Abstract
Doxorubicin (DOX) is an anthracycline drug used for cancer treatment. However, its treatment is contiguous with toxic effects. We examined the nephroprotective potential of A. hydaspica polyphenol-rich ethyl acetate extract (AHE) against DOX persuaded nephrotoxicity. 36 male Sprague Dawley rats were randomly assorted into 6 groups. Control group received saline; DOX group: 3 mg/kg b.w. dosage of DOX intraperitoneally for 6 weeks (single dose/week). In co-treatment groups, 200 and 400 mg/kg b.w AHE was given orally for 6 weeks in concomitant with DOX (3 mg/kg b.w, i.p. injection per week) respectively. Standard group received silymarin 400 mg/kg b.w daily + DOX (single dose/week). Biochemical kidney function tests, oxidative stress markers, genotoxicity, antioxidant enzyme status, and histopathological changes were examined. DOX caused significant body weight loss and decrease kidney weight. DOX-induced marked deterioration in renal function indicators in both urine and serum, i.e., PH, specific gravity, total protein, albumin, urea, creatinine, uric acid, globulin, blood urea nitrogen, etc. Also, DOX treatment increases renal tissue oxidative stress markers, while lower antioxidant enzymes in tissue along with degenerative alterations in the renal tissue compared to control rats. AHE co-treatment ameliorates DOX-prompted changes in serum and urine chemistry. Likewise, AHE treatment decreases sensitive markers of oxidative stress and prevented DNA damages by enhancing antioxidant enzyme levels. DOX induction in rats also caused DNA fragmentation which was restored by AHE co-treatment. Moreover, the histological observations evidenced that AHE effectively rescued the kidney tissue from DOX interceded oxidative damage. Our results suggest that co-treatment of AHE markedly improve DOX-induced deleterious effects in a dose-dependent manner. The potency of AHE co-treatment at 400 mg/kg dose is similar to silymarin. These outcomes revealed that A. hydaspica AHE extract might serve as a potential adjuvant that avoids DOX-induced nephrotoxicity.
Keywords: Doxorubicin, Oxidative stress markers, Genotoxicity, Kidney function, Histopathology, Nephrotoxicity
Abbreviations: DOX, doxorubicin; AHE, Acacia hydaspica ethyl acetate extract; SOD, superoxide dismutase; CAT, catalase; POD, peroxidase; QR, quinone reductase; GPx, glutathione peroxidase; γ-GT, Gamma Glutamyl Transferase; GST, glutathione S transferase; GR, glutathione reductase; H2O2, hydrogen peroxide; NO, nitric oxide; MDA, malondialdehyde; WBCs, white blood cells; RBCs, red blood cells
1. Introduction
Doxorubicin (DOX) is an effective anticancer agent that revered extensive recognition in recent years for the management of several types of cancers. However it's harmful perspective i.e., cardiotoxicity, hepatic damages, and nephrotoxicity have reticent its clinical practice (Su et al., 2015). DOX-impelled renal toxicity may be an element of a multi-organ impairment facilitated primarily due to the free radicals accumulation, ultimately inducing the membrane lipid peroxidation (Ghibu et al., 2012). Stimulation of apoptosis and inflection of nitric oxide (NO) are supplementary sources that may intricate in lethal influences accompanying DOX therapy. The likely role of DOX in NOS metabolism governs through direct or indirect incitement of NO generation as a sequel of augmented free radical production. Free radical generation and/or NO release persuaded by DOX is utterly responsible for the DOX-induced harmfulness (Mizutani et al., 2005, Ayla et al., 2011). Furthermore, DOX can encourage nephrotoxicity via its detrimental effects on renal tissue, as it accrues especially in the kidney; amplified permeability of glomerular capillary and induce tubular degeneration (Lee and Harris, 2011). DOX-prompted harmfulness to other tissues like heart and liver possibly will alter blood supply to the kidney and change the xenobiotic reclamation, henceforth periphrastically executing nephropathy. A lot of antioxidant combinations have been recommended as chemo-deterrent for DOX-persuaded toxicity (Granados-Principal et al., 2010, Wapstra et al., 1999).
Acacia hydaspica R. Parker synonyms Acacia eburnean (family: Leguminosae). The vernacular name of the plant is Pahari Kikar, Kikar; Marmat. The seeds and bark possess high amount of tannins (Chakrabarty and Gangopadhyay, 1996, Jabeen et al., 2009). A. hydaspica exhibited anticancer, antioxidant (Afsar et al., 2016a), anti-inflammatory (Afsar et al., 2015a), cardio-protective (Afsar et al., 2017b, Afsar et al., 2019), protective against CP induced reproductive and hepatic toxicity (Afsar and Razak, 2017, Afsar et al., 2017a). GCMS analysis identified α-Amyrin (5.03%), 1,2-Benzenedicarboxylic acid mono (2-Ethylhexyl) ester (70.65%), Vitamin E (4.56%), Squalene (4%) and 2,6-dimethyl-N-(2-methyl-à-phenyl benzyl) aniline (2.51%) in A. hydaspica (Afsar et al., 2017c). Bioassay-guided isolation identified 7-O-galloyl catechin, catechin, catechin gallate, and methyl gallate as major antioxidant and anticancer phytoconstituents from A. hydaspica ethylacetate fraction (AHE) (Afsar et al., 2016a, Afsar et al., 2018, Afsar et al., 2016). Genus Acacia exposed antioxidant and nephroprotective proficiencies in animal prototypes (Puga et al., 2015). The aqueous extract of A. Senegal showed significant nephroprotective potency against gentamicin (100 mg/kg) induced renal damage by lowering serum creatinine and urea levels in rats (Mohammed, 2015). Administration of A. nilotica significantly inhibits cadmium chloride prompted diminution in serum globulin and albumin concentration, albumin/globulin ratio, SOD, and GPx levels by ameliorating MDA and NO content hence protected renal damages (Koriem et al., 2009). Previous studies reported that green tea polyphenols possess shielding influence counter to CP-persuaded kidney damage in rats. Polyphenols administration before CP inoculation was more operative than post-treatment in lessening CP adverse reactions on the kidney. Epigallocatechin gallate administration prevents CP induced renal damages, diminish the oxidative kidney harms and inflammatory responses (Ahn et al., 2014).
Due to the nephroprotective potential of related species and polyphenolic compounds in animal prototypes and in vivo antioxidant action of A. hydaspica. The present experiment was intended to delineate the protective perspective of the ethyl-acetate extract of A. hydaspica against DOX-prompted renal injuriousness and oxidative trauma in rats. We hypothesized that polyphenol-rich AHE could preclude DOX-induced nephrotoxicity due to its antioxidant properties. The effect of AHE on the antioxidant status, biochemical alterations, DNA damage, and histoarchitecture in DOX-persuaded nephrotoxicity was studied by urine analysis, renal function tests in serum, antioxidant enzymes of renal tissue, oxidative stress, DNA fragmentation and DNA damage in renal tissue, and histological examinations.
2. Materials and methods
2.1. Collection of plant and extract preparation
Aerial parts of A. hydaspica were taken from Kirpa Charah village Islamabad, Pakistan, and were recognized by Dr. Sumaira Sahreen and given Accession No. 0642531. The specimen was submitted in the Herbarium of Pakistan, Museum of Natural History, Islamabad. The detailed process of extract preparation and fractionation has been described in our earlier reports on A. hydaspica (Afsar et al., 2015b), and its ethyl acetate extract (AHE); the most bioactive extract under in vitro and in vivo investigations and having bioactive polyphenols (Afsar et al., 2016, Afsar et al., 2015, Afsar et al., 2017b) was selected for the current investigation.
2.2. Preparation of samples for dosage
Doxorubicin (DOX) injection (Sigma-Aldrich: St. Louis, MO, U.S.A.) was diluted in saline to set quantity for inoculation. A total dose of 18 mg/kg b.w was injected into rats in the course of the experiment (Zhao et al., 2012). Silymarin and AHE were prepared freshly in distilled water just before dosing (Oda and El-Ashmawy, 2012).
2.3. Scheme of the experiment
Sprague Dawley male rats (200–230 g) were obtained from the Primate Facility at Quaid-i-Azam University, Islamabad. The animals were kept under 12 h light/dark cycles at 25 ± 3 °C in conventional steel cages and nourished with regular pellet diet and tap water. The National Institute of animal health guidelines (NIH guidelines) was exactingly followed to conduct the testing efficiently. The ethical board of Quaid-i-Azam University, Islamabad accepted the investigational protocol (Bch#264). 36 rats were randomly separated into six groups (n = 6) and placed in individual steel cages. The study procedure was arranged according to former studies (van Acker et al., 2000, Jalali and Hasanzadeh, 2013, Sakr et al., 2011) with slight adjustments.
