Abstract
Ethylene glycol monomethyl ether (EGME) is a major component of paints, lacquers, inks, and automobile brake fluids. As a result, exposures to humans are inevitable. We therefore, investigated in this study, its effect on testicular cells in a time-course manner in male Wistar rats. Animals were orally administered 50 mg/kg body weight of EGME for duration of 7, 14, and 21 days. Following 7 days of the administration, levels of NO and GSH were significantly reduced, while levels of c-Myc, K-Ras, caspase-3, IL-6, TNF-α, and IL-1β were significantly increased compared with control. At the end of 14 days exposure, GPx, and SOD activities, as well as IL-10 level were significantly decreased, while levels of c-Myc, K-Ras, p53, Bax, caspase-3, IL-6, TNF-α, IL-1β, and GST activity were significantly elevated compared with control. After 21 days of EGME administration, Bcl-2, IL-10, and NO levels were significantly decreased, while levels of c-Myc, K-Ras, p53, Bax, caspase-3, IL-6, TNF-α, IL-1β, MDA and GST activity were significantly increased compared with control. After 7, 14, and 21 days of EGME administrations, testis histopathology showed severe loss of seminiferous tubules, the seminiferous epithelium revealed very few spermatocytes, spermatids, spermatogonia, spermatozoa, and Sertoli cells, while the interstitial tissue is eroded, with scanty abnormal Leydig cells, compared with the control that appeared normal. We therefore, concluded that EGME-induced testicular toxicity as a result of EGME administration could be via the disorganization of the endogenous antioxidant systems as well as up-regulation of pro-inflammatory, apoptotic and oncogenic mediators in rats.
Keywords: Ethylene glycol monomethyl ether, Oxidative stress, Inflammation, Apoptosis, Oncogenes, Histopathology, Testis
Abbreviations: MDA, malondialdehyde; GSH, reduced glutathione; NO, nitric oxide; CAT, catalase; GST, glutathione S-transferase; GPx, glutathione peroxidase; SOD, superoxide dismutase; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin-1 beta; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X; p53, tumor suppressor protein; c-Myc, myelocytomatosis; K-Ras, Kirsten rat sarcoma viral oncogene
1. Introduction
EGME is a member of the chemicals known as the ethylene glycol ethers (EGEs). EGME It is a solvent used industrially and utilized extensively for the manufacture of cellulose acetate, stains, inks, paints, and resins. EGME is also used as an antifreeze in hydraulic fluids and jet fuels, and also as leather and upholstery cleaners [1,2]. EGME is readily absorbed through skin contact and inhalation, posing health risks to diverse animal species including humans [1]. Organs and tissues having cells that are rapidly dividing and high metabolism, such as thymus and testes, are reported to be particularly vulnerable [[3], [4], [5]]. Reproductive tissues, neurological and hematological abnormalities are reported consequences of occupational exposure to EGME in humans [[6], [7], [8], [9], [10], [11]]. Damage to the reproductive system, including alteration in female fertility and testicular atrophy are the outcomes of EGME treatment already reported in laboratory animals [[12], [13], [14], [15], [16]]. Also, EGME can affect the immune system, leading to reduced spleen cell number, decreased thymus weight, and thymic atrophy [17].
Following testicular exposures, EGEs are activated in affected cells by the action of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) respectively [18]. Methoxyacetic acid (MAA), a metabolite of EGME is responsible for the teratogenicity, hematotoxicity, gonadotoxicity, immunotoxicity, and embryotoxicity of EGME [5,[19], [20], [21]]. Severe effects of EGEs on sperm and testes as a whole have been documented in humans [5], but have not been fully investigated. In six EGME-exposed workers, smaller testicular size was reported compared with nine unexposed individuals [7]. In wider research involving 73 painters exposed to EGME and EGEE, high cases of azoospermia, oligozoospermia, and an increased odds ratio (OR) for a smaller sperm count per ejaculate were reported compared to workers not exposed [11]. In animal studies, EGME was reported to cause atrophy of testes and disruption of sperm synthesis [20,22]. The Sertoli cells were also affected [23], and the key target cell where disruption of nuclei or atrophic nuclear chromatin condensation was evident is the pachytene spermatocyte [22].
Previous studies have not checked the time-course effect of EGME on testicular cells. In the light of the above and a follow-up, this present study investigated the time-course effect of EGME on testicular markers of lipid peroxidation (MDA), oxidative stress (CAT, SOD, GPx, GST, GSH, and NO), inflammation (IL-10, IL-6, TNF-α, and IL-1β), apoptosis (caspase-3, p53, Bax, and Bcl-2) and proto-oncogenic markers (c-Myc and K-Ras) in male Wistar rats.
2. Materials and methods
2.1. Chemicals and kits
EGME (C3H8O2; CAS# 109-84-4; 99.5% purity), is a product of BDH Laboratory Supplies, Poole, BH15 1TD, England. Rats IL-10, IL-6, TNF-α, IL-1β, caspase-3, p53, Bax, Bcl-2, c-Myc, and K-Ras enzyme-linked immunosorbent assay (ELISA) kits are manufactured by Cusabio Technology Llc, Houston, TX, USA. The rest of the reagents and chemicals used were obtained from a recognized chemical manufacturing company and were of standard and analytical grade.
2.2. Oral acute toxicity study of EGME
The mean lethal dose (LD50) of EGME was performed according to the method of Lorke [24]. The first phase involved three groups of three rats each and were orally administered 1000, 2000, and 3000 mg/kg body weight of EGME respectively. These doses were administered based on documented findings that the LD50 of EGME in rat is around 1000 mg/kg body weight or more [[25], [26], [27]]. Rats were checked for signs of toxicity and/or mortality for a week. Following the first phase, another three groups of one rat each were orally administered 900, 950, and 980 mg/kg body weight of EGME respectively, based on the outcomes of phase one, and were monitored for signs of toxicity and mortality. LD50 was calculated from the outcomes of the two phases.
2.3. Experimental animals and study design
Male Wistar rats (150 g; n = 20) were bought and kept in cages in the animal house facility of our department, where they were given unrestricted access to food and water. Experimental procedures were conducted by following the established protocol of the Institutional Animal Care and Use Committee that was approved by the Animal Ethical Committee of the Department of Biochemistry, Federal University of Agriculture, Abeokuta, Nigeria. At the expiration of one week of acclimatization, the animals were randomly separated into four groups containing five animals each. Animals in group one were the control and were served only rat chow and water throughout, while animals in groups two, three and four were administered 50 mg/kg EGME orally, once per day for 7, 14, and 21 days respectively. The administered dose is the 1/20th of the mean lethal dose (LD50) obtained in this research study.
