Abstract
Purpose
This study describes the protocol for the design and evaluation of a self-assessment based educational program supporting cancer patients’ return-to-work (RTW), prior to its complete and ongoing implementation.
Methods
We designed a multi-center, randomized controlled trial with three follow-up points. The study population (N = 239) includes recently diagnosed cancer patients who plan to receive active treatment at two university hospitals in Korea. A pre-test is conducted at the point of enrollment for both groups. The intervention group receives a leaflet clarifying misconceptions about RTW and is shown a video clip of patient interviews concerning RTW. The control group receives a booklet about cancer and nutrition, and is not provided with further intervention. After active treatment, the intervention group receives a one-time, face-to-face education session with an oncology nurse. Following the education session, both groups receive three follow-up phone calls. The first follow-up call occurs at the end of intervention and at the end of active treatment for intervention and control groups, respectively. The next two follow-up calls will be conducted one month and a year following the post-test. The primary outcome is whether the patient has RTW or has plans to RTW, and the secondary outcome is knowledge of RTW.
Results
As of April 2020, 239 patients have been enrolled in the trial. Statistical analyses will be conducted upon trial completion in December 2020.
Discussion
We hypothesize that the provision of RTW education near diagnosis will not only enhance patients’ intentions to RTW, but also effectively encourage them to RTW.
Keywords: Oncology, Education, Neoplasms, Return to work, Survivorship
1. Introduction
With recent improvements in cancer survival rates, there has been an increased emphasis on long-term care and management for patients following active treatment. Especially with increased cancer diagnoses among working age and younger people [[1], [2], [3]], and prolonged survival periods after treatment [4], the return-to-work (RTW) of cancer patients has become an important survivorship issue. For individual patients, RTW is often a major goal following treatment due to its association with returning to a “normal life” and a complete recovery [5,6]. Studies suggest that it is also associated with improved quality of life [7]. Furthermore, for society-level agendas, the retaining of cancer patients in the workforce can serve as a marker of economic productivity and sustainability, especially in an aging society [8,9].
Despite such benefits of RTW, unemployment rates remain an unresolved agenda for cancer survivorship [10]. Studies report that 26–53% of cancer survivors lose or quit their jobs during or following the treatment process [11]. Another study shows that the decision to resign occurs prior to initial treatment, suggesting the need for early intervention [12]. Nonetheless, RTW is not an easy task for cancer patients. Physical and psychological burden of cancer and its treatment, such as fatigue, pain, side effects, anxiety, and depression, can interfere with patients’ ability to RTW [[13], [14], [15], [16]]. Patients require assistance to address both personal (physical and psychological) and vocational issues to facilitate the transition back to work.
In recent literature, several studies have documented the development or efficacy of various interventions, including psycho-educational, vocational, and physical support, to facilitate RTW of cancer patients [[17], [18], [19], [20]]. Results from several randomized controlled trials (RCT) suggest that multidisciplinary interventions, as compared to a single type of intervention, are more effective in improving RTW outcomes [17,19].
While RTW interventions were shown to be effective in addressing cancer patients’ vocational issues, there have not yet been RTW interventions in Korea. Further, there is an increasing number of young survivors who are economically active [2]. Five-year survival rates for all types of cancer have improved since 2012, which is comparable to that of other developed countries [2,21]. Forty-seven percent of Korean cancer patients reported losing their jobs following a cancer diagnosis and experienced issues in re-employment [22]. Further, while research is limited, a few studies suggest that Korean cancer patients have unique unmet needs regarding employment and work after diagnosis, including low rates of RTW and public stigma associated with cancer [[23], [24], [25]].
Therefore, we developed the START program, a tailored intervention program that supports the RTW of patients who have recently been diagnosed with cancer in Korea. We aim to evaluate the effectiveness of such programs on improving RTW outcomes through a multi-center, randomized controlled trial (RCT). We hypothesize that the proportion of participants who RTW or have specific plans to RTW will increase after intervention, compared to the control group.
2. Objective
This paper presents the design of a study that aims to evaluate the effectiveness of a return-to-work intervention program for cancer patients in Korea who have recently been diagnosed with cancer.
