Highlights
-
•
Treatment of T-47D breast cancer cells with silvestrol sensitised them to radiation.
-
•
1 nM silvestrol caused a 34% reduction in cells exposed to 2 Gy.
-
•
Clonogenic assays revealed silvestrol had a dose modifying factor of 1.4.
-
•
Radiation was delivered to the tissue culture plate using a clinical LINAC machine.
Keywords: eIF4A, Breast cancer, Silvestrol, Radiotherapy
Abstract
Purpose
eIF4A is an RNA helicase that forms part of the machinery of translation initiation.
Proteomic analysis demonstrated eIF4A expression to be at least two-fold greater in a radioresistant derivative of T-47D breast cancer cells compared to parental cells.
Inhibition of eIF4A has previously been shown to re-sensitize lymphomas to chemotherapeutic agents that cause DNA damage.
The objective of this work is to investigate whether inhibition of eIF4A using silvestrol sensitizes breast cancer cells to radiotherapy in tissue culture, using T-47D as a model system.
Methods and materials
T-47D cells were incubated in medium containing 0 nM to 1 nM silvestrol either for 24 h prior to irradiation at 0 Gy to 10 Gy, delivered by linear accelerator (LINAC) or continually for six days post irradiation. MTT viability and clonogenic assays were used to quantify response.
Results
Pre-treatment of T-47D cells with 1 nM silvestrol caused a 34% reduction (p = 0.014) in viability on irradiation at 2 Gy compared to treatment with a DMSO control, as assessed by MTT assay.
Maintenance of cells in 1 nM silvestrol for six days following irradiation at 2 Gy caused a 58% reduction (p = <0.001) in tumor cell viability.
Clonogenic assays performed on cells maintained in 1 nM silvestrol following irradiation showed a dose modifying factor (DMF) of 1.4 (p = <0.001, one-way ANOVA).
Conclusions
Low concentrations of silvestrol sensitize T-47D breast cancer cells to radiation with minimal effects on unirradiated cells. This highlights the possible usefulness of eIF4A inhibition in potentiating radiation-induced damage at the tumor site without causing systemic toxicity.
1. Introduction
Radiotherapy is a commonly used treatment modality in breast cancer. In 2019, in the US, 49% of women with stage I or II disease received adjuvant radiation therapy along with breast‐conserving surgery and 56% of women with metastatic disease received radiation or chemotherapy alone or in combination [1]. However, disease recurrence following initial therapy is also common, occurring in 20–40% of cases [2]. This is due in part to radioresistance, whereby a subset of cancer cells are refractory to damage from the ionizing radiation.
A number of mechanisms are believed to contribute to the radioresistant phenotype. Briefly, the high-energy X-rays which comprise the radiotherapy beam cause the formation of reactive oxygen species which cause oxidative damage to DNA resulting in single-strand or double-strand breaks (in addition to damage caused by direct energy transfer). However, the tumor microenvironment is often hypoxic, meaning that there are fewer oxygen atoms present to become reactive species [3]. The recurrent population may arise from cancer stem cells, the density of which within a tumor has been shown to correlate with poorer control following radiotherapy [4], [5]. Finally, recurrence may occur as cells repair the radiation-induced DNA damage or invoke cellular checkpoints if the damage is irreparable [6].
The objective of this work was to investigate whether silvestrol, a small molecule inhibitor of the DEAD box, RNA helicase eIF4A (eukaryotic initiation factor 4A), sensitizes breast cancer cells to radiotherapy. Silvestrol is a cyclopenta[b]benzofuran rocaglate originally isolated from Aglaia foveolata [7]. It has shown promising in vitro and in vivo cytotoxic activity in a number of models including breast cancer xenografts [8], [9], [10]. As part of the eIF4F complex, eIF4A processes the secondary structure of messenger RNA molecules to allow the ribosome to bind and scan for the start codon. Proteomic analysis demonstrated that eIF4A expression is at least two-fold greater in a radioresistant derivative of T-47D breast cancer cells compared to parental cells [11].
Inhibition of eIF4A has previously been shown to resensitise Eμ-Myc lymphomas to chemotherapeutic agents that cause DNA damage [12]. Downregulation of eIF4GI, another component of the eIF4F complex, enhances DNA damage in breast cancer cells exposed to ionizing radiation [13].
Here, we report that silvestrol sensitizes T-47D luminal breast cancer cells to the cytotoxic effects of irradiation.
