Abstract
Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) is useful for pathologically diagnosing gastrointestinal stromal tumor (GIST) before surgery. However, its role in mutation analysis remains unclear. To examine the feasibility of analyzing GIST mutations using mRNA obtained with EUS-FNA, we prospectively enrolled 41 patients with subepithelial lesion from which EUS-FNA was successfully acquired tissue sample. Thirty-two, 5, and 4 subepithelial lesions were diagnosed as GISTs, schwannomas, and leiomyomas, respectively. After RNA was extracted from FNA sample, RNA was converted to cDNA. Full-length sequence of the KIT cDNA amplified via the polymerase chain reaction (PCR) was successful in 31 (96.9%) out of 32 GIST and three out of 9 non-GIST (33.3%). The KIT mutation statuses of 31 GISTs in which KIT cDNA was amplified were successfully determined through directional sequencing. Furthermore, 15 of 16 surgically excised GISTs exhibited the same mutation status in both the EUS-FNA and resected samples. In vitro experiment, the minimum number of cells required to amplify full-length of KIT cDNA from RNA was one-tenth of that required to amplify KIT exon11 gene from DNA. This study clarifies that mutation analysis using RNA obtained with EUS-FNA is feasible and reliable. Moreover, our data would support that RNA-based mutation is superior to DNA-based mutation analysis in GIST.
Abbreviations: GIST, gastrointestinal stromal tumor; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration biopsy; PCR, polymerase chain reaction; PDGFRA, platelet-derived growth factor receptor A; SEL, subepithelial lesion; ROSE, rapid on-site evaluation; GIMT, gastrointestinal mesenchymal tumor; ITT, Intention to treat analysis; PP, per protocol analysis
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors affecting the gastrointestinal tract [1]. Ninety percent of GIST possess gene mutations, such as mutations in the KIT or platelet-derived growth factor receptor A (PDGFRA) gene. In addition, 95% of GIST are known to overexpress the KIT protein, which is translated from KIT mRNA [[1], [2], [3], [4]]. Gene mutation analysis is considered to provide useful information for both diagnosing and treating GIST, including for predicting drug responses and recurrence after surgery [[5], [6], [7], [8], [9], [10]]. In general, KIT mutations in GIST are analyzed by examining surgically resected tissue samples. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) is a potentially useful procedure for pathologically diagnosing GIST before surgery [[11], [12], [13]]. However, EUS-FNA samples are generally utilized for pathological examinations because the acquired samples are tiny. A few studies have examined the diagnostic yield of mutation analyses based on examinations of EUS-FNA samples [[14], [15], [16]]. However, the feasibility of performing mutation analyses using EUS-FNA samples have been unclear.
Since the samples obtained with EUS-FNA are usually quite small, the amount of extracted DNA is also extremely low. On the other hand, the fact that almost all GISTs overexpress KIT protein indicates that GISTs contain a large amount of KIT mRNA [17]. Theoretically, there should be a much higher amount of KIT mRNA than KIT DNA in GIST. We hypothesized that analyzing RNA is superior to analyzing DNA for performing mutation analyses of GIST using EUS-FNA samples. The aim of this study was to evaluate the feasibility of mutation analysis of GIST using RNA obtained with EUS-FNA.
Methods
Patients
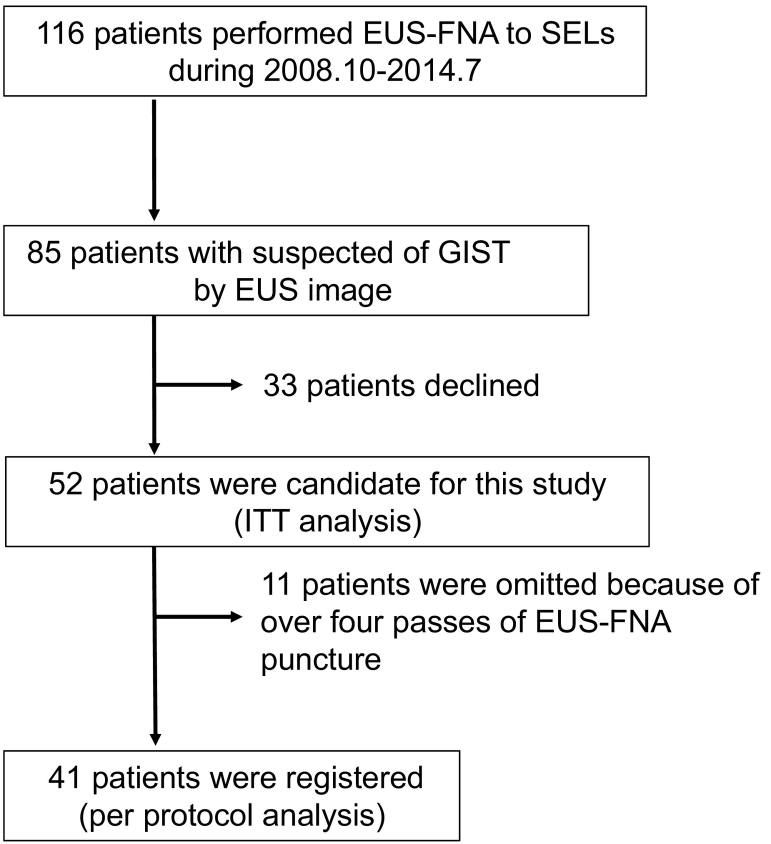
Between October 2008 and September 2014, Gastrointestinal EUS-FNA was performed to 116 cases of subepithelial lesion (SEL) at Nagoya University Hospital. 85 SELs were suspected of GIST from EUS image. We prospectively got informed consent of this study from 52 patients before EUS-FNA. 41 SELs which were acquired tissue within three passes of EUS-FNA puncture, were registered consecutively in this study (Fig. 1).