3. Materials, methods and analysis
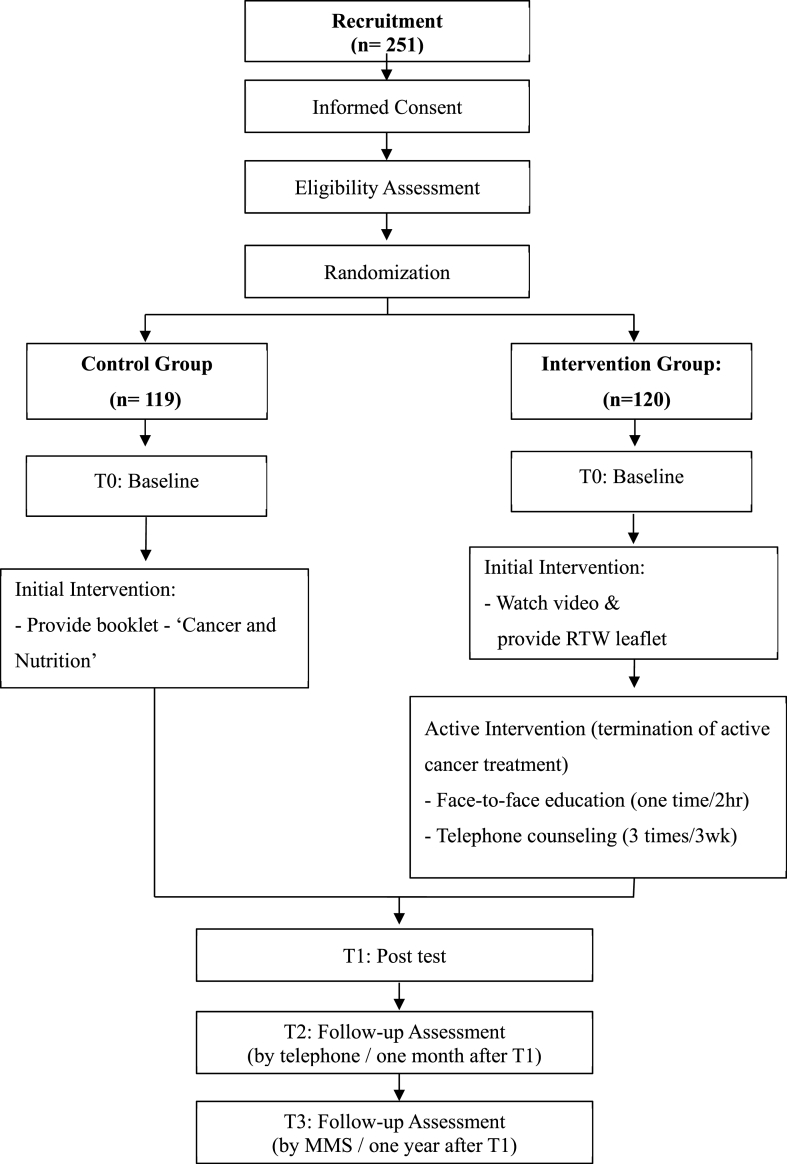
This multi-center clinical trial is being conducted from October 2018 to February 2020 at two university-based cancer centers in Seoul and Suwon, Korea. This study takes the form of a two-armed RCT, with follow-up at one month and one year post-intervention (see Fig. 1). We plan to compare the outcomes of the individually tailored RTW intervention group with the control group who receive usual care and an educational booklet on cancer and nutrition.
Fig. 1.
Flowchart of START RCT
3.1. Study population
Eligibility to participate in the study was as follows: patients who 1) are between the ages of 20 and 69; 2) received or plan to receive active curative treatment, such as surgery, adjuvant chemotherapy, or radiation therapy; 3) discontinued their employment (closure of a privately-owned business or sick leave) within one month after cancer diagnosis; and 4) understood and agreed to the contents of the study. Exclusion criteria were patients who 1) had specific plans to retire or discontinue owning a business within one year, 2) with a treatment period that exceeds 8 months from diagnosis, and 3) were not able to speak and read Korean. We sought to recruit 180 patients from cancer centers in Seoul, and 60 patients from Suwon. However, we were aware that proportions may change later in the study due to unforeseen circumstances at each hospital.
3.2. Sample size calculation
The sample size for the trial was calculated to address the hypothesis that those in the intervention group are more likely to actualize their plans to RTW post-treatment, and thus, discontinue participation in the study, compared to the control group. Based on results from a nationwide survey on cancer patients in Korea [22], we expect that approximately 40% of cancer patients will RTW after cancer diagnosis. To verify our hypotheses, χ2 tests, which assume 80% power and a two-sided α-level of 0.05, have indicated that we should include 102 patients per arm. Expecting approximately 15% losses during follow-up, we increased the sample size to 120 patients per arm, for a total of 240 randomized participants. The sample size was calculated using STATA version 14.0 (StataCorp, Texas).
3.3. Recruitment
Patients are recruited into the study through voluntary participation via recruitment posters on hospital bulletin boards or by referral from treating oncologists. A trained researcher then explains the purpose of the study and confirms eligibility of the study participants. Informed consent is directly obtained from each participant. Participants are informed that they could withdraw from the study at any time.
3.4. Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
3.5. Randomization
3.5.1. Allocation
Patients were randomized in a 1:1 ratio into intervention and control groups. Using an online software (Sealed Envelope Ltd. 2017), an epidemiologist not involved in the study generated random blocks that were sized 2 or 4, and stratified the sample by hospital (Seoul or Suwon), type of cancer (breast, liver, lung, colon, or others), and type of job (white or blue collar).
3.5.2. Blinding
Allocation information was sent in a sealed envelope by independent researchers to coordinators who were responsible for enrolling participants into the study. The envelope was not allowed to be opened until after recruitment was completed. Since the purpose of intervention is education, patients and investigators could not be blinded during the trial. However, data analysts who will analyze the data and investigators who will observe patient outcomes at follow up are to be blinded.
