Abstract
Patients with coronary artery disease remain at increased risk of recurrent life-threatening cardiovascular events even after adequate guideline-based treatment of conventional risk factors, including blood lipid levels. Inflammation is a critical pathway in the pathogenesis of atherosclerosis and is independently associated with risk of recurrent cardiovascular events. Leukotrienes are potent pro-inflammatory and vasoactive mediators synthesized by leukocytes in atherosclerotic lesions. AZD5718 is a novel antagonist of 5-lipoxygenase activating protein that suppresses leukotriene biosynthesis.
FLAVOUR is a phase IIa efficacy and safety study of AZD5718 in patients with myocardial infarction 1–4 weeks before randomization. Stenosis of the left anterior descending coronary artery after percutaneous intervention must be <50%, and Thrombolysis In Myocardial Infarction flow grade must be ≥ 2. Enrolled participants receive standard care plus oral AZD5718 200 mg, 50 mg, or placebo once daily for up to 12 weeks (extended from 4 weeks by protocol amendment). The planned sample size is 100 participants randomized to 12 weeks’ treatment. Change in urine leukotriene E4 levels is the primary efficacy outcome. FLAVOUR also aims to evaluate whether AZD5718 can improve coronary microvascular function, as measured by transthoracic colour Doppler-assisted coronary flow velocity reserve. Centrally pretrained study sonographers use standardized protocols and equipment. Additional outcomes include assessment of comprehensive echocardiographic parameters (including coronary flow, global strain, early diastolic strain rate and left ventricular ejection fraction), arterial stiffness, biomarkers, health-related quality of life, and safety.
Specific anti-inflammatory therapies may represent novel promising treatments to reduce residual risk in patients with coronary artery disease. By combining primary pharmacodynamic and secondary cardiovascular surrogate efficacy outcomes, FLAVOUR aims to investigate the mechanistic basis and potential benefits of AZD5718 treatment in patients with coronary artery disease.
Keywords: 5-Lipoxygenase activating protein, Coronary flow reserve, Coronary flow velocity reserve, Leukotrienes, Myocardial infarction, Echocardiography
1. Introduction
Inflammation plays a causative role in driving the progression of atherosclerosis, independent of blood lipid levels [1]. Survivors of acute coronary syndrome remain at risk of recurrent cardiovascular events, and humoral and cellular biomarkers of inflammation predict myocardial infarction and cardiac death in this population [2]. Despite the effectiveness of standard secondary prevention measures, including antiplatelet therapy, treatment of hypertension, hyperglycaemia and hypercholesterolaemia, and modification of lifestyle risk factors [3], cardiovascular disease remains the leading cause of death worldwide [4,5].
Anti-inflammatory therapies may reduce the residual risk of recurrent cardiovascular events in patients with previous myocardial infarction, independent of the risk reduction achieved by lowering low-density lipoprotein cholesterol levels with statins or other therapies [6]. Results from recent phase 3 cardiovascular outcome trials of canakinumab [7] colchicine [8] and methotrexate [9] in patients with myocardial infarction suggest that anti-inflammatory therapies should target signalling pathways that are mechanistically involved in atherosclerosis [10]. Current evidence highlights the central interleukin-1β, interleukin-6, and C-reactive protein pathway, but many other inflammatory mechanisms influence the development of both large-vessel occlusive disease and coronary microvascular dysfunction associated with atherosclerosis [11].
