Abstract
Honey has been widely used to treat several human pathogens. However, all honeys may not have equal potencies against different human pathogens. The purpose of the current work was to investigate the physico-chemical and antimicrobial qualities of some mono-floral honeys from Ethiopia against some human pathogen bacteria and fungi. In the study, seven different botanical origin honeys were used of which some were from plants known for their medicinal properties. The samples were tested for their major physico-chemical properties (sugar profiles, total free acids, pH, color, electric conductivity and total soluble substances) and their medicinal values as total antioxidant capacity, total phenolic content and antimicrobial properties as minimum inhibitory concentration against some human pathogens, following standard protocols. Generally, the average values of the physico-chemical properties of the samples were within the acceptable ranges of world honey quality values. The average total antioxidant value of the samples was 320.3 ± 15.1 with range of 225.4 ± 12.8–465.7 ± 21.8 μM Fe(II)/100g. Relatively higher values 421.5 ± 23.4 and 465.7 ± 21.8μM Fe(II)/100g recorded for Croton macrostachyus and Vernonia amygalina honeys respectively. The average phenolic contents of the samples varied from 233.3 ± 24.0 to 693.3 ± 26.8 mgGAE/kg and relatively higher values recorded for C. macrostachys and V. amygdalina honeys. The significant proportion of the tested samples showed strong antimicrobial qualities inhibiting the growth of tested pathogens at concentration of 10.5%–28.6% of MIC (% v/v). Honeys from medicinal plants (C. macrostachys and V. amygdalina) relatively showed more antimicrobial properties which could be due to the presence of plant specific phytochemicals which require further investigations.
Keywords: Medicinal plants, Antimicrobial properties, Mono-floral honeys, Antioxidant, Phenolic compounds
1. Introduction
Despites the general similarities in major physico-chemical properties of different honeys; there are no two identical honeys, unless both harvested from the same hive at the same time which indicates the very diverse nature of honeys in their composition and properties. In this regard, honey has been extensively analyzed in terms of its physico-chemical properties, geographical and botanical origins and reported to have significant variations among different honeys (Wang et al., 2009, Alvarez-Suarez et al., 2009, Gomes et al., 2010, Ansari et al., 2018, Kumar et al., 2018, Al-Ghamdi et al., 2019). The harvesting season and associated climatic conditions and postharvest handling techniques contribute for its variations (Alvarez-Suarez et al., 2010b).
Besides its dietary importance, honey has been taken as an essential ingredient of folk medicine and utilized against microbial infections, various diseases and ailments dates back to ancient times (Molan, 1992a, Molan, 1992b, Molan, 2006). Moreover, honey has been assessed for its antimicrobial action (wound healing, and infected surgical wounds), anti-inflammatory property (burns, skin grafting, etc.), anti-oxidant properties and surgical debridement and has been reported for its high therapeutic potentials (Al-Waili, 2004, Van-den Berg et al., 2008, Noori et al., 2012, Ansari et al., 2013, Noori et al., 2013). Moreover, several studies supported the medicinal values of different honeys and the presence of significant variations in their medicinal properties (Molan, 1992a, b; Lusby et al., 2005, Saxena et al., 2010).
The variations in the antimicrobial potencies among the different honeys reported to be more than 100-folds, which is attributed to their botanical and geographical origins (Molan and Cooper, 2000). Such variations in the properties of honeys could be responsible for the broadly varying capabilities of honeys to serve as antioxidants (Lusby et al., 2005; French et al., 2005; Mullai & Menon 2007). The antimicrobial property of honey is mainly attributed to osmolality (sugar), pH, hydrogen peroxide levels and the methylglyoxal (Mavric et al., 2008, Alvarez-Suarez et al., 2010a). Moreover, the honey’s non-peroxide aspects such as: lysozyme, some phytochemicals, phenolic acids and flavonoids are reported to play important role in its antimicrobial properties (Al et al., 2009). Particularly, the presence of phenolic compounds in honey, their anti-oxidant properties and their positive health role have been well documented (Gheldof and Engeseth, 2002, Nicholls and Miraglio, 2003). Moreover, the presence of differences in the amount of phenolic compounds based on its botanical and geographical origins have been also reported (Özkök et al. 2010).
Honeys composition is variable as they vary not only in their physico-chemical properties but also in their biological activities (Khalil et al., 2001). The difference in degrees of antimicrobial properties among different honeys is directly related to their floral source and geographical origins of the floral sources (Mavric et al., 2008, Molan, 1992a, Molan, 1992b). Moreover, the quality of honey is also utmost importance in determining the antimicrobial potential of honey because honey that has been mishandled (overheated or adulterated) may loss its medicinal properties. In addition, some of the physical properties (acidity, phenolic content and antioxidant level) of honey have direct relationships with its antimicrobial properties which are important to assess and correlate the physical properties and medicinal values of honeys.
In the very past and present days’ folk medicines, selection of specific plant origin honeys for specific therapeutic purposes are widely used (Molan, 1992), indicating all honeys may not have equal therapeutic values. In this regard, Iirish et al. (2011) reported that out of 477 honey samples tested only 57% of them have antimicrobial activities, and exceptionally strong antimicrobial properties observed only in honeys derived from three bee plants. The composition of active ingredients in plants depends on different factors, mainly plant bio and chemo-type and environmental situations (Alvarez-Suarez et al., 2010b).
Today the occurrence of antibiotic-resistant pathogens poses a severe risk to public health, thus a high demand for alternative to antibiotics and conventional therapies are increasing more than ever (AL-Waili, et al., 2013). This generally shows the importance of screening of different floral origin honeys and identify the one with more antimicrobial potencies. The purpose of this study was to examine the physico-chemical and antimicrobial properties of some Ethiopian mono-floral honeys against selected drug resistant human pathogen bacteria and fungi isolates using broth microdilution assay. Some of the tested mono-floral honeys were from plants those have been reported for their medicinal properties.
