1. Introduction
The resistance of the pathogenic microorganisms including bacteria, fungi, virus and parasites to antimicrobial agents are increasing constantly due to the extensive use of antibiotics and natural selection of environment (Sib et al., 2019). Recent years, the emergence of resistance to antibiotics increase the morbidity, mortality and challenging health care issues, and expanding all over the world (Mekes et al., 2019). The continuous resistant against current antibiotics is a major reason for increase of multi drug resistance (MDR) effect in microbes (Robert et al., 2018). Among these, MDR Gram negative bacterial (GNB) infections have played a major role worldwide in health care settings (Rajivgandhi et al., 2018a). According to the WHO reports, carbapenem resistant GNB as the number 1 critical list including Acinetobacter baumannii, Klebseilla pneumoniae, Pseudomonas aeruginosa and Proteus mirabilis (WHO, 2017). In 2019, the review of Antimicrobial resistance committee was announced that the carbapenem resistant GNB increase the number of death, it attributed to antimicrobial resistance could increase 1 million each year (Teethaisong et al., 2019). Five fold increased death rate was reported recently in developing nations compared to developed nations.
Emergence of carbapenem resistant (CR) bacterial infections are a life threatening problem due to the production of multiple genetic and physiochemical mechanisms including mutation, horizondal gene transfer, alteration of target proteins, enzyme inactivation of antibiotics, quorum sensing, biofilm formation, extended spectrum beta lactamase production (Rahdar et al., 2019). In particular, carbapenemase producing K. pneumoniae is very most important bacteria among the Enterobacteriaceae family (Cusumano et al., 2019). Carbapenemase is a type of beta-lactamases, it has the ability to hydrolyze oxy amine side chains of the carbapenems antibiotics, uncontrolled efflux pump expression. In addition, the antibiotics could not enter into the outer membrane of bacteria due to the production of porin mutations, and their carbapenamase production is increase the carbapenem resistance efficiency in bacteria (Ece et al., 2018). Various classes of carbapenemases were emerged recently with different mechanisms of carbapenems hydrolysis activity including IMP, VIM, NDM, CMY and PDC beta-lactamases, OXA (Ahmad et al., 2018, Sharma et al., 2019). All of these genes were encoded by plasmid and pose a vigorous spread of carbapenem resistance nature (Hamoud et al., 2014). Different researchers have been reported different kinds of mechanism for carbapenem resistant bacteria, but so far, the exact mechanism is not fully understood (Borer et al., 2012). Therefore, there is an emerging need for developing novel and potent antibiotics targeting the carbapenem resistant GNB, especially CR K. pneumoniae.
The genus Camellia one of the largest medicinal plant that belongs to the Theaceae family (Huawei et al., 2019). It mostly present in East Asia with considerable attraction due to its excellent economic values for human consumption (Yan et al., 2013). Many varieties of Camellia species are available such as C. sinensis, C. grijsii, C. sasanqua, C. sasanqua, C. reticulata C. oleifera and C. japonica. The leaf and buds of the some Camellia species are used in tea production worldwide (Kazuhiko et al., 1986). The extracted seeds of C. japonica and C. oleifera are frequently used in cooking oil and the cultivation and utilization are very high in China (Bin et al., 2019). Some of the species are used to famous ornamental values and produce colorful flowers with long time fluorescence and striking aromas. The high quality of various Camellia species seed oils was highly produced in Taiwan high level in house cooking. In particular, the high content of unsaturated fatty acids contained leic acid and linoleic acid of C. japonica used to human health (Mong et al., 2014). Recent years, 90% of the oil is reported from Camellia species. The rich unsaturated fatty acid sources of Camellia species is the topmost oil compared with other olive oils. It is used in the format of ‘‘omega meals for human. The high level squalene and flavonoids content of the vitamin E is more in Camellia species when compared with other olive oils (Hui et al., 2017). It has highly nutritional and medicinal sources, which used traditionally in stomach ache and wound infections (Eunsun et al., 2007). Recently, some researchers were reports that the Camellia species has high anti-bacteria (Kazuhiko et al., 1986), anti-viral, anti-cancer, skin healing (Mourad et al., 2020) and larvicidal (Na et al., 2018) activities. It act as a excellent drug and protect the liver against CCl4-induced oxidative damage and decreased the body cholesterol level (Huawei et al., 2019, Yan et al., 2013). Sometimes, it increase the oxidative stress responses in human body. In this context, to overcome this crisis, we choose an potential essential oil components from the Chinesh medicinal plant Camellia japonica to combat the CR K. pneumoniae.
2. Materials and methods
2.1. Strain collection
The CR K. pneumoniae strain was procured from Government Head Quarters Hospital, Tiruchirappalli, Tamil Nadu, India, which cause urinary tract infections. The carbapenemase production of selected CR K. pneumoniae strain was confirmed by Nitro-Carba test, followed by previous report of Teethaisong et al., (2019). All the chemicals, reagents, plates and antibiotic discs of this study was procured from Hi-Media Laboratories, Mumbai, India.
2.2. Detection of carbapenom resistant effect
The carbapenemase producing ability of K. pnemoniae was tested against specific antibiotic panel of HX095, HX096, HX066 and HX103 antibiotic discs by disc diffusion method (Clinical & Laboratory Standard Institute Guidelines). The antibiotic discs of aztreonam (AT-30 µg), cefpodoxime (CPD-10 µg), cefpodoxime/Clavulanic acid (CCL-10/5 µg), ceftazidime (CAZ-30 µg), cefotaxime (CTX-30 µg), ceftriaxone (CTR-30 µg) for HX095, cefpodoxime (CPD-10 µg), cefpodoxime/Clavulanic acid (CCL-10/5 µg), ceftazidime (CAZ), ceftazidime/Clavulanic acid (CAC-30/10 µg), cefotaxime (CTX-30 µg) and cefotaxime/Clavulanic acid (CEC-30/10 µg) for HX096, cefepime (CPM-30 µg), cefoperazone (CPZ-75 µg), ceftriaxone (CTR-30 µg), cefoxitin (CX-30 µg), imipenem (IPM-10 µg), ticarcillin/clavulanic acid (TCC-75/10 µg) for HX066 and ciproflaxacin (CIP-5 µg), imipenem (IPM-10 µg), meropenem (MRP-10 µg), ertapenem (ETP-10 µg), cefoperazone/sulbactum (CFS-75/30 µg) piperacillin/tazobactam (PIT-100/10 µg) for HX103 (Subashini et al., 2014).
2.3. Confirmation of carbapenemase production by E-test stripe method
The carbapenemase producing ability of selected K. pneumoniae was determined by E-test MIC stripe assay (Maruthupandy et al., 2018a). Briefly, two different type of gradient concentration E-stripes were used for detection of carbapenemase activity. In first stripe, the upper side coated with specific ESBL enzyme detection mix antibiotics (0.32–4 µg/mL) and lower side coated with specific ESBL enzyme detection antibiotic alone were prepared (0.125–16 µg/mL). Instead, the second stripe coated ESBL enzyme detection mix antibiotics in upper side (0.32–4 µg/mL) and AMC enzyme detection mix antibiotics in lower side were prepared (0.125–16 µg/mL). The MIC value was detected in µg/mL based on the CLSI guidelines (Rajivgandhi et al., 2019b). The interpretation of every zone of inhibition between the strips and calculated their edges were refereed as MIC value. The ESBLs of carbapenemase was indicated as positive, the result of zone exhibited the >8 for Enz MIX, >0.5 for ESBL detection antibiotic alone and >1 for AMC enzyme detection mix were observed.
2.4. Molecular confirmation of CR K. pneumoniae
Based on the carbapenem resistant activity, the genomic DNA of selected CR K. pneumoniae was identified by multi plux PCR using universal primers (Cusumano et al., 2019). The 16S rRNA sequences was extracted by based on the standard protocol including initial denaturation at 95 °C for 10 m, and 35 cycle denaturation temperature at 96 °C for 1 min, annealing at 55 °C for 1 min and extension 72 °C for 2 min, and final extension at 72 °C for 10 min. After, the received PCR product was sequenced by automated sequencer (Shimadzhu, Japan) in Rajivgandhi Centre for Biotechnology (RGCB), Kerala, India. The sequences were aligned carefully and detect the pairwise identities using NBLAST. Then, successive sequences were submitted in NCBI for receive the accession number. After, the accession number was noted and the applied to selected CR K. pneumoniae. In addition, the genetic evidences of carbapenem producucing genes of selected K. pneumoniae was detected by multiplux PCR method (Tayebi et al., 2019).