2. Methods and materials
2.1. Cell lines
T-47D luminal A ER+/PR+ (estrogen and progesterone receptor positive) breast cancer cells were maintained in DMEM (Fisher, MA, USA) containing 10% fetal bovine serum (by volume) and glutamine (2 mM). Vessels were kept in a Sanyo incubator set at 37 °C, 5% CO2.
2.2. Silvestrol
1 mg silvestrol (MedChemExpress, NJ, USA) was suspended in 305.5 µl DMSO to give a stock of 5 mM. This was aliquoted at a range of concentrations and stored at −80 °C.
2.3. Irradiation
Radiation was delivered using a Varian Medical Systems linear accelerator (LINAC machine). An empty 24 well plate containing radiation sensors was irradiated to demonstrate that the whole plate area could be irradiated evenly. This being the case, a protocol was devised in which half the fraction was delivered from above and the other half from below, all at 6 MV.
It was found that greater volume of liquid within the wells enhanced delivery of the radiation.
0 Gy, 2 Gy, 4 Gy, 6 Gy, 8 Gy and 10 Gy were selected as the radiation energies as these are within clinically appropriate limits and have been used previously in experimental work using T-47D cells with a good range of response [11].
Two different treatment schedules were used: pre-treatment with silvestrol and maintenance of cells in medium which contained silvestrol post-irradiation.
In the pre-treatment schedule, a final concentration of 0 pM, 10 pM, 100 pM, 1 000 pM, 10 000 pM or 100 000 pM silvestrol was added to confluent T-47D cells in T-25 flasks. After 16 h, cells were trypsinized and seeded in 24 well plates at 50 000 cells per well in triplicate. Each well was maintained in 1 ml medium plus silvestrol for a further 8 h before being irradiated to give a total of 24 h silvestrol exposure before irradiation. Cells were transported from the incubator to the LINAC machine in a polystyrene box and spent approximately one hour out of the incubator. After irradiation, the medium on all wells was changed for medium containing 1% DMSO only. On day 6 following irradiation, MTT assays were performed.
In the post-treatment schedule, cells maintained in T-25 flasks received medium containing a final concentration of 1% DMSO before being used to seed 24 well plates in triplicate 16 h later. Irradiation took place after 8 h. Following irradiation, medium which contained 0 pM, 10 pM, 100 pM or 1 000 pM silvestrol (in addition to a final concentration of 1% DMSO) was added. Medium containing these additions was changed daily until day 6 on which MTT assays were performed.
2.4. Viability assays
MTT (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assays (Sigma Aldrich, MO, USA) were performed using a standard protocol. Briefly, the medium on each well was replaced with a mixture containing 270 µl fresh medium and 30 µl MTT reagent. Plates were incubated for 2 h before the mixture was changed for 200 µl DMSO. A BMG Labtech FLUOstar Omega spectrometer was then used to measure absorbance at 560 nm.
2.5. Clonogenic assays
These were performed as described previously [14]. 24 well plates were seeded with cells at a density of 50 000 cells per well. Cells were allowed to adhere for 8 h before irradiation. Immediately following irradiation cells were trypsinized and plated at a density of 250 cells per well in 6 well plates in triplicate. Medium containing either 1 000 pM silvestrol (in 1% DMSO) or DMSO only was changed daily. 12 days post-irradiation medium was removed, cells were washed with PBS and stained with 6.0% glutaraldehyde and 0.5% crystal violet. Colonies consisting of 50 cells or more were counted.
2.6. Statistical analysis
Microsoft Excel™ was used for data analysis. Significance of viability assays was determined using paired t-tests assuming equal variance. Clonogenic assays were analyzed using one-way ANOVA.
3. Results
3.1. Optimization
Initial experiments showed a linear response between increasing silvestrol concentration and decreasing cell viability between 0 pM and 10 000 pM (Supplementary data). Beyond this concentration no further reduction in viability was observed. The IC50 of silvestrol was 5.46 nM.