2.4. Sample collections and preparations
Group 1 animals were sacrificed on day 0 before the commencement of EGME administration. EGME was administered for 7, 14, and 21 days, and 24 h after each of these days (days 7, 14, and 21); rats were sacrificed through cervical dislocation. They were handled by the international guidelines for the handling and utilization of laboratory animals [28]. The harvested testes were rinsed in cold saline (0.9% w/v) solution, dried and weighed. A portion of the testis was suspended and homogenized 0.1 M (pH 7.4) phosphate buffer. The homogenate was centrifuged at 5000 rpm for 10 min, and the resulting supernatant was kept in Eppendorf tubes and used for the estimations of the activities, levels or concentrations of biochemical parameters of interest.
2.5. Estimation of biochemical parameters
Testis concentrations of MDA, NO, GSH, and total protein were determined by following the method of Buege and Aust [29], Green et al. [30], Moron et al. [31], and Gornall et al. [32] respectively. Activities of testis GPx, GST, SOD, and CAT were estimated following the method of Rotruck et al. [33], Habig et al. [34], Misra and Fridovich [35], and Sinha [36] respectively.
2.6. Estimations of testicular levels of TNF-α, IL-1β, IL-6, IL-10, caspase-3, p53, Bax, Bcl-2, c-Myc, and K-Ras
All these were determined by following the protocols inserted in each of the ELISA kit manufactured by Cusabio Technology Llc, Houston, TX, USA.
2.7. Histopathological examination
Another section of the testis was excised and suspended in phosphate-buffered formalin solution for 48 h. After the dehydration in elevated concentrations of alcohol and clearance in xylene twice, the testicular tissues were placed in paraffin, excised into sections, stained with hematoxylin-eosin dye, and finally viewed 100× magnification under a microscope.
2.8. Statistical analysis
One-way analysis of variance (ANOVA) was used for data analyses, followed by the Tukey test for multiple comparisons among the groups of rats using Graph Pad Prism program version 6.0. Results were written as mean ± standard error of the mean. A p-value lower than 0.05 (p < 0.05) was taken to be significant statistically.
3. Results
3.1. LD50 study
Animals showed toxicity signs which include decreased food and water consumption and death after about 12 h of EGME administration. Mortality was recorded in phase one after the administration of 1000 and 3000 mg/kg body weight of EGME, while in phase two, no mortality was recorded following administration of 900, 950, and 980 mg/kg body weight of EGME. Consequently, oral LD50 of EGME was calculated using the formula: LD50 = (Do x D100), and was found to be 990 mg/kg in rat, where Do = highest dose that gave no mortality (980 mg/kg) and D100 = lowest dose that produced mortality (1000 mg/kg).
3.2. Effect of EGME on testes relative weight
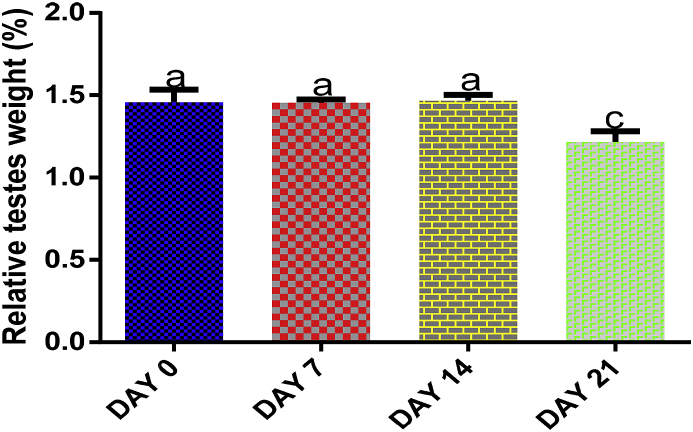
There was no significant (p > 0.05) difference in testes relative weight following 7 and 14 days of EGME exposures, but a significant (p < 0.05) decrease was recorded after 21 days compared with control, 7 and 14 days of administrations (Fig. 1).
Fig. 1.
Time course effect of EGME on relative testes weight. Results are written as mean ± standard error of the mean (n = 5). Bars containing different labels are significant statistically (p < 0.05).
3.3. Time course effect of EGME on testis MDA level
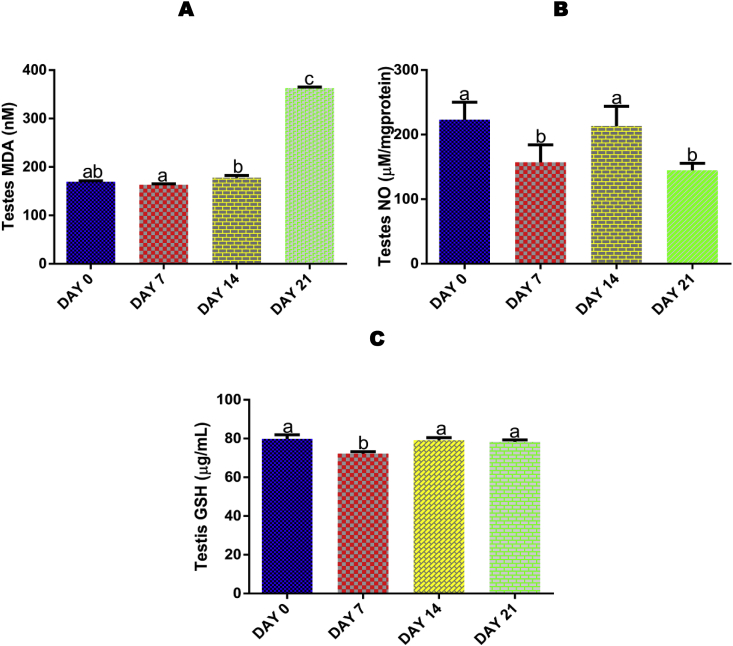
No significant (p > 0.05) effect was recorded for testis MDA after 7 and 14 days, but administrations for 21 days resulted in a significant (p < 0.05) increase compared with control, 7 and 14 days of EGME exposures (Fig. 2A).
Fig. 2.
Time course effect of EGME on testis MDA (2A), NO (2B), and GSH (2C) concentrations. Results are written as mean ± standard error of the mean (n = 5). Bars containing different labels are significant statistically (p < 0.05).
3.4. Time course effect of EGME on testis NO level
Administrations of EGME for 7 and 21 days resulted in a significant (p < 0.05) decrease in testicular NO level compared with control and 14 days of administrations (Fig. 2B).
3.5. Time course effect of EGME on testis GSH level
Administrations of EGME for 7 days significantly (p < 0.05) decreased the testis level of GSH compared with control, 14 and 21 days (Fig. 2C).