2.5. Purification of essential oil (EO) and hydrosol extraction
The fresh cleaned plant seeds of C. japonica was collected from Sun Yat-Sen University campus, Guangzhou, China. Five hundred gram of the fresh seeds were washed with DD·H2O and dried at lab condition in the shade for 20 days. Air dried sample was hydro distilled process using Clevenger apparatus (Shimadzhu, Germany) using n-hexane as a recovery solvent (Shimadzhu, Germany) using n-hexane as a recovery solvent. According to the previous method of Mitali et al. (2019), the 250 g of the sample was placed into 5 L conical flask containing 2 L DD·H2O and then heated at 100 °C temperature for 2–4 h using heating mantle. The condensed liquid nature of the oil was recovered by the experiment and filtered in receiver tube. The water and EOs were extracted separately by using separating funnel and collected in measuring tube. The EOs was dried with anhydrous sodium sulphate for complete water removal. Finally, the purified EOs was recovered and stored at 4 °C for further use.
2.6. GC–MS analysis
Identification of C. japonica EO components was analyzed using GC–MS (TQ 8040-Thermoscientific, Germany) with the modification method of Mancarz et al. (2019). The GC–MS was equipped with GC 2010 plus coupled with triple-quadrupole mass detector, which was attached with an AOC 5000 auto sampler performed in liquid mode. Capillary column size of 25 m × 3 mm for divide, and carrier gas of helium with 1 mL/min flow rate. The n-hexane and 10 μg/mL volume of the sample was used at 1 μg concentration for injection with 1:10 split ratio. The detector and injector temperature was programmed from 40 to 250 °C and initial GC oven temperature was 60 μg for 5 min. The total analysis time was performed with 90 h. The 300 °C of transfer line temperature and MS ion sources were used at 250 °C. The mass range was programmed with 40–400 m/z, which was received by electron ionization at 80 eV. Based on their retention indices (RI) and mass fragmentation patterns, all the chemical components of the oil were assisted by National Institute of Standards and Technology (NIST) of Sun Yat-Sen University mass spectral libraries.
2.7. Anti-bacterial activity
Antibacterial activity of C. japonica EO was performed against CR K. pneumoniae (MN396685) by agar well method (Salem et al., 2018). The 24 h old CR K. pneumoniae (MN396685) culture was spread on the sterile muller hinton agar (MHA) plates. After, various concentrations of EO were added into the wells. Whereas third generation chaphlosporin antibiotic discs CTX and DOR were acted as a positive control for detection of ESBL production. After overnight incubation, the zone of inhibition around the EOs containing wells were calculated in diameter.
2.8. Purification of essential oil by preparative HPLC
Potential anti-bacterial components present in the EO was examined by analytical HPLC for detection of active fraction with the mobile phase of acetonitrile-methanol-0.2 m ammonium acetate-water (45:10:10:35) (Rajivgandhi et al., 2019c). The agar well diffusion method was used to evaluate the essential oil fractions against CR K. pneumoniae (MN396685). Further, excellent anti-bacterial activity of the oil fraction was purified separately by preparative HPLC and confirmed by analytical HPLC. The instrument of preparative HPLC was equipped with C18 coloumn, 140 × 4.6 mm with 4.6 µm of linear gradient and 1 mL/min flow rate at 240 nm. The elution program was set up with 0–10 min of 10–90% A, 10–11 min of 90–100% B and 11–20 min of 100% C and injection time volume was used with 20 µL. At the 40–60 °C temperature was used for entire experiment. Finally, purified sample was further performed separately against CR K. pneumoniae (MN396685) and lyophilized at 40℃ by using lyophilizer for further study. The third generation chaphalosporin piperacillin/tazobactam and methanol was served as a positive and negative control respectively for detection of ESBLs production (Thiago et al., 2018).
2.9. Minimum inhibition concentration
The MIC of HPLC purified EO fraction (PFEO) was determined using micro broth dilution method with 96-well plate (Wang et al., 2017). Aliquot 100 µL of overnight tested bacterial culture into the tryptic soy broth (TSB) containing wells. Various concentration of DMSO diluted PFEO was treated into the wells. Without addition of the oil and media alone containing well used as a control and blank respectively. As same as procedure was used for cefotaxime, imipenem and mirapenem (10% DMSO in the range of 1000–30, 10 and 10 μg/mL concentration respectively), which maintained as a positive control for detection of ESBLs and incubated at 37 °C for 24 h. Lowest concentration of PFEO inhibited the bacteria was noted based on the turbidity of growth and determined the O.D values by spectrophotometer.
2.10. Cell cycle arrest by flow cytometry
Flow cytometric analysis was performed to identify the bacterial viability and growth cell arrest of CR K. pneumoniae (MN396685) cells when treated with MIC of PFEO at 24 h using specific Live/Dead Bac Light kit (Shimadzhu, India) (da Silva et al., 2019). After the time period, the cells were centrifuged at 5000 rpm for 30 m and then rinsed with 1 mL cold water twice, filtered 1 × PBS (pH 7.5). Add 2 ul of Propidium iodide (PI) into the sample tube and vortexed followed by incubated at 30 °C for 10 min. As same as procedure of untreated bacterial cells in the PBS were acted as a control. After incubation, the inhibition range of treated and untreated cells were interpreted based on the PI absorption of flow cytometer (FACS MoFlo XDP Beckman Coulter). Analysis was performed in CyteExpert 2.1 software (Bruker, Japan).
2.11. Invitro anti-carbapenamase activity
Anti-carbapenemase activity of active PFEO was evaluated against CR K. pneumoniae (MN396685) by Carba NP (Carbapenemase Nordmann-Poirel) method (van der Zwaluw et al., 2015). Shortly, the tested bacterial culture was taken into 24-well plate containing Tris-HCl 20 mmol/L lysis buffer (Bacterial Protein Extraction Reagent (B-PERRI), Shimadzu, Germany) and different concentration of PFEO was added into the wells except second well and incubated at 37 °C for 12 h. Based on the CLSI guidelines of 2017, to enhance the enzymatic quantity, bacterial culture one-third to one-half was used and incubated at 37 °C for 12 h supplemented with imipenem. After the 12 h incubation, 100 µL of the indicating solution contain pH indicator and phenol red was added into all the wells and noticed the carbapenemase activity within 2 h due to the color changes of phenol red solution. Result was concluding from hydrolysis of carboxylic derivatives of antibiotics, which decrease the PH value. The first well responds as a control, which containing pathogen and media. Second well responds as an internal control, which containing pathogen and antibiotics to stimulate the carabapenemase. Other wells loaded with the pathogen, imipenem plus the various concentrations of EO. According to the CLSI guidelines, the Carb NP test was interpreted as follows, no color changes of first well indicates that the bacteria did not produce any enzyme. The changes of the red color to yellow/orange in second and third wells indicates that the bacteria was produced carbapenemase. Whereas, the quick decolourization of yellow/orange color after the PFEO treated wells indicates that the PFEO acted as a important role in anti-carbapenemase activity by the hydrolysis of virulence molecules (Literacka et al., 2017).
2.12. Quantitative determination of anti-carbapenemase activity
The quantitative measurement of anti-carbapenemase activity was used to assess the invitro Carba NP test result by colorimeter at 540 nm (Vrioni et al., 2012). In this experiment, the 5 M NaCl solution was replaced nstead of B-PERRI lysis buffer to avoid misinterpretation. The test tube containing a loop of culture was mixed with 100 μl of 5 M NaCl solution and vortexed vigorously for 2 min. Then 50 µL supernatant was collected by centrifugation and 50 µL of corresponding enzymatic suspension were added into the 24-well polystyrene plate and mixed well. In addition, 100 µL of 3 mg/mL solution of imipenem monohydrate solution was added next to control wells. Finally, the different concentration of the PFEO was added in the remaining wells of pathogen plus imipenem at 37 °C for 24 h. Then, phenol red solution (pH 7.8) was added in all the wells and allowed to maintain 2 h (Merck Millipore, Guyancourt, France). After incubation, the color changes were obtained from the wells and determined at 540 nm by the colorimeter.