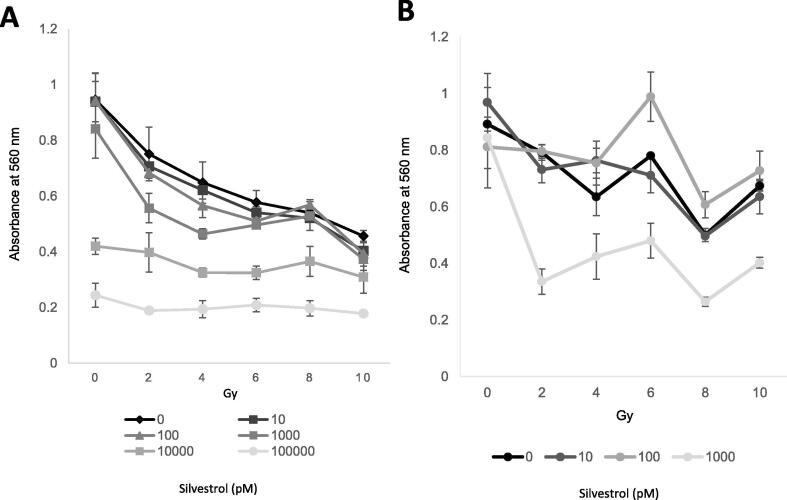
Irradiation of cells pre-treated with 10 nM or 100 nM silvestrol had minimal effect on viability (Fig. 1A). 0 pM, 10 pM, 100 pM and 1 000 pM were therefore chosen as final concentrations of silvestrol for post-irradiation experiments (Fig. 1B).
Fig. 1.
MTT Assay. Silvestrol treatment pre- and post-irradiation reduces cell viability. (A) Pre-treatment with silvestrol for 24 h before irradiation. (B) Maintenance of cells in medium containing silvestrol for 6 days following irradiation. Silvestrol concentrations in pM. Average of 3 repeats. Error bars represent ± S.D.
The final concentration of DMSO in all wells was 1%. MTT assays performed on wells with and without 1% DMSO 6 days after irradiation at 4 Gy revealed DMSO had no significant radioprotective effect at this concentration (Supplementary data).
It was determined that cells reached approximately 50% viability at day 6 post irradiation. This timepoint was therefore used for all further, post-irradiation viability assays.
3.2. Viability assays
Concentrations of 10 000 pM and 100 000 pM caused a reduction in viability irrespective of radiation dose (Fig. 1A).
Pre-treatment with 1 000 pM silvestrol had no effect on mock-irradiated cells but caused a significant reduction in viability at 2 Gy (p = <0.014) and 4 Gy (p = <0.001) (Fig. 1A).
Incubation of cells in 1 000 pM silvestrol for 6 days post-irradiation caused a significant reduction in viability at all doses of radiation (p ≤ 0.024 for all) (Fig. 1B). Viability of cells exposed to 100 pM silvestrol was significantly greater than those exposed to DMSO alone on irradiation with 6 Gy (p = 0.027) and 8 Gy (p = 0.018) (Fig. 1B).
3.3. Clonogenic assay
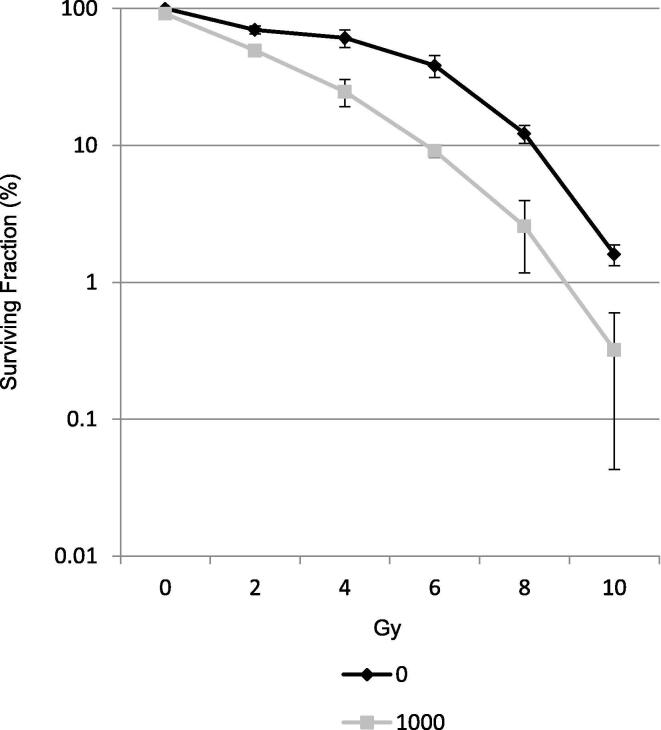
Maintenance of T-47D cells in 1 000 pM silvestrol caused a significant reduction in clonogenicity compared to DMSO alone (p = <0.001, one-way ANOVA) with a dose modifying factor (DMF) of 1.4 (Fig. 2).