Group 1: Control group-administered normal saline for 6 weeks (one dose/week, 0.4 ml, i.p.)
Group II: Drug control was inoculated with DOX (i.p, 3 mg/kg b.w.) for six weeks (single dose/week, so total dose was18 mg/kg b.w. during 6 weeks).
Group III: The plant control group was treated with an oral dose of AHE (400 mg/kg b.w.) daily for 6 weeks.
Group IV: DOX + AHE 200 mg/kg; treated with an oral dose of AHE (200 mg/kg b.w) daily for 6 weeks along with DOX inoculation once per week.
Group V: DOX + AHE 400 mg/kg; treated with an oral dose of AHE (400 mg/kg b.w) for 6 weeks in conjunction with DOX inoculation once per week.
Group VI: DOX + Silymarin; an oral dose of silymarin (400 mg/kg b.w) for 6 weeks in conjunction with DOX inoculation once per week.
The body weights of rats were noted at the beginning and completion of experimentation. 24 h after the last treatment, rats were euthanized by cervical dislocation. Blood was drawn through a direct intra-cardiac puncture and poured in sterile tubes and submitted to centrifugation at 10,000 rpm for 15 min at 4 °C to get the serum. Serum aliquots were placed at −80 °C for further examination. The kidneys were withdrawn and rinsed with ice-cold saline, subsequently, the right kidney was treated with liquid nitrogen and put in storage at −80 °C for biochemical investigations and DNA damage study, while the left kidney was processed in 10% phosphate-buffered formalin for histology.
2.4. Urine analysis
Quantity of red blood cells (RBCs), count, white blood cells (WBCs) count, pH, specific gravity, total protein, albumin, urea, creatinine, and creatinine clearance in urine was examined by using standard diagnostic kits (MediScreen Urine Strips, Orgenics, France).
2.5. Biochemical analysis of serum
Whole protein, albumin, urea, creatinine clearance, and creatinine were examined by standard diagnostic kits (MediScreen kit France). Blood urea nitrogen (BUN), bilirubin, and total protein concentrations were quantified with standard diagnostics kits (AMP Krenngasse 12, 8010 Graz, Australia).
2.6. Biochemical analysis of tissues
2.6.1. Homogenate preparation
Kidney tissue (100 mg) was homogenized in 100 mM KH2PO4 buffer having 1 mM EDTA, pH 7.4. Then it was centrifuged at 12,000g for 30 min (4 °C) to exclude cell debris and the supernatant was stored in aliquots and kept at −20◦ C.
2.6.2. Assessment of tissue protein
Lowry et al method was used for the assessment of tissue total soluble protein content (Lowry et al., 1951).
2.6.3. Enzymatic antioxidant measurement
Quantity of CAT and POD was estimated by the procedure of Afsar et al (Afsar et al., 2017a). The technique of Kakkar et al. was employed for the valuation of SOD action (Kakkar et al., 1984). The amount of quinone reductase in tissues of various experimental groups was calculated as defined previously (Benson et al., 1980). Reduced glutathione quantity was tested as described by Jollow (Jollow et al., 1974). The technique of Habig et al. (Habig et al., 1974) was used for the estimation of GST potency. Glutathione reductase activity in tissue samples was examined as cited by Carlberg and Mannervik (Carlberg and Mannervik, 1975). Glutathione peroxidase function was calculated using a previous protocol (Mohandas et al., 1984). The activity of γ-glutamyl transpeptidase was checked following Orlowski et al scheme (Orlowski et al., 1974).
2.6.4. Assessment of oxidative stress indicators
Iqbal et al. procedure (Iqbal et al., 2005) were implemented for the calculation of lipid peroxidation. The approximation of hydrogen peroxide range in tissue samples was examined by the scheme described earlier (Pick and Mizel, 1981). For the execution of the nitrite assay, Griess reagent was utilized (Green et al., 1982).
2.7. DNA damage analysis
2.7.1. DNA fragmentation test with diphenylamine reaction
DNA fragmentation in renal tissues was analyzed as described previously. 100 mg kidney tissue was homogenized in TTE mixture. 100 µl aliquot (labeled as “B”) was centrifuged for 10 min at 200g (4 °C). The supernatant was collected and labeled as “S”. Then the “S” tubes were centrifuged at 20,000g at 4 °C for 10 min to isolate intact chromatin and this was labeled as “T”. Afterward, 25% TCA (1.0 ml) was pipetted in all of the labeled tubes and kept overnight at 4 °C. Next, the samples were centrifuged for 10 min at 18,000g at 4 °C to recuperate the precipitated DNA. 160 μl of TCA (5%) was added to each tube and heated for 15 min at 90 °C. After that 320 μl of freshly prepared DPA solution was decanted, vortexed, and incubated for 4 hr 37 °C. Optical density was recorded at 600 nm (Smart spec TM Plus, catalog # 170–2525).
2.7.2. Measurement of DNA damage by ladder assay
DNA isolation was carried out by the procedure described previously (Ateşşahín et al.). Renal tissue (100 mg) was rinsed with DNA Buffer before homogenization in 1 ml lysis buffer. Next proteinase K (10 mg/ml, 100 μl) and 10% SDS (240 μl) were poured and agitated slightly and kept overnight in a water bath at 45 °C. Afterward, phenol (0.4 ml) was decanted and agitated for 5–10 min before centrifugation for 5 min (at 3000 rpm, 10 °C). The supernatant was collected and mixed with phenol (1.2 ml) and chloroform/isoamyl alcohol (24:1, 1.2 ml), shake for 5–10 min, and centrifuge at 3000 rpm for 5 min at 10 °C. 25 μl of sodium acetate (pH 5.2, 3 M) and 5 ml ethanol was poured in the supernatant and shake until DNA was precipitated. DNA was splashed with 70% ethanol and then pure DNA was liquefied in TE buffer. The amount of DNA was computed at 260 and 280 nm. 5 μg DNA test sample and 0.5 μg DNA standards were loaded on 1.5% agarose gel. Electrophoresis was executed for 45 min at 100 V batteries (Bio-Rad, electrophoresis apparatus), and DNA was observed below the digital gel doc system (BioRad, Doc XR + System, stain free) and photographed.
2.8. Histopathological examination
Renal tissues from each group were fixed in a fixative comprising 85 ml absolute alcohol, 5 ml glacial acetic acid, and 10 ml of 40% formaldehyde (10 ml). Tissue samples were mounted in paraffin after dehydration steps to make blocks for microtomy. Tissues were sectioned 4–5 µm with a microtome and stained with Hematoxylin-Eosin (H&E). The slides were examined and photographed under a light microscope (DIALUX 20 EB) at 40X.
3. Results
3.1. Acute toxicity assessment
Ethyl-acetate extract of the A. hydaspica exhibited no mortality and toxicity at a maximum concentration of 4000 mg/kg b.w. in acute toxicity experiment after 15 days. Therefore, one-tenth (400 mg/kg b.w) of the highest dose tried in acute toxicity was used for the in vivo study of AHE. For dose-dependent response assessment; 200 mg/kg b.w. the dose was also tested in this investigation.
3.2. Effect of AHE against DOX prompted nephrotoxicity
The protective effect of AHE on DOX-persuaded oxidative stress, biochemical and histopathological alterations in renal tissue were examined by urine analysis, serum analysis, renal tissue antioxidant enzyme status, oxidative stress, and lipid peroxidation. The protective effect exhibited by various treatments was corroborated by analyzing the biochemical and histopathological changes in respective groups.
3.3. Effect on body and kidney weight
The influence of AHE co-administration with DOX on the body and renal tissue weight is reported in Table 1. Data showed noteworthy (p < 0.001) reduction in the absolute body weights of DOX inoculated rats in contrast to both control and AHE treatment rats. Co-administration of AHE results in significant weight gain in DOX treated animals, and 400 mg/kg dose seems to be more effective (p < 0.001) in perfecting the weight loss induced by DOX. AHE co-treatment considerably improved renal weight vacillations in contrast to DOX alone administered group.
Table 1.
Effect of DOX and/or AHE treatment on body weight of rats.
| Treatment (mg/kg) | Body weight (g) |
Kidney weight (g) | |
|---|---|---|---|
| Initial | Final | ||
| Control | 219.0 ± 0.577 | 250.3 ± 0.333b | 2.11 ± 0.03b** |
| DOX | 221.3 ± 0.667 | 226.3 ± 0.661a | 1.88 ± 0.11a** |
| AHE alone | 220.3 ± 0.667 | 248.3 ± 0.671b | 2.10 ± 0.03b** |
| DOX + AHE (200) | 221.7 ± 0.882 | 232.2 ± 0.611a,b | 2.04 ± 0.03 |
| DOX + AHE (400) | 220.3 ± 0.882 | 244.7 ± 0.882a,b,c | 2.1 ± 0.035b** |
| DOX + Sily | 222.0 ± 0.577 | 243.3 ± 0.333a,b | 2.092 ± 0.05b* |
Data expressed as mean ± SEM (n = 6). a: significant difference of final body weight of group Vs. Control group at p < 0.001, b: significant difference of final body weight of group Vs. DOX-treated group at p < 0.001, c: significant difference of final body weight of DOX + AHE (200 mg/kg) treated group Vs. DOX + AHE (400 mg/kg) treated group at p < 0.001.