3. Result
3.1. Detection of carbapenom resistance activity
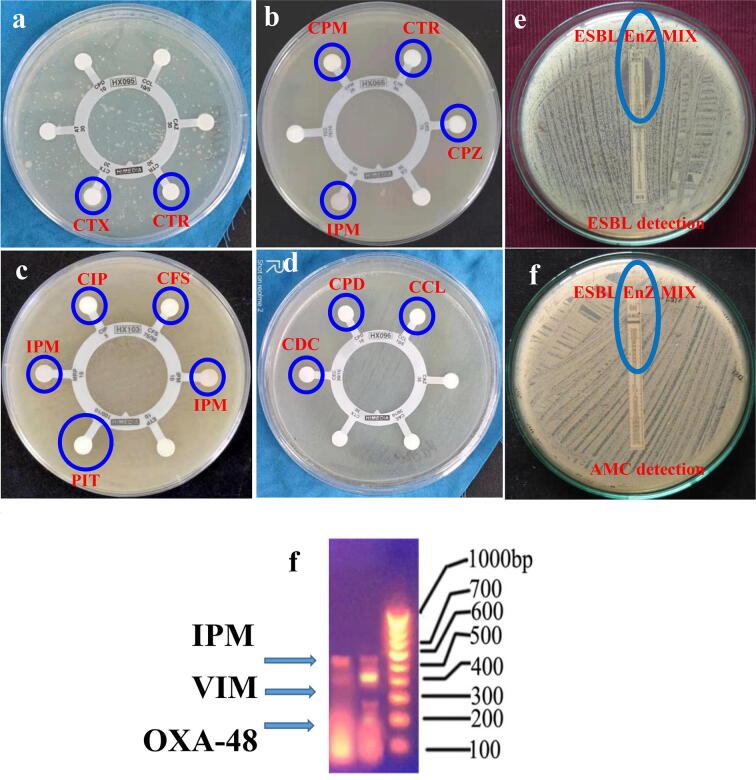
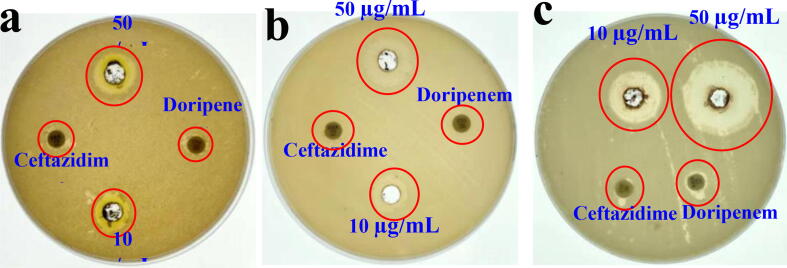
According to the CLSI guidelines, all the Hexa discs results were exhibited more sensitive against tested K. pneumoniae. The specific ESBLs and carbapenemase detection discs of cefpodoxime, ceftazidime, ceftriaxone, cefotaxime, cefpodoxime/Clavulanic acid, ceftazidime/Clavulanic acid, cefoperazone/sulbactum, piperacillin/tazobactam, cefotaxime/Clavulanic acid, ticarcillin/clavulanic acid and imipenem, meropenem, ertapenem were did not produced better inhibition zones against tested K. pneumoniae (Fig. 1a, b). These evidences was suggested that the bacteria has ESBL and carbapenemase producing ability due to the poor performance of performed antibiotics. Especially, the highest concentration of cefoperazone, cefoperazone/sulbactum, ticarcillin/clavulanic acid and piperacillin/tazobactam discs were not act better role against tested bacteria. In addition, the ceftazidime and cefotaxime of specific ESBL identification discs did not correlated with ceftazidime/Clavulanic acid and cefotaxime/Clavulanic acid antibiotics in any places. Similarly, the imipenem, meropenem and ertapenem of specific carbapenem identification discs were also exhibited the with ≤22 mm zone for ceftazidime and ≤27 mm zone for cefotaxime. The result was proved that the tested K. pneumoniae strain was MDR bacteria, especially carbapenemase mediated drug resistant. The CLSI guidelines chart for ESBL and carbapenem resistant bacteria was available in our previously reported article (WHO, 2017).
Fig. 1.
Identification (a–d) and confirmation (e, f) of carbapenem resistant K. pneumoniae by HEXA discs diffusion and MIC stripe methods respectively. Detection of carbapenem resistant genes of IPM, VIM and OXA-48 in selected K. pneumoniae by multiplux PCR analysis (g).
On the other hand, some of the antibiotic discs were observed with low inhibition zones including 6, 2, 6, 6, 8, 4, 4, 4, 16, 4, 20, 10, 6, 8 mm of the zones against CTR, CTX, CPM, CPZ, CTR, IMP, CIP, IPM, MRP, CFS, PIT, CPD, CCL and CEC were observed respectively (Fig. 1c, d). All the antibiotics zones were sensitive to bacteria. They cloud not reach the inhibition level of bacteria due to the target site modifications by chromosomal gene mutations, and inactivate the antibiotics through production of EPS, ESBLs, urease, biofilm formation, QS and efflux pump, penicillinase (Rahdar et al., 2019). All these antibiotics blocked initially and naturalized in their outer membranes by outer membrane proteins (Cusumano et al., 2019). Mechanistically, the GNB cell walls were made up of teichoic acid and EPS, which helped to prevent the external drugs through their harmful chemical productions (Hamoud et al., 2014). Due to this defect, the antibiotics were lost their transferring capacity against bacteria. This result was agreed by previous report of Maruthupandy et al., 2019, Rajivgandhi et al., 2019c, carbapenem resistant GNB was very dangerous. Recently, Ahmad et al. (2018) reported that the carbapenems producing bacteria was shown to be more critical to destroy and kill during the infections time due to a lack of viable treatment choice.
Further, the upper side of ESBL enzyme mix in both the MIC E-stripes exhibited 0.40 and 0. 39 mm zones and lower side of the specific ESBL enzyme detection and AMC ESBL enzymes stripes exhibited with 0.32 and 0.34 mm zone of inhibition were observed against tested K. pneumoniae (Fig. 1e, f). The result was exhibited with >8 mm zone of inhibition. Finally, the MIC E-stripe method was proved, K. pneumoniae was developed more resistant against current antibiotics, especially carbapenemase producer. Our MIC stripe result was correlated with previous report of Rajivgandhi et al., 2018a, Sharma et al., 2019 with ESBL producing P. aeruginosa and K. pneumoniae. The WHO report also mentioned in 2017, >8 mm of the bacterial zone against MIC stripes of ESBL detection antibiotics and AMP antiobiotics were more sensitive (2019). Hence, there is a new chemical derivatives of antibiotic was need to prevent the different kinds of MDR bacteria. Also, increasing scarcity for new idea to detect the appropriate inhibitor for combat the β-lactamase and carbapenemases, which have been very challenging against current antibiotics. Recent years, the medicinal plant and their EO have excellent alternate remedies against various antibiotic resistance infections.
3.2. Molecular identification of selected K. pneumoniae
Based on the NCBI data result, we have found 97.50% similarities with 80% GC content, and 1200 bP for the selected K. pneumoniae and received the accession number of MN396685. In addition, the selected bacteria was named as CR K. pneumoniae (MN396685). Further, the multiplex PCR analysis result was also proved that the selected uropathogens were ESBLs producers, which containing the carbapenemase genes of VIM, IPM and OXA-48 (Fig. 1g).