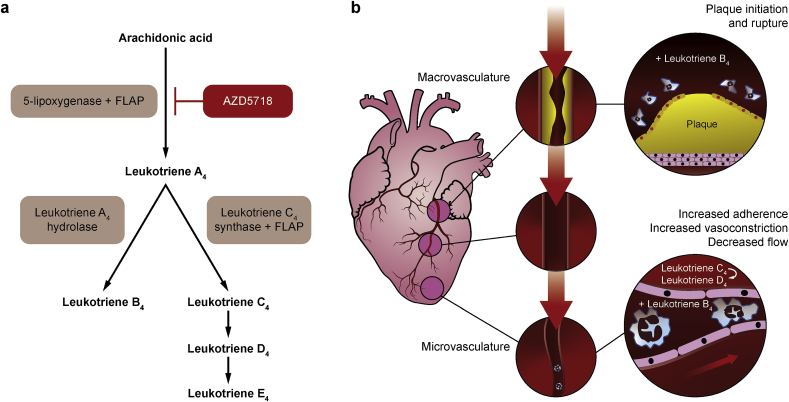
Leukotrienes are potent pro-inflammatory and vasoconstrictive lipid mediators that are produced in atherosclerotic lesions and ischaemic tissue, and are implicated in the pathogenesis of atherosclerotic cardiovascular disease [12]. Leukocytes initiate leukotriene biosynthesis by converting arachidonic acid to leukotriene A4 via 5-lipoxygenase in conjunction with 5-lipoxygenase activating protein (FLAP) (Fig. 1a) [13]. Leukotriene A4 is the precursor for synthesis of leukotriene B4 and the cysteinyl leukotrienes (C4, D4 and E4) by leukocytes and neighbouring cells. Leukotriene B4 stimulates leukocyte recruitment and activation in coronary blood vessels, with involvement in both atherosclerotic plaque initiation and rupture [14]. Leukotriene B4 and the cysteinyl leukotrienes promote vasoconstriction, vessel permeability, and smooth muscle cell proliferation in the coronary microvasculature, leading to endothelial dysfunction (Fig. 1b) [14].
Fig. 1.
a Leukotriene biosynthesis pathway and inhibition by AZD5718. b Role of leukotrienes in cardiovascular disease. FLAP, 5-lipoxygenase activating protein.
Evidence suggests that inhibiting leukotriene biosynthesis may have beneficial effects in patients with coronary artery disease [15]. Single-nucleotide polymorphisms in ALOX5AP (encoding FLAP) were significantly associated with increased risks of spontaneous myocardial infarction and a composite of spontaneous myocardial infarction, stroke or cardiovascular death in a genetic sub-study in patients with acute coronary syndrome in the Platelet Inhibition and Patient Outcomes (PLATO) trial [16]. The 5-lipoxygenase inhibitor atreleuton (VIA-2291) slowed atherosclerotic plaque progression, prevented formation of new lesions, and improved left ventricular ejection fraction in patients with recent acute coronary syndrome in a randomized, double-blind, placebo-controlled, phase II trial [17,18]. Another 5-lipoxygenase inhibitor, zileuton, is approved for treatment of patients with asthma [19,20]. Zileuton improved endothelial function in 10 patients undergoing coronary angiography who had high-risk haplotypes of ALOX5AP and LTA4H (encoding leukotriene A4 hydrolase) [21]. No inhibitor of leukotriene biosynthesis has yet progressed beyond phase II clinical trials in patients with cardiovascular disease, however, and hepatotoxicity may limit development of 5-lipoxygenase inhibitors [15].
AZD5718 is a novel FLAP antagonist that acts at the first step of biosynthesis to block production of all leukotrienes (Fig. 1a), with a half-maximal inhibitory concentration of 39 nM for ex vivo leukotriene B4 production in whole human blood [22]. Multiple oral doses of AZD5718 60–600 mg were well tolerated, and potently reduced both leukotriene E4 levels in urine and leukotriene B4 synthesis by leukocytes ex vivo in a phase I clinical trial in healthy volunteers [22]. AZD5718 inhibited leukotriene B4 production by 90% throughout the day following once-daily administration of 200 mg oral tablets in pharmacodynamic models in healthy volunteers [23]. Oral co-administration of AZD5718 with rosuvastatin had no clinically meaningful effect on the pharmacokinetic profiles of either drug in a phase I drug–drug interaction study, indicating that AZD5718 is a compatible adjunct to standard care in patients with coronary artery disease [23].
By inhibiting leukotriene biosynthesis, AZD5718 may have the potential not only to slow the progression of atherosclerosis, but also to enhance coronary microvascular function and to improve ventricular contractility following myocardial infarction [15]. Based on this rationale, we designed a phase IIa proof-of-principle study to investigate the efficacy and safety of AZD5718 in patients with recent myocardial infarction (FLAVOUR). The study aims primarily to assess inhibition of leukotriene biosynthesis and secondarily to assess the efficacy of AZD5718 compared with placebo in this high-risk patient population, using surrogate outcomes. The comprehensive panel of transthoracic echocardiography efficacy outcomes includes left ventricular ejection fraction as a measure of myocardial contractility and coronary flow velocity reserve (CFVR) as a measure of coronary microvascular function.
