Introduction and aims
Although, it has been success in the generation of animal clones from somatic cells in various animal species, the information related to nuclear reprogramming of cloned embryos is found to be limited. This study aims to compares the effect of both Scriptaid (SCR) and Trichostatin (A) treatments in improving cloning efficiency, and embryos developmental rate of cloned sheep embryos in vitro. Three groups were formed, i.e., one SCR group, second TSA group, with both treatment concentrations of 5 nM, 50 nM, and 500 nM, respectively, and third were control group with 0 nM. Methods: Ovaries of slaughtered sheep were collected and oocytes were recovered from antral follicles using aspiration method and in vitro maturation of oocytes were done. Then zona dissecting with micropipettes and oocyte enucleation were carried out under the micromanipulator. Later nuclear transfer, cell fusion and activation were done via cell fusion machine. Finally the embryo cultured in incubating chamber at the CO2 incubator up to 9 days. The result: In general the results showed that when the concentration increases the cleavage rate increased. The cleavage rates of the SCNT embryos treated with SCR at different concentrations are closely related to cleavage rate of embryos treated with TSA at same concentration; such as 39.47% for 500 nM TSA, 38.09% for 500 nM SCR; 18.6% for 50 nM TSA, 19.17% for 50 nM SCR, and 22.64% for 5 nM TSA, 17.18% for 5 nM SCR. As for the control group, the cleavage rate of the SCNT embryos cleavage ratewere27.47%., 30% and 30.85% respectively for bothtreatments. While there is a significant difference in TSA treatments at an eight-cell stage at the concentration (5 and 50 nM TSA) compared to the all other cleavage cell stages of (500 nM TSA and control). Also their were a differences between (50 nM of TSA) compared to the (50 nM SCR). Also there were a significant differences between the 16 cell stage at the (500 nM TSA) compared to other treatment (5 nM, 50 nM TSA and control). Regarding the SCR there were a significant difference at 8 cell stage between (5 nM SCR), compared to the other treatment (50 nM, 500 nM SCR and control). Also there were a significant difference at 16 cell stage between (50 nM, and 500 nM SCR), compared to the other treatment (5 nM SCR and control). While in the development of the embryos reach to blastocyst stage the SCR and the control group show a higher rate, in compered to TSA that did not show any development to blastocyst stage. The total SCR treatment showed (3/41 = 7.31%), and the total control showed (4/89 = 4.49%) blastula stage. It concludes that SCR improve the final development blastula stage compared to the TSA treatments that did not improved embryos reach to final developmental blastula stages may be due to spices differences or to the toxicity of TSA, especially at higher concentrations.
Keywords: Sheep cloning, Embryo, Somatic cell nuclear transfer, Trichostatin (A) TSA, Scripted (SCR)
1. Introduction
There are several problems limiting the production of embryos in the laboratory, so research directed towards improving the various techniques and steps involved in the process of producing sheep ova maturation and embryos in the laboratory. Attempts are made to overcome these problems to develop and improve the maturation medium from the maturity of the oocytes to the development of embryos in the laboratory by adding supplementation to the ova maturation medium, then fertilization and embryo development (Mishra et al., 2016). Recent reporting of the Red List by International Union for Conservation of Nature (IUCN) has highlighted that twenty-one percent of all mammals are at jeopardy of being extinct globally (Czernik et al., 2019, FAO, 2018). Various studies have highlighted a scientific conviction that this would allow the cell expansion and reestablishment using the popular and unique somatic cell nuclear transfer (SCNT) technology (Hildebrandt et al., 2018, Saragusty et al., 2016). The technology is used to derive cloned embryos with potential applications in both agriculture and regenerative medicine and can be utilized for the genetic cloning of the endangered species (Martínez-Ibarra et al., 2019, Gupta et al., 2018). Ogorevc and his co-worker 2016 have demonstrated SCNT particular use for rescuing the genetically valuable animals at farms or for their transgenic generation.
Precisely, SCNT involves a series of complex procedures including the culture of donor cells, in vitro maturation (IVM) of oocytes, enucleation, nucleus injection, fusion, activation, in vitro culture (IVC) of reconstructed embryos and embryo transfer. If any part of these steps is suboptimal, the production of cloned embryos or animals can be influenced (Hildebrandt et al., 2018). Although, it has been success in the generation of animal clones from somatic cells in various animal species such as mice (Mizutani et al., 2015), cats (Evecen et al., 2016), rabbits (Yuan et al., 2016), sheep (Ogorevc et al., 2016), pigs (Sheets et al., 2016), goats (Burgstaller and Brem, 2017), and cattle (De Bem et al., 2017) but the information related to nuclear reprogramming of cloned embryos is found to be limited (Demir et al., 2019). In the same context, a study by Watanabe and Nagai 2011 and (Yuan et al., 2016) showed that the survival rate of the cloned births lies below four percent, excluding cattle. It has been resonated that this may be due to the inadequate synchronization of the recipient cells with the donor cell, which may be due to activation difficulty of the oocyte-somatic cell as well as in vitro culture conditions, cloned embryos and oocytes. Recognized as the HDAC inhibitor, the trichostatin-A (TSA) advanced the acetylated histones number as well as the DNA demethylation. Histones hyperacetylation was promoted due to TSA through the histone inhibitions’ enzyme deacetylase for the freshly formulated embryo (Oliveiraet al., 2011. Another major reason for investigating TSA cloning was due to the adult ICR mouse successful cloning for the first time which previously has never been cloned directly.