Fig. 1.
Flow of patients evaluated in this study.
Among them, 16 patients with GIST who underwent surgery after EUS-FNA gave their consent for their resected GISTs to be analyzed as well. The protocols for the collection of the tumor samples and clinical information were approved by the institutional review board at Nagoya University Hospital (IRB approval No. 2008–0611). Written informed consent was obtained from all patients.
Sample collection
All EUS-FNA procedures were performed in the course of clinical practice to determine the pathological diagnoses of SEL using an oblique-viewing echoendoscope (GF-UCT240AL-5; Olympus, Japan) with an ultrasound processor with a color Doppler function (EU-ME1; Olympus, Japan). Puncturing was carried out using disposable 22-G puncture needles (EZ Shot2: NA-220H-8022; Olympus, Japan). Endoscopists checked FNA sample visually without rapid on-site evaluation (ROSE) after three puncture passes. When white string tissues were seen, the procedure was finished with registration of this study. If tissue was not obtained within three passes, the procedure was continued until tissue sample was obtained. But such a case was omitted from this study to avoid additional punctures. A part of the obtained sample, which was equivalent to tissue obtained in single session, was assigned for use in RNA extraction.
The sample was immediately immersed in RNAlater™ stabilization solution (Invitrogen, Carlsbad, CA) and was stored at −80 °C until just before RNA extraction step.
RNA extraction and cDNA reverse transcription
Each stored sample was deeply frozen with liquid nitrogen and homogenized in the mortar. Total RNA was extracted using 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA), 0.2 ml of chloroform, 0.5 ml of isopropanol and 1 ml of 75% ethanol according to the manufacturer's instruction. After extracted RNA was diluted by 20 μl of RNA-free water, RNA concentration and absorbance at wavelengths of 260/280 nm were measured with the Nano-Drop 1000™ system (Thermo Fisher Scientific, Waltham, MA) to evaluate the quality and quantity of total RNA. For cDNA synthesis, 3 μl of total RNA was reverse transcribed using PrimeScript™ Reverse Transcriptase (Takara Bio, Inc., Shiga, Japan) according to the manufacturer's protocol.
Amplification of KIT cDNA
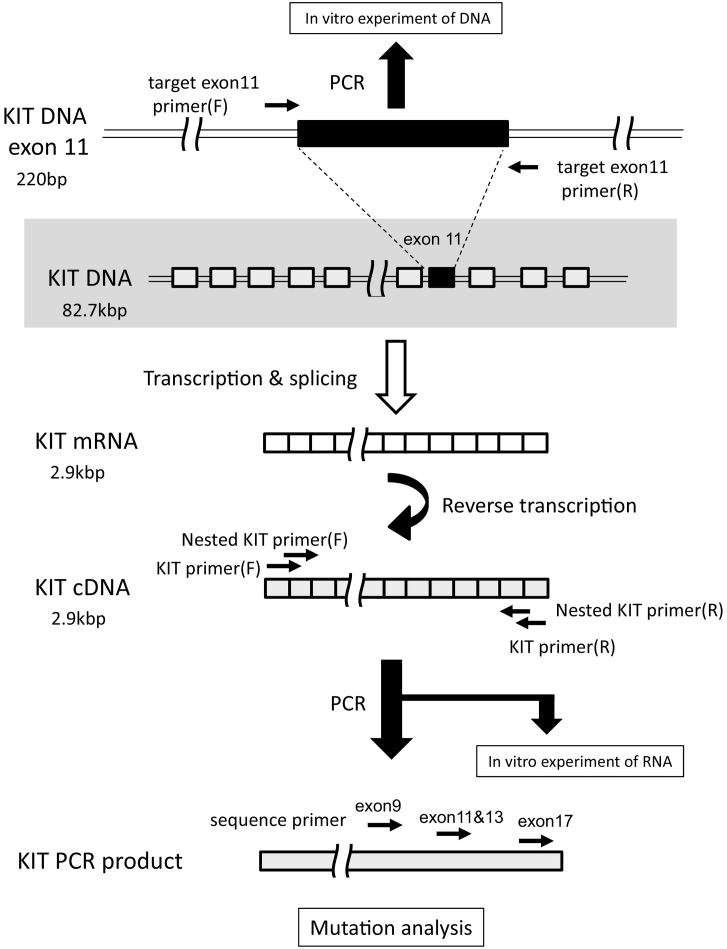
Full-length KIT cDNA was amplified with 40 cycles of polymerase chain reaction (PCR) using appropriate primers (Table 1). Template cDNA which was equivalent to 200 ng of RNA was mixed with 2 μl of dNTP, 0.5 μl of each primer, 0.5 μl of KOD FX (TOYOBO CO., LTD, Osaka, Japan), 25 μl of KOD FX buffer and water. A total reaction volume of 50 μl was prepared for PCR. One PCR cycling condition was 1 min of denaturation at 95 °C, 0.5 min of annealing at 55 °C and 3 min of extension at 72 °C. After PCR amplification, agarose gel electrophoresis was used to confirm whether the amplification had succeeded or failed. In case of failure to KIT amplification by 1st PCR, nested PCR was performed and then checked again by agarose gel electrophoresis. The schematic location where amplified using each primer of KIT was shown in Fig. 2.
Table 1.