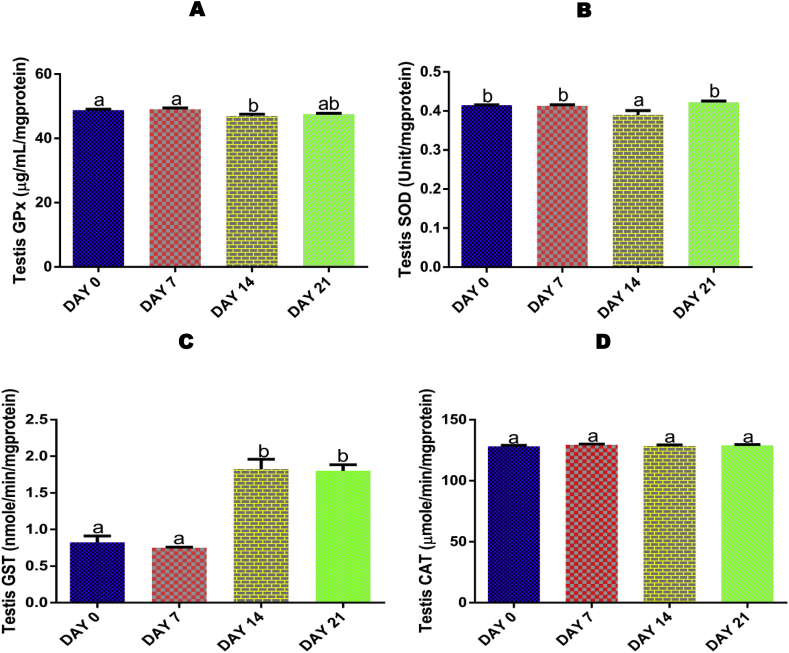
3.6. Time course effect of EGME on testis activity of GPx
Administrations of EGME for 14 days resulted in a significant (p < 0.05) decrease in testis activity of GPx compared with control and 7 days (Fig. 3A).
Fig. 3.
Time course effect of EGME on testis GPx (3A), GST (3B), SOD (3C), and CAT (3D) activities. Results are written as mean ± standard error of the mean (n = 5). Bars containing different labels are significant statistically (p < 0.05).
3.7. Time course effect of EGME on testis SOD activity
The testis SOD activity was significantly (p < 0.05) decreased following 14 days of EGME administrations compared with control, 7 and 21 days (Fig. 3B).
3.8. Time course effect of EGME on testis GST activity
For GST, both 14 and 21 days of exposure to EGME significantly (p < 0.05) increased the testicular activity of the antioxidant enzyme compared with control and 7 days of exposure (Fig. 3C).
3.9. Time course effect of EGME on testis CAT activity
Exposure to EGME for 7, 14 and 21 days did not have any significantly (p > 0.05) effect on the activity of testis CAT compared with control (Fig. 3D).
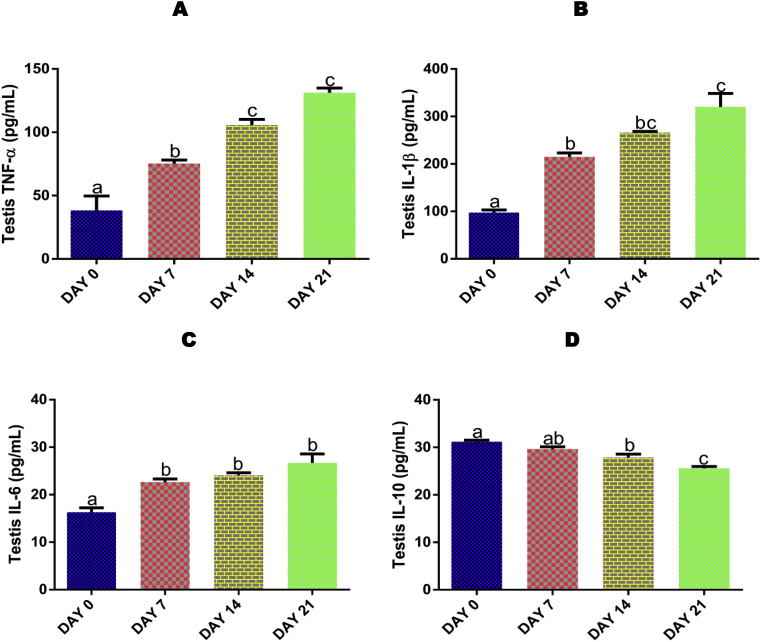
3.10. Time course effect of EGME on testis TNF-α, IL-1β, IL-6, and IL-10 levels
EGME administrations for 7, 14 and 21 days resulted in a significant (p < 0.05) increase in TNF-α (Fig. 4A), IL-β (Fig. 4B), and IL-6 (Fig. 4C) levels, as well as a significant (p < 0.05) decrease (after 14 and 21 days) in IL-10 (Fig. 4D) level in a time-dependent manner compared with control.
Fig. 4.
Time course effect of EGME on testis TNF-α (4A), IL-1β (4B), IL-6 (4C), and IL-10 (4D) levels. Results are written as mean ± standard error of the mean. Bars containing different labels are significant statistically (p < 0.05).
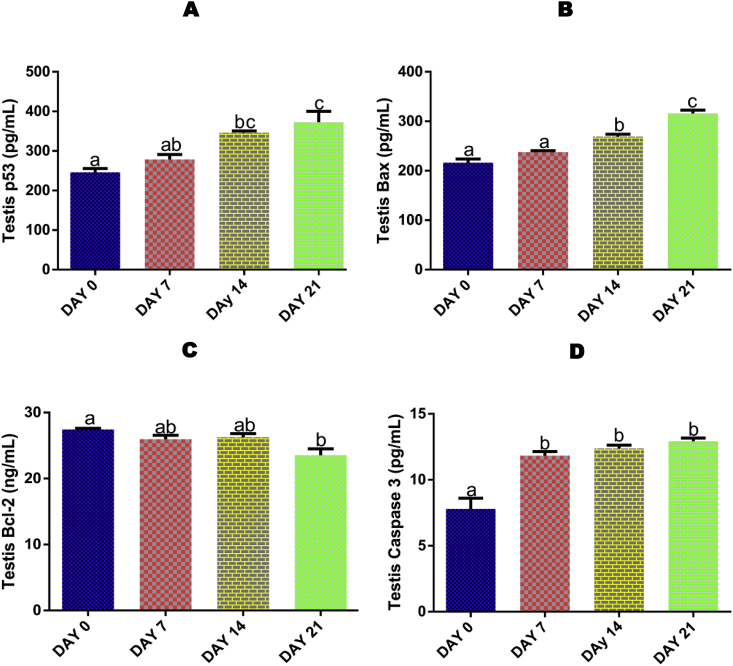
3.11. Time course effect of EGME on testis caspase 3, p53, Bax, and Bcl-2 levels
For testis p53 (Fig. 5A) and Bax (Fig. 5B), a significant (p < 0.05) increase was recorded after 14 and 21 days of EGME exposure, while Bcl-2 (Fig. 5C) was significantly (p < 0.05) decreased after 21 days compared with control. Also, after 7, 14 and 21 days of EGME administrations, testicular level of caspase-3 (Fig. 5D) was significantly (p < 0.05) increased compared with control.
Fig. 5.
Time course effect of EGME on testis p53 (5A), Bax (5B), Bcl-2 (5C) and caspase-3 (5D) levels. Results are written as mean ± standard error of the mean. Bars containing different labels are significant statistically (p < 0.05).