Earlier studies have indicated that SCNT efficiency is the same since its initial development, such as only one percent of the reconstructed embryos can give rise to live-born animals (Tan et al., 2016). Moreover, nuclear transfer (NT) has resulted in severe developmental problems, including a high rate of abortion during early gestation and increased perinatal death (Maiorka et al., 2015). It is, however, unclear whether the developmental failures of cloned embryos are because of incomplete nuclear reprogramming or for the overall cloning procedure itself. To enhance the efficiency of cloning, studies have shown increased use of histone deacetylase inhibitors (HDACis) (Akagi et al., 2013; and Alhimaidi et al., 2014). So far, Trichostatin-A (TSA), a histone deacetylase inhibitor, has shown noticeable improvement in mouse cloning (Azuma et al., 2018). This TSA treatment led to a more than fivefold increase in the success rate of mouse cloning with no abnormality.
Studies indicate that TSA does induce hyperacetylation of histones and suppresses remethylation of DNA, leading to the un-methylated status of injected nuclei in oocytes (Mishra et al., 2016). Further, this TSA cloning technique led to the first success in cloning the adult Institute of Cancer Research (ICR) mouse, an outbred strain that was directly cloned for the first time in history (Czernik et al., 2019). Beside mouse, some studies showed that TSA treatment resulted in higher pre-implantation embryonic development in pigs (Wang et al., 2018), cattle (Ding et al., 2008), and rabbits (Zeng et al., 2017). The use of HDACi has been found to improve the epigenetic reprogramming and facilitate embryo development following SCNT in mouse and other species. The other technique Scriptaid (SCR) has been devised for the development of the embryos for effective cloning (Wen et al., 2014). Researchers start in study Scriptaid (SCR) and TSA, and investigate the role of time, concentration, and other parameters on the SCNT embryos development and therefore increase cloning efficiency in different animal species. Some reports have demonstrated that scripted treatment after somatic cell nuclear transfer (NT) improves the success rate of bovine embryo cloning (Ding et al., 2008 and Xu et al., 2013). To the best of the author’s knowledge, followed by an assessment of the literature, the reports on the comparison of the two technologies have been rare (Lee et al., 2016; and Srirattana et al., 2014). Therefore, this study aims to compare the effect of the TSA and SCR in the development of the SCNT cloned Namey sheep embryos in vitro.
2. Methods
2.1. Ovaries collection and oocytes recovery
The study collected the sheep ovaries in a bottle containing normal saline from the central slaughterhouse in Riyadh, Saudi Arabia. The ovaries were transported to the laboratory at room temperature within 2–3 h where they washed twice in fresh normal saline to recover primary oocytes antral follicles using aspiration method. The method included a 19-gauge needle that was attached to a 10 ml syringe constituting of 0.5 ml TCM-199 Hank’s salt‘s (Sigma M7653) supplemented with 10% FBS (Sigma F4135), 0.3 mM Sodium Pyruvate (Sigma P5280), 25 μg/ ml Gentamycin sulphate (Gibco 15710049) and 143 μg/ml Heparin sodium salt (Sigma H3149).
Following the process of aspiration, the syringe content was emptied in a 15 ml conical tube (BD Falcon 352099), and the oocytes are allowed five minutes to settle to the bottom of the conical tube. The supernatant is then emptied whereas the cumulus-oocyte complex (COC) and other follicular debris were transferred into a 90 mm sterile Petri dish (Medical Disposable Manufacturing Company 1200) for subsequent searching using glass Pasteur pipette (Normax 5426015). The selection of compact cumulus investment and uniform cytoplasm was made which were transferred to a 60 mm Petri dish (BD Falcon 351007) containing five drops, each with 100 μl size and covered with embryo-tested mineral oil (Sigma M5310), 20 oocytes were transferred into droplets for washing (Al-Mutary et al., 2019).