Primer design.
| Target cDNA | Forward | Reverse |
|---|---|---|
| KIT | AGCTCGGATCCCATCGCTA | CTGCTCAGACATCGTCGTGCAC |
| nested KIT | AGCTACCGCGATGAGAGGCGCT | GTCGTGCACAAGCAGAGGCTG |
| PDGFRA | ATCCGGCGTTCCTGGTCTTAG | CAGGAAGCTGTCTTCCACCAG |
| Sequencing target | Forward | |
|---|---|---|
| KIT | exon 9 | GTGAATGGCATGCTCCAATGT |
| exons 11 & 13 | TCACTCCTTTGCTGATTGGT | |
| exon 17 | ATCATGGAGGATGACGAGTTG | |
| PDGFRA | exon 12 | ATCTCACTTATTGTCCTGGTT |
| exon 14 | AGCCGGTCCCAACCTGTCATG | |
| exon 18 | AGCTTCACCTATCAAGTTGCC | |
| PCR in vitro experiment | ||
|---|---|---|
| Target DNA | Forward | Reverse |
| KIT exon11 | CCAGAGTGCTCTAATGACTG | CTGTTATGTGTACCCAAAAAGG |
Fig. 2.
The location of each primers on schematic images of DNA, mRNA, cDNA and PCR product related with KIT gene.
Mutation analysis
Using the amplified full-length KIT cDNA as a template, exons 9, 11, 13, and 17 were analyzed using the directional sequencing method on an ABI 310 system (Applied Biosystems, Foster City, CA). Sequence primer was shown in Table 1 and Fig. 2). 2 μl of PCR product was mixed with 4 μl of 50 times diluted primer, 1 μl of Big Dye terminator 3.1 Ready Reaction Mix (Applied Biosystems, Foster City, CA), 4 μl of Sequencing buffer and water. A total volume of 20 μl was performed sequencing reaction over 25 cycles of the following step (10 s of denaturation at 96 °C, 5 s of annealing at 50 °C and 1 min of extension at 60 °C). In GISTs which were negative for KIT mutations, exons 12, 14, and 18 of the PDGFRA gene were further analyzed after PCR amplification of full-length PDGFRA cDNA. GIST that did not possess KIT or PDGFRA mutation was defined as wild-type GIST.
Pathological diagnosis
Immunohistochemical staining was performed to distinguish GIST from other mesenchymal tumors in addition to hematoxylin and eosin staining. For the immunohistochemical staining, commercially available primary antibodies against KIT (polyclonal, 1:500; MBL, Nagoya, Japan), CD34 (clone QBEnd10, 1:600; Dako, Glostrup, Denmark), S100 (polyclonal, 1:1000; Dako), alpha smooth muscle actin (clone 1A4, 1:1000; Dako) and MIB-1(clone MIB-1, 1:1000; Dako) were utilized.
In vitro experiment
To examine the minimum number of cells required to amplify target KIT gene via PCR, the GIST-T1 cell line (Cosmo Bio, Tokyo, Japan) which is Immunohistochemically positive for c-KIT and has KIT mutation in exon 11 (codon 560–578 deletion), was utilized [18]. After being subjected to trypsin treatment and serum neutralization, the suspended cells were counted using a hemocytometer. The density of the suspended cells was adjusted to 500,000 cells/100 μl, and the suspension was concentrated or diluted to create suspensions with various numbers of cells. Consequently, suspensions with cell counts of 1 × 103, 5 × 103, 1 × 104, 5 × 104, 1 × 105, and 2 × 105 were subjected to gene extraction. DNA was extracted using the illustra™ tissue and cells genomicPrep mini spin kit (GE Healthcare, Buckinghamshire, England, UK). RNA extraction was performed as described previously. KIT genomic DNA was amplified over 40 cycles of PCR using appropriate primers which target KIT exon 11 gene (Table 1). Full-length of KIT cDNA was amplified after RNA was reverse transcribed to cDNA. The detailed method was described in previous paragraphs. The schematic locations of cDNA or DNA where amplified were shown in Fig. 2. For each PCR product, agarose gel electrophoresis was used to confirm whether the PCR amplification had succeeded or failed. All experimental procedures were repeated three times with same condition.
Statistical analysis
The correlation between the quantity and quality of the extracted RNA was evaluated using Spearman's rank correlation coefficient. The success rates of KIT amplification during the first PCR and/or nested PCR was described with both per protocol analysis (PP) and Intention to treat analysis (ITT). The success rate of KIT amplification was compared between GIST and non-GIST using Fisher's exact test in per protocol analysis. Statistical significance was defined as P < .05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 24 (IBM, Armonk, NY, USA).
Results
Patient characteristics
Although 52 patients were candidate for this study, 11 patients were omitted because of over four passes of EUS-FNA puncture(Fig. 1). The characteristics of 41 patients enrolled in this study are shown in Table 2. Thirty-two of 41 tumors were pathologically diagnosed with GISTs. Five and four tumors were diagnosed with schwannomas and leiomyomas, respectively.
Table 2.
Patients' characteristics.