Fig. 2.
Clonogenic Assay. Silvestrol treatment post irradiation reduces clonogenicity. Maintenance of T-47D cells in 1 000 pM silvestrol caused a significant reduction in clonogenicity compared to DMSO alone (p = <0.001, one-way ANOVA) with a dose modifying factor (DMF) of 1.4. Silvestrol concentrations in pM. Average of 3 repeats. Error bars represent ± S.D.
4. Discussion
In this study, we demonstrate that treatment of T-47D cells with 1 000 pM silvestrol reduces tumor cell viability and clonogenicity on exposure to clinically appropriate radiation doses. Notably, this concentration of silvestrol had minimal effect on unirradiated T-47D in both clonogenic and MTT viability assays. This possibly indicates that eIF4A inhibition could be used to potentiate the local effects of radiation at the tumor site without systemic toxicity.
A clearer dose response was apparent in cells maintained in silvestrol post-irradiation (Fig. 1B) compared to those that received pre-treatment (Fig. 1A). This may reflect the longer duration of exposure (24 h pre-irradiation vs. 6 days post-irradiation) but it may also be caused by attenuation of the DNA damage response.
In a previous study on cervical cancer, the double strand break (DSB) marker γ-H2AX became elevated 30 min after irradiation with 10 Gy, reducing significantly after six hours [15]. This reduction in γ-H2AX was not seen in cells treated with shRNAs targeting eIF4AI [15]. This highlights the potential involvement of eIF4A in the post-irradiation DNA damage response.
Maintenance of cells in medium containing 100 pM silvestrol post-irradiation led to an increase in viability, relative to the control, on irradiation with 6 or 8 Gy. At present there is no clear association between eIF4A inhibition and radioprotection.
4.1. Clinical application
Silvestrol has favorable pharmacokinetic properties in mouse models [16] and there is ongoing interest in discovery and characterization of other members of the rocaglate family [17].
The fact that exposure to silvestrol both pre- and post-irradiation results in radiosensitization (Fig. 1A, B) means that a course of the compound may work well when administered alongside a radiotherapy regime consisting of daily fractions.
There is intense interest in the discovery of radiosensitizing agents for breast cancer [18] and this study highlights the potential of translation-initiation as another avenue for target discovery.
4.2. Limitations and future work
The T-47D cell line was chosen for this study because radioresistant derivatives have been demonstrated to overexpress eIF4A [11]. Further studies are needed to establish whether silvestrol also acts as a radiosensitizer in cells lines which do not overexpress eIF4A in response to radiation exposure. It would also be interesting to investigate the effect of silvestrol in irradiated non-cancer cells to explore its potential for off-target effects.
In addition to inhibition of eIF4A isoforms I and II, rocaglates have been shown to cause caspase-mediated apoptosis and suppress anaerobic respiration in tumor cells [8], [19]. It is therefore not possible to attribute the effects observed to suppression of eIF4A activity. Repeat studies with other small molecule eIF4A inhibitors (such as hippuristanol), gene silencing or gene editing could be used to test whether the effects observed are due to eIF4A inhibition specifically, rather than any of the other effects of silvestrol. Other rocaglates could also be investigated.
The cohort of genes dependent on eIF4A for efficient expression (the eIF4A signature) is being defined [20], [21], [22]. The sensitizing effect of silvestrol on irradiated breast cancer cells may be due to the downregulation of a small number, or even only one, of these genes. Analysis of the genes that comprise the eIF4A signature could reveal more precise radiation-sensitizing targets. There is also evidence that some rocaglates have gene-specific effects as they inhibit translation initiation by stabilizing eIF4A-polypurine complexes specifically [23], [24].