3.3. Composition of essential oil
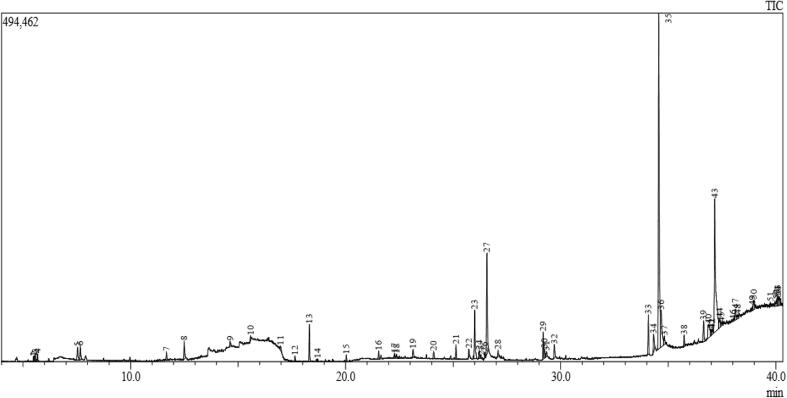
The hydrodistillation method by Clevenger apparatus of C. japonica seeds given sweet and pleasant aroma dark green yellow oil with a yield of 0.17% based on the dry weight. The result of GC–MS was revealed various other compounds also present in the EO of C. japonica seeds (Fig. 2). The GC–MS result was exhibited with 53 compounds and it indicating 90% of the total oil. On basis of the NIST identification, all the compounds were listed in Table. 1. Among the identified compounds, the α-terpineol, α-terpinolene, α-pinene, β-pinene, α-terpenyl acetate, spathulenol of the oil moieties were present more in the C. japonica seeds. The EOs variations of present C. japonica was different to that obtained from C. japonica seed, Japan (Yang et al., 2015). On the other hand, the chemical composition of C. japonica was significantly varied from compound moieties due to the influence of genetic factors, plant material, and harvesting time. In this study, we found most of the carboxylic acid, hydrocarbons, alcohol and esters with significant biological activity. Previously many researchers were reported with various biological activities of C. japonica seeds from various places, they are effectively presented in Table. 2.
Fig. 2.
Identification of essential oil present in the Camellia japonica by GC–MS.
Table 1.
GC–MS analysis of identified compounds of C. japonica seeds essential oil.
| Peaks | RT | Compound name | Area | Area (%) | RSI | RI |
|---|---|---|---|---|---|---|
| 1 | 12.08 | α-Atlantone | 1321 | 3.0 | 997 | 966 |
| 2 | 8.90 | Β-Asarone | 1541 | 2.9 | 932 | 898 |
| 3 | 11.26 | β-Biotol | 1761 | 1.8 | 864 | 832 |
| 4 | 19.02 | α-Costol | 2190 | 2.1 | 901 | 906 |
| 5 | 6.17 | α-Terpineol | 8098 | 1.4 | 887 | 905 |
| 6 | α-Cadalene | 3476 | 2.0 | 799 | 803 | |
| 7 | 26.585 | β-Amyrin | 30,776 | 1.16 | 980 | 933 |
| 8 | 36.642 | Β-Camphane | 53,014 | 0.2 | 970 | 926 |
| 9 | 37.16 | Β-Elemene | 609,741 | 2.3 | 790 | 763 |
| 10 | 27.786 | Anethole | 53,217 | 0.2 | 864 | 840 |
| 11 | 30.60 | Diazepin | 45,671 | 0.6 | 930 | 946 |
| 12 | 25.01 | α-Pinene | 4563 | 0.9 | 899 | 832 |
| 13 | 12.12 | Β-Farnesene | 3567 | 0.6 | 964 | 940 |
| 14 | 16.01 | Caffeine | 2345 | 0.9 | 939 | 936 |
| 15 | 21.14 | Junipene | 4567 | 0.4 | 960 | 889 |
| 16 | 33.11 | Α-Eudesmol | 1340 | 0.8 | 9.23 | 900 |
| 17 | 37.01 | p-Cyamene | 2345 | 1.2 | 886 | 879 |
| 1819 | 22.03 | Furans | 4562 | 0.9 | 901 | 901 |
| 20 | 9.32 | Pluegone | 1265 | 1.5 | 878 | 900 |
| 21 | 28.02 | Indolizine | 1381 | 1.8 | 900 | 886 |
| 22 | 13.15 | Isophorone | 1598 | 2.0 | 933 | 864 |
| 23 | 19.01 | Fenchol | 3456 | 2.2 | 921 | 910 |
| 24 | 20.34 | Erucic acid | 23,451 | 3.1 | 890 | 904 |
| 25 | 19.64 | cis-Vaccenic acid | 256,781 | 2.2 | 908 | 902 |
| 26 | 12.34 | Palmitoleic acid | 654,389 | 1.6 | 901 | 884 |
| 27 | 13.43 | Eicosenoic acid | 23,452 | 3.0 | 904 | 803 |
| 28 | 11 | Tetracosenoic acid | 34,256 | 2.4 | 991 | 809 |
| 29 | 32 | 1-Mono-oleoylglycerol | 346,781 | 2.8 | 899 | 865 |
| 30 | 19.01 | Linolenic acd | 298,764 | 2.0 | 990 | 843 |
| 31 | 14.05 | cis-13-Eicosenoic acid | 190,845 | 2.4 | 834 | 874 |
| 32 | 13.65 | Myristic acid | 324,871 | 0.6 | 867 | 865 |
| 32 | 11.21 | Fluorobenzylamine | 400,918 | 0.9 | 980 | 835 |
| 33 | 9.10 | Pthalic acid | 239,085 | 1.5 | 964 | 890 |
| 34 | 9.16 | cis-10-Nonadecenoic acid | 340,967 | 1.9 | 933 | 900 |
| 35 | 11.20 | n-Hexadecanoic acid | 239,074 | 1.6 | 902 | 991 |
| 36 | 13.01 | Formamide, | 134,781 | 1.8 | 976 | 990 |
| 37 | 11.20 | 6-Octadecanoic acid, | 256,876 | 1.3 | 935 | 912 |
| 38 | 14.05 | 9-Octadecenamide | 982,365 | 1.6 | 907 | 910 |
| 39 | 12.07 | 1-Allylazetidine | 264,539 | 1.4 | 901 | 901 |
| 40 | 11.26 | 9-H-Pyrido[3,4-b]indole | 340,671 | 1.2 | 909 | 900 |
Table 2.
Previous identification of C. japonica effect against various biomedical applications.