| All patients | GIST | Non-GIST | |
|---|---|---|---|
| No. | 41 | 32 | 9 |
| Gender | |||
| Male | 22 | 18 | 4 |
| Female | 19 | 14 | 5 |
| Age, y, mean (SD) | 65.7 ± 11.5 | 65.5 ± 11.1 | 66.3 ± 13.2 |
| Tumor size (mm), median, range | 25 (16–240) | 27.5 (16–240) | 25 (20–43) |
| Tumor location | |||
| Esophagus | 3 | 1 | 2 |
| Stomach | 37 | 30 | 7 |
| Duodenum | 1 | 1 | 0 |
| Pathological diagnosis | |||
| GIST | 32 | 32 | – |
| Schwannoma | 5 | – | 5 |
| Leiomyoma | 4 | – | 4 |
Quantity and quality of RNA
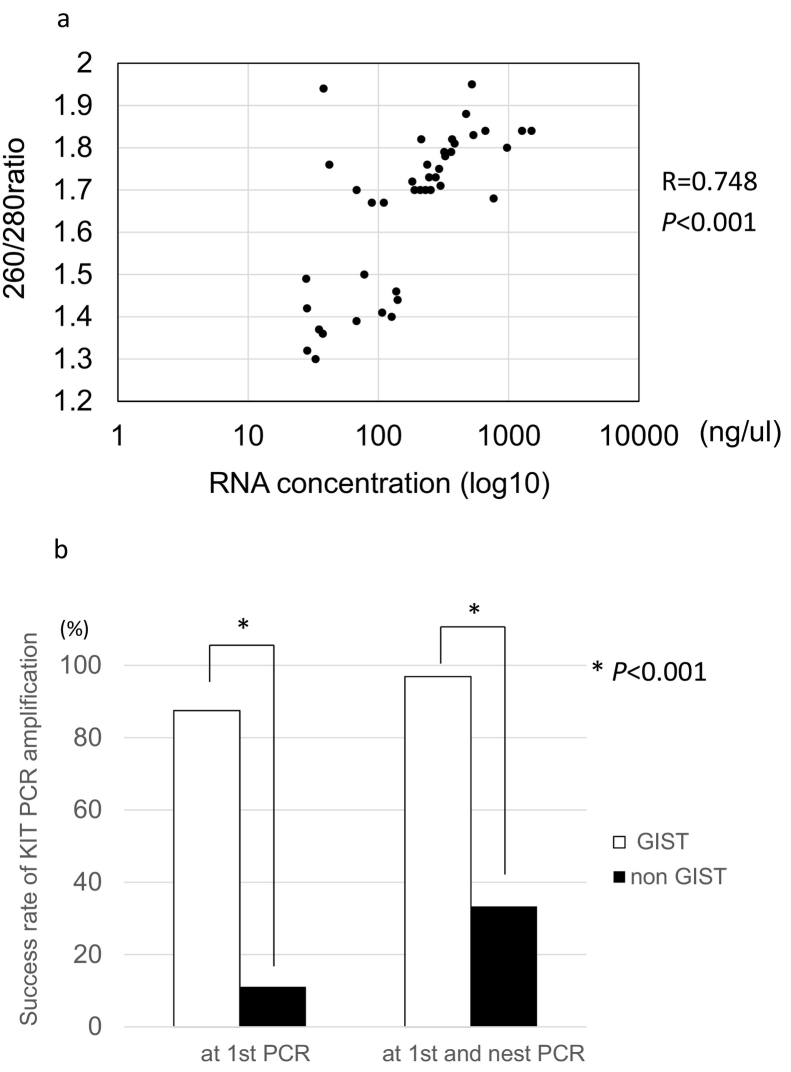
RNA extraction was successfully achieved from all samples. The median RNA concentration was 213.2 ng/ul (range: 28–1498). The median 260 nm/280 nm absorbance ratio, which indicates RNA quality, was 1.71 (range: 1.30–1.95). The correlation between RNA concentration and 260 nm/280 nm absorbance ratio is shown in Fig. 3a. There is a strong correlation between the RNA concentration and the 260 nm/280 nm absorbance ratio (R = 0.748, P < .0001).
Fig. 3.
The correlation between the RNA concentration and 260 nm/280 nm absorbance ratio of the RNA extracted from EUS-FNA samples obtained from 41 SEL (Fig. 3a).
A comparison of the success rate of KIT amplification between GIST and non-GIST (Fig. 3b).
Success rate of KIT amplification
All samples were subjected to cDNA reverse transcription and PCR amplification. The success rate of the first PCR was 70.7% (PP), 55.8%(ITT), and the overall success rate of both the 1st PCR and nested PCR was 83.0%(PP), 65.4%(ITT).
Among 32 GISTs, the success rate of the first PCR was 87.5%, and the overall success rate of both the 1st PCR and nested PCR was 96.9%. On the other hand, the success rate of the 1st PCR was 11.1%, and the overall success rate of both the 1st PCR and nested PCR was 33.3% among non-GISTs. The success rate of KIT amplification was significantly higher among GISTs than among non-GISTs (P < .001) (Fig. 3b).
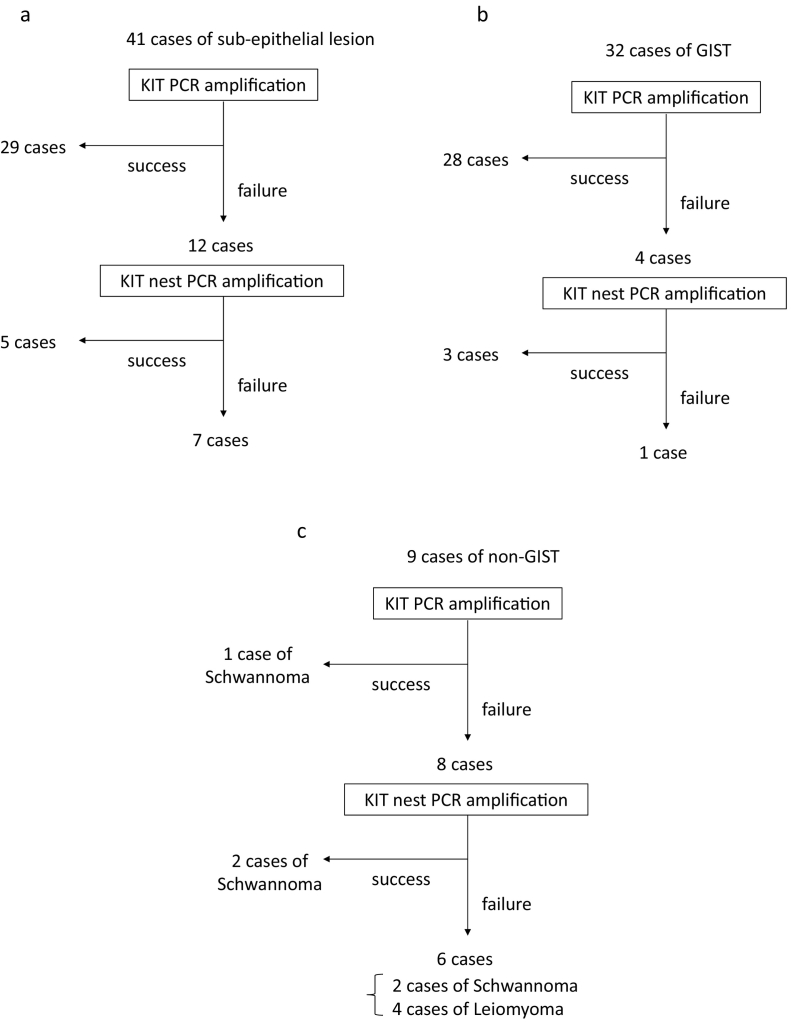
The KIT amplification flowcharts for all SELs, GISTs, and non-GISTs are shown in Fig. 4a, b, and c, respectively. c-kit cDNA was successfully amplified by 1st PCR in 29 out of 41 SELs. c-kit cDNA was successfully amplified from 5 of the remaining 12 SELs via nested PCR. In 7 SELs, KIT amplification was failed. Three non-GISTs from which the KIT gene was amplified were all schwannomas. We compared RNA concentration and 260 nm/280 nm absorbance ratios between the samples which were succeed by 1st PCR and those which were failed by 1st PCR. However, neither of these parameters differed significantly between two groups (Supplementary Table 1).