2. Materials and methods
2.1. Honey samples and their classification
Honey samples were collected from farm gates targeting the harvesting periods of the specific mono-floral honeys from various apiaries in the different regions of Ethiopia. In the study, six mono- and one poly floral honey samples were collected. For each honey type, six independent samples were collected. The samples were kept in sterile screw capped glass jars and stored in refrigerator below 5 °C until analysis.
2.2. Melissopalynological analysis
The botanical origins of the honey samples were authenticated based on the analysis of the honey pollen grains following the methodology of Louveaux et al. (1978). The identification of the pollen grains was made based on the pollen reference slides of honey plants of the target areas and also related publication (Atlas of pollen grains of major honey source plants) (Nuru, 2007). In addition to the pollen analysis, sensorial and physico-chemical properties were applied to differentiate the honey types. Moreover, the honey samples were collected from the farm gates considering the harvesting season and geographical regions of the targeted honey samples.
2.3. Physico-chemical analysis
The major compositions of honey such as: sugar profiles, total minerals (ash) content, acidity (total free acid) as meq.acid/kg of honey, pH and electric conductivity were determined following the International Honey Commission (2009) protocols. Every sample was tested in triplicate for every parameter and their average values were taken.
2.3.1. Sugar profile analysis
The major honey sugars (fructose, glucose and sucrose) contents were investigated using HPLC following International Honey Commission, (2009) protocols. Standard sugars (fructose, glucose, sucrose) were obtained from Sigma chemicals (Germany). Before the analysis, the appropriateness of the methods, validation parameters (average recover values, limits of detection, quantification and the linearity response index) were confirmed.
2.3.2. Total free acid
The total free acid in the honeys was determined based on the following protocols: 10 g of honey sample was dissolved in 75 ml of carbon dioxide-free water in a 250 ml beaker. The solution was stirred with the magnetic stirrer. The honey solution was titrated with 0.1 M NaOH to pH 8.30, using Hanna® pH measuring device. The result was expressed as free acidity, (Milliequivalents of acid/kg honey = ml of 0.1 M NaOH × 10).
2.3.3. PH determination
The honey pH was determined by dissolving the samples in Milli-Q water (10% w/v) and measuring its pH using 3510 pH meter (Jenway® Instrument UK).
2.3.4. Electrical conductivity (EC)
For EC test, a 20% (w/v) honey water solution was prepared using Milli-Q water. EC was determined by a conductivity meter (HI98311 Hanna Instruments, Mauritius). The result was presented as mS/cm.
2.3.5. Color
The honey samples colour was determined using digital honey colorimeter (Portable Photometer, Hanna®, Instrument USA). Before the analysis the debris and air bubbles were removed. The results were expressed in mm P-fund (0–150 mm).
2.3.6. Total soluble substance (TSS)
The TSS as oBrix was determined through measuring, the total soluble substances in the honeys using a refractometer (Atago, No. 3840, Japan). During testing some honey drops were smeared on the prism of the refractometer and a reading was made directly from the scale. For the values above or below the standard temperature of 20 °C were corrected through adding or subtracting 0.00023/0C as correction factor. The results were expressed in oBrix as total soluble substance (%) = 100 – % moisture content).
2.3.7. Total antioxidant capacity (TAC) determination
Total antioxidant capacity of the honey samples was investigated chemically applying FRAP (Ferric Reducing Antioxidant Power) assay following Benzie and Strain, 1996, Alvarez-Suarez et al., 2010b protocols.
2.3.8. Total phenolic content
The total phenolic contents of the honey samples were investigated based on Folin-Ciocalteau reactive, following the Singleton et al., 1999, Alvarez-Suarez et al., 2010a, Alvarez-Suarez et al., 2010b protocols. For each sample 5 g of liquid honey was dissolved in 50 ml of distilled water and the solution was filtered using Whatman No. 1 filter paper (0.5 ml). Then the honey solution was added to 2.5 ml of 0.2 N Folin-Ciocalteau reagent and mixed for 5 min then 2 ml 0.7 M Sodium carbonate (Na2CO3) was added. The solution was incubated in dark at 25 °C for 2 h. The absorbance of the reaction mixture was determined at 760 nm using Agilent Cary 60 UV–Vis (© Agilent Technologies, Inc). The calibration curve was determined using standard Gallic acid following Özkök et al. (2010) protocol. Results were expressed as mg Gallic Acid Equivalents/Kg of honey (mgGAE/Kg of honey).
2.4. Antimicrobial assays
2.4.1. Pathogenic isolates
Cultures of various human pathogenic strains (isolated from human specimens) which are reported to be resistance to common antibiotics were obtained from Department of Microbiology, College of Pharmacy, King Saud University, Riyadh. The pathogen strains include: Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiella pneumonia, Salmonella typhimurum, Micrococcus luteus, Staphylococcus epidermidis, Bacillus cereus, Aspergillus nidulans, and Enterobacter aerogene. The isolates were identified by the standard bacteriological techniques.
2.4.2. Determination of minimum inhibitory concentration (MIC)
The antimicrobial activities and Minimum Inhibitory Concentration (MIC) of the honey samples were determined against some gram negative bacteria (Escherichia coli, Salmonella typhimurum, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterobactor aerogenes), gram positive bacteria (Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermis, Bacillus cereus, Micrococcus luteus) and some common human fungal pathogens (Candida albicans, Candida tropicans and Aspergillus Nodules). The antimicrobial activities and minimum inhibitory concentration were determined following Franch et al. (2005) & Alvarez-Suarez et al. (2010b) protocols with some modifications. A dilution series with honey concentrations that ranges from 5 to 50% v/v with an increment of 5% were added aseptically into sterile test tubes. The required honey concentration with nutrient broth solutions were mixed by stirring with small vortexing. Specimen of each microorganism was taken from pure culture grown in 10 ml nutrient broth. These specimens were cultured in broth containing different concentrations of individual honey by using a standard loop (10 μl). Control plates of nutrient without honey were included in each susceptibility assay to check the viability and density of the bacterial cells.