3.6. Intervention
3.6.1. Intervention group
The START program includes three components: 1) a brief leaflet and video education at diagnosis (enrollment), 2) a face-to-face education session at completion of active treatment, and 3) three telephone counseling sessions following treatment.
At enrollment, those in the intervention group receive a 3-fold leaflet and are shown a 5-min video clip on a tablet device. The leaflet aims to provide information about misconceptions concerning cancer patients' RTW through provision of the five most frequently asked questions about RTW, a brief checklist for RTW, and access to resources for cancer patients to assist with RTW. The video consists of brief interviews with healthcare professionals and other cancer survivors who discuss cancer patients’ RTW. In the video, healthcare professionals provide encouragement and advice concerning RTW and clarify the misconceptions that patients may have concerning RTW. Also, the video features interviews with cancer survivors who talk about their experiences of having successfully returned to work.
Once patients complete their planned treatment, they are asked to attend a face-to-face education session with a trained oncology nurse. During the education session, the nurse guides the patients through a series of educational materials titled ‘RTW guide for cancer patients,’ which consists of a one-page handout and three booklets. The education materials have been developed by the research team to address the vocational needs of cancer patients through a systematic literature review, qualitative interviews, and network analyses of online cancer communities. The individual content of the educational materials are as follows: (1) First, the nurse reviews the one-page handout titled “Debunking Myths of RTW,” which addresses six major misconceptions of working despite cancer, such as “Will working after cancer diagnosis cause recurrence?” and “Is it mandatory that I rest during cancer treatment?’’. The nurse encourages patients that, unless they are working in a directly hazardous environment, having a daily work routine may have more benefits than harm, and suggests that the patient consult a healthcare professional about working again. (2) Next, the nurse guides the patient through a booklet titled “Checking Myself,” which is an interactive guide that helps patients self-assess the pros and cons of RTW, workload, work environment, and one's physical and mental capability to carry out tasks at work. The last two booklets are on how to maintain (3) physical health and (4) mental health while working. The physical health guide allows patients to create their own exercise plans and provides dietary and lifestyle tips on sustaining physical health. The mental health guide provides realistic suggestions for managing stressful situations such as conflicts with coworkers/employers, requests for adjustments or reductions in workload, or the job search process.
After the group education session, patients receive three weekly individual telephone counseling sessions conducted by the oncology nurse who provides face-to-face education. During the first session, the nurse addresses the patient's level of fatigue and provides counseling and feedback on the patient's self-assessment for their physical readiness to RTW. The second session addresses the patient's mental and emotional readiness to RTW. The nurse also checks how the patient has been coping with their stress and fear of recurrence. At the final counseling session, the nurse provides feedback and counseling concerning the patient's vocational barriers to RTW, as well as reminders from the learning materials that had been addressed at the face-to-face education session.
3.6.2. Control group
Participants in the control group are provided with a booklet titled “Cancer and Nutrition” at enrollment. They do not receive any further education at follow up. Once the RCT trial comes to an end, copies of all the educational materials used for the intervention group are then sent to those in the control group.
3.7. Procedure
Data on outcomes are collected at four time points during the study: baseline (T0), at the end of intervention (T1), one-month post-intervention (T2), and finally one-year post-intervention (T3). The flowchart of the RCT procedure is described in Fig. 1. Baseline data (pre-test) collection occurs immediately following enrollment. Then, the first follow-up data (post-test) is collected at the end of the intervention for the intervention group and after termination of active treatment for the control group. Finally, both control and intervention groups are contacted by a blinded researcher via telephone, one month and one year following the post-test. In addition, we asked the intervention group how satisfied they were and what component of the intervention they liked most at T2. Information on measured outcomes at each time point is described in Table 1.
Table 1.
Outcomes measured at each time point.
| Outcomes | T0a (Baseline) | T1a (Post-test) | T2a (1 month after intervention) | T3a (1 year after intervention) |
|---|---|---|---|---|
| Plan for return to work | O | O | O | O |
| Return to work | O | O | O | |
| Knowledge of RTW | O | O | ||
| Work Ability Index | O | O | Ob | |
| Brief Fatigue Index | O | O | ||
| EORTC QLQ C30c | O | O | Od | |
| Fear of cancer recurrence | O | O | ||
| Spiritual well-being | O | O | ||
| Working As Meaning Inventory | O | O | ||
| Socioeconomic characteristics | O | O | ||
| Work-related characteristics | O | O | ||
| Disease characteristics | O | O |
T0 is baseline (at diagnosis),T1 is right after the intervention, T2 is 1 month after the intervention and T3 is 1 year after the intervention.
Only work ability was measured.
EORTC QLQC30: European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire.
Only global health and QoL was measured.