Fig. 4.
Flowcharts of KIT amplification from SEL (Fig. 4a)
After each SEL had been pathologically diagnosed as a GIST or non-GIST, the flowchart for GIST (Fig. 4b) or non-GIST (Fig. 4c) was followed.
Gene mutations in GIST
The results of GIST mutation analysis are shown in Table 3. All 31 GISTs in which full-length KIT cDNA was amplified were revealed their KIT mutation statuses completely. Twenty-seven GISTs carried KIT mutations. KIT exon 11 mutations were detected in 25 of the 27 GISTs with KIT mutations (92.6%). Regarding the types of mutations found in exon 11, insertions, point mutations, and deletions were detected in 6, 12, and 7 GISTs, respectively. Of the remaining two GISTs, one duodenal GIST exhibited an insertion in exon 9, and the other had a point mutation in exon 13. Two GISTs carried point mutations in exon 18 of PDFGRA. The remaining two GISTs that have neither KIT nor PDGFRA mutations were wild type.
Table 3.
Characteristics of mutation status in patients with GIST (n = 32).
| No. | Amplification |
Mutation status |
||||
|---|---|---|---|---|---|---|
| 1st PCR | Nested PCR | Gene | Exon | Codonb, c | Type | |
| 1 | Failure | 〇 | None | – | – | – |
| 2 | 〇 | KIT | 11 | V559D | PM | |
| 3 | 〇 | KIT | 9 | 503AY504 | I | |
| 4 | 〇 | KIT | 13 | K642E | PM | |
| 5 | 〇 | PDGFRA | 18 | D842V | PM | |
| 6 | 〇 | KIT | 11 | 550–558 | D | |
| 7 | 〇 | KIT | 11 | 555 | D | |
| 8 | 〇 | KIT | 11 | W557R | PM | |
| 9 | 〇 | KIT | 11 | 579DPTQLPYD580 | I | |
| 10 | 〇 | KIT | 11 | W557R | PM | |
| 11 | 〇 | PDGFRA | 18 | D842V | PM | |
| 12 | 〇 | KIT | 11 | 580DH581 | I | |
| 13 | 〇 | KIT | 11 | V560E | PM | |
| 14 | 〇 | KIT | 11 | 573DP574 | I | |
| 15 | 〇 | KIT | 11 | 585YDHKWEFP586 | I | |
| 16 | 〇 | KIT | 11 | V560D | PM | |
| 17 | 〇 | KIT | 11 | L577P | PM | |
| 18 | 〇 | KIT | 11 | V561D | PM | |
| 19 | 〇 | KIT | 11 | L577P | PM | |
| 20 | 〇 | KIT | 11 | V560D | PM | |
| 21 | 〇 | KIT | 11 | L576P | PM | |
| 22 | 〇 | KIT | 11 | 561–562 | D | |
| 23 | Failure | 〇 | KIT | 11 | 560 | D |
| 24 | 〇 | KIT | 11 | V559D | PM | |
| 25 | 〇 | KIT | 11 | 558 | D | |
| 26 | 〇 | KIT | 11 | 580DPTQLPYDH581 | I | |
| 27 | 〇 | KIT | 11 | 560 | D | |
| 28 | Failure | 〇 | None | – | – | – |
| 29a | Failure | Failure | None | – | – | – |
| 30 | 〇 | KIT | 11 | V559D | PM | |
| 31 | 〇 | KIT | 11 | 585LPYDHKWEFP586 | I | |
| 32 | 〇 | KIT | 11 | 557–558 | D | |
Mutation status of No.29 was analyzed using surgical tissue sample because of failure to amplification by FNA sample.
Each letter indicates the amino acid that was inserted or substituted due to the gene mutation (e.g., V559D indicates that valine was substituted for aspartic acid at codon 559 due to a point mutation, and 503AY504 indicates that alanine and tyrosine were inserted between codons 503 and 504).
The numbers in the codon column indicate deleted codons (e.g., 557–558 indicates that codons 557 and 558 were deleted).
Abbreviations. D, deletion; PM, point mutation; I, insertion.