The cultures were incubated at 37 °C for 24 h. Then a loopful (10 μl) of the cultures of each of the specimens of microorganisms was streaked onto agar plates. The streaked plates were incubated aerobically at 37 °C and inspected after 24 h to measure MIC. Then the growth of the bacterial cells was assessed as partial or total inhibition and recorded for each strain. The minimum inhibitory concentration was considered to be the lowest honey concentration at which bacterial growth was totally inhibited. The testing was repeated three times for each strain at each concentration level. The average value for the minimum inhibitory concentration was taken from the three replicates for each strain for each concentration level.
3. Results and discussion
3.1. Pollen analysis
In general, all the six honey samples were classified as mono-floral honeys based on consisting of a greater proportion of pollen grains counts from the respective species (Table 1) and also supported with sensorial and physic-chemical properties and conformity in its harvesting season and geographical origins.
Table 1.
Melissopalynologicala analysis of the honey samples.
| Floral origin | Quantitative melissopalynological result of the honey samples |
|---|---|
| Schefflera abyssinica | S. abyssinica honey samples were characterized by the low pollen representation, that range between 25 and 30% with the presence of other species pollen also. But the honey samples were authenticated based on sensorial and physico-chemical analysis and its distinctive harvesting season and regions. |
| Eucalyptus globules | Eucalyptus globules honey samples were characterized by relatively high pollen content accounting for ≥ 45% of the typical pollen grains of the respective species. |
| Guizotia scabra | G. scabra honey samples were characterized by predominant ≥ 45% content of the typical G. scabra pollen grains. |
| Vernonia amygdalina | V. amygdalina honey samples were characterized by low pollen representation, that ranges between 25 and 30% of the V. amygdalina pollen grains with the presence of other species pollen. |
| Croton macrostachyus | C. macrostachyus honey samples were characterized by low pollen representation between 25 and 30% with the presence of other species pollen, but the honey samples were authenticated based on sensorial and physicochemical analysis, |
| Becium grandiflorum | B. grandiflorum honey samples were characterized by under representation (≤ 25%) of its pollen, because of the flower morphology (long filament that keeps the dehiscence of anther faraway from its nectar location, which limits the direct contamination of the nectar by its pollen. The honey samples were further confirmed based on sensorial and physico-chemical analysis and its distinctive harvesting season and geographical origins. |
| Multi-floral honey | The multi-floral honeys were characterized by the presence of very diverse pollen grains from various cultivated oil crops pulses and annual weeds. |
*The mono-floral honeys considered in this study, besides their pollen analysis, distinctiveness in geographical location and harvesting seasons, it has been easily differentiated by experts familiar with the honeys, by assessing their peculiar tastes, aroma, color, structure of their crystals.
3.2. Physico-chemical analysis
The mean and standard deviations of the physico-chemical properties of the honey samples are shown in Table 2. The average major sugars: sucrose, glucose and fructose contents of the honey samples were 3.7 ± 0.9, 30.3 ± 2.5 & 37.1 ± 2.6 respectively and the results are closer to the reports of Ouchemoukh et al., 2010, Nuru et al., 2017. The mean of EC, acidity, mineral (ash) content and pH values of the honey samples were 0.5 ± 0.14, 27.0 ± 3.07, 0.4 ± 0.15 & 3.8 ± 0.25 respectively and the results are within the values reported by Saxena et al., 2010, Feás et al., 2010.
Table 2.
The mean and standard deviation of physicochemical and antioxidant properties of the tested honey samples (n = 6).
| Botanical sources of the samples | Sucrose % | Glucose % | Fructose % | TSS | EC (mS/cm) | Acidity meq/kg | ash % | PH | Color mm | FRAP (μM Fe(II)/100 g of honey) |
|---|---|---|---|---|---|---|---|---|---|---|
| Schefflera abyssinica | 2.0 ± 0.5a | 29.5 ± 2.2a | 36.5 ± 3.2b | 79.5 ± 3.3a | 0.3 ± 0.1a | 19.9 ± 2.3 | 0.2 ± 0.1a | 3.7 ± 0.3b | 13.5 ± 2.1b | 285.6 ± 10.2b |
| Eucalyptus globules | 3.1 ± 1.1b | 30.5 ± 1.9b | 35.2 ± 3.5a | 82.2 ± 2.5b | 0.4 ± 0.1a | 20.2 ± 2.5 | 0.4 ± 0.2b | 3.4 ± 0.2a | 20.1 ± 2.4c | 310.9 ± 17.5b |