3.4. Renal function markers in urine
Proper kidney functioning is usually depicted by the composition of urine; therefore urine analysis was performed to notice the variability in kidney function of animals in different treatment groups. The protective efficacy of AHE against DOX provoked alterations in the various urine biomarkers of renal impairment are shown in Table 1, Table 2. DOX treatment ensued noteworthy (p < 0.0001) augmentation in urine specific gravity, RBCs and WBCs count, and concentration of urea in the urine while urine PH was dropped significantly (p < 0.0001) in the DOX treated group in comparison to the levels in normal control rats. Co-administration of AHE to DOX treated rats noticeably (p < 0.001) attenuated the above parameters of urine in a dose-dependent manner, thus improving the kidney functions (Table 2).
Table 2.
Physical analysis of urine in various treatment groups.
| Treatment (mg/kg) | pH | Specific gravity | RBC/µl | WBC/µl | Urea (mg/dl) |
|---|---|---|---|---|---|
| Control | 7.110 ± 0.050b | 1.042 ± 0.011b | 0.046 ± 0.01b | 15.83 ± 0.291b | 12.77 ± 0.590b |
| DOX | 6.020 ± 0.032a | 1.487 ± 0.019a | 13.35 ± 0.05a | 75.17 ± 0.318a | 41.37 ± 0.876a |
| AHE alone | 7.107 ± 0.029b | 1.041 ± 0.012b | 0.047 ± 0.011b | 15.77 ± 0.233b | 12.57 ± 0.484b |
| DOX + AHE (200) | 6.78 ± 0.103a**,b,d* | 1.19 ± 0.02a,b,d | 5.930 ± 0.09a,b,d | 40.67 ± 0.278a,b,d | 24.55 ± 0.553a,b,d |
| DOX + AHE (400) | 7.057 ± 0.024b,c* | 1.06 ± 0.02b,c** | 0.647 ± 0.022a,b,c | 16.07 ± 0.120b,c | 14.43 ± 0.338b,c |
| DOX + Sily | 7.053 ± 0.038b | 1.053 ± 0.020b | 0.618 ± 0.014a,b | 16.17 ± 0.260b | 14.37 ± 0.318b |
Values expressed as mean ± SEM. a: Significance at p < 0.0001 Vs. control group, b: Significance at p < 0.0001 Vs. Doxorubicin (DOX) group, c: Significance at p < 0.0001 of DOX + AHE 400 mg/kg group Vs. DOX + AHE 200 mg/kg group. d: Significance at p < 0.0001 of AHE co-treatment groups Vs DOX + Sily group. **: Significant difference at p < 0.05 and p < 0.001 respectively. Non-significant difference (p > 0.05) was recorded between control and AHE alone treated group in all parameters. (One way ANOVA followed by Tukey’s multiple comparison tests). Sily-Silymarin.
DOX administration markedly increased the levels of urine creatinine, urinary albumin, and urinary protein while, decreased creatinine clearance capacity as compared to control animals (Table 3). Of the different urinary biomarkers evaluated, an increase in urinary albumin and creatinine revealed high prognostic measure for prediction of advanced DOX-induced nephrotoxicity, defined as primary glomerular damage and secondary tubular injury. Urine albumin and creatinine were more sensitive biomarkers for nephrotoxicity then serum creatinine and serum BUN. AHE co-administration dose-dependently attenuated the DOX-induced toxicity by significantly ameliorating the above parameters compared to animals receiving only DOX. Relatively significant (p < 0.0001) ameliorating effects were recorded with AHE high dose (400 mg/kg b.w) in comparison with low dose group. The levels of creatinine and creatinine clearance restored to that of the control group in the DOX + AHE high dose group, however urinary albumin and protein content were remained significantly (p < 0.05 and p < 0.0001, respectively) high in contrast to control group. DOX + AHE 400 mg/kg b.w. group displayed similar ameliorative effects on urine biomarkers to DOX + silymarin treated group. Oral doses of AHE alone did not induce any difference in urine profile in comparison to control rats.
Table 3.
Effect of Doxorubicin (DOX) and different treatments of AHE on urine creatinine, creatinine clearance, albumin and proteinuria.
| Treatment (mg/kg) | Creatinine (mg/dl) | Creatinine clearance (ml/min) | Albumin (mg/dl) | Urinary Protein (mg/dl) |
|---|---|---|---|---|
| Control | 0.4033 ± 0.055b | 0.8252 ± 0.064b** | 7.337 ± 0.171b | 23.29 ± 0.761b |
| DOX | 1.387 ± 0.019a | 0.4002 ± 0.043a** | 15.83 ± 0.418a | 54.35 ± 0.805a |
| AHE alone | 0.4007 ± 0.055b | 0.8265 ± 0.065b** | 7.280 ± 0.141b | 23.29 ± 0.025b |
| DOX + AHE (200) | 0.900 ± 0.029a,b,d | 0.5990 ± 0.055 | 11.57 ± 0.348a,b,d | 34.50 ± 0.433a,b,d** |
| DOX + AHE (400) | 0.5077 ± 0.037b,c | 0.7575 ± 0.075b* | 8.817 ± 0.235a*,b,c | 27.60 ± 0.432a,b,c |
| DOX + Sily | 0.497 ± 0.035b | 0.7584 ± 0.071b* | 8.833 ± 0.167a*,b | 27.17 ± 0.029a**,b |
Values expressed as mean ± SEM. a: Significance at p < 0.0001 Vs. control group, b: Significance at p < 0.0001 Vs. Doxorubicin (DOX) group, c: Significance at p < 0.0001 of DOX + AHE 400 mg/kg group Vs. DOX + AHE 200 mg/kg group. d: Significance at p < 0.0001 of AHE co-treatment groups Vs DOX + Sily group. *, **: Significant difference at p < 0.05 and p < 0.001 respectively. Non-significant difference (p > 0.05) was recorded between control and AHE alone treated group in all parameters. (One way ANOVA followed by Tukey’s multiple comparison tests). Sily-Silymarin.
3.5. Effect of AHE on serum kidney function tests
Like urine profile, serum profile also provides an idea about the kidney function. The level of serum creatinine, urea, uric acid, and BUN was measured to assess DOX-induced nephrotoxicity. DOX treatment significantly (p < 0.0001) decreased serum albumin, protein, and globulin concentration while the quantity of serum urea, uric acid, creatinine, NO, and BUN were considerably (p < 0.0001) elevated in divergence to control group (Table 4, Table 5). AHE combine treatment perfected the adverse effect of DOX markedly (p < 0.001) by improving the albumin, protein, and globulin and preventing the rise in serum urea, uric acid, creatinine, NO and BUN in a dose-dependent manner. AHE high dose significantly (p < 0.0001) restored the levels of the above-mentioned parameters in comparison to low dose treatment. Administration of silymarin significantly improved the DOX mediated deteriorations in serum biomarkers. Treatment of experimental animals with a high dose of AHE retained the concentration of serum albumin, globulin, creatinine, and uric acid similar to the levels in the control group, while serum protein, BUN, urea and NO content remained significantly different from the control group. AHE alone treatment at 400 mg/kg b.w. showed paralleled response to the control group, depicting the non-lethal influence of plant extract.
Table 4.