2.2. In vitro maturation of oocytes
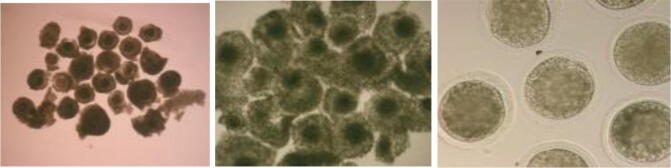
The chosen oocytes were matured for 22 h in TCM-199 Earle‘s salt‘s (Sigma M4530). This were supplemented with 10% FBS, 0.3 mM Sodium Pyruvate, 1 μg/ ml Estradiol-17β (Sigma E2758), 0.023 IU/ml LH (Sigma L5269), 0.02 IU/ml FSH (Sigma F8174) and 25 μg/ ml Gentamycin sulphate, at 38.5oC and exposed to 5% carbon dioxide in air atmosphere at maximum humidity. The oocytes were cultured in 35 mm Petri dish (BD Falcon, 351008) containing five drops, each with 50 μl size and covered with embryo tested mineral oil, 10–15 oocytes were cultured in a droplet (Fig. 1a); (Al-Mutary et al., 2019).
Fig. 1a.
1-Ova with follicle cells before and 2-after In vitro maturation (IVM) of ova 22 h.3- an ova with polar bodies after 22 h (IVM).
2.3. Zona dissecting with Micropipettes
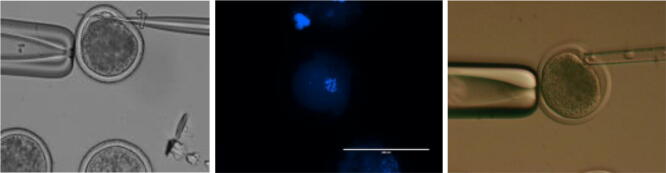
After 22 h of maturation, cumulus-enclosed oocytes were transferred into 35 mm Petri dish contain hyaluronidase enzyme (300 IU/ml) (Sigma H2126) covered with mineral oil. This was done to remove cumulus cells mechanically using glass Pasteur pipette. Denuded oocytes were washed in TCM-199 Hank’s salt‘s supplemented with 10% FBS and Gentamycin, then groups of 10 oocytes were transferred to a micromanipulation droplet overlaid with mineral oil. Only oocytes with the extruded first polar body were selected for zona dissection using the cutting pipette (glass capillary with filament) (Narishige GD-1). Orientate the oocyte until the first polar body (FPB) in the per vitelline space (PVS) become evident in 12-o’clock position after which the oocyte is aligned with the holding pipette (Sunlight Medical SHP-100-30), and the cutting pipette was pushed through the ZP at one point and out again at another point under the FPB. The oocyte is pushed along to a thicker section of the cutting pipette, against which the holding pipette can be rubbed in order to tear a slit in the zonapellucida (Fig. 1b) (Alhimaidi et al., 2014).
Fig. 1b.
1-Ova cutting and enucleating 2-the removed nucleus stained and checked under fluorescent microscopy, 3-the transfer of somatic cell to enucleated oocytes.
2.4. Oocyte enucleation
To remove the oocytes nucleus, zona dissected oocytes were transferred into TCM-199 Hank’s salt‘s supplemented with 10% FBS and 5 μg/ml cytochalasin-B (Sigma C6762). The time duration for this was fifteen-minute following which a slight pressure with rolling was applied on the oocytes using the cutting pipettes to remove a small amount of cytoplasm enclosed in plasma membrane directly beneath the FPB outside the ZP. Enucleation was confirmed by separation the outcome cytoplasm from the oocyte, and cultured the first one in TCM-199 Earle‘s salt is supplemented with 10% FBS and 10 μg/ml Hoechst 33,342 (Sigma B2261) for 30 min. This cytoplasm was then exposed to UV light (Evos® FL imaging System AMF4300) and checking for the presence of a metaphase (Fig. 1b) (Siriboon et al., 2018).
2.5. Preparation of Fibroblast donor cells
Fibroblast donor cells were derived from freshly slaughtered sheep, which possessed a normal diploid karyotype. The collected sample was used for SCNT at passages 4–6. Fibroblast cells were cultured in Dulbecco‘s modified minimum essential medium (DMEM) (Sigma D6046) supplemented with 10% FBS and 25 μg/ml Gentamycin sulphate using multi-well cell culture plates (BD Falcon 353047) at 38.5oC and exposed to 5% carbon dioxide air atmosphere at maximum humidity. Cells were grown to 80% confluence, and before NT cells were disaggregated by 0.25% trypsin-EDTA solution (Gibco 25200) (Fig. 1b) (Alhimaidi et al., 2014).