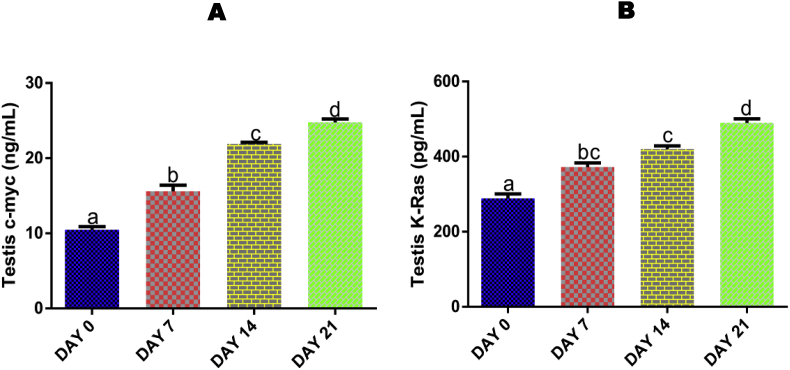
3.12. Time course effect of EGME on testis levels of c-Myc and K-Ras
Administrations of EGME for 7, 14 and 21 days significantly increased the testicular levels of c-Myc (Fig. 6A) and K-Ras (Fig. 6B) in a time-dependent manner compared with control.
Fig. 6.
Time course effect of EGME on testis c-myc (6A) and K-Ras (6B) levels. Results are written as mean ± standard error of the mean. Bars containing different labels are significant statistically (p < 0.05).
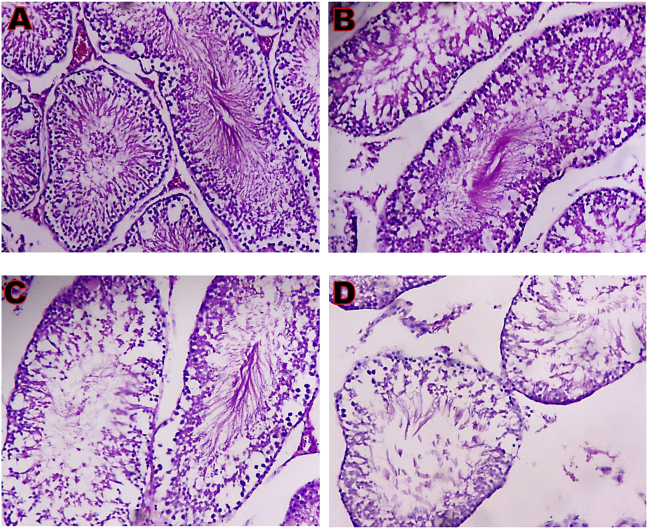
3.13. Time course effect of EGME on testis histopathology
Testis microphotograph of control showed the seminiferous epithelium and seminiferous tubules consisting of spermatocytes, spermatogonia, spermatozoa, spermatids, and Sertoli cells, while the Leydig cells in the interstitia are appearing normal (Fig. 7). After 7 days of exposure, there was a mild loss of seminiferous tubules, and the seminiferous epithelium consisting of few spermatozoa, spermatocytes, spermatogonia, Sertoli cells, and spermatids, while the interstitia have normal Leydig cells (Fig. 7). For 14 days of exposure, there was a severe loss of the seminiferous tubules, the seminiferous epithelium consisting of spermatids, spermatogonia, spermatocytes, Sertoli cells, and loss of spermatozoa, as well as disrupted interstitial tissue with few Leydig cells (Fig. 7). Following 21 days of exposure, there was a severe loss of seminiferous tubules, the seminiferous epithelium consisting of very few Sertoli cells, spermatogonia, spermatids, spermatocytes, and spermatozoa. The interstitial tissue is eroded, having scanty abnormal Leydig cells (Fig. 7.).
Fig. 7.
Testis microphotographs (x 100) showing (A) normal architecture; (B) mild loss of seminiferous tubules, the seminiferous epithelium consisting of few spermatogonia, spermatocytes, spermatids, spermatozoa and Sertoli cells; (C) loss of seminiferous tubules, the seminiferous epithelium consisting of spermatogonia, spermatocytes, and spermatids. There is a loss of spermatozoa and Sertoli cells, and a disrupted interstitial tissue with few Leydig cells; and (D) severe loss of seminiferous tubules, the seminiferous epithelium consisting of very few spermatogonia, spermatocytes, spermatids, spermatozoa, and Sertoli cells, while the interstitial tissue are eroded, having scanty abnormal Leydig cells. A = Day 0; B = Day 7; C = Day 14; D = Day 21.
4. Discussion
Greater doses of EGME cause 100% mortality and toxicity to the reproductive system, for all the major routes of exposure. The degree of EGME toxicity on the fetus is completely dependent on the period of exposure [37,38]. Following exposures in this present study, relative testes weight was significantly decreased (Fig. 1) after 21 days of EGME administrations, an indication of testicular toxicity in the rats over time [5,39].
Reactive oxygen species (ROS) generation and lipid peroxidation are the key players in testicular pathology and physiology [40]. Excessive ROS production that overcomes the mopping of these ROS by endogenous antioxidants is linked to male infertility [41]. It has been reported that the administration of EGEs leads to a steady production of ROS [42] and alterations in the anti-oxidative systems by either raising or lowering the levels of antioxidant defense systems through ROS production [43]. In this study, the significant increase in testis MDA concentration after 21 days of EGME administrations (Fig. 2A) may be an indication of oxidative stress following the generation of reactive oxygen species. The generated free radicals may be responsible for the attack of electron-rich components of biological membranes, leading to their destruction and thereby jeopardizing the cellular integrity and functions [44,45]. In a study where furan was administered to rats, testicular MDA concentration was found to increase significantly [46], while cisplatin triggered testicular and epididymal oxidative stress in rats [47].
NO is a major and vital biological molecule formed in cells, involved in the regulation of many important physiological processes, including immune response, blood pressure and neural communication [48]. Overproduction of cellular NO can cause the generation of peroxynitrite that can eventually destroy tissues [49,50]. From this study, the significant decrease in testicular NO level (Fig. 2B) after 7 and 21 days may be attributed to EGME-induced oxidative stress that may have led to excessive production of ROS. Increased ROS concentrations lower functional NO concentrations via chemical deactivation. This is referred to as NO mopping, a major outcome of oxidative stress [48].