2. Methods and analysis
2.1. Overview
FLAVOUR is an ongoing 12-week, randomized, placebo-controlled, multicentre, parallel-group, phase IIa clinical trial of the efficacy, safety and tolerability of AZD5718 in patients with coronary artery disease who have recently had a myocardial infarction. The study started in October 2017.
2.2. Protocol amendment to extend treatment duration
Enrolment into the study began with a 4-week treatment period as specified in the original protocol. Following completion of long-term animal toxicity studies, a protocol amendment in April 2018 extended the treatment period to 12 weeks for subsequently enrolled patients, to enable longer-term assessment of efficacy and safety. The amendment introduced two extra study visits and new 12-week secondary endpoints (Table 1). The planned sample size was increased from 100 to approximately 138, because the 38 patients already enrolled under the original protocol had a 4-week treatment period. This ensured that the same statistical power as originally planned is available for the new 12-week primary and key secondary endpoints (Table 1).
Table 1.
Objectives.
| Objective | Assessment |
|---|---|
| Primarya |
|
| Key secondary (ranked)a |
|
| Other secondaryc |
|
| Exploratoryc |
|
HRQoL, health-related quality of life.
Primary and key secondary outcomes are hierarchically ranked for inferential statistical analysis in sequence to control the overall false positive rate; the study was powered for these outcomes only (see Statistical methods).
Outcomes at 12 weeks were introduced in a protocol amendment that extended the study treatment duration from 4 to 12 weeks.
Other secondary and exploratory outcomes were not included in the power calculation or the hierarchical testing procedure.
2.3. Objectives
The primary objective is to assess the effect of AZD5718 treatment on urine leukotriene E4 levels in patients with recent myocardial infarction. Assessing the effect of AZD5718 on echocardiographic CFVR is one of the key secondary objectives (Table 1). The primary and key secondary endpoints are ranked for hierarchical testing (see Statistical methods). Other secondary objectives and exploratory objectives are listed in Table 1, and include assessing the safety and pharmacokinetics of AZD5718, as well as additional efficacy parameters.
2.4. Participants
Eligible patients are men and women 18–75 years of age with a body mass index of 18–35 kg/m2, who have had a myocardial infarction with or without ST-segment elevation from 7 to 28 days before randomization. Diagnosis of myocardial infarction is based on the fourth universal definition [24], with mandatory coronary angiography (analysed at the study site). Residual stenosis of the left anterior descending coronary artery must be below 50% and the Thrombolysis In Myocardial Infarction (TIMI) flow grade [25] must be 2 or above after percutaneous coronary intervention (if performed). Bypass graft of the left anterior descending coronary artery is an exclusion criterion. To be included, women must be surgically sterile or postmenopausal (amenorrhea for ≥12 months) and men must be surgically sterile or be using barrier contraception to prevent pregnancy in partners. The complete list of exclusion criteria is provided in Table 2.
Table 2.
Exclusion criteria.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NYHA, New York Heart Association; ULN, upper limit of normal.
Defined as Canadian Cardiovascular Society grade ≥3 at study visit 1 or 2.
Estimated using the Cockcroft and Gault equation.
Including in detention, under guardianship, under trusteeship, and committed to an institution.