Effect of Doxorubicin (DOX) and different treatments of AHE on serum protein, albumin, globulin, BUN, serum nitrite profile.
| Treatment (mg/kg) | Serum proteins (mg/dl) | Albumin (mg/dl) | Globulin (mg/dl) | BUN (mg/dl) | Serum nitrite (µM/ml) |
|---|---|---|---|---|---|
| Control | 78.06 ± 0.361b | 19.70 ± 0.889b | 58.36 ± 1.246b | 9.167 ± 0.033b | 41.50 ± 0.764b |
| DOX | 37.95 ± 0.396a | 9.367 ± 0.73a | 28.59 ± 1.115a | 23.17 ± 0.561a | 82.37 ± 0.797a |
| AHE alone | 79.06 ± 0.554b | 19.93 ± 0.636b | 59.12 ± 1.078b | 9.133 ± 0.033b | 41.20 ± 0.611b |
| DOX + AHE (200) | 52.19 ± 0.384a,b,d | 15.23 ± 0.176a**,b | 36.95 ± 0.40a,b,d | 17.07 ± 0.23a,b,d | 62.67 ± 0.921a,b,d |
| DOX + AHE (400) | 73.32 ± 0.439a,b,c | 17.78 ± 0.174b | 55.54 ± 0.425b,c | 12.53 ± 0.484a,b,c | 48.13 ± 0.962a,b,c |
| DOX + Sily | 74.62 ± 0.50a**,b | 17.87 ± 0.233b | 56.75 ± 0.333b | 12.37 ± 0.367a,b | 47.23 ± 0.535a**,b |
Values expressed as mean ± SEM. a: Significance at p < 0.0001 Vs. control group, b: Significance at p < 0.0001 Vs. Doxorubicin (DOX) group, c: Significance at p < 0.0001 of DOX + AHE 400 mg/kg group Vs. DOX + AHE 200 mg/kg group. d: Significance at p < 0.0001 of AHE co-treatment groups Vs DOX + Sily group. **: Significant difference at p < 0.05 and p < 0.001 respectively. Non-significant difference (p > 0.05) was recorded between control and AHE alone treated group in all parameters. (One way ANOVA followed by Tukey’s multiple comparison tests). Sily-Silymarin.
Table 5.
Effect of Doxorubicin (DOX) and different treatments of AHE on serum creatinine, urea and uric acid profile.
| Treatment (mg/kg) | Serum creatinine (mg/dl) | Urea (mg/dl) | Uric acid (mg/dl) |
|---|---|---|---|
| Control | 0.437 ± 0.032b | 24.03 ± 0.549b | 0.407 ± 0.043b |
| DOX | 2.433 ± 0.089a | 69.33 ± 0.333a | 0.810 ± 0.027a |
| AHE alone | 0.433 ± 0.033b | 23.73 ± 0.857b | 0.403 ± 0.044b |
| DOX + AHE (200) | 1.203 ± 0.009a,b,d | 45.40 ± 0.839a,b,d | 0.707 ± 0.023a,d** |
| DOX + AHE (400) | 0.607 ± 0.018b,c | 30.17 ± 0.441a,b,c | 0.477 ± 0.038b,c** |
| DOX + Sily | 0.593 ± 0.023b | 30.03 ± 0.578a,b | 0.470 ± 0.036b |
Values expressed as mean ± SEM. a: Significance at p < 0.0001 Vs. control group, b: Significance at p < 0.0001 Vs. Doxorubicin (DOX) group, c: Significance at p < 0.0001 of DOX + AHE 400 mg/kg group Vs. DOX + AHE 200 mg/kg group. d: Significance at P < 0.0001 of AHE co-treatment groups Vs DOX + Sily group. *, **: Significant difference at p < 0.05 and p < 0.001 respectively. Non-significant difference (p > 0.05) was recorded etween control and AHE alone treated group in all parameters. (One way ANOVA followed by Tukey’s multiple comparison tests). Sily-Silymarin.
3.6. Effect of AHE on renal antioxidants
Table 6, Table 7 make evident DOX-induced insufficiency of renal antioxidant enzymes and protective effect of AHE to reduce renal damage with the restoration of renal antioxidant levels. Treatment with DOX-induced a remarkable (p < 0.0001) diminution in renal POD, SOD, CAT, and QR quantity compared to control rats. Simultaneous inoculation of DOX with a high dose of AHE expressively (p < 0.0001) restored renal POD and SOD to the levels statistically similar to control values. However, the level of CAT and QR showed a significant difference to control and DMSO alone treated groups.
Table 6.
Effect of Doxorubicin (DOX) and different treatments of AHE on renal antioxidant enzymes.
| Treatment (mg/kg) | POD (U/min) | SOD (U/mg protein) | CAT (U/min) | QR (nM/min/mg protein) |
|---|---|---|---|---|
| Control | 10.85 ± 0.150b | 1.752 ± 0.059b | 35.50 ± 0.289b | 93.49 ± 0.526b |
| DOX | 5.570 ± 0.341a | 0.581 ± 0.040a | 18.43 ± 0.251a | 63.55 ± 1.275a |
| AHE alone | 11.30 ± 0.185b | 1.778 ± 0.066b | 35.93 ± 0.479b | 93.84 ± 0.140b |
| DOX + AHE (200) | 8.060 ± 0.017a,b,d | 1.153 ± 0.088a,b | 26.96 ± 0.248a,b,d | 73.32 ± 0.667a,b,d |
| DOX + AHE (400) | 10.07 ± 0.306b,c | 1.647 ± 0.083b,c** | 30.08 ± 0.107a,b,c | 83.22 ± 0.491a,b,c |
| DOX + Sily | 10.02 ± 0.096b | 1.595 ± 0.055b | 30.19 ± 0.335a,b | 83.11 ± 0.537 a,b |
Values expressed as mean ± SEM. a: Significance at p < 0.0001 Vs. control group, b: Significance at p < 0.0001 Vs. Doxorubicin (DOX) group, c: Significance at p < 0.0001 of DOX + AHE 400 mg/kg group Vs. DOX + AHE 200 mg/kg group. d: Significance at p < 0.0001 of AHE co-treatment groups Vs DOX + Sily group. **: Significant difference at p < 0.05 and p < 0.001 respectively. Non-significant difference (p > 0.05) was recorded between control and AHE alone treated group in all parameters. (One way ANOVA followed by Tukey’s multiple comparison tests). Sily-Silymarin.
Table 7.
Effect of Doxorubicin (DOX) and different treatments of AHE on renal GSH profile and phase II antioxidants.
| Treatment (mg/kg) | GSH (µM/g tissue) | GR (nM/min/mg protein) | GST (nM/min/mg protein) | γ-GT (nM/min/mg Protein) | GPx (nM/min/mg Protein) |
|---|---|---|---|---|---|
| Control | 17.41 ± 0.529b | 150.4 ± 0.725b | 139.3 ± 0.355b | 394.4 ± 1.246b | 129.7 ± 1.300b |
| DOX | 9.572 ± 0.323a | 96.22 ± 1.480a | 95.60 ± 0.956a | 107.9 ± 1.156a | 76.85 ± 0.864a |
| AHE alone | 18.38 ± 0.673b | 150.6 ± 0.409b | 139.5 ± 0.442b | 394.8 ± 1.379b | 130.1 ± 1.101b |
| DOX + AHE (200) | 12.90 ± 0.145a,b,d** | 120.2 ± 0.799a,b,d | 110.6 ± 0.298a,b,d | 215.2 ± 0.672a,b,d | 93.00 ± 1.034a,b,d |
| DOX + AHE (400) | 16.63 ± 0.302b,c** | 139.1 ± 1.146a,b,c | 132.5 ± 0.853a,b,c | 362.9 ± 0.610a,b,c | 120.3 ± 1.052a,b,c |
| DOX + Sily | 16.00 ± 0.145b | 138.0 ± 1.201 a,b | 131.8 ± 1.049 a,b | 364.5 ± 0.691a,b | 119.6 ± 0.985 a,b |
Values expressed as mean ± SEM. a: Significance at p < 0.0001 Vs. control group, b: Significance at p < 0.0001 Vs. Doxorubicin (DOX) group, c: Significance at p < 0.0001 of DOX + AHE 400 mg/kg group Vs. DOX + AHE 200 mg/kg group. d: Significance at p < 0.0001 of AHE co-treatment groups Vs DOX + Sily group. **: Significant difference at p < 0.05 and p < 0.001 respectively. Non-significant difference (p > 0.05) was recorded between control and AHE alone treated group in all parameters. (One way ANOVA followed by Tukey’s multiple comparison tests). Sily-Silymarin.
DOX inoculation caused substantial (p < 0.0001) depletion in renal tissue content of GSH, GR, GST, γ-GT and GPx. The administration of AHE with DOX significantly (p < 0.001) enhanced the level of renal antioxidant enzymes in contrast to only DOX-treated rats, in a dose-dependent manner. Similar levels of GSH, GR, GST, γ-GT, and GPx were recorded in AHE high dose and standard drug silymarin treated groups. Glutathione exhaustion is one of the problems resulting in renal injury, results show that co-administration of AHE at high dose retained level of GSH to normal control values, while the level of activity of GR, GST, γ-GT, and GPx showed a significant difference in contrast to control group. AHE co-treatment with DOX-induced improvement of renal enzymes in a dose-dependent way, indicating that AHE high dose provided better protection as compared to low dose. The high dose of AHE, without DOX treatment, showed no change in renal antioxidant enzymes in comparison to the control group.