| S. no | Place | Source | Compound name | Activities | References |
|---|---|---|---|---|---|
| 1 | Japan | Leaves | Camellenodial,Camellenodiol-3-o-6′-methoxy-β-D-glucuronopyranoside | Anti-viral | Yang et al., 2015 |
| 2 | Korea | Leaves | Steroidal saponins | Acetylcholinesterase inhibition | Kim et al., 2014 |
| 3 | Korea | Leaves | Quercetin, quercetin-3-O-glucoside, quercitrin and kaempferol, | Anti-oxidant | |
| 4 | Korea | Leaves | Camelliatannin A, H and F | Anti-HIV | Park et al., 2002 |
| 5 | Germany | Seeds | Cyclooctadepsipeptides | Anthelmintics | Harder et al., 2003 |
| 6 | Germany | Seeds | Cyclooctadepsipeptides | Anthelmintics | Jeschke et al., 2005 |
| 7 | Korea | Leaves | Mthanolic Extract | Anti-microbial and anti-biofilm | |
| 8 | Japan | Leaves | Class of Camelliasaponins (A1, A2, B1, B2, C1, and C2), | Anti-microbial | |
| 9 | USA | Leaves | Fumaric acid | Anti-microbial | |
| 10 | China | Seeds | Essential oil | Anti-bacterial | Current Study |
3.4. Anti-bacterial activity
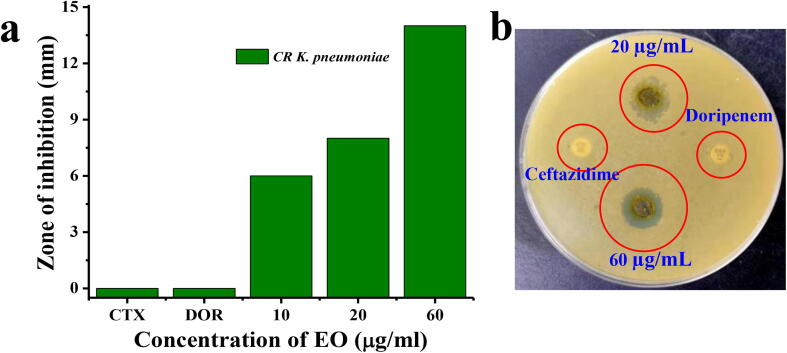
Anti-bacterial effect of EO and third generation cephalosporin antibiotics cefotaxime and doripenem against tested CR K. pneumoniae (MN396685) are presented in Fig. 2. The result was showed with moderate anti-bacterial efficiency against tested CR K. pneumoniae (MN396685), with inhibition ranging diameter of 16 mm zone at 60 µg/mL (Fig. 3a, b). Whereas, the ceftazidime and doripenem was exhibited with no zone of inhibition. The result was suggested that the CR K. pneumoniae (MN396685) was more sensitive to C. japonica EO. The interaction between the EO and bacteria may be attributed to the presence of n-hexadecanoic acid as a sole sources, in fact palmitoleic acid, eicosenoic acid, tetracosenoic acid, cis-13-Eicosenoic acid, Subashini, 1-mono-oleoylglycerol, myristic acid, pthalic acid, cis-10-nonadecenoic acid, 6-octadecanoic acid, 9-octadecanemide, 1-allylazetidine have been known to possess anti-bacterial, anti-cancer, anti-viral and larvicidl activities (Jeschke et al., 2005, Kim et al., 2014, Yoshikawa et al., 1994). This result was agreed by Park et al. (2002) and his team, reported that EO of C. japonica are known to be active against bacterial strains. Mechanistically, some plant essential oil constituents were surrounded with bioactive compound moieties, that used to performed highly in inside of the essential oil with low efficiency (Gupta et al., 2017; Yang et al., 2016). They played a major role in inside of the bacterial that leads to cell wall destruction, QS inactivation, enzyme degradation and biofilm inhibition activity (Soon-Young et al., 2019). Therefore, C. japonica EO of our study was found to be generally active against CR K. pneumoniae (MN396685).
Fig. 3.
Anti-bacterial activity of crude essential oil against CR K. pneumoniae (a, b).
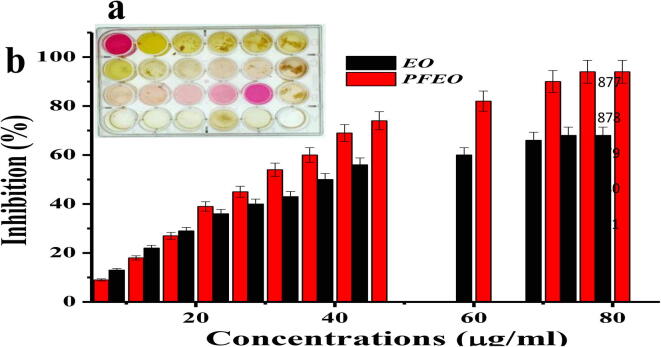
3.5. Purification of active molecules by preparative HPLC
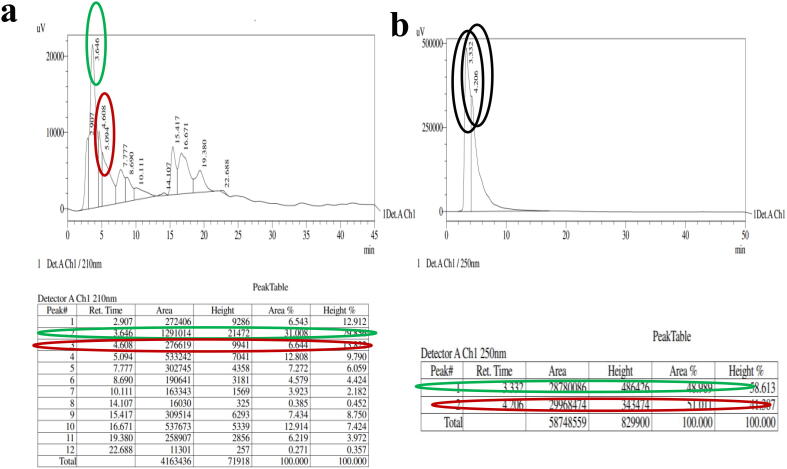
The exact anti-bacterial compound from the EO was purified by using preparative HPLC using n-Hexane as a active solvent (Rho et al., 2019). After purification, the purified EO material was analyzed by analyticle HPLC for identified the different groups of compounds. In result, there are 12 different peaks were identified based on the retention time, area, height, area percentages and height percentages (Fig. 4a). Among 12 fractions, fractions 2 and 3 only exhibited 24 mm and 18 mm inhibition zone against CR K. pneumoniae (MN396685) was determined at 50 µg/mL in agar well diffusion method (Fig. 5a, b). Whereas the other fractions did not shown better zone of inhibition against same bacteria. The mixed fractions of 2 and 3 were showed 32 mm zone of inhibition at 40 µg/mL (Fig. 5c). It was very excellent anti-bacterial activity compared with previous reports (Jeschke et al., 2005, Kim et al., 2014, Yoshikawa et al., 1994), and this mixed fractions were purified separately by preparative HPLC using same mobile phase, retention, flow rate, temperature and fraction time, and confirmed by analytical HPLC (Fig. 4b). This purification result was also suggested that the EO was composed with some bioactive compounds compound derivatives which available in inside of the essential oil (Saeed et al., 2017). Therefore, the present result was suggested that the plant C. japonica EO could act as an effective inhibitor against CR K. pneumoniae (MN396685).
Fig. 4.
Detection of anti-bacterial fractions (a) and preparative HPLC purified anti-bacterial fractions (b) from crud essential oil by analytical HPLC.
Fig. 5.
Anti-bacterial effect of purified fractions (a, b) and enhanced anti-bacterial effect of mixed fractions (c) against CR K. pneumoniae by agar well diffusion method.
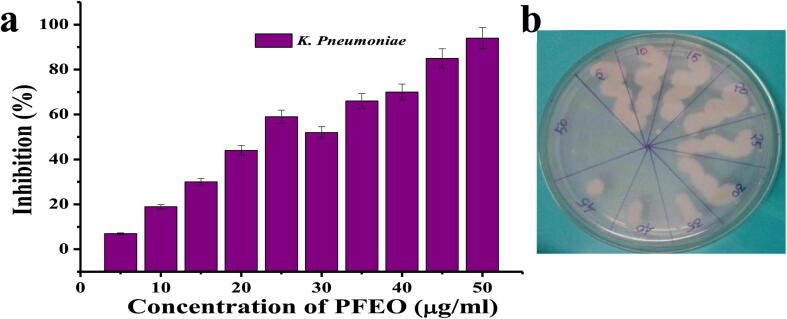
3.6. Minimum inhibition concentration
No any visible turbidity was detected at 50 µg/mL PFEO treated well when compared with untreated control of first well. Similarly, the percentage of microtitre plate result was exhibited the inhibition rate of 10% for 5 µg/mL and 52% for 30 µg/mL concentration (Fig. 6a). Also, PFEO was found to be significantly reduced soon after starting the action of PFEO, which immediately went to decline phase. Based on the observation, the MIC of PFEO was fixed as 50 µg/mL against tested bacteria. The MBC result was exhibited same as MIC also observed and shown in Fig. 6b. Our result was indicated that the PFEO inhibit the CR K. pneumoniae (MN396685) at concentration dependent manner. Further, the validation results of MBC was confirmed the MIC values at same concentration in MHA plate. In mechanistic approach, the PFEO may targeted the region of special outer membrane in CR K. pneumoniae (MN396685) which can block a number of anions and harmful materials to come into cytoplasm (Harder et al., 2003). The lipid bilayer surrounded teichoic acid and peptidoglycon of GNB outer membrane produced the negative charge in surface of the cell wall, which attracted transferred positive chemical substances of PFEO through outer membrane and leads to cell destruction (Yoshikawa et al., 1994). The continuous action of PFEO damaged the integrated membrane and preventing the proliferation of bacteria and bacteria loss their virulence (KIm et al., 2014). The invitro assay result was supported to disk diffusion assay result and also evidenced that the PFEO of C. japonica was more effective against CR K. pneumoniae (MN396685).