Enough evidence has been gathered on the deleterious effects of environmental contaminants and toxicants on the male reproductive system [51,52]. Free radicals are research focus due to their contributions in cellular pathogenesis and physiology of various disorders including male and female infertility [53,54]. GSH, a key multifunctional non-enzymatic antioxidant, is also a protein in which diverse that are thiol-dependent depend on. GPx is a key peroxidase enzyme that is involved in the detoxification of hydroperoxides by catalyzing the GSH-dependent reduction of hydrogen peroxide and lipid hydroperoxides [55]. GST belongs to one of the phase two enzymes of drug metabolism that catalyzes the conjugation of GSH with xenobiotics to form water-soluble products, readily excreted from the body [56]. The mutual and synergistic actions between SOD and CAT, another two endogenous antioxidant enzymes, against the overwhelming generation of ROS helps to detoxify superoxide and peroxyl radicals by converting them into non-toxic forms [56]. The significant decrease in testicular GSH level (Fig. 2C), GPx (Fig. 3A) and SOD (Fig. 3B) activities, as well as significant increase in GST (Fig. 3C) activity following EGME administrations, can be attributed to testicular response to EGME-induced free radical generation and oxidative stress. It has been reported that an increase in the activity of GST is known to serve as protective responses to eliminate xenobiotics [43,57]. Also reported was that exposure to ethylene glycol monoethyl ether (EGEE), a member of the ethylene glycol ether family, resulted into a decrease in the testicular level of GSH [19,56,58], activities of GPx [58], and SOD [19,58], as well as a significant increase in testicular GST [19,58] activity in rats. Following the dismutation of the superoxide radicals, the resulting H2O2 is broken down to H2O and O2 by CAT [59]. GSH is the substrate for GPx, and as the former is been oxidized, there is a concomitant conversion of H2O2 to non-toxic products [60]. Also, the non-significant effect of EGME on testis CAT activity recorded in this study (Fig. 3D), suggested that scavenging of H2O2 may be through the glutathione family of antioxidants as stated above, and not by CAT.
Cytokines are involved in immune cell activities [61]. Within the testes, numerous factors including tumor necrosis factor and interleukins coordinate immune cell function. These cytokines are also formed by non-immune cells to promote and maintain sperm production [61]. In this study, the significant increase in testicular levels of TNF-α (Fig. 4A), IL-1β (Fig. 4B), and IL-6 (Fig. 4C), as well as decreased level of IL-10 (Fig. 4D) as a result of 7, 14 and 21 days of EGME administrations, is an indication of EGME-induced testicular damage, causing the spermatogenic and testicular somatic cells to produce the inflammatory cytokines in response to the damage. Also, it could be as a result of the immune response, leading to the stimulation and secretion of inflammatory cytokines by immune cells to the affected site where they initiate inflammation [62]. In our previous study, we reported that administration of methyl cellosolve significantly increase the levels of inflammatory cytokines after 7, 14 and 21 days of administration in rats [63]. Also, Khosravi et al. [64] reported an increase in the levels of inflammatory cytokines in streptozotocin-induced diabetic rats.
Apoptosis is a process of programmed cell death characterized by biochemical and morphological alterations, as well as changes in genomic expression [65,66]. p53 is actively involved in the response to cellular discomfort by serving as a major hindrance to carcinogenesis [67]. A significant increase in testicular p53 level (Fig. 5A) after 14 and 21 days of exposure is an indication of EGME-induced testicular cell damage. The damage could be attributed to EGME-induced testicular oxidative stress and inflammation recorded in this study, which may have stimulated p53 activation. Upon activation, p53 may have initiated cell cycle arrest and activation of apoptotic genes to facilitate testicular apoptosis. Apoptosis is a strictly regulated process controlled by several signaling pathways, such as the mitochondrial pathway and caspase cascade [[68], [69], [70]]. p53 is a positive regulator of the Bad, Bak, and Bax pro-apoptotic proteins to stop Bcl-2 capture. Free Bad, Bak and Bax eventually attach to the mitochondrial membrane to cause mitochondrial membrane damage and cellular apoptosis [[71], [72], [73]]. This study reveals a significant increase in testicular Bax (Fig. 5B) (after 14 and 21 days) and a decrease in Bcl-2 (Fig. 5C) levels (after 21 days) following EGME administrations, suggesting a p53-induced testicular programmed cell death. In response to testicular damage, Activated p53 recorded in the study, may have up-regulated Bax and down-regulated Bcl-2 expressions, which are two players of apoptosis, and p53 targets. An increase in the cytosolic level of free Bax may have subsequently attacked the mitochondrial membrane; creating pores in it, causing mitochondrial membrane damage and the release of cytochrome c that propagate the testicular apoptosis with other downstream mediators of apoptosis (Apaf-1, caspases-3 and 9). Past studies have reported that p53 stimulates the transcription of Bak and Bax, which controls the outflow of cytochrome c from the mitochondrion, resulting in cellular apoptosis by stimulating the excision of caspase-3 and caspase-9 [72,73]. Cellular apoptosis is a complex biological process related to complex signaling pathway responses. The activation of cysteine proteases, particularly the caspases, is a major intracellular coordinator of cellular apoptosis [74,75]. In this study, an increased level of testis caspase-3 (Fig. 5D) was recorded after 7, 14 and 21 days of EGME administrations. The released cytochrome c into the cytoplasm following the attack of Bax on the mitochondrial membrane may have interacted with downstream apoptotic mediators (Apaf-1, caspase-9) to form apoptosome that cleaved the executioner caspases including caspase-3, thereby facilitating the testicular apoptotic process. The result of caspase-3 obtained in this study is corroborated by the findings of Adedara et al. [76] who reported that exposure to ethylene glycol monoethyl ether (EGEE) resulted into a significant increase in the expressions of testicular stress-inducible proteins (active caspases, Fas and Fas-L) in rats, while 14 and 21 days of methyl cellosolve in rats led to the significant increase in renal caspase-3 in rats [63].
K-Ras is a proto-oncogene and the widely accepted mutated oncogene, that is constantly associated with some of the worst forms of cancer [77]. It has been reported that Myc is essential for K-Ras-driven cancer [78] and activation of Ras stabilizes Myc [79], enabling Myc to render cells vulnerable to DNA damage and apoptosis [80]. c-Myc is another proto-oncogene, a strong pleiotropic transcription factor known to coordinate cell cycle growth, progression, adhesion, differentiation, metabolism, proliferation, and apoptosis [[81], [82], [83]]. Testicular c-Myc (Fig. 6A) and K-Ras (Fig. 6B) levels after 7, 14 and 21 days of EGME administrations were significantly increased, which may be an indication of EGME-induced mutations in these oncogenes by amplification or translocation to areas of high transcriptional activities, resulting into their activations and subsequent generation of reactive oxygen species that may have resulted into DNA damage. Activation of these oncogenes may further explain the significant increase in the levels of apoptotic mediators, p53, Bax, caspase-3, recorded in this study that facilitated apoptosis and prevented tumor initiation and progression that may ensue as a result of uncontrolled proliferation of damaged cells.
Testis histopathology (Fig. 7) revealed the toxic effect of EGME on the testicular cells. The severe loss of the seminiferous tubules, a very few spermatogonia, spermatocytes, spermatids, spermatozoa, and Sertoli cells, as well as the erosion of the interstitial tissue with scanty abnormal Leydig cells, confirmed the outcomes of other findings in this study, and a clear indication that exposure to EGME over time may result into male infertility, hence, difficulty in childbearing.