3.7. Protective effect of AHE on renal tissue protein and oxidative stress markers
Renal MDA content serves as a marker of renal lipid peroxidation, nitrite/nitrate proportion was taken as an indicator of renal NO concentrations; H2O2 and NO are important markers of oxidative stress. DOX administration significantly decreased renal tissue protein content and augmented tissue MDA and NO levels in comparison to control. AHE in high dose retrieved the renal protein content, NO, and MDA to the levels statistically insignificant from the control group, while the level of H2O2 remained significantly different in comparison to control animal tissues. Furthermore, co-administration of AHE high dose showed similar effects to standard drug silymarin by preventing the DOX-induced deterioration. On the other hand, giving AHE in both low and high doses to DOX-inoculated animals improved above said parameters markedly when compared to the DOX alone treated group, but still, the levels were significantly different from control animals. AHE administration alone in high doses showed no substantial influence on renal tissue protein content, H2O2, NO, and MDA content in comparison to their levels in the tissue of control animals (Table 8). These results indicated that DOX-induced renal damage involved inconsistency in the ratio of renal tissue oxidant and antioxidant, and AHE showed protection against DOX-induced renal impairments via reversing the altered levels of above-investigated parameters, suggesting a potential role for AHE in DOX-induced nephrotoxicity.
Table 8.
Effect of Doxorubicin (DOX) and different treatments of AHE on renal tissue protein, oxidative stress markers and lipid peroxidation.
| Treatment (mg/kg) | Protein (µg/mg Tissue) | H2O2 (nM/min/mg Tissue) | Nitrite (content µM/ml) | TBAR (nM/min/mg protein) |
|---|---|---|---|---|
| Control | 2.530 ± 0.056b | 1.743 ± 0.039b | 47.84 ± 1.156b | 5.026 ± 0.301b |
| DOX | 0.807 ± 0.225 a | 4.946 ± 0.051a | 85.00 ± 1.055a | 11.92 ± 0.321a |
| AHE alone | 2.470 ± 0.019b | 1.707 ± 0.013b | 45.46 ± 1.302b | 5.007 ± 0.183b |
| DOX + AHE (200) | 1.60 ± 0.043 a,b**,d* | 3.381 ± 0.036a,b,d | 66.73 ± 1.620a,b,d | 8.643 ± 0.279a,b,d |
| DOX + AHE (400) | 2.288 ± 0.076b,c** | 2.04 ± 0.047a**,b, c | 50.84 ± 1.051b,c | 6.119 ± 0.077b,c |
| DOX + Sily | 2.215 ± 0.039b | 2.109 ± 0.07a**,c | 49.13 ± 1.075b | 6.171 ± 0.191a*,b |
Values expressed as mean ± SEM. a: Significance at p < 0.0001 Vs. control group, b: Significance at p < 0.0001 Vs. Doxorubicin (DOX) group, c: Significance at p < 0.0001 of DOX + AHE 400 mg/kg group Vs. DOX + AHE 200 mg/kg group. d: Significance at p < 0.0001 of AHE co-treatment groups Vs DOX + Sily group. *, **: Significant difference at p < 0.05 and p < 0.001 respectively. Non-significant difference (p > 0.05) was recorded between control and AHE alone treated group in all parameters. (One way ANOVA followed by Tukey’s multiple comparison tests). Sily-Silymarin.
3.8. Molecular analysis of DNA damage
3.8.1. DNA ladder assay
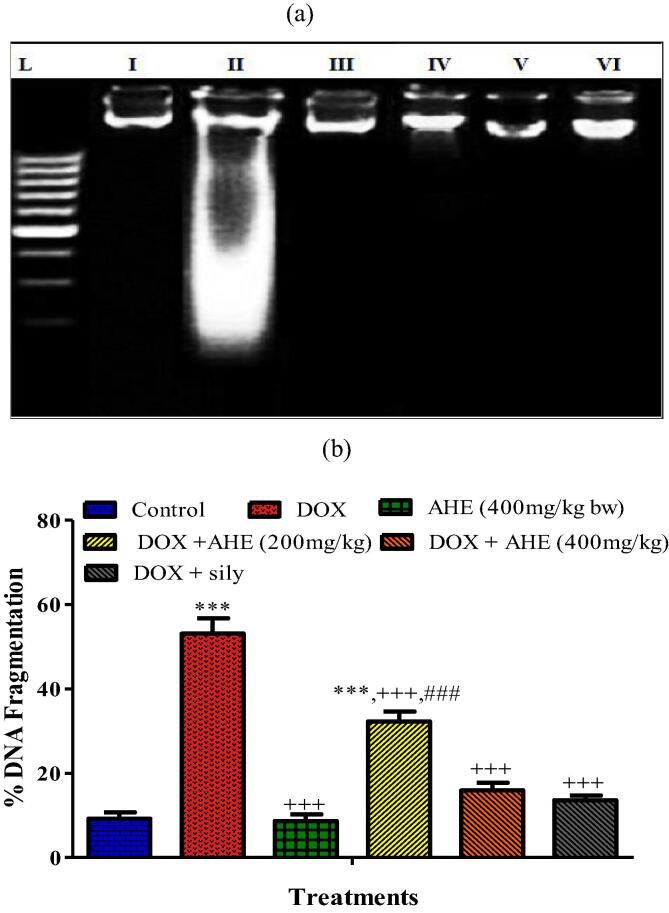
DNA was isolated from the renal tissues and dissimilar banding conformations were distinguished in Fig. 1a. The genomic DNA presented a distinct sharp band without dilapidation and tail configuration in the control group. In the case of DOX treated rats, a typical band fragmentation of DNA can be implicit, which was not present for the control group. AHE conducts indicated noticeable restoring of the DNA damage. DNA isolated from the group co-treated with AHE and DOX bared revamped DNA. The group treated with AHE alone did not show any sort of DNA injuries.
Fig. 1.
a: Lanes from left (L) low molecular weight marker pattern in a ladder, (1) Control, (2) DOX (3) AHE alone (4) DOX + AHE (200 mg/kg), (5) DOX + AHE (400 mg/kg), (6) DOX + Sily. b: Percent DNA fragmentation in different treatment groups.
3.8.2. AHE preclude DOX-prompted % DNA fragmentation
DNA fragmentation (%) exhibited discernible variations in all experimental groups (Fig. 1 b). The AHE treated groups repaired the DOX persuaded DNA damage and reduced the % DNA fragmentation displaying the shielding effects at the genetic level. High dose co-administration of AHE upturned the extent of DNA fragmentation (%) adjacent to the control group.
3.8.3. Histopathology of kidney
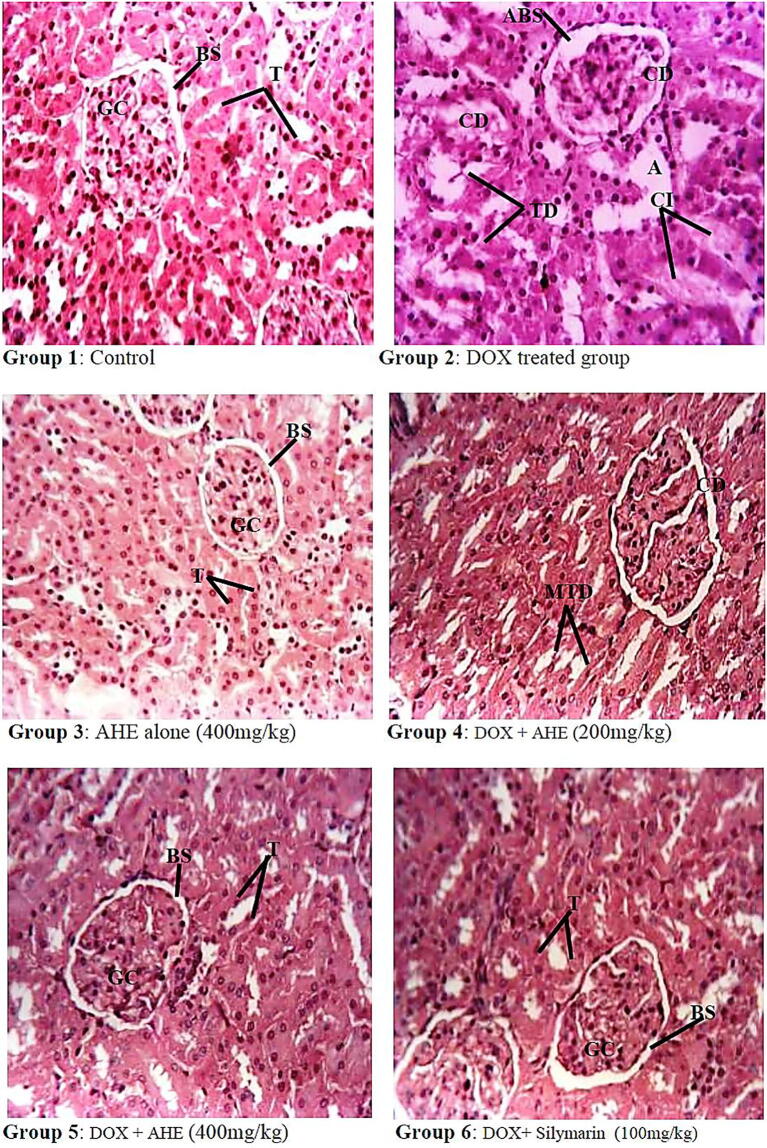
Light Microscopic sections of renal histology of different treatment groups are shown in Fig. 2. Histopathological investigation revealed normal morphology of renal glomeruli and cortical tubules in control and AHE alone (400 mg/kg b.w) treated groups. DOX treatment resulted in degenerative changes viz. glomerular atrophy or disappearance; dilated Bowman’s capsules and capillaries; and marked disintegration of renal tubules with exfoliated cells, protein casts, and cystic dilatation; blood congestion in the capillary loops; cuboidal or round shape parietal layer of Bowman’s membrane; inflammatory cell infiltrations; vocalization in the endothelial cell cytoplasm of proximal tubules and degenerated or lost microvillus. Concomitant administration of AHE with DOX leads to the abrogation of DOX-impelled renal tissue damages in a dose-dependent pattern. DOX + AHE 400 mg/kg b.w. group showed better preserved cellular and tubular structure with the regeneration of renal epithelial cell lining of cortical tubules and reinstatement of regular morphology of renal cortex when compared with DOX alone and AHE 200 mg/kg b.w. groups. The low dose (200 mg/kg b.w.) of AHE however, did not completely reverse morphological alterations observed in DOX alone group and showed moderately degenerated renal tubules, mild atrophy with capsule distortion and tubular dilations of some tubules.