3.8. Outcomes and measurements
3.8.1. Primary outcome
The primary outcome measure of the study is whether the patient has returned to work or has plans to RTW at post-test. Patients are asked about their current employment status, whether they are 1) currently working, 2) on sick leave or leave of absence (temporarily closed, for self-employed patients), 3) using vacation days, or 4) resigned (permanently closed, for self-employed patients). For those who report that they are currently working, we consider them as having returned-to-work. For those who are not currently working, we assess whether they have plans to RTW, by asking “Do you have specific plans to return to work?“. If patients answer “yes,” we assess specific planned dates to RTW. For those who do not have plans to RTW, we ask them to state their reasons for not having plans.
3.8.2. Secondary outcome
3.8.2.1. Knowledge of return-to-work
Knowledge of RTW is measured using a 10-item questionnaire that assesses the patient's agreement with common misconceptions related to working through cancer. The items are scored from 1 to 4 (1 = not at all; 4 = very much). As there are no valid instruments for assessing knowledge of RTW, the research team developed items for the questionnaire ahead of trial. A group of experts (two oncology nurses, two social scientists, two general physicians, and one epidemiologist) have come together to review previous qualitative and quantitative literature and have pooled items related to misconceptions of RTW. Then, they conducted semi-structured interviews with 50 cancer patients on their knowledge of RTW to select items for the questionnaire.
3.8.3. Other outcomes
3.8.3.1. Work-related information
At T0, respondents are asked about the age they first started working, their most recent occupation, their most recent occupation's employment period, and their employment type (permanent, temporary contract, self-employed, etc.). At T1, respondents are asked about their employment type and whether they are still in the same occupation as before the cancer diagnosis.
Work ability is measured using a section from the Work Ability Index (WAI), an instrument developed for occupational health services and to assess workers' work ability [26]. In this study, we utilize three items that measure respondents' ability to work, which are current work ability compared with the lifetime best, work ability in relation to the demands of the job, and estimated work impairment due to disease [26]. The overall score of the original scale ranges from 7 to 49 and are classified as follows: poor, moderate, good and excellent [26]. However we measure the current work ability compared with the lifetime best on a scale of 0–10 (0 = completely unable to work; 10 = work ability is at its best) and the remaining two items are used to confirm patients’ physical and mental demands. All items are measured at follow-up assessments (T2, T3).
3.8.3.2. Clinical information
Clinical information, which includes cancer type and stage, comorbidities, diagnosis date, surgery date, treatment type (surgical procedure by cancer type), adjuvant therapy type (chemotherapy, radiation therapy, targeted therapy, etc.), and psychiatric treatment, were obtained from the electronic medical record (EMR) system.
3.8.3.3. Other variables
We have also collected data on additional variables that could influence RTW, including fatigue, QoL, fear of cancer recurrence (FCR), spiritual well-being, and meaning of work.
Fatigue is measured using the Brief Fatigue Inventory (BFI) [27], for which the Korean version of the inventory has been validated in previous literature [28]. The BFI is a 9-item questionnaire that measures cancer-related fatigue and all items are scored from 0 to 10 (0 = no fatigue or does not interfere; 10 = as bad as you can imagine or completely interferes). The total score ranges from 0 to 90. Mean score is calculated and higher score means more fatigue.
Quality of life is measured using the EORTC-QLQ C30, a measurement tool developed to assess the quality of life of cancer patients. The tool has been validated among Korean speaking cancer patients [29]. It contains 30 items, and includes scales for global health status, functional scales, and a symptoms scale. Scores range from 0 to 100. Mean score is calculated from a range of 0–100, where higher scores on global health status, functional scales and a lower score on symptoms scale indicate a higher quality of life. Only two items of the tool are measured at follow up (T2, T3): overall health and QoL in the past week.
Fear of cancer recurrence is measured using nine items of the Korean version of the Fear of Cancer Recurrence Inventory (K-FCRI) [30]. Out of the 42 items, 9 items that measure severity of fear, and that comprise the severity subscale, are used in this study. Each item is scored on a Likert scale from 0 to 4 (0 = not at all or never; 4 = a great deal or all the time). Mean score is calculated from an overall score ranging from 0 to 36. Higher score indicates a higher level of fear of cancer recurrence.
Spiritual well-being is measured using four items from the Korean version of the Quality of Life-Cancer Patient (QOL-CS-K), which measures spiritual well-being [31]. Each item is scored on a Likert scale from 0 to 4 (0 = not at all or never; 4 = a great deal or all the time). Mean score is calculated from an overall score ranging from 0 to 16, where a higher score on the scale indicates a higher spiritual well-being
Meaning of work is assessed using the Work and Meaning Inventory (WAMI) [32], which has been translated to Korean in previous literature [33,34]. The WAMI measures the subjective experience of meaning in one's work to benefit the greater good. It consists of 10 questions and is scored from 1 to 5 (1 = absolutely untrue; 5 = absolutely true), and has been found to be reliable among Korean workers. Mean score is calculated from a range of 10–50, where a higher score indicates participants finding greater value in their work.