Sixteen GISTs were surgically resected after EUS-FNA. The surgical specimens from these cases were subjected to gene mutation analysis using the same method as was employed for the EUS-FNA samples. The gene mutation analysis was successful in all 16 cases. One GIST in which KIT amplification from the EUS-FNA sample failed was confirmed to be a wild-type GIST through the surgical sample. Complete correspondences between the mutation statuses of the surgical and EUS-FNA samples were seen in the remaining 15 GISTs.
Comparison of the minimum number of cells required for PCR-based amplification of the KIT gene between DNA and RNA samples
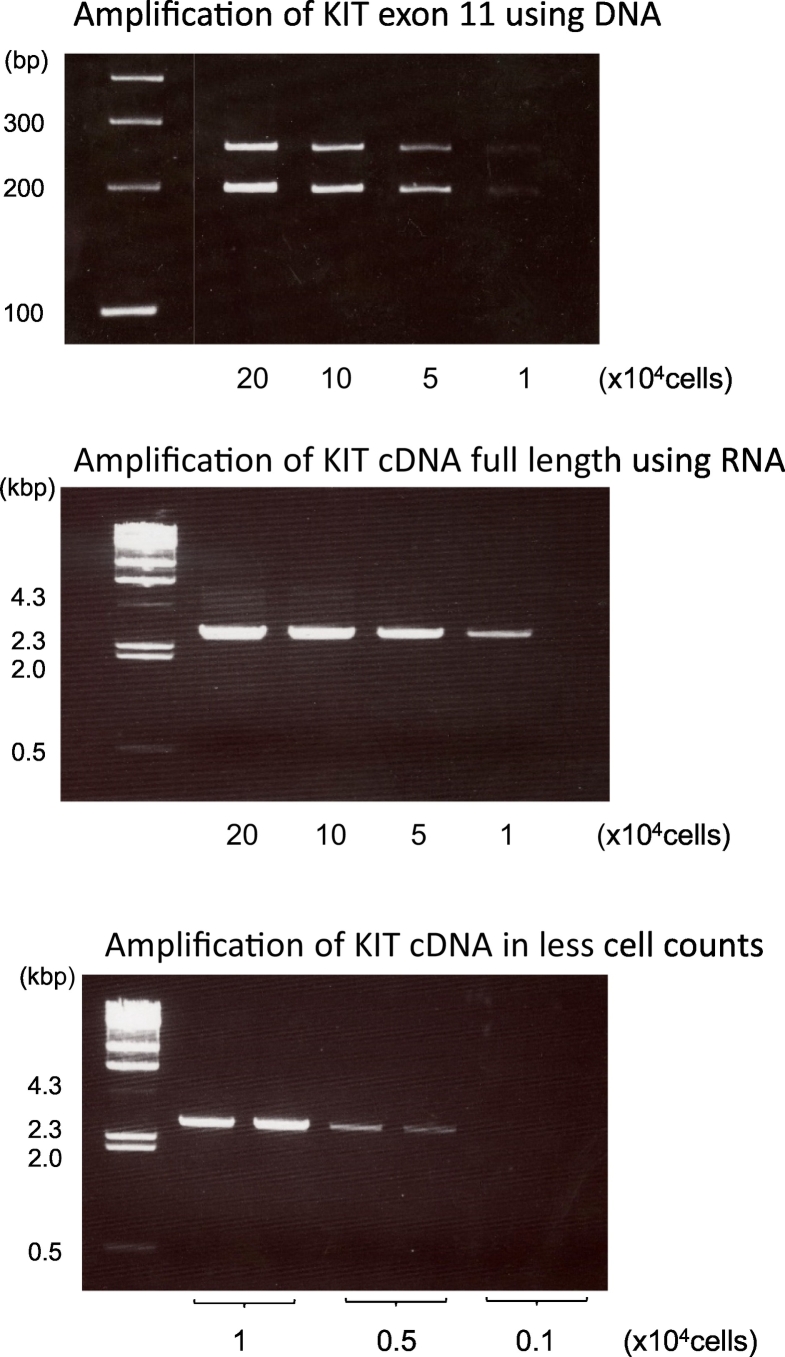
The minimum number of cells required for KIT amplification from DNA was 5 × 10 [4] (Fig. 5). In the case of RNA, which was converted to cDNA, the minimum number of cells required was 5 × 10 [3], which was one-tenth of the value for DNA samples. The results of this experiment indicate that using RNA is superior to the conventional DNA method for mutation analyses of small samples, such as those obtained with EUS-FNA.
Fig. 5.
The PCR products amplified using the KIT primers at each cell density.
The upper panel shows the KIT exon 11 products amplified from DNA. Because of the deletion of 57 bases in exon 11, two bands appeared. The middle panel shows those amplified from full-length KIT cDNA, which is an alternative to RNA. The minimum number of cells required to amplify cDNA was investigated, and the PCR products amplified from less than 10,000 cells are shown in the lower panel.
Discussion
Although EUS-FNA is used to pathologically diagnose SEL, gene mutation analysis using EUS-FNA samples has been quite limited. Because the FNA sample which is tiny tissue has limitations regarding tumor purity and DNA quantity, such gene analyses have the concerns that extracted DNA is insufficient or is not from a tumor. Here, we clarified the feasibility of mutation analysis using RNA extracted from EUS-FNA samples. First, we confirmed that RNA could be extracted from all EUS-FNA samples collected from SEL and that there was a positive correlation between the quantity and quality of extracted RNA. Secondly, we found that the success rate of KIT amplification was significantly higher among GIST than among non-GIST (96% vs. 33%). However, there was no relationship between the success rate and the quality or quantity of extracted RNA, even among GIST. In addition, 15 surgical samples out of 16 GISTs showed that the mutation status was consistent with EUS-FNA sample. Finally, an in vitro experiment using GIST-T1 cell line indicated that the minimum number of cells required to amplify KIT gene from RNA was one tenth of that required to amplify it from DNA.