| Guizotia scabra | 4.5 ± 0.8c | 28.6 ± 3.4a | 37.0 ± 2.6b | 81.9 ± 1.8b | 0.6 ± 0.2b | 28.9 ± 3.6 | 0.5 ± 0.2b | 4.2 ± 0.3c | 50.8 ± 3.5d | 225.4 ± 12.8a |
| Vernonia amygdalina | 4.4 ± 0.5c | 29.0 ± 2.5a | 39.5 ± 1.1c | 77.1 ± 4.2a | 0.8 ± 0.2b | 39.8 ± 4.2 | 0.3 ± 0.1a | 4.1 ± 0.1c | 115.5 ± 7.2f | 465.7 ± 21.8c |
| Croton macrostachyus | 2.3 ± 1.5a | 31.4 ± 1.6b | 38.8 ± 2.4c | 78.2 ± 2.9a | 0.5 ± 0.1b | 37.0 ± 4.1 | 0.3 ± 0.1a | 3.5 ± 0.2a | 93.3 ± 5.3e | 421.5 ± 23.4c |
| Becium grandiflorum | 4.4 ± 1.3c | 33.6 ± 2.9b | 37.0 ± 2.3b | 84.0 ± 3.1b | 0.3 ± 0.1a | 12.4 ± 1.3 | 0.4 ± 0.2b | 3.7 ± 0.3b | 6.1 ± 1.3a | 295.7 ± 8.6b |
| Multi-floral honey | 4.2 ± 0.7c | 29.4 ± 3.1a | 35.6 ± 3.4a | 81.7 ± 2.4b | 0.6 ± 0.2b | 31.0 ± 3.5 | 0.5 ± 0.2b | 4.1 ± 0.4c | 52.6 ± 4.0d | 237.1 ± 11.7a |
| Mean | 3.7 ± 0.9 | 30.3 ± 2.5 | 37.1 ± 2.6 | 80.7 ± 2.9 | 0.5 ± 0.14 | 27.0 ± 3.1 | 0.4 ± 0.15 | 3.8 ± 0.25 | 50.3 ± 3.7 | 320.3 ± 15.1 |
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.001 | < 0.01 | < 0.01 | < 0.0001 | < 0.0001 |
The average colors of the honey samples were varying from 6.1 ± 1.3 mm to 115.5 ± 7.2 mm P-fund scale. Relatively, darker color with high P-fund value of 115.5 ± 7.2 mm and 93.3 ± 5.3 mm recorded for Vernonia amygalina and Croton macrostachys honeys respectively while very light color with P-fund value of 6.1 ± 1.3 mm and 13.5 ± 2.1 mm recorded for Becium grandiflorum and Schefflera abyssinica honey samples respectively (Table 2). The significant variations in physico-chemical properties the different floral origin honeys (Table 2) could be due to the variations in their botanical and geographical origins of the samples. Generally, the mean values of the physico-chemical properties of the honey samples were within the acceptable ranges of honey quality standards set by Codex., 2001, E. C., 2001.
3.3. Antioxidant
The average total antioxidant capacities of the honey samples as Ferric Reducing Antioxidant Power (FRAP) were varying 225.4 ± 12.8–465.7 ± 21.8 FRAP (μM Fe(II)/100 g with a mean of 320.3 ± 15.1 μM Fe(II)/100 g (Table 2). Relatively lower FRAP values of 225.4 ± 12.8 & 237.1 ± 11.7 μM Fe(II)/100 g recorded for Guizotia scabra and multi-floral honeys while relatively higher FRAP values of 421.5 ± 23.4 and 465.7 ± 21.8 μM Fe(II)/100 g recorded for C. macrostachyus and V. amygalina honeys respectively. Generally, all the honey samples have possessed strong antioxidant properties and the values were closer or within the values reported by Alvarez-Suarez et al., 2010b, Bundit et al., 2016 for Cuban and Thai honeys respectively. The antioxidant capacities of the V. amygalina and C. macrostachyus honeys were within the ranges of the antioxidant values reported for Manuka honey (Bundit et al., 2016). The differences in antioxidant properties of the honey samples could be due to the variations in phytochemicals of the respective plants and their geographical origins. The presence of variations in antioxidant properties of different honeys as a result of their floral sources, seasonal and environmental factors are well reported (Estevinho et al., 2008, Jantakee and Tragoolpua, 2015).
3.4. Phenolic contents
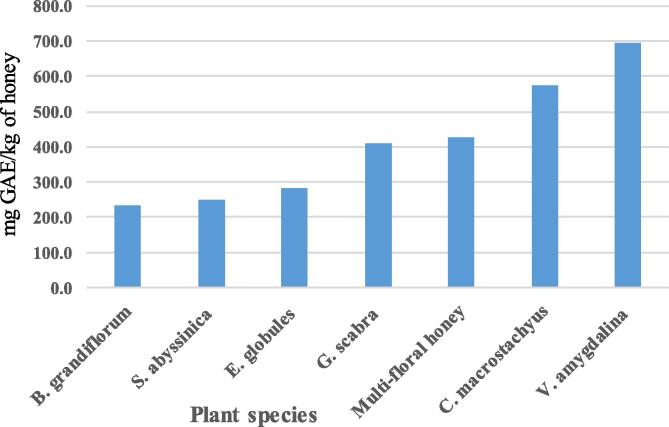
In general, the total phenolic contents of the honey samples obtained in this study were high and varied from 233.3 ± 24.0 mg GAE/kg to 693.3 ± 26.8 mg GAE/kg (Fig. 1). Relatively higher phenolic contents of 574.2 ± 40.8 mg GAE/kg and 693.3 ± 26.8 mg GAE/kg were recorded for C. macrostachys and V. amygdalina honeys respectively. The phenolic content obtained in this study were closer or within the reported ranges of phenolics (325.9–1147.5 mg GAE/kg of honey) for Burkina Fasan’s honeys (Meda et al. 2005); 213.9–595.8 mg GAE/kg of Cuban honeys (Alvarez-Suarez et al., 2010a, Alvarez-Suarez et al., 2010b) and 250–548 mg GAE/kg for some Brazilian honeys (Potis et al., 2014). The darker honeys from V. amgdalina and C. macrostachys have higher phenolic contents than lighter color honeys. It was also obtained strong correlation (r2 = 0.925, P < 0.0001) between phenolic contents and the color values of the honey samples. Similarly, the presence of higher phenolic contents of darker color honeys than lighter honeys and their strong correlations are well documented Alvarez-Suarez et al., 2010a, Alvarez-Suarez et al., 2010b for Cuban honeys and Isla et al. (2011) for Argentina honeys.