3.9. Demographic/socioeconomic variables
Demographic variables measured at T0 are assessed, including residence, marital status, education level, cohabitation status, household income and main household income producer, insurance type (regular or Medicaid), ownership of private insurance, smoking history, and drinking history. At T1, meanwhile, coverage by private insurance, healthcare costs (direct/indirect), major source of funding for treatment, and smoking and drinking habits are assessed. In addition, we asked participants in the intervention about overall satisfaction of the intervention and the most helpful component of the intervention at T2.
3.10. Data management
Personal information collected in this study will not be shared with any third party during or after the trial and will only be utilized for statistical analysis. All responses are coded with a distinct patient identifier number to avoid identification of personal information. Hard copies of the questionnaires are stored in a locked file cabinet in a locked room.
3.11. Statistical analysis
All analyses will be conducted using the intention-to-treat principle, as study patients will be assigned to their randomized group irrespective of compliance with the study intervention. Continuous variables will be summarized as means and standard deviations, and categorical variables as proportions. Differences in baseline characteristics between the intervention and control groups will be compared with t-tests and χ2 tests for continuous and categorical variables, respectively. For the analysis of primary outcomes, we will compare the proportion of participants who return to work or have plans to RTW using χ2 tests. Missing data will be replaced by last-observation-carried-forward. To test our hypothesis that the proportion of those who RTW or have plans to RTW will increase following intervention, linear mixed regression models to calculate least square estimates of slopes will be used to assess longitudinal within-subject change. We do not plan to perform any interim analyses. P-values of <0.05 will be considered statistically significant. All analyses will be conducted using STATA version 14.0 (StataCorp, Texas).
4. Ethics and dissemination
The study has been approved by the institutional review board and ethics committee at Samsung Medical Center (No. SMC 2018-08-034) and Ajou University Medical Center (No. AJIRB-MED-SUR-18-375). The results of the trial will be submitted for publication in peer-reviewed academic journals, and disseminated at relevant conferences, workshops and websites to be used by cancer patients, hospitals with cancer treatment centers, and healthcare professionals such as oncologists and oncologic nurses. The intervention tools used in the study will be refined based on the feedback patients provide upon completion of the study and will be made available on websites and in print.
5. Discussion
This protocol outlines the methodology of the RCT study designed to assess the START program, a tailored, self-assessment based RTW intervention for patients who have recently been diagnosed with cancer.
Previous RTW interventions have mainly targeted patients that have completed treatment or were receiving treatment [[35], [36], [37]]. The timing of enrollment into intervention and duration of intervention have been identified as important factors in designing RTW interventions for patients with lower back pain [38]. According to results from our preliminary qualitative interviews and quantitative survey, many patients decided to stop working as soon as they suspected a diagnosis of cancer, even before confirmation of the diagnosis. Further, at the end of treatment, patients reported difficulties in re-employment. Therefore, to prevent patients from resigning from their jobs prematurely, we sought to provide intervention immediately after diagnosis. By preventing premature resignation or prolonged sick leave, early intervention for RTW has shown to benefit patients’ RTW in various non-cancer populations [39,40]. However, it is difficult to deliver work-related information to patients who have recently been diagnosed with cancer, as they are still coping with the shock and confusion regarding news of the diagnosis. Therefore, we provide a leaflet and video clip, validated by oncologists, which could deliver work-related information in a concise and captivating manner. The remaining parts of the intervention are rolled out following termination of active treatment, and when the patient is ready to consider working again.
Another advantage of this intervention is its self-assessment component that empowers patients to determine their own work ability and adjust the timing of their RTW. Deciding when to begin working again is one of the major concerns of cancer patients in the RTW process [41]. The vocational needs of cancer patients can vary depending on job type, job environment, and health condition of the patient. The diverse needs of each cancer patient cannot be addressed with a one-size-fits-all approach. A self-assessment-based intervention allows for patients to make tailored RTW plans and decisions by evaluating their own work ability and work demands. The self-assessment component of the START program allows more patients to participate in RTW planning regardless of their occupations or health condition.
Furthermore, built on evidence through qualitative interviews of patients and network analyses of online cancer communities, the START program reflects the actual voices of cancer patients. The program provides education and counseling for real problems that cancer patients face in the RTW process including communication, fatigue, and cancer-related worries. Studies have found that cancer patients are exposed to false information that encourages patients to quit their jobs [24,42]. The program also addresses these misconceptions that patients may have regarding returning to work.
While the START program is a package of interventions (educational information at diagnosis, face-to-face education at end of treatment, and telephone counseling sessions), each intervention can stand on its own as a separate intervention. While the intervention is designed for all three components to be consecutively delivered to a single patient, it may not be feasible for implementation in clinical settings. Therefore, when we designed each part of the intervention, we ensured that the contents of each intervention can be delivered independently. For example, the three follow-up telephone counseling sessions can stand alone as a single RTW intervention. We believe this flexibility improves compliance to the intervention and sustainability in busy clinical settings.