Fig. 2.
Histopathological effect of Doxorubicin and protective effect of AHE in rat kidney (H&E staining, magnification 40X). Group 1: Renal section from control rats showing normal morphology. Group 2: renal sections from DOX-treated rats show degenerative changes, atrophy, capsule distortion, and cellular infiltrations. Group 3: Represents renal section from AHE alone treated rats. Group 4: AHE Low dose treatment showed mild tubular dilations. Group 5: AHE high dose treatment results in significant protection against DOX-induced renal injury. Group 6: Showed a protective effect of Silymarin treatment. AHE-A. hydaspica ethyl acetate fraction, DOX- Doxorubicin, GC-Glomerular capsule, BS-Bowman’s space, T- Tubules, CI- Cellular infiltrations, ABS-alteration in Bowman’s space, TD-Tubule dilation, CD- Capsule distortion, VC-vascular congestions, A- Atrophy.
4. Discussion
The deleterious consequence of DOX-induced nephrotoxicity is mainly governed by selective damaging of proximal tubule cells via mechanisms that continue to be the focus (Grant et al., 2019). Severe renal tubular impairments as a result of chemotherapy lead to acute renal failure (Ruggiero et al., 2017). Inflammation enhances the production of ROS, oxidative stress, apoptosis, and decrease in antioxidant enzymes in the kidney might be the underlying causes of DOX induces renal injury (Abdelmeguid et al., 2010, Carvalho et al., 2009). Drug-induced amelioration on kidney function and tubular lesions can be easily evaluated by using rat models as their intra‐renal enzyme dissemination is analogous to that in humans (Saad et al., 2009). The current study was proposed to discern the perspective of AHE to prevent the chemotherapeutic drugs induced renal toxicity, which might enhance the antitumor efficacy DOX.
Urine analyses point out the grade of kidney functional capacity and acid-base balance. The elevated level of urine creatinine, urea, protein, and albumin, and decreased creatinine clearance are general indicators of kidney injury impelled by drug treatment(Dhondup and Qian, 2017). Specifically abnormal proteinuria and hematuria in DOX treated groups indicate nephrotoxicity. Urine specific gravity associated with urine osmolality and provides critical details of hydration status. DOX altered urine specific gravity and PH. Co-treatment with AHE ameliorates DOX-induced alterations in a dose-dependent way.
Inoculation of DOX to rats resulted in lessening in glomerular filtration rate, which is linked with increased serum creatinine, BUN, urea, and uric acid. Current outcomes are in correspondence with earlier outcomes in different studies revealing that boost in the serum levels of renal damage biomarkers concomitant to compromised renal architecture, tubular blockade (El-Sheikh et al., 2012). Such functional instabilities in DOX exposed rats point toward the ability of this drugs to prevent protein synthesis in the tubular cells or to recruit lipid peroxidation and grounds free radical formation in renal tissue (Naziroğlu et al., 2004, Ateşşahín et al., 2007, Naqshbandi et al., 2012). Contrariwise, co-treatment with DOX significantly improved the kidney function biomarkers in serum. Moreover DOX administration decrease kidney tissue protein content and AHE treatments result in significant restoration of kidney tissue protein content.
The decline of SOD, CAT, POD, QR, GPx, γ-GT, GST, and GR activities accompanied by a decrement of GSH content was unveiled after CP and DOX injection, resulting in the reduced ability of the kidney to scavenge toxic H2O2 and lipid peroxides. These findings were also noticed by other researchers (El-Sheikh et al., 2012). In the present investigation, AHE was able to significantly restore the above mentioned antioxidant enzyme activities in kidney tissues. Reno-protective actions of AHE against DOX-induced nephrotoxicity may be due to its unique composition, as AHE fraction of A. hydaspica is rich in flavonoids (polyphenolics, tannins, and so forth), and flavonoids have been reported to possess both antioxidant and anti-inflammatory activities via quenching free radicals and impeding lipid peroxidation (Nijveldt et al., 2001). Similarly, green tea intake revealed a marked reduction in the cisplatin-induced oxidative stress via increases in the activities of renal SOD and catalase (Khan et al., 2009). Likewise, Wang and colleagues demonstrated that green tea polyphenols proficiently scavenge ROS production initiated by lead exposure, thus diminished ROS-interceded inflammatory cytokines discharge through ERK/JNK/p38 pathways(Wang et al., 2016). Epigallocatechin-3-gallate (EGCG), the main catechin of AHE, is also the chief constituent of green tea extract, is recognized as a dominant antioxidant and ROS scavenger. Numerous studies have shown EGCG has a prospective role in chronic kidney disease models. It is proposed that EGCG modulates cellular and molecular mechanisms via inflammation-allied NF-кB and Nrf2 signaling pathway (Bao and Peng, 2016, Kanlaya and Thongboonkerd, 2019). Similarly, Tatlidede and his colleagues demonstrate the protective effect of resveratrol (flavonoid) against DOX prompted cardiotoxicity by lessening oxidative injury (Tatlidede et al., 2009). Among a lot of inducers of oxidative stress, renal MDA and NO were testified to be increased after DOX inoculation. Outcomes of present research also indicate that there was a significant upsurge in MDA, NO, and H2O2 quantity in the renal tissue of rats that received DOX alone in contrast to the control group. These results were in covenant with preceding studies, which demonstrated an increase in lipid peroxidation and subsidence of antioxidant defense system in the kidney after DOX treatment (Abdel Moneim, 2014, El-Sheikh et al., 2012). Results reveal that AHE could restore renal injury induced by DOX treatments and confirmed the important role of AHE antioxidant property against drug-induced nephrotoxicity, in particular, via enhancing the antioxidant defense system. Consistently, Abdel Moneim et al. reported that A. indica may inhibit lipid peroxidation via quenching free radicals and augmenting intracellular concentration of glutathione owing to the presence of flavonoids (Abdel Moneim et al., 2014). AHE co-treatment works more efficiently at 400 mg/kg b.w dose compared to 200 mg/kg b.w dose to ameliorate the toxic outcomes of DOX. Induction of nephrotoxicity induced by chemotherapeutic drugs is anticipated to be a quick outcome encompassing reaction proteins in the renal tubules. Hence the nephron-protective mediator must be given at the same time with the chemotherapeutic drug to prevent its side effects. The examination of renal histoarchitecture is necessary to corroborate the biochemical findings of experimental groups. Glomerular capillary tufts size contraction, Bowman’s capsule dilation, deterioration, necrosis and detachment of the epithelial cell lining of proximal tubules, flaking of the apical microvilli and accretion of consistent exudates inside the distended tubular lumina of both distal and proximal convoluted tubules were witnessed at the cortico-medullary area in the kidney sections of DOX inoculated rats. Some of the tubules were entirely collapsed and cellular intricacies have vanished. Analogous histopathological findings were demonstrated previously in the kidney of rats treated with altered doses of DOX (Nasr and Saleh, 2014, Abdelmeguid et al., 2010, Ayla et al., 2011, El-Sheikh et al., 2012). It was assumed that histopathological changes may be linked with the absorption power of renal tubules that initiate functional congestion of nephrons with consequent kidney malfunction (Khan et al., 2012). AHE treatment significantly ameliorates the damaging effects of DOX on kidney morphology. Previous research approves that silymarin possesses excellent renoprotective effect against drug and chemical-induced renal alterations and regarded as a potentially pragmatic candidate in combined chemotherapy regimens by functioning as a potent quencher of radical species in the kidney thus precluding the toxic influences at both the histological and biochemical levels (Shahbazi et al., 2012, Ahmed et al., 2019, Nouri and Heidarian, 2019).