2.5. Randomization and blinding
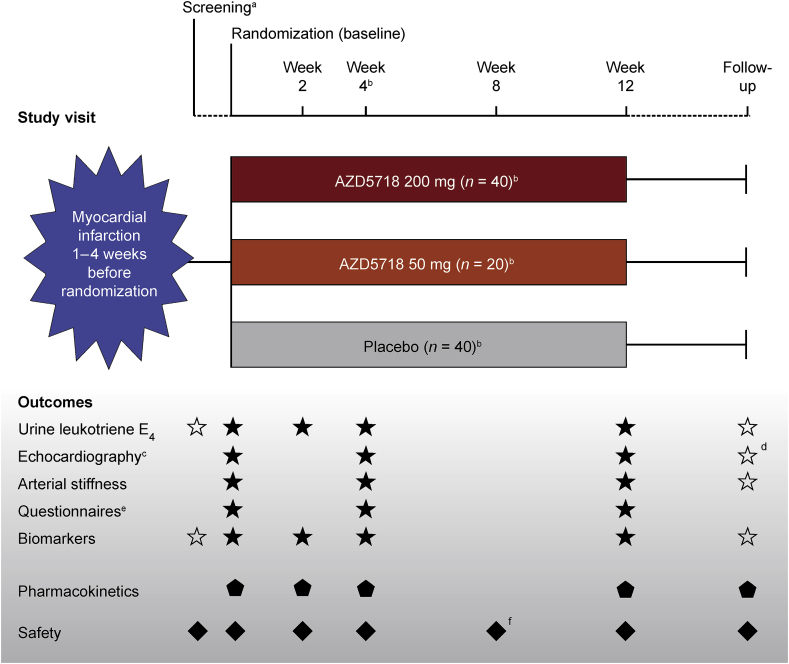
Enrolled patients are randomized 2:1:2 to receive AZD5718 200 mg, AZD5718 50 mg or matching placebo as once-daily oral tablets (Fig. 2). Efficacy assessments will be based on comparisons of the AZD5718 200 mg group versus the placebo group; the smaller 50 mg group serves only to guide dose selection in future studies. Randomization is stratified by type of myocardial infarction (with or without ST-segment elevation). Randomization block sequences are generated by the contract research organization (PAREXEL) using an algorithm provided by the sponsor (AstraZeneca).
Fig. 2.
Study design. Stars indicate efficacy assessments, pentagons indicate pharmacokinetic assessments, and diamonds indicate safety assessments. Empty (white) stars indicate efficacy assessments that were not used in statistical analyses of AZD5718 versus placebo. a Screening assessments take place during days 1–5 after myocardial infarction and include TIMI coronary flow grade, GRACE risk score, and plasma sampling for biomarkers on days 1, 2, 3, and 5 (if feasible). b Study duration originally 4 weeks (n = 38) before amendment to 12 weeks (n = 100; total N = 138). c Includes CFVR measurement (key secondary efficacy outcome). d Without adenosine stress. e Patient-reported HRQoL, functioning, and symptoms of dyspnoea and fatigue. f Adverse events only (other tests optional). CFVR, coronary flow velocity reserve; GRACE, Global Registry of Acute Coronary Events; HRQoL, health-related quality of life; TIMI, Thrombolysis In Myocardial Infarction.
Participants, study site staff and the sponsor study team will remain blinded to treatment assignment throughout the study. Investigators will remain blinded unless they need to know a patient's assigned treatment in a medical emergency. Placebo matches either AZD5718 50 mg or 200 mg tablets, so the identity of the treatment cannot be discerned. To reduce tablet burden, the tablets do not match between doses. The study is therefore double-blind for AZD5718 versus placebo, but single-blind for assignment to placebo/AZD5718 50 mg or placebo/AZD5718 200 mg.
2.6. Procedures
Potentially eligible patients are screened in hospital within 5 days after their myocardial infarction (Fig. 2). Participants are enrolled and randomized within 7–28 days of their myocardial infarction to receive AZD5718 or matching placebo once daily for 12 weeks (or 4 weeks for those enrolled under the original protocol). AZD5718 and placebo are provided as matching film-coated tablets to be taken every morning with 2 dL of water. Participants’ adherence to the regimen is monitored using diary cards. Participants are scheduled to attend study visits at randomization (baseline), at weeks 2, 4, 8 and 12 of treatment, and at follow-up (4 weeks after the last dose). Safety and efficacy are assessed throughout the study (Fig. 2).
2.7. Efficacy outcomes
2.7.1. Urine leukotriene E4 levels
The percentage change in creatinine-normalized urine leukotriene E4 levels from baseline to week 4 is the primary outcome and change from baseline to week 12 is the first of three key secondary outcomes (Table 1). The primary efficacy measure was based on the change from baseline to week 4, reflecting the original duration of the study and the rapid decrease in urine leukotriene E4 levels observed in phase I studies in healthy volunteers [22,23].