Finally, future studies should quantify eIF4A expression in response to inhibition and radiation exposure as overexpression in these conditions may provide further mechanistic evidence for the importance of eIF4A in the radiation response.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Isaac Schapera Trust, the Experimental Cancer Medicine Centre at King's College London, the King’s Health Partners/ King’s College London Cancer Research UK Cancer Centre and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2020.07.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Miller K.D., Nogueira L., Mariotto A.B. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 2.Gerber B., Freund M., Reimer T. Recurrent breast cancer: treatment strategies for maintaining and prolonging good quality of life. Deutsches Arzteblatt. 2010;107:85–91. doi: 10.3238/arztebl.2010.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker H.E., Paget J.T., Khan A.A. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaromina A., Krause M., Thames H. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother Oncol. 2007;83:304–310. doi: 10.1016/j.radonc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Baumann M., Krause M. CD44: a cancer stem cell-related biomarker with predictive potential for radiotherapy. Clin Cancer Res. 2010;16:5091–5093. doi: 10.1158/1078-0432.CCR-10-2244. [DOI] [PubMed] [Google Scholar]
- 6.Mladenov E., Magin S., Soni A., Iliakis G. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy. Front Oncol. 2013;3:113. doi: 10.3389/fonc.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang B.Y., Su B.N., Chai H. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J Org Chem. 2004;69:3350–3358. doi: 10.1021/jo040008h. [DOI] [PubMed] [Google Scholar]
- 8.Kim S., Hwang B.Y., Su B.N. Silvestrol, a potential anticancer rocaglate derivative from Aglaia foveolata, induces apoptosis in LNCaP cells through the mitochondrial/apoptosome pathway without activation of executioner caspase-3 or -7. Anticancer Res. 2007;27:2175–2183. PMID: 17695501. [PMC free article] [PubMed] [Google Scholar]
- 9.Kogure T., Kinghorn A.D., Yan I. Therapeutic potential of the translation inhibitor silvestrol in hepatocellular cancer. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0076136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cencic R., Carrier M., Galicia-Vázquez G. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, Silvestrol. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith L., Qutob O., Watson M.B. Proteomic identification of putative biomarkers of radiotherapy resistance: a possible role for the 26S proteasome? Neoplasia. 2009;11:1194–1207. doi: 10.1593/neo.09902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cencic R., Robert F., Galicia-Vázquez G. Modifying chemotherapy response by targeted inhibition of eukaryotic initiation factor 4A. Blood Cancer J. 2013;3:128. doi: 10.1038/bcj.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badura M., Braunstein S., Zavadil J., Schneidera R.J. Biochemistry DNA damage and eIF4G1 in breast cancer cells reprogram translation for survival and DNA repair mRNAs. Proc Natl Acad Sci. 2012;109:18767–18772. doi: 10.1073/pnas.1203853109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franken N.A., Rodermond H.M., Stap J. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 15.Liang S., Ju X., Zhou Y. Downregulation of eukaryotic initiation factor 4A1 improves radiosensitivity by delaying DNA double strand break repair in cervical cancer. Oncol Lett. 2017;14(6):6976–6982. doi: 10.3892/ol.2017.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saradhi U.V.R.V., Gupta S.V., Chiu M. Characterization of silvestrol pharmacokinetics in mice using liquid chromatography-tandem mass spectrometry. AAPS J. 2011;13(3):347–356. doi: 10.1208/s12248-011-9273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu J., Zhang W., Cencic R. Amidino-Rocaglates: a potent class of eIF4A inhibitors. Cell Chem Biol. 2019;26(11):1586–1593.e3. doi: 10.1016/j.chembiol.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z., Yang Z., Yu X. Highlights on molecular targets for radiosensitization of breast cancer cells: current research status and prospects. Cancer Med. 2018;7(7):3110–3117. doi: 10.1002/cam4.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santagata S., Mendillo M.L., Tang Y. Tight coordination of protein translation and heat shock factor 1 activation supports the anabolic malignant state. Science. 2013;341(6143):1238303. doi: 10.1126/science.1238303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio C.A., Weisburd B., Holderfield M. Transcriptome-wide characterization of the eIF4A signature highlights plasticity in translation regulation. Genome Biol. 2014;15(10):476. doi: 10.1186/s13059-014-0476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe A.L., Singh K., Zhong Y. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature. 2014;513(7516):65–70. doi: 10.1038/nature13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki S., Floor S.N., Ingolia N.T. Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature. 2016;534(7608):558–561. doi: 10.1038/nature17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manier S., Huynh D., Shen Y.J. Inhibiting the oncogenic translation program is an effective therapeutic strategy in multiple myeloma. Sci Transl Med. 2017;9(389):eaal2668. doi: 10.1126/scitranslmed.aal2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu J., Zhang W., Cencic R. Rocaglates induce gain-of-function alterations to eIF4A and eIF4F. Cell Rep. 2020;30(8):2481–2488.e5. doi: 10.1016/j.celrep.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.