There are several limitations in this study. First, only one oncology nurse will be used for the main intervention. Currently, this oncology nurse is specially trained for RTW intervention. In future interventions, involvement of more oncology nurses or physicians are recommended. Secondly, there could be many patients who might not be able to participate in the interventions and follow ups, especially in the intervention group. We hypothesize that those in the intervention group are more likely to work due to early exposure to RTW information at diagnosis. As such, those who are attending work may find it difficult to attend educational sessions held at the hospital, resulting in higher dropout rates. Nighttime sessions or video chat would facilitate attendance of the program. It would be worth to try to develop and test an online-based intervention in the future. Thirdly, due to voluntary participation, the trial may attract participants with already strong intentions to RTW, even prior to the intervention. However, we believe the randomization process will alleviate any potential self-selection biases. Fourth, while control group receive initial educational material, they would receive less attention and time compared to the intervention group who received a group session and 3 individual nurse phone calls resulting in a potential confounder. Lastly, we might not include some relevant socio-demographic or clinical variables for RTW such as the number and age of the children.
This study was the first to develop a systematic RTW intervention for cancer patients in Korea. We anticipate the successful implementation of the intervention in hospital settings will assist employment pursuits among cancer patients in their decision to RTW. We believe that the intervention is an efficient hospital-based program that can provide realistic information to cancer patients— in an environment where accurate information, policies, and national systems relating to the RTW of cancer patients are scarce.
Ethics approval and consent to participate
The institutional review board and ethics committee at Samsung Medical Center (No. SMC 2018-08-034) and Ajou University Medical Center (No. AJIRB-MED-SUR-18-375) approved the study. Patients sign an informed consent form before participating.
Consent for publication
Not applicable.
Availability of data
Not applicable.
Author contributions
KB: developed the intervention and the educational materials. DK: Supervision, had supervised the sample size calculation and randomization, and provided statistical analysis consultations. YJ: Writing - original draft, helped to draft the manuscript. JC: obtained funding for this study and was the principal investigator of the START project. KI: developed the intervention and the educational materialsJY: Writing - original draft, helped to draft the manuscript. SK: recruited patients for previous quantitative study at Chonnam National University Hwasun Hospital and recommended this project. JA: recruited patients at Samsung Madical Center. SN: recruited patients at Samsung Madical Center. YS: recruited patients at Samsung Madical Center, Also. MC: recruited patients and conducted the trial at Ajou University. JH: Writing - original draft, recruited patients and conducted the trial at Ajou University. All co-authors have read and commented on the draft manuscript.
Funding
This study was supported by a grant from the National R&D program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (No. 1720220) and Goldman Sachs Gives.
Current trial status
Recruitment, intervention, and follow-up are being simultaneously conducted depending on the enrollment date and treatment schedule of patients. The first patient has already been included in the trial as of October 2018.
Trial registration
The trial, as of October 12, 2018, is registered at the Clinical Research Information Service (Korean Trial Registry), with the trial number KCT0003262.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Special thanks to Sungkyunkwan University (Seo Heui Jeon), Samsung Medical Center (Ayoung Lee), and Ajou University Medical Center (Eunae Jeon, Mijin Jung).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2020.100633.
Contributor Information
Jaesung Heo, Email: md.js.informatics@gmail.com.
Juhee Cho, Email: jcho@skku.edu.
Abbreviations
- RTW
Return to work
- RCT
Randomized controlled trial
- CRIS
Clinical Research Information Service
- QoL
Quality of life
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Short P.F., Vasey J.J., Tunceli K. Employment pathways in a large cohort of adult cancer survivors. Cancer. 2005;103(6):1292–1301. doi: 10.1002/cncr.20912. [DOI] [PubMed] [Google Scholar]
- 2.Center NC National cancer statistics in Korea 2016. https://www.cancer.go.kr/lay1/S1T639C642/contents.do Edited by Registry KCC; 2017 [cited 2020 Jan 30]. Available from:
- 3.Vuik F.E., Nieuwenburg S.A., Bardou M., Lansdorp-Vogelaar I., Dinis-Ribeiro M., Bento M.J. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820–1826. doi: 10.1136/gutjnl-2018-317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro C.L. Cancer survivorship. N. Engl. J. Med. 2018;379(25):2438–2450. doi: 10.1056/NEJMra1712502. [DOI] [PubMed] [Google Scholar]