These findings ratified that biochemical and histological variations induced by DOX treatment may be ameliorated by AHE due to the occurrence of active antioxidant polyphenolic metabolites (mainly 7-O-galloyl catechin, catechin, and methyl gallate). These compounds might boost cellular antioxidant enzymes, augmented cells GSH amount, and quenched the radical species (Rahman et al., 2006, Murakami et al., 2002, Hsieh et al., 2004). Therefore, AHE might be a significant candidate providing excellent protective effect in combination with chemotherapy due to its antioxidant and radical species quenching potential.
5. Conclusion
By our knowledge, this is the first study indicating the protective effect of A. hydaspica on DOX provoked kidney damage in rats. The outcomes revealed the ameliorating potential of AHE against DOX persuaded kidney injury via suppression of DOX mediated oxidative deterioration in renal tubular cells, preservation of kidney function biomarkers, and prevention of DNA damage. Combine administration of AHE at upper tested dose AHE offered added protection against DOX-induced kidney damage. The mechanism of nephroprotective action by AHE fraction could be due to the antioxidant and free radical scavenging activities of its active metabolites. The present results suggest that AHE might be a potential therapeutic in preventing DOX nephrotoxic effects. Although AHE showed its protection in our study, it warrants further research for use as therapeutic agents.
Limitations of the study
Further investigations are a prerequisite to endorse the current outcomes using molecular tools and immune-histochemical techniques. There is a necessity for advanced investigations to create an empirical basis for the consumption of A. hydaspica as supplementary therapy with DOX, therefore, the details mechanism of A. hydaspica effects on anti-inflammatory and anti-apoptotic signaling, the reformative proficiency of the kidneys, and renal cellular transport should be evaluated. Furthermore, due to lack of funding presently we were unable to analyze the details of the mechanism of protection in the xenograft mice model. Therefore, investigating the detailed mechanism of protection and how A. hydapica preserve the anticancer potential of DOX in tumor models will be the focus of further investigations.
Declarations
Ethics approval and Consent to participate
The investigational procedure for the use of animal was sanctioned (Bch#0256) by the ethical board of Quaid-i-Azam University, Islamabad Pakistan.
Availability of data and materials
All the data are contained in the manuscript.
Funding
We are gratifying the Deanship of Scientific Research at King Saud University for its funding of this research through Research Group number (RGP-193).
CRediT authorship contribution statement
Tayyaba Afsar: Conceptulization, Data curation, Formal analysis, Investigation, Software, Methodology, Writing original draft, Writing - review & editing. Suhail Razak: Project administration, Validation, Visualisation, Methodology, Resources, Funding acquisition, Writing - review & editing. Ali Almjawal: Resources, Visualisation, Validation, Writing - review & editing. Dara Al-Disi: Resources, Visualisation, Funding acquisition, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors proclaim that they have no competing interests.
Acknowledgements
The authors would like to extend their heartfelt thankfulness to the Deanship of Scientific Research at King Saud University, KSA for its support through the research group no. (RGP-193).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Tayyaba Afsar, Email: tayyaba_sona@yahoo.com.
Suhail Razak, Email: smarazi@ksu.edu.sa.
Ali Almajwal, Email: aalmajwal@ksu.edu.sa.
Dara Al-Disi, Email: daldisi@ksu.edu.sa.
References
- Abdel Moneim, A.E., Othman, M.S., Aref, A.M., 2014. Azadirachta indica Attenuates Cisplatin-Induced Nephrotoxicity and Oxidative Stress. BioMed Res. Int., 2014. [DOI] [PMC free article] [PubMed]
- Abdelmeguid N.E., Chmaisse H.N., Zeinab N.A. Protective effect of silymarin on cisplatin-induced nephrotoxicity in rats. Pak. J. Nutr. 2010;9:624–636. [Google Scholar]
- Afsar T., Khan M.R., Razak S., Ullah S., Mirza B. Antipyretic, anti-inflammatory and analgesic activity of Acacia hydaspica R. Parker and its phytochemical analysis. BMC Complement Altern. Med. 2015;15:136. doi: 10.1186/s12906-015-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar T., Razak S. Modulatory influence of Acacia hydaspica R. Parker ethyl acetate extract against cisplatin inveigled hepatic injury and dyslipidemia in rats. BMC Complement Altern. Med. 2017;17:307. doi: 10.1186/s12906-017-1824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar T., Razak S., Almajwal A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer. 2017;17:883. doi: 10.1186/s12885-017-3898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar, T., Razak, S., Almajwal, A., Shabbir, M., Khan, M. R. J. B. C. & Medicine, A., 2019. Evaluating the protective potency of Acacia hydaspica R. Parker on histological and biochemical changes induced by Cisplatin in the cardiac tissue of rats. 19, 182. [DOI] [PMC free article] [PubMed]
- Afsar T., Razak S., Batoo K.M., Khan M.R. Acacia hydaspica R. Parker prevents doxorubicin-induced cardiac injury by attenuation of oxidative stress and structural Cardiomyocyte alterations in rats. BMC Complement Altern. Med. 2017;17:554. doi: 10.1186/s12906-017-2061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar T., Razak S., Khan M.R., Almajwal A. Anti-depressant and anxiolytic potential of Acacia hydaspica R. Parker aerial parts extract: Modulation of brain antioxidant enzyme status. BMC Complement Altern. Med. 2017;17:228. doi: 10.1186/s12906-017-1671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar T., Razak S., Khan M.R., Mawash S., Almajwal A., Shabir M., Haq I.U. Evaluation of antioxidant, anti-hemolytic and anticancer activity of various solvent extracts of Acacia hydaspica R. Parker aerial parts. BMC Complement Altern. Med. 2016;16:258. doi: 10.1186/s12906-016-1240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar T., Razak S., Shabbir M., Khan M.R. Antioxidant activity of polyphenolic compounds isolated from ethyl-acetate fraction of Acacia hydaspica R. Parker. Chem. Central J. 2018;12:5. doi: 10.1186/s13065-018-0373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar T., Trembley J.H., Salomon C.E., Razak S., Khan M.R., Ahmed K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia Hydaspica: involvement of multiple signal transduction pathways. Sci. Rep. 2016;6:23077. doi: 10.1038/srep23077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M.A., Tayawi H.M., Ibrahim M.K. Protective effect of Silymarin against kidney injury induced by carbon tetrachloride in male rats. Iraqi J. Veter. Sci. 2019;33:127–130. [Google Scholar]
- Ahn T.-G., Kim H.-K., Park S.-W., Kim S.-A., Lee B.-R., Han S.J. Protective effects of green tea polyphenol against cisplatin-induced nephrotoxicity in rats. Obstetr. Gynecol. Sci. 2014;57:464–470. doi: 10.5468/ogs.2014.57.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateşşahín A., Çeríbaşi A.O., Yuce A., Bulmus Ö., Çikim G. Role of Ellagic Acid against Cisplatin-Induced Nephrotoxicity and Oxidative Stress in Rats. Basic Clin. Pharmacol. Toxicol. 2007;100:121–126. doi: 10.1111/j.1742-7843.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- Ayla, S., Seckin, I., Tanriverdi, G., Cengiz, M., Eser, M., Soner, B., Oktem, G., 2011. Doxorubicin induced nephrotoxicity: protective effect of nicotinamide. Int. J. Cell Biol.. [DOI] [PMC free article] [PubMed]
- Bao H., Peng A. The Green Tea Polyphenol (—)-epigallocatechin-3-gallate and its beneficial roles in chronic kidney disease. J. Trans. Int. Med. 2016;4:99–103. doi: 10.1515/jtim-2016-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A.M., Hunkeler M.J., Talalay P. Increase of NAD (P) H: quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc. Natl. Acad. Sci. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- Carvalho C., Santos R.X., Cardoso S., Correia S., Oliveira P.J., Santos M.S., Moreira P.I. Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- Chakrabarty T., Gangopadhyay M. The genus Acacia P. Miller (Leguminosae: Mimosoideae) in India. J. Econ. Taxonomic Botany. 1996;20:599–633. [Google Scholar]