2.6. Nuclear Transfer, fusion and activation
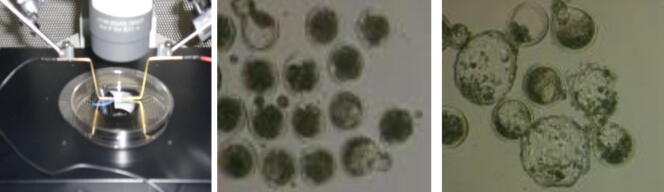
A single somatic cell was deposited into the per vitelline space of each enucleated oocyte using polar body biopsy pipettes (Sunlight Medical SPB-15X-30). After NT, couplets were directly being fused in Zimmermann’s cell fusion medium by applying single AC pulse of 0.2 KV/cm for one second followed by single DC pulse 2.5 KV/cm for 50 μs at room temperature. Selected fused couplets after fusion divided into three major groups, depending on the type of HDACi, control group, TSA groups, and SCR groups. In each major group, except the control, the couplets divided into three minor groups, depending on the HDACi concentrations, 1st group (5 nM), 2nd group (50 nM) and 3rd group (500 nM).
Following fusion, selected fused SCNT were activated using 5 μM of ionomycin (Sigma I0634) supplemented with one of the three concentrations of TSA or SCR for 5 min at room temperature. Immediately after ionomycin treatments, couplets were incubated in synthetic oviduct fluid medium (SOF medium) (Cassion IVL05) supplemented with 4 mg/ml BSA (Sigma A6003), 10 μl/ml MEM-essential amino acid (50x) (Sigma B6766), 10 μl/ml MEM Non-essential amino acid (100x) (Sigma M7145), 25 μg/ ml Gentamycin sulphate (Gibco 15710049), 10 μg/ml Cycloheximide (Sigma C1988) and the same treatment concentration that used with ionomycin treatment for 6 h at 38.5oC with 5% CO2 using 35 mm Petri dish covered with embryo tested mineral oil (Fig. 1b, Fig. 1c) (Alhimaidi et al., 2014).
Fig. 1c.
1-Cell fusion of Somatic cell and oocytes, 2-then developed of cloned sheep embryos at different stage with 3-hatched blastula.
2.7. Embryo culture
After 6 h of activation, embryos were cultured for 42 h in SOF medium supplemented with 4 mg/ml BSA, 10 μl/ml MEM essential amino acid (50x), 10 μl/ml MEM Non– essential amino acid (100x) and 25 μg/ ml Gentamycin sulphate. It also included the same treatment concentration that was used with cycloheximide treatment in a humidified atmosphere at 38.5oC of 5% oxygen (O2), 5% carbon dioxide (CO2) and 90% nitrogen (N2) using 35 mm Petri dish covered with embryo tested mineral oil. Following the 48 h of treatment the embryos were culture further up to 120 h in SOF medium supplemented with 4 mg/ml BSA, 10 μl/ml MEM essential amino acid (50x), 10 μl/ml MEM Non– essential amino acid (100x) and 25 μg/ ml Gentamycin sulphate in the same previous conditions (Fig. 1c) (Alhimaidi et al., 2014).
2.8. Statistical analysis
The study has carried out non-parametric two independent samples test (Mann-Whitney test) between the embryo’s numbers obtained by two different concentrations from the same treatment factor (Trichostatin (A)TSA or Scripted SCR). Similarly, the same test was used for the determination of the embryo’s numbers obtained by two treatment factors (TSA and SCR) at the same concentration. Statistical analysis was performed using IBM SPSS Statistics windows software program v21.0. The differences were considered significant at P values < 0.05.
3. Results
The net total of the oocytes utilized in this study were 588 oocytes. The oocytes for the scripted (SCR) treatment were 179; whereas, for Trichostatin (A)(TSA) treatment these were 134, each with three different concentrations. For the control group, the total number of oocytes for both treatments were 275 ova. The findings revealed a close association of the cleavage rates (from 1 cell to morula stage) of the SCNT embryos treated with Scripted at different concentrations with TSA at the same concentration. The total cleavage rat in general were not significantly difference between the different treated groups, for SCR 22.9%, the TSA 26.12% and the control 29.45%. The cleavage rate at each treated group was 22.64% for 5 nM TSA,18.6% for 50 nM TSA, and 39.47% for 500 nM TSA is higher than the other TSA group. While the SCR it was 17.18% for 5 nM SCR, 19.17% for 50 nM SCR and 38.09% for 500 nM SCR also higher than the other SCR group at (P < 0.05). As for the control group, the cleavage rate of the SCNT embryos was 30.85% for 0 nM, 30% for 0 nM and 27.47% 0nM respectively for all treatments Table 1.
Table 1.