We therefore, concluded that EGME-induced testicular toxicity may be via the disorganization of the endogenous antioxidant systems, causing the up-regulation of pro-inflammatory, apoptotic and oncogenic mediators in rats.
Funding organization
None.
CRediT authorship contribution statement
Oluwatobi T. Somade: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration. Babajide O. Ajayi: Methodology, Investigation, Resources, Supervision, Project administration. Olubisi E. Adeyi: Methodology, Investigation, Resources, Supervision, Project administration. Anuoluwapo A. Adeshina: Investigation, Resources. Adewale S. James: Methodology, Investigation, Resources, Project administration. Peter F. Ayodele: Methodology, Investigation, Resources, Project administration.
Declaration of competing interest
None to declare.
References
- 1.Takei M., Ando Y., Saitoh W., Tanimoto T., Kiyosawa N., Manabe S., Sanbuissho A., Okazaki O., Iwabuchi H., Yamoto T., Adam K.P., Weiel J.E., Ryals J.A., Milburn M.V., Guo L. Ethylene glycol monomethyl ether–induced toxicity is mediated through the inhibition of flavoprotein dehydrogenase enzyme family. Toxicol Sci. 2010;118(2):643–652. doi: 10.1093/toxsci/kfq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Ketttenis P. The historic and current use of glycol ethers: a picture of change. Toxicol Lett. 2005;156:5–11. doi: 10.1016/j.toxlet.2003.12.076. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi G., Waxman D.J. Toxicity of ethylene glycol monomethyl ether: impact on testicular gene expression. Int J Androl. 2008;31:269–274. doi: 10.1111/j.1365-2605.2007.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boatman R.J. International industry initiatives to improve the glycol ether health effects knowledge base. Toxicol Lett. 2005;156:39–50. doi: 10.1016/j.toxlet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Johanson G. Toxicity review of ethylene glycol monomethyl ether and its acetate ester. Crit Rev Toxicol. 2000;30:307–345. doi: 10.1080/10408440091159220. [DOI] [PubMed] [Google Scholar]
- 6.Cohen R. Reversible subacute ethylene glycol monomethyl ether toxicity associated with microfilm production: a case report. Am J Ind Med. 1984;6:441–446. doi: 10.1002/ajim.4700060607. [DOI] [PubMed] [Google Scholar]
- 7.Cook R.R., Bodner K.M., Kolesar R.C., Uhlmann C.S., VanPeenen P.F., Dickson G.S. A cross-sectional study of ethylene glycol monomethyl ether process employees. Arch Environ Health. 1982;37:346–351. doi: 10.1080/00039896.1982.10667589. [DOI] [PubMed] [Google Scholar]
- 8.El-Zein R.A., Abdel-Rahman S.Z., Morris D.L., Legator M.S. Exposure to ethylene glycol monomethyl ether: clinical and cytogenetic findings. Arch Environ Health. 2002;57:371–376. doi: 10.1080/00039890209601424. [DOI] [PubMed] [Google Scholar]
- 9.Larese F., Fiorito A., De Zotti R. The possible haematological effects of glycol monomethyl ether in a frame factory. Br J Ind Med. 1992;49:131–133. doi: 10.1136/oem.49.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch L.S., Cullen M.R. Effect of exposure to ethylene glycol ethers on shipyard painters: III. Hematologic effects. Am J Ind Med. 1988;14:527–536. doi: 10.1002/ajim.4700140504. [DOI] [PubMed] [Google Scholar]
- 11.Welch L.S., Schrader S.M., Turner T.W., Cullen M.R. Effects of exposure to ethylene glycol ethers on shipyard painters. II. Male reproduction. Am J Ind Med. 1988;14:509–526. doi: 10.1002/ajim.4700140503. [DOI] [PubMed] [Google Scholar]
- 12.Berndtson W.E., Foote R.H. Disruption of spermatogenesis in rabbits consuming ethylene glycol monomethyl ether. Reprod Toxicol. 1997;11:29–36. doi: 10.1016/s0890-6238(96)00194-3. [DOI] [PubMed] [Google Scholar]
- 13.Chapin R.E., Lamb J.C. Effects of ethylene glycol monomethyl ether on various parameters of testicular function in the F344 rat. Environ Health Perspect. 1984;57:219–224. doi: 10.1289/ehp.8457219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodo T., Taketa Y., Sugiyama M., Inomata A., Sonoda J., Okuda Y., Mineshima H., Hosokawa S., Aoki T. Collaborative work on evaluation of ovarian toxicity. 11) Two- or four-week repeated-dose studies and fertility study of ethylene glycol monomethyl ether in female rats. J Toxicol Sci. 2009;34(1):SP121–S128. doi: 10.2131/jts.34.s121. [DOI] [PubMed] [Google Scholar]
- 15.Doe J.E., Samuels D.M., Tinston D.J., de Silva Wickramaratne G.A. Comparative aspects of the reproductive toxicology by inhalation in rats of ethylene glycol monomethyl ether and propylene glycol monomethyl ether. Toxicol Appl Pharmacol. 1983;69:43–47. doi: 10.1016/0041-008x(83)90117-5. [DOI] [PubMed] [Google Scholar]
- 16.Hanley T.R., Young J.T., John J.A., Rao K.S. Ethylene glycol monomethyl ether (EGME) and propylene glycol monomethyl ether (PGME): inhalation fertility and teratogenicity studies in rats, mice and rabbits. Environ Health Perspect. 1984;57:7–12. doi: 10.1289/ehp.84577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exon J.H., Mather G.G., Bussiere J.L., Olson D.P., Talcott P.A. Effects of subchronic exposure of rats to 2-methoxyethanol or 2- butoxyethanol: thymic atrophy and immunotoxicity. Fund Appl Toxicol. 1991;16:830–840. doi: 10.1016/0272-0590(91)90168-4. [DOI] [PubMed] [Google Scholar]
- 18.Aasmoe L., Winberg J.O., Aarbakke J. The role of liver alcohol dehydrogenase isoenzymes in the oxidation of glycolethers in male and female rats. Toxicol Appl Pharmacol. 1998;150:86–90. doi: 10.1006/taap.1998.8410. [DOI] [PubMed] [Google Scholar]
- 19.Adedara I.A., Farombi E.O. Induction of oxidative damage in the testes and spermatozoa and hematotoxicity in rats exposed to multiple doses of ethylene glycol monoethyl ether. Hum Exp Toxicol. 2010;29(10):801–812. doi: 10.1177/0960327109360115. [DOI] [PubMed] [Google Scholar]
- 20.Foster P.M., Creasy D.M., Foster J.R., Thomas L.V., Cook M.W., Gangolli S.D. Testicular toxicity of ethylene glycol monomethyl and monoethyl ethers in the rat. Toxicol Appl Pharmacol. 1983;69:385–399. doi: 10.1016/0041-008x(83)90262-4. [DOI] [PubMed] [Google Scholar]
- 21.Starek-Swiechowicz B., Miranowicz-Dzierzawska K., Szymczak W., Budziszewska B., Starek A. Hematological effects of exposure to mixtures of selected ethylene glycol alkyl ethers in rats. Pharmacol Rep. 2012;64:166–178. doi: 10.1016/s1734-1140(12)70743-0. [DOI] [PubMed] [Google Scholar]