Fig. 1.
The average phenolic contents (in mg GAE/kg) of different botanical origin honeys.
3.5. Microbial assay
Most of the studied honey samples showed strong antimicrobial properties in inhibiting the growth of tested human pathogens at relatively lower concentration which averagely range from 10.5 ± 0.5–28.6 ± 0.9 of MIC (% v/v) (Table 3). In regarded to the gram negative bacteria, the average MIC (% v/v) of the studied honeys samples averagely varied from 14.0 ± 0.7–25.5 ± 1.1% v/v (Table 3).
Table 3.
The antimicrobial property of different mono-floral honeys as Minimum Inhibitory Concentration (MIC) that necessary to inhibit 100% of the microbial growth in vitro expressed as mean and standard deviation of v/v % solution (n = 7).
| Pathogenic strains | Schefflera Abyssinica | Eucalyptus globulus | Guizotia scabra | Vernonia amygdalina | Croton macrostachyus | Becium grandiflorum | Multi-floral |
|---|---|---|---|---|---|---|---|
| Gram (─) bacteria | |||||||
| Escherichia coli | 25.5 ± 0.5 | 20.5 ± 0.9 | 25.2 ± 0.8 | 15.5 ± 0.3 | 17.5 ± 0.8 | 21.5 ± 0.9 | 25.2 ± 0.3 |
| Salmonella typhimurum | 24.6 ± 0.5 | 18.5 ± 0.5 | 23.4 ± 0.5 | 15.2 ± 0.2 | 10.5 ± 0.5 | 22.5 ± 1.0 | 24.4 ± 0.5 |
| Pseudomonas aeruginosa | 24.5 ± 1.0 | 18.5 ± 0.6 | 26.2 ± 0.6 | 14.5 ± 1.0 | 18.5 ± 0.5 | 20.5 ± 1.0 | 23.5 ± 1.0 |
| Klebsiella pneumonia | 24.5 ± 1.0 | 20.5 ± 1.0 | 24.5 ± 0.7 | 10.5 ± 0.6 | 17.5 ± 0.6 | 20.5 ± 1.0 | 25.5 ± 1.1 |
| Enterobactor aerogenes | 23.5 ± 0.6 | 22.2 ± 0.6 | 23.2 ± 1.3 | 14.5 ± 1.3 | 17.5 ± 1.0 | 20.5 ± 0.8 | 24.4 ± 1.1 |
| Group mean | 24.5 ± 0.72 | 20.0 ± 0.7 | 24.5 ± 0.8 | 14.0 ± 0.7 | 16.3 ± 0.68 | 21.1 ± 0.9 | 24.6 ± 0.8 |
| Gram (+) bacteria | |||||||
| Bacillus subtilis | 26.5 ± 1.3 | 20.5 ± 1.0 | 27.5 ± 1.0 | 16.5 ± 0.5 | 18.5 ± 0.9 | 24.5 ± 0.9 | 25.5 ± 0.7 |
| Staphylococcus aureus | 25.5 ± 0.9 | 22.5 ± 0.7 | 25.5 ± 1.0 | 15.5 ± 1.0 | 10.5 ± 0.9 | 23.5 ± 0.6 | 26.4 ± 0.4 |
| Staphylococcus epidermis | 25.5 ± 0.7 | 20.5 ± 1.1 | 24.5 ± 0.9 | 10.5 ± 0.5 | 18.5 ± 0.5 | 20.5 ± 1.2 | 25.6 ± 0.9 |
| Bacillus cereus | 27.5 ± 0.7 | 24.5 ± 1.0 | 26.5 ± 0.3 | 16.5 ± 0.6 | 17.5 ± 1.0 | 22.5 ± 0.6 | 26.5 ± 0.7 |
| Micrococcus luteus | 25.5 ± 1.0 | 24.5 ± 0.7 | 25.5 ± 1.0 | 15.5 ± 1.0 | 18.5 ± 1.0 | 21.5 ± 0.5 | 24.4 ± 1.2 |
| Group mean | 26.1 ± 0.92 | 22.5 ± 0.9 | 25.9 ± 0.84 | 14.9 ± 0.72 | 16.7 ± 0.86 | 22.5 ± 0.76 | 25.7 ± 0.78 |
| Fungal pathogens | |||||||
| Candida albicans | 28.6 ± 0.9 | 25.5 ± 0.1 | 26.5 ± 1.1 | 18.5 ± 0.9 | 20.0 ± 0.4 | 25.5 ± 0.5 | 26.5 ± 1.0 |
| Candida tropicans | 27.5 ± 1.0 | 24.0 ± 0.6 | 25.5 ± 0.2 | 16.5 ± 0.1 | 20.5 ± 1.0 | 26.5 ± 0.3 | 27.5 ± 0.1 |
| Aspergillus Nodules | 27.5 ± 0.3 | 22.5 ± 0.1 | 26.5 ± 0.1 | 15.5 ± 0.1 | 20.0 ± 0.5 | 23.5 ± 0.7 | 26.5 ± 1.1 |
| Group mean | 27.9 ± 0.73 | 24.0 ± 0.27 | 26.2 ± 0.47 | 16.8 ± 0.37 | 20.2 ± 0.63 | 25.2 ± 0.5 | 26.8 ± 0.73 |
However, specific honeys from V. amygdalina, C. macrostachyes, E. globules and B. grandiflorum have showed relatively stronger antimicrobial properties in inhibiting the growth of the tested gram negative bacteria at an averagely of 14.0 ± 0.7, 16.3 ± 0.68, 20.0 ± 0.7 and 21.1 ± 0.9 MIC (% v/v) respectively. Among the gram negative bacteria, Salmonella typhimurum and Klebsiella pneumonia become more susceptible to C. macrostachyes and V. amygdalina honeys at 10.5 ± 0.5 and 10.5 ± 0.6 MIC (% v/v) respectively.