Fig. 6.
Minimum inhibition concentration (a) and minimum bactericidal concentration (b) of active purified essential oil (c) against CR K. pneumoniae.
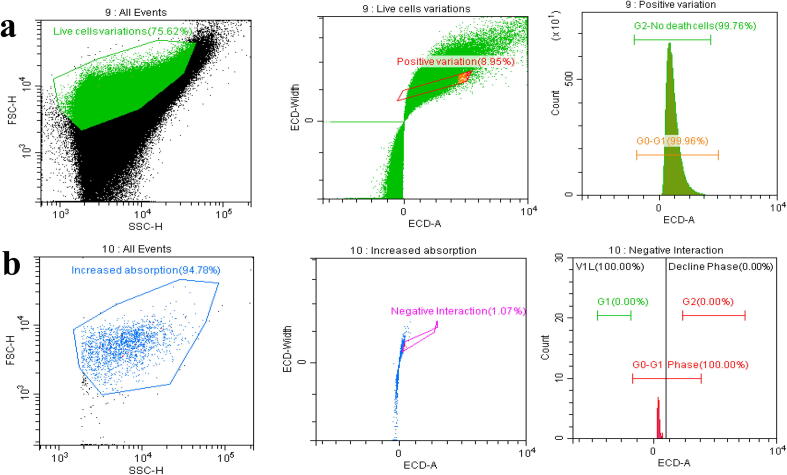
3.7. Cell cycle arrest by flow cytometry
The invitro plate assay of viability was indicated false positive result due to the virulence factors stimulation, While they continue rest and reproduced enzyme activity after certain time interval (Elisha et al., 2017). To overcome this problem, the MIC od PFEO was treated cells was stained with AO/EB stains and examined under flow cytometry, which given as a real time assessment of bacterial viability (da Silva et al., 2019). In staining result, the entire region of the places were covered with PI observed live and dead bacterial cells, indicating a shift of arranged peak in P1. The percentages differentiation of PI absorbance in dead and live cell cells were highlighting in the P2 region. Inhibition range of the dead cells were also observed in P3 region due to the decreased growth of the bacteria. Fig. 7b of the P1, P2 and P3 of the scatter plot was indicated that the cells were significantly stained with PI and 98% of the cells were died due to the effect of PFEO. Whereas, the tightly arranged cells of P1, P2 and P3 of Fig. 7a, indicated that the cells were could not observed the PI dye properly and exhibited with well developed bacterial growth. The result was clearly confirmed that the PFEO of C. japonica EO has anti-bacterial activity against CR K. pneumoniae (MN396685).
Fig. 7.
Cell cycle arrest of control (a) and purified EO fraction treated (b) CR K. pneumoniae growth.
3.8. Invitro anti-carbapenamase activity
According to the CLSI guideline, Carba NP test is an important key test for indentify the carbapenemase in Enterobacteriaceae (Seifert et al., 2018). It is a rapid and highly sensitive phenotypic screening test, which extensively used to the detect the carbapenemase producing Enterobacteriaceae and some times for Pseudomonas sp (Jia et al., 2019). hydrolysis of β-lactam ring in imipenem treated culture was the important identification method (Maruthupandy et al., 2018a). In the epidemiological settings, it is very most important test for confirmation of carbapenemase-producing pathogens, a strong positive reaction is used as the criterion for a positive result (Shaker et al., 2017). As per the guidelines, after added with phenol red, the internal control of imipenem containing well was completely changed their color from red to dark yellow within 2 h. Contrary, the control sample was exhibited with red color continuously. In the result of PFEO treated wells were could modify the yellow color to red color at 75 µg/mL. Initially, the moderate color changes were observed until 30 ug/mL concentration. After 35 µg/mL, the bacteria was lost their carbapenemase-producing ability completely due to the effect of PFEO. Therefore, our result was confirmed that the PFEO of C. japonica has more anti-carbapenemase activity against CR K. pneumoniae (MN396685) (Fig. 8a). Our result was agreed by and the hydrolytic degradation of carbapenemase using Carba NP test method advance than any other method, owing to its superior specificity and given uninterpretable results within 2 h. Recently, the complex mechanisms of antimicrobial resistance carbapenemase-producing bacteria was reported by Rajivgandhi et al. (2019c) and the absence of outer membrane permeability was leads to overproduction of cephalosporins and ESBLs or express a broad-spectrum β-lactamase without carbapenemase activity (ESBLs, plasmid and chromosome-encoded cephalosporinases.
Fig. 8.
Anti-carbapenemase activity of HPLC purified EO fraction treated and untreated CR K. pneumoniae by invitro Carba NP test (a) and quantification of carbapenemase inhibition percentages at various concentration of HPLC purified EO fraction (b) by colorimetric analysis.
3.9. Quantitative determination of anti-carbapenemase activity
After incubation with PFEO treatment, all the wells of red color was changed to red color at increasing concentration, At 75 µg/mL, the red bright red color was produced in the treated well when compared with other wells. In this point, the result was indicated that the 75 µg/mL of the PFEO was very effective against CR K. pneumoniae (MN396685) and involved in hydrolysis of β-lactam ring cleavage and stimulate the β-lactamase inhibition ability. Contrary, the internal control of untreated well was remained yellow color due to the carbapenamase production. It also suggested that the antibiotics imipenem could not undergo the β-lactam ring cleavage activity due to the production of carbapenemase (van der Zwaluw et al., 2015). This result was validated by using colorimetry detection of treated and untreated cells quantitatively. After the colorimetric OD value, the inhibition effect of 94% was observed against CR K. pneumoniae (MN396685) at 75 µg/mL. When compared with control and other wells, this inhibition rate was very high at 75 ug/mL. Based on the colorimetric variation, all the wells and their inhibition percentages were presented in Fig. 8b.
The mechanistic approach of carbapenemase inhibition depends on the oxido-reductase process (Literacka et al., 2017). When the PFEO stimulate the oxidation, the phenoloin was reduced, and changed the color (Vrioni et al., 2012). Phenoloin is a acid derivatives formed by penicillins, which is hydrolytic opening of the β-lactum ring (Maruthupandy et al., 2019). However, free phenol was easily attached with enzyme molecules and stimulate the color variation, which indicates that the PFEO has anti-carbapenemase activity (Shaaban et al., 2017). Further, these evidences were confirmed by measurement of the transferred molecules into the phenol red added by using colorimeter analysis (Seifert et al., 2018). In addition, the use of 5 M NaCL solution of quantitative method was also more advantages in colorimeter method. The sufficient NaCL concentration alter the pH slightly and its hyperosmotic properties lead to efficient lysis of the bacteria (Panacek et al., 2016).
4. Conclusion
The current was conclude, the plant C. japonica EO has chemical composition of diverse biological importance. The destructive effect of the purified C. japonica EO on carbapenem resistant bacterial membrane was observed with collapsed turbidity by minimum inhibition concentration assay. The more death cells of the bacteria was observed by SyTO 9/PI of CLSM and confirmed by flow cytometry. Finally, the carbapenemase enzyme inactivation in tested CR K. pneumoniae (MN396685) was stimulated by treatment of Camellia japonica EO. Overall, this finding is very promising and declare that the EO isolated from C. japonica seeds can be used as a potential natural preservative ingredients in medical industry and would be helpful in the treatment of MDR infection.