- Dhondup T., Qian Q. Electrolyte and acid-base disorders in chronic kidney disease and end-stage kidney failure. Blood Purificat. 2017;43:179–188. doi: 10.1159/000452725. [DOI] [PubMed] [Google Scholar]
- El-Sheikh A.A., Morsy M.A., Mahmoud M.M., Rifaai R.A., Abdelrahman A.M. Effect of coenzyme-Q10 on doxorubicin-induced nephrotoxicity in rats. Adv. Pharm. Sci. 2012 doi: 10.1155/2012/981461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibu S., Delemasure S., Richard C., Guilland J.-C., Martin L., Gambert S., Rochette L., Vergely C. General oxidative stress during doxorubicin-induced cardiotoxicity in rats: absence of cardioprotection and low antioxidant efficiency of alpha-lipoic acid. Biochimie. 2012;94:932–939. doi: 10.1016/j.biochi.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Granados-Principal S., Quiles J.L., Ramirez-Tortosa C.L., Sanchez-Rovira P., Ramirez-Tortosa M. New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients. Food Chem. Toxicol. 2010;48:1425–1438. doi: 10.1016/j.fct.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Grant M.K., Seelig D.M., Sharkey L.C., Choi W.S., Abdelgawad I.Y., Zordoky B.N. Sexual dimorphism of acute doxorubicin-induced nephrotoxicity in C57Bl/6 mice. PloS One. 2019;14:e0212486. doi: 10.1371/journal.pone.0212486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hsieh T.-J., Liu T.-Z., Chia Y.-C., Chern C.-L., Lu F.-J., Chuang M.-C., Mau S.-Y., Chen S.-H., Syu Y.-H., Chen C.-H. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem. Toxicol. 2004;42:843–850. doi: 10.1016/j.fct.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Iqbal S., Bhanger M., Anwar F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005;93:265–272. [Google Scholar]
- Jabeen A., Khan M.A., Ahmad M., Zafar M., Ahmad F. Indigenous uses of economically important flora of Margallah hills national park, Islamabad, Pakistan. African J. Biotechnol. 2009;8 [Google Scholar]
- Jalali A.S., Hasanzadeh S. Crataegus monogyna fruit aqueous extract as a protective agent against doxorubicin-induced reproductive toxicity in male rats. Avicenna J. Phytomed. 2013;3:159. [PMC free article] [PubMed] [Google Scholar]
- Jollow D., Mitchell J., Zampaglione N.A., Gillette J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kakkar P., Das B., Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Kanlaya R., Thongboonkerd V. Protective effects of epigallocatechin-3-gallate from green tea in various kidney diseases. Adv. Nutrit. 2019;10:112–121. doi: 10.1093/advances/nmy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.A., Khan M.R., Sahreen S. Protective effects of rutin against potassium bromate induced nephrotoxicity in rats. BMC Complement. Alternative Med. 2012;12:204. doi: 10.1186/1472-6882-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A., Priyamvada S., Khan W., Khan S., Farooq N., Yusufi A.N. Studies on the protective effect of green tea against cisplatin induced nephrotoxicity. Pharmacol. Res. 2009;60:382–391. doi: 10.1016/j.phrs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Koriem K.M., Farrag A.R.H., Badawy M.A., El-Toumy S.A. Role of some Egyptian medicinal plants against liver and kidney toxicity induced by cadmium chloride. Toxicol. Mech. Methods. 2009;19:524–534. doi: 10.1080/15376510903121145. [DOI] [PubMed] [Google Scholar]
- Lee V.W., Harris D.C. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology. 2011;16:30–38. doi: 10.1111/j.1440-1797.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mizutani H., Tada-Oikawa S., Hiraku Y., Kojima M., Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–1453. doi: 10.1016/j.lfs.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Mohammed, E.E., 2015. Nephro-protective Effect of Acacia senegal and Hyphaene thebacia Against Drug-induced Renal Damage in Rats. University of Khartoum.
- Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- Murakami C., Hirakawa Y., Inui H., Nakano Y., Yoshida H. Effect of tea catechins on cellular lipid peroxidation and cytotoxicity in HepG2 cells. Biosci. Biotechnol. Biochem. 2002;66:1559–1562. doi: 10.1271/bbb.66.1559. [DOI] [PubMed] [Google Scholar]
- Naqshbandi A., Khan M.W., Rizwan S., Ur Rehman S., Khan F. Studies on the protective effect of dietary fish oil on cisplatin induced nephrotoxicity in rats. Food Chem. Toxicol. 2012;50:265–273. doi: 10.1016/j.fct.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Nasr A.Y., Saleh H.A. Aged garlic extract protects against oxidative stress and renal changes in cisplatin-treated adult male rats. Cancer Cell In. 2014;14:92–104. doi: 10.1186/s12935-014-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroğlu, M., Karaoğlu, A., Aksoy, A.O., 2004. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology, 195, 221-230. [DOI] [PubMed]
- Nijveldt R.J., van Nood E., van Hoorn D.E., Boelens P.G., van Norren K., van Leeuwen P.A. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutrit. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Nouri A., Heidarian E. Nephroprotective effect of silymarin against diclofenacinduced renal damage and oxidative stress in male rats. J. Herbmed Pharmacol. 2019 [Google Scholar]
- Oda S.S., El-Ashmawy I.M. Protective effect of silymarin on mercury-induced acute nephro-hepatotoxicity in rats. Studies. 2012;35:36. [Google Scholar]
- Orlowski M., Sessa G., Green J.P. γ-Glutamyl transpeptidase in brain capillaries: possible site of a blood-brain barrier for amino acids. Science. 1974;184:66–68. doi: 10.1126/science.184.4132.66. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Puga C.D., Hilario M.C., Mendoza J.G.E., Campos O.M., Jijón E.M., Martínez M.D., Izazaga M.A.Á., Solano J.Á.L., Chaverri J.P. Antioxidant activity and protection against oxidative-induced damage of Acacia shaffneri and Acacia farnesiana pods extracts: in vitro and in vivo assays. BMC Complement Altern. Med. 2015;15:435. doi: 10.1186/s12906-015-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ruggiero A., Ferrara P., Attinà G., Rizzo D., Riccardi R. Renal toxicity and chemotherapy in children with cancer. Brit. J. Clin. Pharmacol. 2017;83:2605–2614. doi: 10.1111/bcp.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.A., Youssef M.I., El-Shennawy L.K. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: the protective effect of grape seed proanthocyanidin extract. Food Chem. Toxicol. 2009;47:1499–1506. doi: 10.1016/j.fct.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Sakr S.A., Mahran H.A., Lamfon H.A. Protective effect of ginger (Zingiber officinale) on adriamycin-induced hepatotoxicity in albino rats. J. Med. Plant Res. 2011;5:133–140. [Google Scholar]
- Shahbazi F., Dashti-Khavidaki S., Khalili H., Lessan-Pezeshki M. Potential renoprotective effects of silymarin against nephrotoxic drugs: a review of literature. J. Pharm. Pharm. Sci. 2012;15:112–123. doi: 10.18433/j3f88s. [DOI] [PubMed] [Google Scholar]
- Su Z., Ye J., Qin Z., Ding X. Protective effects of madecassoside against Doxorubicin induced nephrotoxicity in vivo and in vitro. Sci. Rep. 2015;5:18314. doi: 10.1038/srep18314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatlidede E., Sehirli Ö., Velioglu-Ögünç A., Cetinel S., Yegen B.Ç., Yarat A., Süleymanoglu S., Sener G. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radical Res. 2009;43:195–205. doi: 10.1080/10715760802673008. [DOI] [PubMed] [Google Scholar]
- van Acker F.A., van Acker S.A., Kramer K., Haenen G.R., Bast A., van der Vijgh W.J. 7-monohydroxyethylrutoside protects against chronic doxorubicin-induced cardiotoxicity when administered only once per week. Clin. Cancer Res. 2000;6:1337–1341. [PubMed] [Google Scholar]
- Wang H., Li D., Hu Z., Zhao S., Zheng Z., Li W. Protective effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol. Cells. 2016;39:508. doi: 10.14348/molcells.2016.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapstra F.H., van Goor H., de Jong P.E., Navis G., de Zeeuw D. Dose of doxorubicin determines severity of renal damage and responsiveness to ACE-inhibition in experimental nephrosis. J. Pharmacol. Toxicol. Methods. 1999;41:69–73. doi: 10.1016/s1056-8719(99)00015-5. [DOI] [PubMed] [Google Scholar]
- Zhao X., Zhang J., Tong N., Chen Y., Luo Y. Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol. Pharm. Bull. 2012;35:796–800. doi: 10.1248/bpb.35.796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are contained in the manuscript.