Mutation analysis is useful not only for diagnosing GIST but also for predicting the effects of molecular targeting medicines and the risk of recurrence after surgery [[4], [5], [6], [7], [8], [9], [10],19,20]. However, such analyses have been exclusively performed using DNA extracted from surgically resected samples. In unresected GIST due to metastasis, there is no opportunity to get mutation information although it contributes to assume the effectiveness of tyrosine kinase inhibitors.
In this study, we proved that mutation analyses can be conducted using RNA extracted from EUS-FNA specimens collected from GIST. Clinically, RNA-based mutation analysis in unresectable GIST would offer informative data that can aid drug selection.
The feasibility of KIT analysis using RNA was shown in almost all GIST but a few in non-GIST. We speculate that it was resulted due to the abundant KIT mRNA in GIST. However, it is interesting that one-third of non-GIST showed KIT amplification by PCR. There are two possibilities why KIT was amplified even though non-GIST generally does not express KIT protein. One is that gastrointestinal mesenchymal tumors (GIMTs) including leiomyoma and schwannoma expressed small amount of KIT mRNA which cannot detect by protein level such as immunohistochemical staining. The other is that intestinal cells of Cajal was contained in GIMTs by chance. Anyway, we think these does not disturb clinical management because all cases which were diagnosed of leiomyoma or schwannoma did not possess KIT mutation. Regarding PDGFRA mutation, 4 GISTs without KIT mutation were analyzed after amplification of full-length PDGFRA cDNA. All 4 succeeded amplification and read sequence although PDGFRA expression has not examined pathologically to diagnose of GIST. It may be also advantage of RNA analysis that both sequences are performed using EUS-FNA sample.
There have been a few reports about mutation analysis using EUS-FNA specimens [[14], [15], [16],21]. These mutation analyses were performed using DNA, rather than RNA. Gomes et al. reported that they were able to perform molecular analysis using DNA from 51 out of 85 cell samples. Thus, the success rate of this approach was only 60% [14]. On the other hand, a previous study described mutation analysis of GIST using EUS-guided biopsy samples. In the latter study, in which the analyses were performed using extracted DNA, it was shown that the success rate of sequencing was 98%. Interestingly, no EUS-FNA samples were subjected to sequencing because of their poor quality and small size [16]. Recently, Gleeson et al. examined the gene mutations in EUS-FNA samples using targeted next-generation sequencing. Sequencing results were successfully obtained in 19 out of 20 (95%) subjects. However, the 20 subjects were limited among 32 subjects which had fulfilled the cell number criteria after cytologic smears [21].
To the best of our knowledge, there have not been any reports about mutation analysis using RNA, even with regard to analyses of surgical samples. The present study is the first to demonstrate that gene mutation analysis using RNA obtained with EUS-FNA is feasible. In addition, it indicated that RNA analysis, rather than DNA analysis, has a great advantage in GIST, which contains lots of KIT mRNA in each cell. In other words, the tumors in which RNA sequencing is less likely to be successful are what KIT is no or less expressed, so less of a problem if the assay fails. Utilizing RNA for gene analysis would be of benefit, especially when a sample is a small volume or a high percentage of admixed non-tumor cells. In the present study, the success rate of the mutation analysis was 96%. When EUS-FNA samples are not suitable for immunostaining or pathological evaluation in SEL, RNA based mutation analysis would aid the diagnosis of GIST. Furthermore, our result would support the utility of RNA based genetic testing using the clinical samples in other tumors that overexpress some mRNA with a mutation.
This study had a few limitations. Firstly, it was conducted at a single center and involved a relatively small sample size. Secondly, this study contains a selection bias because the registration was done after confirming EUS-FNA sample was obtained within 3 passes. Thirdly, EUS-FNA has been less common for SELs in the latest clinical practice. Due to the development of puncture needles, the name of the examination has been changing to Endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB), which offers more volume of specimens than EUS-FNA. However, the needle we used in this study belongs to the conventional type for EUS-FNA. We believe that mutation analysis using RNA is also applicable to EUS-FNB. Fourthly, the comparison of RNA- and DNA-based gene analyses was not performed simultaneously because the EUS-FNA samples were too small to allow two analyses to be performed. To directly prove the superiority of RNA analysis over DNA analysis, comparisons of mutation analysis success rates should be conducted simultaneously using DNA and RNA from the same samples in surgically resected tissue. Even consideration with these limitations, this study has revealed a novel efficient approach to mutation analyses in GIST using a combination with RNA and EUS-FNA.
In conclusion, mutation analysis using RNA obtained with EUS-FNA was found to be highly feasible and provided reliable results. This approach is suggested to be superior to DNA-based mutation analysis of GIST using EUS-FNA samples.
The following is the supplementary data related to this article.
Quality and quantity of total RNA.
CRediT authorship contribution statement
Kohei Funasaka: Conceptualization, Methodology, Data curation, Writing - original draft, Formal analysis. Ryoji Miyahara: Investigation. Kazuhiro Furukawa: Investigation. Tsunaki Sawada: Resources. Keiko Maeda: Resources. Takeshi Yamamura: Resources. Takuya Ishikawa: Resources. Eizaburo Ohno: Resources. Masanao Nakamura: Resources. Hiroki Kawashima: Resources. Yoshiki Hirooka: Supervision. Naoki Ohmiya: Supervision. Mitsuhiro Fujishiro: Supervision.
Acknowledgments
Acknowledgements
The authors wish to thank Dr. Takeshi Senga for his valuable help with the molecular biological technique.