Moreover, the same V. amygdalina, C. macrostachyes, Eucalyptus globules and B. grandiflorum honeys have showed relatively strong antimicrobial properties in inhibiting the growth of the tested gram positive bacteria at an average of 14.9 ± 0.72, 16.7 ± 0.86, 22.5 ± 0.9 & 22.5 ± 0.76 MIC (% v/v) respectively. Among the gram positive bacteria, Staphylococcus aureus and Staphylococcus epidermis become more susceptible to C. macrostachys and V. amygdalina, honeys at 10.5 ± 0.9 and 10.5 ± 0.5 MIC (% v/v) respectively. The MIC results of the current study were closer to the findings of Mullai and Menon (2007) who reported MIC of 11–20% v/v for isolates of Pseudomonas aeruginosa. Moreover, most of the current study results were within the MIC range of 10–25% v/v reported for the antimicrobial properties of Argentina honeys against different gram positive and gram negative bacteria [(Isla et al., 2011). However, the MIC of the current study results were relatively higher from the findings of Alvarez-Suarez et al. (2010b) who reported MIC 2.5–10.4%v/v for gram positive bacteria and 7.2–16% v/v for gram negative bacteria for Cuban mono-floral honeys. Moreover, the MIC values of the current results were higher than the report of Andargachew et al. (2004) who recorded MIC (6.25% and 7.5% v/v) activities of honeys against some human pathogens: P. aeruginosa, E. coli and S. aureus respectively. Moreover, Sherlock et al. (2010) reported MIC of 6.3% and 12.5% v/v for Ulmo (Chile) and Manuka honeys respectively. The variations among results could be due to the qualities, freshness and the botanical and geographical sources of the honeys. The existences of large variation in the antimicrobial properties of natural honeys due to their spatial and temporal variations in sources of nectar is well documented (Saxena et al., 2010, Alvarez-Suarez et al., 2010a, Alvarez-Suarez et al., 2010b, Mandal and Mandal, 2011, Isla et al., 2011). On other hand relatively higher MIC of 32 ± 8.3% to 58 ± 13% v/v of honey was recorded against drug resistant bacteria and pathogenic fungi (AL-Waili et al., 2013).
Regarding the antifungal pathogens, V. amygdalina, C. macrostachyes and E. globules honeys showed relatively stronger potency in inhibiting the growth of the tested human fungal pathogens at an average MIC (v/v%) of 16.8 ± 0.37, 20.2 ± 0.63 and 24.0 ± 0.27 respectively. The study indicated that fungi were less susceptible than bacteria. The less susceptibility of fungi to different honey samples than bacteria were also reported (AL-Waili et al., 2013).
Generally, honeys from V. amygdalina, C. macrostachyes, E. globules and B. grandiflorum showed relatively stronger antimicrobial potencies compered to others. These could be due to the medicinal properties of the plants and the presence of some floral specific bioactive substances in their honeys. In this regard, besides the widely utilization of the different parts of the above plants in traditional medicines, the strong antimicrobial properties of the different parts of the plants have been well documented. For instance: the medicinal potentials of C. macrostachyus leaves, barks, stem and fruits for treatment of wider range of human and animal diseases and aliments (Maroyi, 2017), the strong antimicrobial properties of C. macrostachyus extracts against wide range of human pathogens (2010; Mesfin et al. 2010); the antimicrobial and the various medicinal properties of V. amygdalina leaves extracts have been well documented (Ogbulie et al., 2007, Ijeh and Ejike, 2011).
Moreover, the antimicrobial properties of different eucalyptus oils against different pathogenic micro-organisms (Gilles et al., 2010) and the significantly more effectiveness of Eucalyptus marginata honey against Candida spp are reported (Irish et al., 2006). Moreover, the wound healing and antimicrobial properties of leaf extract of B. grandiflorum are well documented (Khalid, 2017). These generally may indicate that honey obtained from medicinal plants may have more medicinal values.
The presence of significant variations in medicinal properties among the different botanical origin honeys are well established (Lusby et al., 2005, Molan, 1992a, Saxena et al., 2010). In this regard the variations in antimicrobial potencies among the different honeys reported to be more than 100-folds, which is attributed to their botanical and geographical origins (Molan and Cooper, 2000). The difference in levels of antimicrobial activities and the functional properties in human health promotion among the different honeys have been associated to their floral source, geographical location, climatic conditions and the plant bio and chemo-types (Alvarez-Suarez et al., 2010a, Alvarez-Suarez et al., 2010b, Mavric et al., 2008, Molan, 1992a, Molan, 1992b). Moreover, the more antimicrobial properties of some of the honeys could be due to their higher phenolic content and antioxidant properties. The strong relations of antimicrobial properties of honeys with their antioxidant properties and phenolic contents well discussed (Isla et al., 2011). Particularly phenolic compounds are believed to defend against diseases like cancer, cardiovascular disorders and, neurodegenerative diseases by cleaning up potentially damaging free radicals occurring in our body (Özkök et al., 2010).
In the very past and present days’ folk medicines, selection of specific plant origin honeys for specific therapeutic purposes are widely used (Molan, 1992), indicating that all honeys may not have equal therapeutic values. In this regard, Irish, et al. (2011) reported that exceptionally strong antimicrobial properties observed only in honeys derived from three out of 477 bee plants.
4. Conclusion
Based on the current research findings and the available literature supports, honeys derived from plants those have medicinal properties may have more medicinal values. Hence, more research would be important to focus on honeys from medicinal plants and to establish the possible relations between the bioactive substances in plant parts and their nectars.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research and College of Food and Agricultural Sciences Research Center, King Saud University Riyadh, for providing material supports of this research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nuru Adgaba, Email: nuruadgaba@gmail.com.