Acknowledgement
All the authors gratefully acknowledge the National Natural Science Foundation of China (Project Approval Number: 41950410573) and Postdoctoral Science Foundation of China (Project Approval Number: 2019M663213) for financial support for this work. The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/70), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad N., Ali S.M., Khan A.U. Molecular characterization of novel sequence type of carbapenem-resistant New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae in the neonatal intensive care unit of an Indian hospital. Int. J. Antimicrob. Agents. 2018;53:525–529. doi: 10.1016/j.ijantimicag.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Bin H., Cheng L., Wen Q., Zhiqing Z., Zhang Y., Liu A., Liu R., Jia Z., Yin X., Han Y., Zhu Q., Luo Shuxiang L. A method for extracting oil from tea (Camelia sinensis) seed by microwave incombination with ultrasonic and evaluation of its quality. Indus. Crop. Prod. 2019;131:234–242. [Google Scholar]
- Borer A., Saidel-Odes L., Eskira S., Nativ R., Riesenberg K., Livshiz-Riven I., Schlaeffer F., Sherf M., Peled N. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am. J. Infect. Control. 2012;40:421–425. doi: 10.1016/j.ajic.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Cusumano J.A., Caffrey A.R., Daffinee K.E., Luther M.K., Lopes V., La Plante K.L. Weak biofilm formation among carbapenem-resistant Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2019;95 doi: 10.1016/j.diagmicrobio.2019.114877. [DOI] [PubMed] [Google Scholar]
- da Silva P.M., da Silva B.R., de Oliveira Silva J.N., de Moura M.C., Soares T., Feitosa A.P.S., Brayner F.A., Alves L.C., Paiva P.M.G., Damborg P., Ingmer H., Napoleão T.H. Punica granatum sarcotesta lectin (PgTeL) has antibacterial activity and synergistic effects with antibiotics against β-lactamase- producing Escherichia coli. Int. J. Biol. Macromol. 2019;135:931–939. doi: 10.1016/j.ijbiomac.2019.06.011. [DOI] [PubMed] [Google Scholar]
- Ece G., Tunc E., Otlu B., Aslan D., Ece C. Detection of blaOXA-48 and clonal relationship in carbapenem resistant K. pneumoniae isolates at a tertiary care center in Western Turkey. J. Infect. Public Health. 2018;11:640–642. doi: 10.1016/j.jiph.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Elisha I.L., Botha F.S., McGaw L.J., Eloff J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017;17:133. doi: 10.1186/s12906-017-1645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eunsun J., Jongsung L., Jihoon B., Kwangsun J., Jiyoung L., Sungran H., Saebom K., Jaesook K., Deokhoon P. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007;112:127–131. doi: 10.1016/j.jep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Hamoud R., Zimmermann S., Reichling J., Wink M. Synergistic interactions in two-drug and three-drug combinations (thymol, EDT and vancomycin) against multi drug resistant bacteria including E. coli. Phytomed. 2014;21:443–447. doi: 10.1016/j.phymed.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Harder A., Schmitt-Wrede H.P., Krücken J., Marinovski P., Wunderlich F., Willson J., Amliwala K., Holden-Dye L., Walker R. Cyclooctadepsipeptides–an anthelmintically active class of compounds exhibiting a novel mode of action. Int. J. Antimicrob. Agents. 2003;22:318–331. doi: 10.1016/s0924-8579(03)00219-x. [DOI] [PubMed] [Google Scholar]
- Huawei L., Liping W., Luping Z., Mengji C., Ruhui L. Characterization of three new viruses of the family Betaflexi viridae associated with camellia ring spot disease. Virus Res. 2019;272 doi: 10.1016/j.virusres.2019.197668. [DOI] [PubMed] [Google Scholar]
- Hui H., En-Hua X., Hai-Bin Z., Qiu-Yang Y., Li-Zhi G. De novo transcriptome sequencing of Camellia sasanqua and the analysis of major candidate genes related to floral traits. Plant. Physiol. Biochem. 2017;120:103–111. doi: 10.1016/j.plaphy.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Jeschke R., Iinuma K., Harder A., Schindler M., Murakami T. Influenceofthe cyclooctadepsipeptides PF1022A and PF1022E as natural products on the design of semi-synthetic anthelmintics such as emodepside. Parasitol. Res. 2005;97:S11–S16. doi: 10.1007/s00436-005-1439-y. [DOI] [PubMed] [Google Scholar]
- Jiya J., Anju K.N., Nanda-Kumar K., Oluwatobi O., Sabu T. Novel bio compactable silver nanowires and nanocubes: An effective treatment against carbapenem and vancomycin resistant strains isolated from cancer patients. J. Sau. Chem. Soc. 2019;2019 doi: 10.1016/j.jscs.2019.06.004. [DOI] [Google Scholar]
- Kazuhiko O., Yoshikatsu S., Akio W., Morimas M., Tsukasa I. Relationship between inhibitory activity of myrmicacin analogues on Camellia japonica pollen germination and their lipophilicity. Phytochem. 1986;25:941–943. [Google Scholar]
- Kim J.K., Kim C.R., Lim H.J., Nam S.H., Joo O.S., Shin D.H., Shin E.C. An optimized extraction technique for acetylcholinesterase inhibitors from the Camellia japonica seed cake by using response surface methodology. Biosci. Biotechnol. Biochem. 2014;78:1237–1241. doi: 10.1080/09168451.2014.915723. [DOI] [PubMed] [Google Scholar]
- Literacka E., Herda M., Baraniak A., Żabicka D., Hryniewicz W., Skoczyńska A., Gniadkowski M. Evaluation of the Carba NP test for carbapenemase detection in Enterobacteriaceae, Pseudomonas spp. and Acinetobacter spp., and its practical use in the routine work of a national reference laboratory for susceptibility testing. Eur. J. Clin. Microbiol. Infect Dis. 2017;36:2281–2287. doi: 10.1007/s10096-017-3062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarz, G. F. F., Laressa, C.L., Thaís, A. M. S., Melina de S.P., Daianyde S., Maria, R,M,P., Lauro, M.S., Tomoe, N., Rosiane, G.M., 2019. Chemical composition and biological activity of Liquidambar styraciflua L. leaf essential oil, Indus. Crops. Prod., 138, 111446.
