;94(2):559–563. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]
- Yakout S.M., Alkahtani S.A., Al-Disi D. Coexistence of pre-sarcopenia and metabolic syndrome in Arab Men. Calcif. Tissue Int. 2019;104(2):130–136. doi: 10.1007/s00223-018-0477-2. [DOI] [PubMed] [Google Scholar]