Comparison between the effects of different concentrations from both SCR and TSA treatments on the cleavage blastocyst rates of SCNT sheep embryos.
| No & type of treatment | Concentration of treatment & No of ova | No and % of cleavage rate | Degenerating ova no & % | Total cleavage % | Total Blastula stage | Total degenerating |
|---|---|---|---|---|---|---|
|
Scripted SCR 179 ova |
5 nM = 64 ova | 11 = 17.18% | 53 = 82.82% | 41/179 = 22.91% | 3/41 = 7.31%* | 138/179 77.09% |
| 50 nM = 73 ova | 14 = 19.17% | 59 = 80.82% | ||||
| 500 nM = 42 ova | 16 = 38.09%* | 26 = 61.91% | ||||
|
Trichostatin (A) TSA 134 ova |
5 nM = 53 ova | 12 = 22.64% | 41 = 77.36% | 35/134 = 28.69% | 0/35 = 0% | 87/134 = 71.31% |
| 50 nM = 43 ova | 8 = 18.6% | 35 = 81.4% | ||||
| 500 nM = 38 ova | 15 = 39.47%* | 23 = 60.53% | ||||
| Control 275 ova | 0 nM = 94 ova | 29 = 30.85%* | 65 = 69.15% | 89/275 = 32.36% | 4/89 = 4.49%* | 186/275 = 67.64% |
| 0 nM = 90 ova | 27 = 30.%* | 63 = 70.0% | ||||
| 0 nM = 91 ova | 25 = 27.47%* | 66 = 72.53% |
(*) Significantly difference at p < 0.05 compared to the others without star at the same type of treatment or columens.
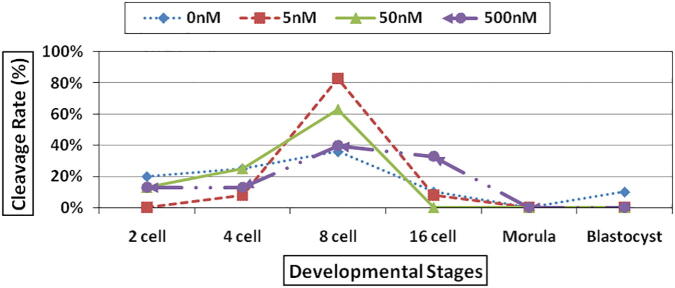
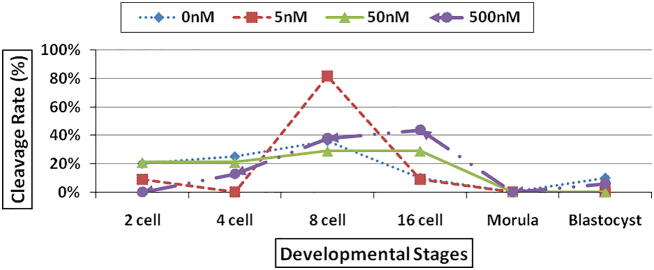
In the different cleavage rate the result in general reveals that when the treatment concentration increases the number of developed embryos increase (from 1 cell up to 8 cells stage). There was a significant difference (at P < 0.05) in TSA treatments at the eight-cell stage between (5 & 50 nM) as compared to (control & 500 nM). While there was a significant difference (at P < 0.05) in TSA treatments at the 16-cell stage between (500 nM) compared to the (control, 5 nM and 50 nM) (Fig. 2).
Fig. 2.
Cleavage rate of SCNT cloned sheep embryos in deferent developmental stages treated by using different concentrations of TSA: 0 nM TSA (control), 5 nM TSA, 50 nM TSA and 500 nM TSA.
There is a significant difference (at P < 0.05) in SCR treatments at the 8-cell stage between (5 nM SCR) compared the other SCR treatment. In addition the 16 cell stage SCR (5 nM & 500 nM) showed higher cleavage rat compared to (control and 5 nM SCR (Fig. 3). The concentration found to be directly related to the development of embryos, such as an increase in concentration leads to the development of embryos.
Fig. 3.
Cleavage rate of SCNT cloned sheep embryos in different developmental stages treated by different concentrations of SCR 0.nM SCR (control), 5 nM SCR, 50 nM SCR and 500 nM SCR.
Considering the total blastocyst stage formation rate for the SCR group 3/41 = 7.31%, it was found to be for the 5 nM SCR (1/11 = 9.09%); for the 50 nM SCR (0/14 = 0 %), whereas for 500 nM SCR treatment (2/16 = 12.5), The total control group blastocyst stage it was found to be (4/81 = 4.93%,) While no embryos were developed for the blastocyst stage at any concentration of TSA treatments table (Fig. 2, Fig. 3) Table 1.