Ahmad Al-Ghamdi, Email: aalkhazim@gmail.com.
References
- Al M.L., Daniel D., Moise A., Boris O., Laslo L., Bogdanov S. Physicochemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009;112(4):863–867. [Google Scholar]
- Al-Ghamdi A., Mohammed S.E.A., Ansari M.J., Adgaba N. Comparison of physic-chemical properties and effects of heating regimes on stored Apis mellifera and Apis florea honey. Saudi J. Biol. Sci. 2019;26(4):845–848. doi: 10.1016/j.sjbs.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Suarez J.M., Tulipani S., Díaz D., Estevez Y., Romandini S., Giampieri S., Damiani E., Astolfi P., Bompadre S., Battino M. Antioxidant and antimicrobial capacity of several mono-floral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010;48(8–9):2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Alvarez-Suarez J.M., Tulipani S., Romandini S., Bertoli E., Battino M. Contribution of honey in nutrition and human health: a review. Mediterr. J. Nutr. Metab. 2010;3(1):15–23. doi: 10.1007/s12349-009-0051-6. [DOI] [Google Scholar]
- Alvarez-Suarez J.M., Tulipani S., Romandini S., Vidal A., Battino M. Methodological aspects about determination of phenolic compounds and in vitro evaluation of antioxidant capacity in the honey: A review. Curr. Anal. Chem. 2009;5(4):293–302. [Google Scholar]
- AL-Waili, N., Al-Ghamdi, A. Ansari, M.J., Al-Attal, Y., Al-Mubarak, A., Salom, K., 2013. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi, Arch. Med. Res. 44 (2013) 307–316. [DOI] [PubMed]
- Al-Waili Noori S. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J. Med. Food. 2004;7(2):210–222. doi: 10.1089/1096620041224139. [DOI] [PubMed] [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Khan K.A., Adgaba N., El-Ahmady S.H., Gad H.A., Roshan A., Meo S.A., Kolyali S. Validation of botanical origins and geographical sources of some Saudi honeys using ultraviolet spectroscopy and chemometric analysis. Saudi J. Biol. Sci. 2018;25(2):377–382. doi: 10.1016/j.sjbs.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Al-Waili N.S., Sharma D., Nuru A., Al-Attal Y. Effect of jujube honey on Candida albicans growth and biofilm formation. Arch. Med. Res. 2013;44(5):352–360. doi: 10.1016/j.arcmed.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Benzie Iris F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bundit T., Anothai T., Pattaramart P., Roongpet T., Chuleeporn S. Comparison of antioxidant contents of thai honeys to manuka honey. Malaysian J. Nutrition. 2016;22(3):413–420. [Google Scholar]
- Codex., 2001. Codex Alimentarius standard for honey 12-1981. Revised Codex standard for honey. Standards and standard methods (Vol. 11). Retrieved December, 2014, from http:// www.codexalimentarius.net.
- E. C., 2001. Council directive 2001/110/EC of 20 December 2001 relating honey. Official Journal of the European Communities 12.1.2002 L10/47-52.
- Estevinho L., Prereira A.P., Moreira L., Dias L.G., Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008;46(12):3774–3779. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Feás X., Pires J., Iglesias A., Estevinho M.L. Characterization of artisanal honey produced on the Northwest of Portugal by melissopalynological and physico-chemical data. Food Chem. Toxicol. 2010;48(2010):3462–3470. doi: 10.1016/j.fct.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Franch V.M., Cooper R.A., Molan P.C. The antibacterial activity of honey against coagulase- negative staphylococci. J. Antimicrob. Chemother. 2005;56(1):228–231. doi: 10.1093/jac/dki193. [DOI] [PubMed] [Google Scholar]
- Gheldof N., Engeseth N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food. Chem. 2002;50(10):3050–3055. doi: 10.1021/jf0114637. [DOI] [PubMed] [Google Scholar]
- Gilles M, Zhao J., An M., Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chemistry. 2010;119(2):731–737. doi: 10.1016/j.foodchem.2009.07.021. [DOI] [Google Scholar]
- Gomes S., Dias L.G., Moreira L.L., Rodrigues P., Estevinho L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010;48(2010):544–548. doi: 10.1016/j.fct.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Ijeh I.I., Ejike C.E.C.C. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J. Med. Plant Res. 2011;5(7):1051–1061. http://www.academicjournals.org/JMPR [Google Scholar]
- International Honey Commission, 2009. Harmonized methods of the International Honey Commission, pp.63. http://www.bee-hexagon.net/en/network.htm.
- Irish J., Blair S., Carter D.A. The antibacterial activity of honey derived from Australian Flora. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0018229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish, J., Carter, D.A., Shokohi, T., Blair, S.E., 2006. Honey has an antifungal effect against Candida species. Med. Mycol. 2006; 44(3), 289–91. DOI: 10.1080/13693780500417037. [DOI] [PubMed]
- Isla M.I., Craig A., Ordoñez R., Zampini C., Sayago J., Bedascarrasbure E., Alvarez A., Salomón V., Maldonado L. Physico chemical and bioactive properties of honeys from Northwestern LWT. Food Sci. Technol. 2011;44:1922–1930. doi: 10.1016/j.lwt.2011.04.003. [DOI] [Google Scholar]
- Jantakee K., Tragoolpua Y. Activities of different types of Thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals. Biol. Res. 2015;48(1):4. doi: 10.1186/0717-6287-48-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid, B., 2017. Evaluation of wound healing activity of 70% Eethanol leaf extract of Becium grandiflorum Lam. (Lamiaceae) in Mice, MSc. Thesis, School of Pharmacy, Addis Ababa, Ethiopia, P. 68.