- 22.Chapin R.E., Dutton S.L., Ross M.D., Sumrell B.M., Lamb J.C. The effects of ethylene glycol monomethyl ether on testicular histology in F344 rats. J Androl. 1984;5:369–380. doi: 10.1002/j.1939-4640.1984.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 23.Creasy D.M., Beech L.M., Gray T.J., Butler W.H. An ultrastructural study of ethylene glycol monomethyl ether-induced spermatocyte injury in the rat. Exp Mol Pathol. 1986;45:311–322. doi: 10.1016/0014-4800(86)90020-1. [DOI] [PubMed] [Google Scholar]
- 24.Lorke D. A new approach to tropical acute toxicity testing. Arch Toxicol. 1983;53:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 25.Smyth H.F., Jr., Seaton J., Fischer L. The single dose toxicity of some glycols and derivatives. J Ind Hyg Toxicol. 1941;23:259–268. [Google Scholar]
- 26.Carpenter C.P., Pozzani U.C., Weil C.S., Nair J.H., 3rd, Keck G.A., Smyth H.F., Jr. The toxicity of butyl cellosolve solvent. AMA Archives of Industrial Health. 1956;14(2):114–131. [PubMed] [Google Scholar]
- 27.ECETOC . European Centre for Ecotoxicology and Toxicology of Chemicals; Brussels: 1995. The toxicology of glycol ethers and its relevance to man; p. 350. (ECETOC Technical Report No. 64) [Google Scholar]
- 28.NRC . National Academy Press; Washington, DC: 1996. Guide for the Care and use of laboratory animals. [Google Scholar]
- 29.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 30.Green L.C., Wagner D.A., Glogowski J., Skiper P.L., Wishnock J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 31.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 32.Gornall A.G., Bardawill C.J., David M.M. Determination of serum protein by biuret method. J Biol Chem. 1949;117:751–766. [PubMed] [Google Scholar]
- 33.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 34.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 35.Misra H.P., Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 36.Sinha A.K. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 37.IPCS . World Health Organization; Geneva: 1990. Environmental health criteria 115. 2–Methoxyethanol, 2–ethoxyethanol, and their acetates, international programme on chemical safety. [Google Scholar]
- 38.ECETOC . European Centre for Ecotoxicology and Toxicology of Chemicals; Brussels: 1995. The toxicology of glycol ethers and its relevance to man; p. 350. (ECETOC Technical Report No. 64) [Google Scholar]
- 39.Starek-Swiechowicz B., Szymczak W., Budziszewska B., Starek A. Testicular effect of a mixture of 2-methoxyethanol and 2-ethoxyethanol in rats. Pharm Represent. 2015;67:289–293. doi: 10.1016/j.pharep.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Murugesan P., Muthusamy T., Balasubramanian K., Arunakaran J. Studies on protective role of vitamin C and E against polychlorinated biphenyls (Aroclor 1254) – induced oxidative damage in Leydig cells. Free Radic Res. 2005;39:1259–1272. doi: 10.1080/10715760500308154. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki A., Gagnon C. Formation of reactive oxygen species in spermatozoa in infertile men. Fertil Steril. 1992;57:409–418. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- 42.Jurczyk A.P., Galecki P., Jankowska B., Meissner E., Szram S., Fijalkowski P., Blaszczyk J., Kedziora J., Smigielski J. Effect of ethylene glycol on antioxidative enzymes and lipid peroxidation activity in erythrocytes. Arch Med Sadowej Kryminol. 2002;52:147–153. [PubMed] [Google Scholar]
- 43.Celik I., Suzek H. Effect of subacute treatment of ethylene glycol on serum marker enzymes and erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Chem Biol Interact. 2007;167:145–152. doi: 10.1016/j.cbi.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Somade O.T., Akinloye O.A., Adeyeye M.O., Fabunmi G.D., Idowu O.O., Badmus F.O., Salaudeen B.O. Quercetin, a natural phytochemical and antioxidant protects against sodium azide-induced hepatic and splenic oxidative stress in rats. J Invest Biochem. 2015;4:69–74. [Google Scholar]
- 45.Somade O.T., Olorode S.K., Olaniyan T.O., Faokunla O. Quercetin, a polyphenolic phytochemical prevents sodium azide-induced extrahepatic oxidative stress in rats. Cog Biol. 2016;2 1200798. [Google Scholar]
- 46.Abd El-Hakim Y.M., Mohamed W.A., El-Metwally A.E. Spirulina platensis attenuates furan reprotoxicity by regulating oxidative stress, inflammation, and apoptosis in testis of rats. Ecotoxicol Environ Saf. 2018;161:25–33. doi: 10.1016/j.ecoenv.2018.05.073. [DOI] [PubMed] [Google Scholar]
- 47.Nna V.U., Ujah G.A., Suleiman J.B., Mohamed M., Nwokocha C., Akpan T.J., Ekuma H.C., Fubara V.V., Kekung-Asu C.B., Osim E.E. Tert-butylhydroquinone preserve testicular steroidogenesis and spermatogenesis in cisplatin-intoxicated rats by targeting oxidative stress, inflammation and apoptosis. Toxicology. 2020:152528. doi: 10.1016/j.tox.2020.152528. [DOI] [PubMed] [Google Scholar]
- 48.Pierini D., Bryan N.S. Nitric oxide availability as a marker of oxidative stress. Methods Mol Biol. 2014;1208:63–71. doi: 10.1007/978-1-4939-1441-8_5. [DOI] [PubMed] [Google Scholar]
- 49.Ajayi B.O., Adedara I.A., Farombi E.O. Benzo(a)pyrene induces oxidative stress, pro-inflammatory cytokines, expression of nuclear factor-kappa B and deregulation of wnt/beta-catenin signaling in colons of BALB/c mice. Food Chem Toxicol. 2016;95:42–51. doi: 10.1016/j.fct.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Ignarro L.J., Cirino G., Casini A., Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Aitken R.J., Koopman P., Lewis S.E. Seeds of concern. Nature. 2004;432:48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- 52.Mathur P.P., Saradha B., Vaithinathan S. Impact of environmental toxicants on testicular function. Immunol Endocr Metab Agents Med Chem. 2008;8:79–90. [Google Scholar]
- 53.Mehta A., Verma R.S., Srivastava N. Chlorpyrifos induced alterations in the levels of hydrogen peroxide, nitrate and nitrite in rat brain and liver. Pestic Biochem Physiol. 2009;94:55–59. [Google Scholar]
- 54.Farombi E.O., Adedara I.A., Akinrinde S.A., Ojo O.O., Eboh A.S. Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rat. Andrologia. 2012;44:273–284. doi: 10.1111/j.1439-0272.2012.01279.x. [DOI] [PubMed] [Google Scholar]