- 5.Spelten E.R., Sprangers M.A., Verbeek J.H. Factors reported to influence the return to work of cancer survivors: a literature review. Psycho Oncol. 2002;11(2):124–131. doi: 10.1002/pon.585. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy F., Haslam C., Munir F., Pryce J. Returning to work following cancer: a qualitative exploratory study into the experience of returning to work following cancer. Eur. J. Canc. Care. 2007;16(1):17–25. doi: 10.1111/j.1365-2354.2007.00729.x. [DOI] [PubMed] [Google Scholar]
- 7.Lamore K., Dubois T., Rothe U., Leonardi M., Girard I., Manuwald U. Return to work interventions for cancer survivors: a systematic review and a methodological critique. Int. J. Environ. Res. Publ. Health. 2019;16(8) doi: 10.3390/ijerph16081343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mewes J.C., Steuten L.M., Groeneveld I.F., de Boer A.G., Frings-Dresen M.H., Mj I.J. Return-to-work intervention for cancer survivors: budget impact and allocation of costs and returns in The Netherlands and six major EU-countries. BMC Canc. 2015;15:899. doi: 10.1186/s12885-015-1912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaronson N.K., Mattioli V., Minton O., Weis J., Johansen C., Dalton S.O. Beyond treatment - psychosocial and behavioural issues in cancer survivorship research and practice. EJC Suppl. 2014;12(1):54–64. doi: 10.1016/j.ejcsup.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer A.G.E.M., Taskila T., Ojajärvi A., van Dijk F.J.H., Verbeek J.H.A.M. Cancer survivors and unemployment: a meta-analysis and meta-regression. J. Am. Med. Assoc. 2009;301(7):753–762. doi: 10.1001/jama.2009.187. [DOI] [PubMed] [Google Scholar]
- 11.Mehnert A. Employment and work-related issues in cancer survivors. Crit. Rev. Oncol. Hematol. 2011;77(2):109–130. doi: 10.1016/j.critrevonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi M., Tsuchiya M., Horio Y., Funazaki H., Aogi K., Miyauchi K. Job resignation after cancer diagnosis among working survivors in Japan: timing, reasons and change of information needs over time. Jpn. J. Clin. Oncol. 2017;48(1):43–51. doi: 10.1093/jjco/hyx143. [DOI] [PubMed] [Google Scholar]
- 13.de Boer A.G., Verbeek J.H., Spelten E.R., Uitterhoeve A.L., Ansink A.C., de Reijke T.M. Work ability and return-to-work in cancer patients. Br. J. Canc. 2008;98(8):1342–1347. doi: 10.1038/sj.bjc.6604302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouknight R.R., Bradley C.J., Luo Z. Correlates of return to work for breast cancer survivors. J. Clin. Oncol. 2006;24(3):345–353. doi: 10.1200/JCO.2004.00.4929. [DOI] [PubMed] [Google Scholar]
- 15.Bower J.E., Meyerowitz B.E., Desmond K.A., Bernaards C.A., Rowland J.H., Ganz P.A. Perceptions of positive meaning and vulnerability following breast cancer: predictors and outcomes among long-term breast cancer survivors. Ann. Behav. Med. 2005;29(3):236–245. doi: 10.1207/s15324796abm2903_10. [DOI] [PubMed] [Google Scholar]
- 16.Yang E.J., Kim S.W., Heo C.Y., Lim J.Y. Longitudinal changes in sexual problems related to cancer treatment in Korean breast cancer survivors: a prospective cohort study. Support. Care Canc. 2011;19(7):909–918. doi: 10.1007/s00520-010-0885-y. [DOI] [PubMed] [Google Scholar]
- 17.Lepore S.J., Helgeson V.S., Eton D.T., Schulz R. Improving quality of life in men with prostate cancer: a randomized controlled trial of group education interventions. Health Psychol. 2003;22(5):443–452. doi: 10.1037/0278-6133.22.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamminga S.J., Verbeek J.H., Bos M.M., Fons G., Kitzen J.J., Plaisier P.W. Effectiveness of a hospital-based work support intervention for female cancer patients - a multi-centre randomised controlled trial. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Egmond M.P., Duijts S.F., Jonker M.A., van der Beek A.J., Anema J.R. Effectiveness of a tailored return to work program for cancer survivors with job loss: results of a randomized controlled trial. Acta Oncol. 2016;55(9–10):1210–1219. doi: 10.1080/0284186X.2016.1213417. [DOI] [PubMed] [Google Scholar]
- 20.Leensen M.C.J., Groeneveld I.F., Heide I.V., Rejda T., van Veldhoven P.L.J., Berkel S.V. Return to work of cancer patients after a multidisciplinary intervention including occupational counselling and physical exercise in cancer patients: a prospective study in The Netherlands. BMJ open. 2017;7(6) doi: 10.1136/bmjopen-2016-014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung K.W., Won Y.J., Kong H.J., Lee E.S. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51(2):417–430. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J.H., Park E.C., Park J.H., Kim S.G., Lee S.Y. Job loss and re-employment of cancer patients in Korean employees: a nationwide retrospective cohort study. J. Clin. Oncol. 2008;26(8):1302–1309. doi: 10.1200/JCO.2007.14.2984. [DOI] [PubMed] [Google Scholar]