In general most of the ova are in the SCNT were degenerating about 60–80% in all treated and control groups. In the SCR 3groups vary between 61 and 82%, the total is 138/179 (77.1%). Compared to the TSA 3 groups vary between 60 and 81%, with a total of 99/134 (73.88%) while in the control 3groups it vary between 69 and 72% with a total 194/275 (70.55%.) Table 1.
4. Discussion
The study applied an experimental approach to compare the effects of Scriptaid and Trichostatin (A) in vitro development of cloned sheep embryos. The experimental results showed that Scriptaid and Trichostatin (A) produce about similar results in the in vitrocleavage rate development of the cloned sheep embryos. The findings revealed that TSA treatments did not improve embryos to reach the final developmental stages. The reason for its ineffectiveness could be due to its toxicity at higher concentrations. This has been referred by various studies such as (Siriboon et al., 2018) has reported similar findings stating the TSA treatment did not improve an overall number of cells in cloned porcine blastocysts which led to its ineffectiveness. Similarly, these results have been correlated by (Meng et al., 2009) who found that rabbit offspring result from TSA-treated embryos died within an hour to 19 days while four pups of the untreated group have grown into adulthood and three of them produced offspring. The blastocyst development rate was found highest for the SCR group and control, whereas, it was low, mostly zero for the TSA group. This may be due to specific species or the treatment time for the TSA. Also, the embryos for the treatment groups were found to be low as compared to the experimental group, which may account to the toxicity particularly present at higher concentrations.
The results of the treatment showed that the blastocyst development rate was higher in the SCR and control group. Conflicting with findings of the previous research results on mice and cattle, the study resonates that this may be due to the treatment on a particular species, or the time duration for SCR and TSA treatment. This is consistent with the results of (Wu et al., 2008) who concluded that donor cells treated with 50 ng/ml TSA resulted in significantly lower blastocyst development (9.9% vs. 20%) in bovine. The research by Akagi et al., 2014) also examined the effect of treatment with HDACi SCR on the improvement of cattle SCNT embryos development. The study reported that SCR treatment could improve the post-implantation development of SCNT embryos (Alhimaidi et al., 2014). Compared to the (Hu et al., 2012) their results showed the treatment of cloned sheep embryos with 50 nM TSA for 24 h after activation could significantly improve blastocyst rate compared to the control (23.3 vs. 16.7% respectively). Hu et al., 2016 study showed enhanced sheep cloning efficiency which did not agreed with the result of our study. Accordingly, (Wen et al., 2014) find that the HDACi SCR was significantly more effective than TSA to enhance the developmental potential of cloned sheep embryos. Also, SCR modified the histone acetylation status of these embryos, which subsequently enhanced their nuclear reprogramming and development potential (Alhimaidi et al., 2014). The Efficiency per reconstructed oocyte in the SCNT is 0.3 of sheep (Wilmut et al., 1997) were less than our result, could be, because we count the present of the blastocyst to the developed embryo only, not to the all total reconstructed ova. Regarding the high rate of ova degeneration in the SCNT, it is, however, unclear whether the developmental failures of cloned embryos are because of incomplete nuclear reprogramming or for the overall cloning procedure itself or the free radicals effect in vitro.
Funding
This research project was funded by the Deanship of Scientific Research at King Saud University, Saudi Arabi, through research group No (RGP-1440-051).
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RGP-1440-051).Our gratitude and very deep appreciation to the administrating staff at the Environmental Health Care agency and the Central Riyadh slaughtered house for their help and assistance in collecting the sheep ovaries samples.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Muath Q Al-Ghadi, Email: malghadi@ksu.edu.sa.
Ahmad R Alhimaidi, Email: ahimaidi@ksu.edu.sa.sa.
Daisaku Iwamoto, Email: bd700id@waka.kindai.ac.jp.
Mohsen G. AL-Mutary, Email: al-mutary2006@hotmail.com.
Aiman A Ammari, Email: aimanammari@gmail.com.
Kazuhiro O Saeki, Email: saeki@waka.kindai.ac.jp.
Mohammed S. Aleissa, Email: dr.aleissaa@gmail.com.