- Maruthupandy M., Rajivgandhi G., Muneeswaran T., Song J.M., Manoharan N. Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microb. Pathog. 2018;121:224–231. doi: 10.1016/j.micpath.2018.05.041. [DOI] [PubMed] [Google Scholar]
- Maruthupandy M., Rajivgandhi G., Kadaikunnan S., Veeramani T., Alharbi S., Muneeswaran T., Jamal K., Khaled M., Li W.J., Alanzi K.F. Anti-biofilm investigation of graphene/chitosan nanocomposites againstbiofilm producing P. aeruginosa and K. Pneumoniae. Carbohyd. Polym. 2019;2019:115646. [Google Scholar]
- Mekes E., Zahlane K., Ait Said L., Tadlaoui Ouafi A., Barakate M. The clinical and epidemiological risk factors of infections due to multi-drug resistant bacteria in an adult intensive care unit of University Hospital Center in Marrakesh-Morocco. J. Infect. Public Health. 2019;S1876–0341 doi: 10.1016/j.jiph.2019.08.012. [DOI] [PubMed] [Google Scholar]
- Mitali M., Proshanta G., Ahindra N. Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: Process optimization, GC-MS analysis and anti-microbial activity. Ind. Crops. Prod. 2019;138 [Google Scholar]
- Mong H.I., Su M., Chih S., Kuan-Hung L. Chemical composition of seed oils in native Taiwanese Camellia species. Food Chem. 2014;156:369–373. doi: 10.1016/j.foodchem.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Mourad K., Ilias M., Meryem E.J., Abdelaziz B., Yvan V.H. Recent advances in untargeted and targeted approaches applied in herbal-extracts and essential-oils fingerprinting - A review. J. Pharmaceut. Biomed. lnt. Anal. 2020;177 doi: 10.1016/j.jpba.2019.112849. [DOI] [PubMed] [Google Scholar]
- Na G., Tuantuan T., Ning R., Youying T., Bo L. Saponins from seeds of Genus Camellia: phytochemistry and bioactivity. Phytochem. 2018;149:42–55. doi: 10.1016/j.phytochem.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Panacek A., Smekalova M., Vecerova R., Bogdanova K., Roderova M., Kolar M., Kilianova M., Hradilova S., Froning J.P., Havrdova M., Prucek R., Zboril R., Kvítek L. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multi esistant Enterobacteriaceae. Colloids Surf. B Biointerf. 2016;142:392–399. doi: 10.1016/j.colsurfb.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Park J.C., Hur J.M., Park J.G., Hatano T., Yoshida T., Miyashiro H., Min B.S., Hattori M. Inhibitory effects of Korean medicinal plants and camellia tannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytother. Res. 2002;16:422–426. doi: 10.1002/ptr.919. [DOI] [PubMed] [Google Scholar]
- Rahdar H.A., Malekabad E.S., Dadashi A.R., Takei E., Keikha M., Kazemian H., Karami-Zarandi M. Correlation between biofilm formation and carbapenem resistance among clinical isolates of Klebsiella pneumoniae. Ethiop. J. Health Sci. 2019;2:745–750. doi: 10.4314/ejhs.v29i6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajivgandhi G., Maruthupandy M., Manoharan N. Detection of TEM and CTX-M genes from ciprofloxacin resistant Proteus mirabilis and Escherichia coli isolated on urinary tract infections (UTIs) Microb. Pathog. 2018;121:123–130. doi: 10.1016/j.micpath.2018.05.024. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G., Maruthupandy M., Veeramani T., Quero F., Li W.J. Anti-ESBL investigation of chitosan/silver nanocomposites against carbapenem resistant Pseudomonas aeruginosa. Int. J. Biol. Macromol. 2019;132:1221–1234. doi: 10.1016/j.ijbiomac.2019.03.238. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G., Ramachandran G., Maruthupandy M., Vaseeharan B., Manoharan N. Molecular identification and structural characterization of marine endophytic actinomycetes Nocardiopsis sp. GRG 2 (KT235641) and its antibacterial efficacy against isolated ESBL producing bacteria. Microb. Pathog. 2019;126:138–148. doi: 10.1016/j.micpath.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Rho T., Choi S.J., Kil H.W., Ko J., Yoon K.D. Separation of nine novel triterpene saponins from Camellia japonica seeds using high-performance countercurrent chromatography and reversed-phase high-performance liquid chromatography. Phytochem. Anal. 2019;30:226–236. doi: 10.1002/pca.2808. [DOI] [PubMed] [Google Scholar]
- Robert S., Joan W., Philip C., John S., Jeremy G., Jonathan F., Carlos D., John B., Mark P. Multi-drug resistant gram negative bacteria colonization and infection inburned children: lessons learned from a 20-year experience. Burns Open. 2018;2:43–46. [Google Scholar]
- Saeed M., Naveed M., Arif M., Kakar M.U., Manzoor R., Abd El-Hack M.E., Alagawany M., Tiwari R., Khandia R., Munjal A., Karthik K., Dhama K., Iqbal H.M.N., Dadar M., Sun C. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans-A comprehensive review. Biomed. Pharmacother. 2017;95:1260–1275. doi: 10.1016/j.biopha.2017.09.024. [DOI] [PubMed] [Google Scholar]
- Salem N., Kefi S., Tabben O., Ayed A., Jallouli S., Feres N., Hammami M., Khammassi S., Hrigua I., Nefisi S., Sghaier A., Limama F., Elkahoui S. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crops. Prod. 2018;124:115–125. [Google Scholar]
- Seifert H., Stefanik D., Sutcliffe J.A., Higgins P.G. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents. 2018;51(1):62–64. doi: 10.1016/j.ijantimicag.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Shaaban M.I., Shaker M.A., Mady F.M. Imipenem/cilastatin encapsulated polymeric nanoparticles for destroying carbapenem-resistant bacterial isolates. J. Nanobiotech. 2017;15:29. doi: 10.1186/s12951-017-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker M.A., Shaaban M.I. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: In vitro antibacterial study. Int. J. Pharm. 2017;525:71–84. doi: 10.1016/j.ijpharm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Sharma D., Garg A., Kumar M., Khan A.U. Proteome profiling of carbapenem-resistant K. pneumoniae clinical isolate (NDM-4): exploring the mechanism of resistance and potential drug targets. J. Proteomics. 2019;200:102–110. doi: 10.1016/j.jprot.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Sib E., Voigt A.M., Wilbring G., Schreiber C., Faerber H.A., Skutlarek D., Parcina M., Mahn R., Wolf D., Brossart P., Geiser F., Engelhart S., Exner M., Bierbaum G., Schmithausen R.M. Antibiotic resistant bacteria and resistance genes in biofilms in clinical wastewater networks. Int. J. Hyg. Environ. Healt. 2019;222:655–662. doi: 10.1016/j.ijheh.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Soon-Young L., Chun-Sik B., Nam-Sook S., Chang-Su N., Hah Young Y., Deuk-Sil O., Min-Suk B., Myung-Sang K., Seung-Sik C., Dae-Hun P. Camellia japonica oil suppressed asthma occurrence via GATA-3 & IL-4 pathway and its effective and major component is oleic acid. Phytomed. 2019;57:84–94. doi: 10.1016/j.phymed.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Subashini J., Khanna V.G., Kannabiran K. Anti-ESBL activity of silver nanoparticles biosynthesized using soil Streptomyces species. Bioprod. Biosyst. Eng. 2014;37:999–1006. doi: 10.1007/s00449-013-1070-8. [DOI] [PubMed] [Google Scholar]
- Tayebi Z., Hosseini Doust R., Karim Rahimi M., Siadat S.D., Goudarzi M. Distribution of different carbapenemase genes in carbapenem-resistant Acinetobacter baumannii strains isolated from intensive care: a two year multi-center study in Tehran, Iran. Gene Rep. 2019;15 [Google Scholar]
- Teethaisong Y., Nakouti I., Evans K., Eumkeb G., Hobbs G. Nitro-Carba test, a novel and simple chromogenic phenotypic method for rapid screening of carbapenemase-producing Enterobacteriaceae. J. Glob. Antimicrob. Resist. 2019;18:22–25. doi: 10.1016/j.jgar.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Thiago P.C., Pinheiro R.E.E., Melo E.S., Soares M.J.S., Souza J.S.N., de Andrade T.B., Gomes T.L., Coutinho H.D.M. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strainsIndustrial. Indus. Crops Prod. 2018;112:70–74. [Google Scholar]
- Van der Zwaluw K., de Haan A., Pluister G.N., Bootsma H.J., de Neeling A.J., Schouls L.M. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrioni G., Daniil I., Voulgari E., Ranellou K., Koumaki V., Ghirardi S., Kimouli M., Zambardi G., Tsakris A. Comparative evaluation of a prototype chromogenic medium (ChromID CARBA) for detecting carbapenemase-producing Enterobacteriaceae in surveillance rectal swabs. J. Clin. Microbiol. 2012;50:1841–1846. doi: 10.1128/JCM.06848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wei F., Song C., Jiang B., Tiana S., Yia J.W., Yu C., Song Z., Sun L., Bao Y., Wu Y., Huang Y., Li Y. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Indus. Crop. Prod. 2017;109:358–366. [Google Scholar]
- WHO, 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics, http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ 1–7.
- Yan T., Chun-Yan Wu., Li-Zhi G. Characterization of chloroplast microsatellite loc, from whole chloroplast genome of Camellia taliensis and their utilization for evaluating genetic diversity of Camellia reticulata (Theaceae) Biochem. Syst. Eco. 2013;50:207–211. [Google Scholar]
- Yang J.L., Ha T.K., Dhodary B., Pyo E., Nguyen N.H., Cho H., Kim E., Oh W.K. Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J. Med. Chem. 2015;58:1268–1280. doi: 10.1021/jm501567f. [DOI] [PubMed] [Google Scholar]
- Yang C., Liu X., Chen Z., Lin Y., Wang S. Comparison of oil content and fatty acid profile of ten new Camellia oleifera cultivars. J Lipids. 2016 doi: 10.1155/2016/3982486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Harada E., Murakami T., Matsuda H., Yamahara J., Murakami N. Camelliasaponins B1, B2, C1 and C2, new type inhibitors of ethanol absorption in rats from the seeds of Camellia japonica L. Chem. Pharmaceut. Bullet. 1994;42:742–744. doi: 10.1248/cpb.42.742. [DOI] [PubMed] [Google Scholar]