- 23.Yoo S.H., Yun Y.H., Park S., Kim Y.A., Park S.Y., Bae D.S. The correlates of unemployment and its association with quality of life in cervical cancer survivors. J Gynecol Oncol. 2013;24(4):367–375. doi: 10.3802/jgo.2013.24.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho J., Choi E.K., Kim S.Y., Shin D.W., Cho B.L., Kim C.H. Association between cancer stigma and depression among cancer survivors: a nationwide survey in Korea. Psycho Oncol. 2013;22(10):2372–2378. doi: 10.1002/pon.3302. [DOI] [PubMed] [Google Scholar]
- 25.Shim H.Y., Shin J.Y., Kim J.H., Kim S.Y., Yang H.K., Park J.H. Negative public attitudes towards cancer survivors returning to work: a nationwide survey in Korea. Cancer Res Treat. 2016;48(2):815–824. doi: 10.4143/crt.2015.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilmarinen J. The work ability Index (WAI) Occup. Med. (Lond.) 2007;57(2) 160-160. [Google Scholar]
- 27.Mendoza T.R., Wang X.S., Cleeland C.S., Morrissey M., Johnson B.A., Wendt J.K. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 28.Yun Y.H., Wang X.S., Lee J.S., Roh J.W., Lee C.G., Lee W.S. Validation study of the Korean version of the brief fatigue inventory. J. Pain Symptom Manag. 2005;29(2):165–172. doi: 10.1016/j.jpainsymman.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Yun Y.H., Park Y.S., Lee E.S., Bang S.M., Heo D.S., Park S.Y. Validation of the Korean version of the EORTC QLQ-C30. Qual. Life Res. 2004;13(4):863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 30.Shin J., Goo A., Ko H., Kim J.H., Lim S.U., Lee H.K. Validation study for the Korean version of fear of cancer recurrence inventory. J. Kor. Med. Sci. 2017;32(11):1792–1799. doi: 10.3346/jkms.2017.32.11.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho J., Kang D., Kim I.R., Kim W.S., Ferrell B., Kim S.J. Validation of the Korean version of the quality of life-cancer survivors (QOL-CS-K) questionnaire in lymphoma survivors. Cancer Res Treat. 2018;50(1):204–211. doi: 10.4143/crt.2017.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steger M.F., Dik B.J., Duffy R.D. Measuring meaningful work: the work and meaning inventory (WAMI) J. Career Assess. 2012;20(3):322–337. [Google Scholar]
- 33.Lee J.S., Seo Y.S. The relation beween employees' job burnout and job satisfaction: moderating effects of meaning of work and working environments. Korean J. Counsel. Psychother. 2014;26(4):1109–1129. [Google Scholar]
- 34.Yang D.J., Kang D., Kim Y.K., Kim Y.H., Yang Y.A., Cha S.M. Reliability of self-administered Work Ability Index questionnaire among Korean workers. Ergonomics. 2013;56(11):1652–1657. doi: 10.1080/00140139.2013.835073. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard G., Gray N.M., Ayansina D., Evans J.M., Kyle R.G. Case management vocational rehabilitation for women with breast cancer after surgery: a feasibility study incorporating a pilot randomised controlled trial. Trials. 2013;14:175. doi: 10.1186/1745-6215-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieuwenhuijsen K., Bos-Ransdorp B., Uitterhoeve L.L., Sprangers M.A., Verbeek J.H. Enhanced provider communication and patient education regarding return to work in cancer survivors following curative treatment: a pilot study. J. Occup. Rehabil. 2006;16(4):647–657. doi: 10.1007/s10926-006-9057-9. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher L., Armaou M., Rolf P., Sadhra S., Sutton A.J., Zarkar A. Usefulness and engagement with a guided workbook intervention (WorkPlan) to support work related goals among cancer survivors. BMC Psychol. 2017;5(1):34. doi: 10.1186/s40359-017-0203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Duijn M., Eijkemans M.J., Koes B.W., Koopmanschap M.A., Burton K.A., Burdorf A. The effects of timing on the cost-effectiveness of interventions for workers on sick leave due to low back pain. Occup. Environ. Med. 2010;67(11):744–750. doi: 10.1136/oem.2009.049874. [DOI] [PubMed] [Google Scholar]
- 39.Vargas-Prada S., Demou E., Lalloo D., Avila-Palencia I., Sanati K.A., Sampere M. Effectiveness of very early workplace interventions to reduce sickness absence: a systematic review of the literature and meta-analysis. Scand. J. Work. Environ. Health. 2016;42(4):261–272. doi: 10.5271/sjweh.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoefsmit N., Houkes I., Nijhuis F.J. Intervention characteristics that facilitate return to work after sickness absence: a systematic literature review. J. Occup. Rehabil. 2012;22(4):462–477. doi: 10.1007/s10926-012-9359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamminga S.J., Braspenning A.M., Haste A., Sharp L., Frings-Dresen M.H.W., de Boer A.G.E.M. Barriers to and facilitators of implementing programs for return to work (RTW) of cancer survivors in four European countries: a qualitative study. J. Occup. Rehabil. 2019;29(3):550–559. doi: 10.1007/s10926-018-9818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho J., Smith K., Choi E.K., Kim I.R., Chang Y.J., Park H.Y. Public attitudes toward cancer and cancer patients: a national survey in Korea. Psycho Oncol. 2013;22(3):605–613. doi: 10.1002/pon.3041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.