References
- Akagi S., Geshi M., Nagai T. Recent progress in bovine somatic cell nuclear transfer. Anim. Sci. J. 2013;84:191–199. doi: 10.1111/asj.12035. [DOI] [PubMed] [Google Scholar]
- Akagi S., Matsukawa K., Takahashi S. Factors affecting the development of somatic cell nuclear transfer embryos in cattle. J. Reprod. Dev. 2014;60:329–335. doi: 10.1262/jrd.2014-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhimaidi A., Algady M., Iwamoto D., Almutary M., Alfuraje M., Alzeer F., Barakat I., Kandeel S., Iritani A. The enhancement of Scripted in the development of cloned sheep embryos. Int. J. Sci. Res. 2014;3(12):538–541. [Google Scholar]
- Al-Mutary M., Al-Ghadi M., Al-himaidi A., Iwamoto D., Al-anazi Y., Ammari A., Ahmad J., Al-Khedhairy A. Using RT-PCR and glutathione level to study the effect of follicular fluid on in vitromaturation and gene expression of sheep oocytes. Saudi J. Biolog. Sci. 2019;26(6):1216–1222. doi: 10.1016/j.sjbs.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma R., Miyamoto K., Oikawa M., Yamada M., Anzai M. Combinational treatment of trichostatin a and vitamin C improves the efficiency of cloning mice by somatic cell nuclear transfer. JoVE (J. Visual. Exper.). 2018;26 doi: 10.3791/57036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgstaller J.P., Brem G. Aging of cloned animals: A mini-review. Gerontology. 2017;63:417–425. doi: 10.1159/000452444. [DOI] [PubMed] [Google Scholar]
- Czernik M., Anzalone D.A., Palazzese L., Oikawa M., Loi P. Somatic cell nuclear transfer: failures, successes, and the challenges ahead. Int. J. Dev. Biol. 2019;63:123–130. doi: 10.1387/ijdb.180324mc. [DOI] [PubMed] [Google Scholar]
- De Bem T.H., da Silveira J.C., Sampaio R.V., Sangalli J.R., Oliveira M.L., Ferreira R.M. Low levels of exosomal-miRNAs in maternal blood are associated with early pregnancy loss in cloned cattle. Sci. Rep. 2017;7:14319. doi: 10.1038/s41598-017-14616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir K., Pabuccuoğlu S., Cirit Ü., Evecen M., Karaman E., ÖzdaşÖb Effects of serum starvation and ionomycin activation on the development of somatic cell nuclear transfer embryos in sheep. Vet. FakDerg. 2019;66:37–42. doi: 10.1501/vetfak_0000002885. [DOI] [Google Scholar]
- Ding X., Wang Y., Zhang D., Guo Z., Zhang Y. Increased pre-implantation development of cloned bovine embryos treated with 5-aza-2′-deoxycytidine and trichostatin A. Theriogenology. 2008;70:622–630. doi: 10.1016/j.theriogenology.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Evecen M., Pabuccuoğlu S., DEMiR K., Yağcıoğlu S., Can A., Ertürk E. Somatic Cloning in Cats Using MI or MII Oocytes. KafkasUniv Vet FakDerg. 2016;6:923–928. [Google Scholar]
- FAO. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture, 2018. (B.D. Scherf, D. Pilling, eds.). FAO Commission on Genetic Resources for Food and Agriculture Assessments. Rome, Italy.
- Gupta, A., Chaudhary, M., Goyal, R.K., Yadav, V., Chandra, S., Sinha, S., 2018. Recent advances in reproductive biotechnologies in small ruminants.
- Hildebrandt T.B., Hermes R., Colleoni S., Diecke S., Holtze S., Renfree M.B. Embryos and embryonic stem cells from the white rhinoceros. Nat. Commun. 2018;9:2589. doi: 10.1038/s41467-018-04959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ni W., Chen C., Sai W., Hazi W., He Z. Comparison between the effects of valproic acid and trichostatin A on in vitro development of sheep somatic cell nuclear transfer embryos. J. Anim. Vet. Adv. 2012;11:1868–1872. doi: 10.3923/javaa.2012.1868.1872. [DOI] [Google Scholar]
- Lee J.H., Chun J.L., Lee J.H., Kim K.J., Kim E.Y., Han K.W. Improvement of porcine scnt embryo development using histone deacetylase inhibitors. J. Faculty Agric., Kyushu Univ. 2016;61:115–120. [Google Scholar]
- Maiorka P.C., Favaron P.O., Mess A.M., dos Santos C.R., Alberto M.L., Meirelles F.V. Vascular alterations underlie developmental problems manifested in cloned cattle before or after birth. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0106663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Ibarra J.L., Espinoza-Mendoza E.A., Rangel-Santos R., Ambriz-García D.A., Navarro-Maldonado M.D. Effect of resveratrol on the in vitro maturation of ovine (Ovisaries) oocytes and the subsequent development of handmade cloned embryos. Veterinaria México. 2019;5:1–4. doi: 10.22201/fmvz.24486760e.2018.4.491. [DOI] [Google Scholar]