Grant number
This research was supported by the JSPS KAKENHI Grant-in-Aid for Young Scientists (B) (grant number: JP24790689).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Miettinen M., Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archiv: an international journal of pathology. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 2.Rubin B.P., Singer S., Tsao C., Duensing A., Lux M.L., Ruiz R., Hibbard M.K., Chen C.J., Xiao S., Tuveson D.A., Demetri G.D., Fletcher C.D., Fletcher J.A. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. [PubMed] [Google Scholar]
- 3.Hirota S., Nishida T., Isozaki K., Taniguchi M., Nakamura J., Okazaki T., Kitamura Y. Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J. Pathol. 2001;193:505–510. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH818>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Lasota J., Jasinski M., Sarlomo-Rikala M., Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am. J. Pathol. 1999;154:53–60. doi: 10.1016/S0002-9440(10)65250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer S., Rubin B.P., Lux M.L., Chen C.J., Demetri G.D., Fletcher C.D., Fletcher J.A. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J. Clin. Oncol. 2002;20:3898–3905. doi: 10.1200/JCO.2002.03.095. [DOI] [PubMed] [Google Scholar]
- 6.Kim T.W., Lee H., Kang Y.K., Choe M.S., Ryu M.H., Chang H.M., Kim J.S., Yook J.H., Kim B.S., Lee J.S. Prognostic significance of c-kit mutation in localized gastrointestinal stromal tumors. Clin. Cancer Res. 2004;10:3076–3081. doi: 10.1158/1078-0432.ccr-03-0581. [DOI] [PubMed] [Google Scholar]
- 7.Martin J, Poveda A, Llombart-Bosch A, Ramos R, Lopez-Guerrero JA, Garcia del Muro J, Maurel J, Calabuig S, Gutierrez A, Gonzalez de Sande JL, Martinez J, De Juan A, Lainez N, Losa F, Alija V, Escudero P, Casado A, Garcia P, Blanco R, Buesa JM, Spanish Group for Sarcoma R. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol. 2005;23:6190–6198. [DOI] [PubMed]
- 8.Andersson J., Bumming P., Meis-Kindblom J.M., Sihto H., Nupponen N., Joensuu H., Oden A., Gustavsson B., Kindblom L.G., Nilsson B. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology. 2006;130:1573–1581. doi: 10.1053/j.gastro.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich M.C., Corless C.L., Blanke C.D., Demetri G.D., Joensuu H., Roberts P.J., Eisenberg B.L., von Mehren M., Fletcher C.D., Sandau K., McDougall K., Ou W.B., Chen C.J., Fletcher J.A. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J. Clin. Oncol. 2006;24:4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi M., Nishida T., Hirota S., Isozaki K., Ito T., Nomura T., Matsuda H., Kitamura Y. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999;59:4297–4300. [PubMed] [Google Scholar]
- 11.Ando N., Goto H., Niwa Y., Hirooka Y., Ohmiya N., Nagasaka T., Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest. Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 12.Akahoshi K., Sumida Y., Matsui N., Oya M., Akinaga R., Kubokawa M., Motomura Y., Honda K., Watanabe M., Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J. Gastroenterol. 2007;13:2077–2082. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okubo K., Yamao K., Nakamura T., Tajika M., Sawaki A., Hara K., Kawai H., Yamamura Y., Mochizuki Y., Koshikawa T., Inada K. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J. Gastroenterol. 2004;39:747–753. doi: 10.1007/s00535-004-1383-0. [DOI] [PubMed] [Google Scholar]
- 14.Gomes A.L., Bardales R.H., Milanezi F., Reis R.M., Schmitt F. Molecular analysis of c-Kit and PDGFRA in GISTs diagnosed by EUS. Am. J. Clin. Pathol. 2007;127:89–96. doi: 10.1309/M1EC8JE9ACAMJACU. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt F.C., Gomes A.L., Milanezi F., Reis R., Bardales R.H. Mutations in gastrointestinal stromal tumors diagnosed by endoscopic ultrasound-guided fine needle aspiration. Minerva Med. 2007;98:385–388. [PubMed] [Google Scholar]
- 16.Hedenstrom P., Nilsson B., Demir A., Andersson C., Enlund F., Nilsson O., Sadik R. Characterizing gastrointestinal stromal tumors and evaluating neoadjuvant imatinib by sequencing of endoscopic ultrasound-biopsies. World J. Gastroenterol. 2017;23:5925–5935. doi: 10.3748/wjg.v23.i32.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabone S., Theou N., Wozniak A., Saffroy R., Deville L., Julie C., Callard P., Lavergne-Slove A., Debiec-Rychter M., Lemoine A., Emile J.F. KIT overexpression and amplification in gastrointestinal stromal tumors (GISTs) Biochim. Biophys. Acta. 1741;2005:165–172. doi: 10.1016/j.bbadis.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi T., Sonobe H., Toyonaga S., Yamasaki I., Shuin T., Takano A., Araki K., Akimaru K., Yuri K. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stromal tumor. Lab. Investig. 2002;82:663–665. doi: 10.1038/labinvest.3780461. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa T., Matsuno Y., Shimoda T., Hirohashi S. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum. Pathol. 2002;33:669–676. doi: 10.1053/hupa.2002.124116. [DOI] [PubMed] [Google Scholar]
- 20.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson F.C., Kipp B.R., Kerr S.E., Voss J.S., Graham R.P., Campion M.B., Minot D.M., Tu Z.J., Klee E.W., Lazaridis K.N., Henry M.R., Levy M.J. Kinase genotype analysis of gastric gastrointestinal stromal tumor cytology samples using targeted next-generation sequencing. Clin. Gastroenterol. Hepatol. 2015;13:202–206. doi: 10.1016/j.cgh.2014.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality and quantity of total RNA.