- Khalil M.I., Motallib M.A., Anisuzzaman A.S.M., Sathi Z.S., Hye M.A., Shahjahan M. Antibacterial activities of different brands of unifloral honey available at the Northern Region of Bangladesh. J. Med. Sci. 2001;1:389–392. [Google Scholar]
- Kumar A., Gill J.P.S., Bedi J.S., Manav M., Ansari M.J., Walia G.S. Sensorial and physicochemical analysis of Indian honeys for assessment of quality and floral origins. Food Res. Int. 2018;108:571–583. doi: 10.1016/j.foodres.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Louveaux J., Maurizio A., Vorwohl G. Methods of melissopalynology. Bee World. 1978;59:139–157. doi: 10.1080/0005772X.1978.11097714. [DOI] [Google Scholar]
- Lusby P.E., Coombes A.L., Wilkinson J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res. 2005;36:464–467. doi: 10.1016/j.arcmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Mandal M.D., Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pacific J. Tropical Biomed. 2011;1(2):154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of croton macrostachyus Hochst. Ex Delile: A comprehensive review”. Evidence-Based Complementary Alternative Med. 2017;20:1–17. doi: 10.1155/2017/1694671. Article ID 1694671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavric E., Wittmann S., Barth G., Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008;52:483–489. doi: 10.1002/mnfr.200700282. [DOI] [PubMed] [Google Scholar]
- Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91(2005):571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Mesfin T., Aberra G., Asfaw D. In vitro anti-Neisseria gonorrhoeae activity of Albizia gummifera and Croton macrostachyus”. Revista Cenic Ciencias Biologicas. 2010;41(4):1–11. [Google Scholar]
- Molan P.C. The antibacterial activity of honey, (part, 1, the nature of the antibacterial activity) Bee World. 1992;73(1):5–28. [Google Scholar]
- Molan P.C. The antibacterial activity of honey, (part, 2) Variation in the potency of the antibacterial activity. Bee World. 1992;73(2):60–75. [Google Scholar]
- Molan P.C., Cooper R.A. Honey and sugar as a dressing for wounds and ulcers. Trop. Doct. 2000;30(4):249–250. doi: 10.1177/004947550003000429. [DOI] [PubMed] [Google Scholar]
- Molan P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Lower Extremity Wounds. 2006;5(1):40–54. doi: 10.1177/1534734605286014. [DOI] [PubMed] [Google Scholar]
- Mullai V., Menon T. Bactericidal activity of different types of honey against clinical and environmental isolates of Pseudomonas aeruginosa. J. Alternative Complementary Med. 2007;13(4):439–441. doi: 10.1089/acm.2007.6366. [DOI] [PubMed] [Google Scholar]
- Nicholls J., Miraglio A.M. Honey and healthy diets. Cereal Foods World. 2003;48(3):116–119. [Google Scholar]
- Noori A.L., Al Ghamdi A., Ansari M.J., Al-Attal Y., Al-Mubarak A., Salom K. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multi-resistant bacteria and pathogenic fungi. Arch. Med. Res. 2013;44(4):307–316. doi: 10.1016/j.arcmed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Noori A.L., Al-Ghamdi A., Ansari M.J., Al-Attal Y., Salom K. Synergistic effects of honey and propolis toward drug multi-resistant staphylococcus aureus, escherichia coli and candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012;9(9):793–800. doi: 10.7150/ijms.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuru A. Ethiopian Society of Animal production, Addis Ababa; Ethiopia: 2007. Atlas of pollen grains of major honeybee flora of Ethiopia; p. 152. [Google Scholar]
- Nuru A., Ahmed A.A., Awraris G., Yilma T., Abera B., Ansari M.J., Radloff S.E., Sharma D. Characterization of honeys by their botanical and geographical origins based on physico-chemical properties and chemo-metrics analysis. Food Measurement. 2017;11:1106–1117. doi: 10.1007/s11694-017-9487-4. [DOI] [Google Scholar]
- Ogbulie J.N., Ogueke C.C., Nwanebu F.C. Antibacterial properties of Uvaria chamae, Congronema latifolium, Garcinia kola, Vernonia amygdalina and Aframomium melegueta. Afr. J. Biotechnol. 2007;6(13):1549–1553. [Google Scholar]
- Ouchemoukh S., Schweitzer P., Bey M.B., Djoudad-Kadji H., Louaileche H. HPLC sugar profiles of Algerian honeys. Food Chem. 2010;121(2010):561–568. doi: 10.1016/j.foodchem.2009.12.047. [DOI] [Google Scholar]
- Özkök, A., D’arcy, B., Sorkun, K., 2010. Total phenolic acid and total flavonoid content of Turkish pine honeydew honey. J. ApiProduct ApiMedical Sci. 2 (2): 65 - 71 DOI 10.3896/IBRA.4.02.2.01.
- Saxena S., Gautam S., Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010;118(2):391–397. doi: 10.1016/j.foodchem.2009.05.001. [DOI] [Google Scholar]
- Sherlock O., Dolan A., Athman R., Power A., Gethin G., Cowman S., Hilary Humphreys H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complementary Alternative Med. 2010;10:47. doi: 10.1186/1472-6882-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Van-den Berg A.J., Van-den Worm E., Van-Ufford H.C., Halkes S.B., Hoekstra M.J., Beukelman C.J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J. Wound Care. 2008;17(4):172–178. doi: 10.12968/jowc.2008.17.4.28839. [DOI] [PubMed] [Google Scholar]
- Wang J., Kliks M., Qu W.Y., Jun S.J., Shi G.Y., Li Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI TOF MS. J. Agric. Food Chem. 2009;57(21):10081–10088. doi: 10.1021/jf902286p. [DOI] [PubMed] [Google Scholar]