- Meng Q., Polgar Z., Liu J., Dinnyes A. Live birth of somatic cell-cloned rabbits following trichostatin A treatment and cotransfer of parthenogenetic embryos. Cloning Stem Cells. 2009;11:203–208. doi: 10.1089/clo.2008.0072. [DOI] [PubMed] [Google Scholar]
- Mishra A., Reddy I.J., Gupta P.S.P., Mondal S. L- carnitine mediated reduction in oxidative stress and alteration in transcript level of antioxidant enzymes in sheep embryos produced in vitro. Reprod. Domest. Anim. 2016;51:311–321. doi: 10.1111/rda.12682. [DOI] [PubMed] [Google Scholar]
- Mizutani E., Oikawa M., Kassai H., Inoue K., Shiura H., Hirasawa R. Generation of cloned mice from adult neurons by direct nuclear transfer. Biol. Reprod. 2015;92:81–91. doi: 10.1095/biolreprod.114.123455. [DOI] [PubMed] [Google Scholar]
- Ogorevc J., Orehek S., Dovč P. Cellular reprogramming in farm animals: an overview of iPSC generation in the mammalian farm animal species. J. Anim.Sci.Biotechnol. 2016;7:10. doi: 10.1186/s40104-016-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C.S., Oliveira L.Z., Saraiva N.Z., Monteiro F.M., Garcia J.M. Efeitos da tricostatina A sobre a acetilação de histonas, proliferaçãocelular e diferenciação de célulastroncoembrionáriasmurinas. Acta Sci. Vet. 2011;39:1–9. [Google Scholar]
- Saragusty J., Diecke S., Drukker M., Durrant B., Friedrich Ben-Nun I., Galli C. Rewinding the process of mammalian extinction. Zoo Biol. 2016;35:280–292. doi: 10.1002/zoo.21284. [DOI] [PubMed] [Google Scholar]
- Sheets T., Park C.H., Park K.E., Powell A., Donovan D., Telugu B. Somatic cell nuclear transfer followed by CRIPSR/Cas9 microinjection results in highly efficient genome editing in cloned pigs. Int. J. Mol. Sci. 2016;17:2031. doi: 10.3390/ijms17122031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriboon C., Li T.S., Yu C.W., Chern J.W., Ju J.C. Novel histone deacetylase inhibitors and embryo aggregation enhance cloned embryo development and ES cell derivation in pigs. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0204588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srirattana K., Ketudat-Cairns M., Nagai T., Kaneda M., Parnpai R. Effects of trichostatin A on In vitro development and DNA methylation level of the satellite I region of swamp buffalo (Bubalusbubalis) cloned embryos. J. Reprod. Dev. 2014;60:336–341. doi: 10.1262/jrd.2013-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Proudfoot C., Lillico S.G., Whitelaw C.B. Gene targeting, genome editing: from Dolly to editors. Transgenic Res. 2016;25:273–287. doi: 10.1007/s11248-016-9932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Cui W., Meng C., Zhang J., Li Y., Qian Y. MC1568 enhances histone acetylation during oocyte meiosis and improves the development of somatic cell nuclear transfer embryos in pig. Cell Reprogram. 2018;20:55–65. doi: 10.1089/cell.2017.0023. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Nagai T. Survival of embryos and calves derived from somatic cell nuclear transfer in cattle: a nationwide survey in Japan. Anim. Sci. J. 2011;82:360–365. doi: 10.1111/j.1740-0929.2010.00846.x. [DOI] [PubMed] [Google Scholar]
- Wen B.Q., Li J., Li J.J., Tian S.J., Sun S.C., Qi X. The histone deacetylase inhibitor Scriptaid improves in vitro developmental competence of ovine somatic cell nuclear transferred embryos. Theriogenology. 2014;81:332–339. doi: 10.1016/j.theriogenology.2013.09.032. [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke A.E., McWhir J., Kind A.J., Campbell K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Wu X., Li Y., Li G.P., Yang D., Yue Y., Wang L. Trichostatin A improved epigenetic modifications of transfected cells but did not improve subsequent cloned embryo development. AnimBiotechnol. 2008;19:211–224. doi: 10.1038/cr.2008.234. [DOI] [PubMed] [Google Scholar]
- Xu W., Li Z., Yu B., He X., Shi J., Zhou R. Effects of DNMT1 and HDAC inhibitors on gene-specific methylation reprogramming during porcine somatic cell nuclear transfer. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0064705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Sui T., Chen M., Deng J., Huang Y., Zeng J. CRISPR/Cas9-mediated GJA8 knockout in rabbits recapitulates human congenital cataracts. Sci. Rep. 2016;6:22024. doi: 10.1038/srep22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng N., Wang H., Xu Y., Wu Y., Wu M. Effect of histone deacetylase inhibitor, trichostatin A, on cartilage regeneration from free perichondrial grafts in rabbits. Trop. J. Pharm. Res. 2017;16:1253–1257. doi: 10.4314/tjpr.v16i6.7. [DOI] [Google Scholar]