- 55.Jee J.H., Kang J.C. Biochemical changes of enzymatic defense system after phenanthrene exposure in olive flounder, Paralichthys olivaceus. Physiol Res. 2005;54:585–591. [PubMed] [Google Scholar]
- 56.Adedara I.A., Farombi E.O. Kolaviron protects against ethylene glycol monoethyl ether-induced toxicity in boar spermatozoa. Andrologia. 2014;46:399–407. doi: 10.1111/and.12095. [DOI] [PubMed] [Google Scholar]
- 57.Smith G.J., Litwack G.C. Role of ligandin and the Glutathione-S-transferases in binding steroid metabolites, carcinogens and other compounds. Rev Biochem Toxicol. 1980;2:1–47. [Google Scholar]
- 58.Adedara I.A., Farombi E.O. Chemoprotection of ethylene glycol monoethyl ether-induced reproductive toxicity in male rats by kolaviron, isolated biflavonoid from Garcinia kola seed. Hum Exp Toxicol. 2012;31(5):506–517. doi: 10.1177/0960327111424301. [DOI] [PubMed] [Google Scholar]
- 59.Tomlin C.D. tenth ed. The British Crop Protection Council; Surrey, UK: 1994. The E-pesticide manual. [Google Scholar]
- 60.Somade O.T., Adeniji K.D., Adesina A.A., Olurinde O.J. Oral acute toxicity study as well as tissues oxidative stress and histopathological disorders in edible camphor administered rats. Exp Toxicol Pathol. 2017;69:99–108. doi: 10.1016/j.etp.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Loveland K.L., Klein B., Pueschl D., Indumathy S., Bergmann M., Loveland E., Hedger M.P., Schuppe H.C. Cytokines in male fertility and reproductive pathologies: immunoregulation and beyond. Front Endocrinol. 2017;8:307. doi: 10.3389/fendo.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somade O.T., Ajayi B.O., Safiriyu O.A., Oyabunmi O.S., Akamo A.J. Renal and testicular up-regulation of pro-inflammatory chemokines (RANTES and CCL2) and cytokines (TNF-α, IL-1β, IL-6) following acute edible camphor administration is through activation of NFkB in rats. Toxicol Rep. 2019;6:759–767. doi: 10.1016/j.toxrep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somade O.T., Ajayi B.O., Olushola M.O., Omoseebi E.O. Methyl cellosolve-induced renal oxidative stress and time-dependent up-regulation of pro-inflammatory cytokines, apoptotic, and oncogenic markers in rats. Toxicol Rep. 2020;7:779–787. doi: 10.1016/j.toxrep.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khosravi Z., Sedaghat R., Baluchnejadmojarad T., Roghani M. Diosgenin ameliorates testicular damage in streptozotocin-diabetic rats through attenuation of apoptosis, oxidative stress, and inflammation. Int Immunopharm. 2019;70:37–46. doi: 10.1016/j.intimp.2019.01.047. [DOI] [PubMed] [Google Scholar]
- 65.Kajta K. Apoptosis in the central nervous system: mechanisms and protective strategies. Pol J Pharmacol. 2004;56:689–700. [PubMed] [Google Scholar]
- 66.Philchenkov A. Caspases: potential targets for regulating cell death. J Cell Mol Med. 2004;8:432–444. doi: 10.1111/j.1582-4934.2004.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haupt S., Berger M., Goldberg Z., Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 68.Thornberry N.A., Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 69.Herr I., Debatin K.M. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 70.Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;26:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 71.Cheng E.H., Wei M.C., Weiler S., Flavell R.A., Mak T.W., Lindsten T., Korsmeyer S.J. Bcl-2, Bcl-X(L) sequester BH3 domain-only molecules preventing BAX and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 72.Degenhardt K., Chen G., Lindsten T., White E. BAX and BAK mediate p53 independent suppression of tumorigenesis. Canc Cell. 2002;2:193–203. doi: 10.1016/s1535-6108(02)00126-5. [DOI] [PubMed] [Google Scholar]
- 73.Henry H., Thomas A., Shen Y., White E. Regulation of the mitochondrial checkpoint in p53 mediated apoptosis confers resistance to cell death. Oncogene. 2002;21:748–760. doi: 10.1038/sj.onc.1205125. [DOI] [PubMed] [Google Scholar]
- 74.Mu R., Lu N., Wang J., Yin Y., Ding Y., Zhang X., Gui H., Sun Q., Duan H., Zhang L., Zhang Y., Ke X., Guo Q. An oxidative analogue of gambogic acid-induced apoptosis of human hepatocellular carcinoma cell line HepG2 is involved in its anticancer activity in vitro. Eur J Canc Prev. 2010;19:61–67. doi: 10.1097/CEJ.0b013e328333fb22. [DOI] [PubMed] [Google Scholar]
- 75.Alenzi F.Q., Alenazi B.Q., Al-Anazy F.H., Mubaraki A.M., Salem M.L., Al-Jabri A.A., Lotfy M., Bamaga M.S., Alrabia M.W., Wyse R.K. The role of caspase activation and mitochondrial depolarisation in cultured human apoptotic eosinophils. Saudi J Biol Sci. 2010;17:29–36. doi: 10.1016/j.sjbs.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adedara I.A., Mathur P.P., Farombi E.O. Kolaviron prevents ethylene glycol monoethyl ether-induced testicular apoptosis via down-regulation of stress proteins, Fas/Fas-L and caspases expressions in rats. Toxicol Mech Methods. 2013;23(9):689–696. doi: 10.3109/15376516.2013.843107. [DOI] [PubMed] [Google Scholar]
- 77.Ischenko I., Zhi J., Hayman M.J., Petrenko O. K-Ras-dependent suppression of MYC enhances the sensitivity of cancer cells to cytotoxic agents. Oncotarget. 2017;8(11):17995–18009. doi: 10.18632/oncotarget.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eilers M., Eisenman R.N. Myc’s broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee T., Yao G., Nevins J., You L. Sensing and integration of Erk and PI3K signals by Myc. PLoS Comput Biol. 2008;4 doi: 10.1371/journal.pcbi.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shortt J., Johnstone R.W. Oncogenes in cell survival and cell death. Cold Spring Harbor Perspect Biol. 2012;4(12) doi: 10.1101/cshperspect.a009829. a009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitfield J.R., Soucek L. Tumor microenvironment: becoming sick of myc. Cell Mol Life Sci. 2012;69:931–934. doi: 10.1007/s00018-011-0860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer N., Penn L.Z. Reflecting on 25 years with myc. Nat Rev Canc. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 83.Miller D.M., Thomas S.D., Islam A., Muench D., Sedoris K. C-myc and cancer metabolism. Clin Canc Res. 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]