2.7.2. Echocardiography
CFVR is measured as the ratio of hyperaemic to resting diastolic blood flow velocity in the left anterior descending coronary artery. Hyperaemia is induced by adenosine infusion (140 μg/kg/min for up to 5 min). Changes in CFVR from baseline to week 12 and week 4 are the second and third of the three key secondary outcomes, respectively (Table 1). Ranking change to week 12 above change to week 4 in the key secondary efficacy outcomes reflects the extension of the study to allow detection of potential longer-term improvement in myocardial microvascular function.
Other secondary echocardiographic efficacy parameters include the change from baseline in measures of coronary flow (diastolic flow velocity in the left anterior descending coronary artery) and measures of left ventricular functional reserve (global longitudinal strain, global circumferential strain, early diastolic strain rate and left ventricular ejection fraction). Exploratory echocardiographic parameters include 15 strain measures and 11 3D functional measures assessed at rest and during hyperaemia. Standard cardiac and Doppler-assisted imaging parameters, such as the ratio between early mitral inflow velocity and early diastolic mitral annular velocity (E/e′), are also assessed.
Study sonographers were trained and certified in person at Sahlgrenska University Hospital (Gothenburg, Sweden) prior to the study. The sponsor provided a detailed protocol for comprehensive transthoracic echocardiographic examinations, together with dedicated pre-configured equipment (Siemens Acuson SC2000 PRIME Ultrasound System, Siemens Healthineers, Mountain View, CA, USA). Imaging includes B-mode, colour Doppler and tissue Doppler modalities in 2D and 3D. High-frame-rate cine loops and still images are captured at the study site. All echocardiographic data are analysed off-line by the core laboratory at Sahlgrenska University Hospital.
2.7.3. Arterial stiffness
Changes in arterial stiffness from baseline to 4 and 12 weeks are exploratory efficacy outcomes. Carotid–femoral pulse wave velocity is measured using a femoral cuff and carotid tonometer. Brachial pulse wave analysis is conducted using a brachial cuff.
2.7.4. Biomarkers
Whole blood and plasma are collected for exploratory analyses of cardiovascular disease and inflammation biomarkers. Samples are collected during screening (days 1, 2, 3 and 5 after myocardial infarction, if feasible) and throughout the study (Fig. 2). Inflammatory biomarkers include interleukin-1β, interleukin-6, high-sensitivity C-reactive protein and growth differentiation factor 15.
2.8. Safety outcomes
Safety is assessed throughout the study (Fig. 2). Safety assessments include clinical laboratory tests (chemistry, haematology, coagulation, serology and urinalysis), physical examinations, monitoring of vital signs (pulse and blood pressure) and body weight, electrocardiography and recording of adverse events.
2.9. Questionnaires
Participants complete questionnaires assessing their health-related quality of life (HRQoL), functioning, and symptoms of dyspnoea and fatigue as exploratory efficacy outcomes. HRQoL is assessed with the standardized 5-dimension EuroQol questionnaire (EQ-5D) [26]; functional health and well-being are assessed in eight domains with the 36-item Short Form Health Survey (SF-36) version 2 [27]; dyspnoea is assessed using the Rose Dyspnea Scale (4 items) [28]; and fatigue during usual daily activities is assessed using the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System (13 items) [29].
2.10. Statistical methods
2.10.1. Sample size calculation
The study is powered to compare AZD5718 200 mg with placebo for the primary and the three key secondary efficacy outcomes of urine leukotriene E4 levels and CFVR (Table 1). Other endpoints were not included in the power calculation, including other echocardiographic outcomes. A sample size of 33 evaluable patients per group will provide more than 99% power to detect an 80% decrease in urine leukotriene E4 levels, and 80% power to detect a 20% increase in CFVR in the AZD5718 200 mg group versus the placebo group (both with one-sided α = 0.05). Approximately 40 patients per group are being recruited to retain at least 33 evaluable patients per group at week 12, assuming an approximate 18% discontinuation rate. The AZD5718 50 mg group will include approximately 20 patients and serves only to guide dose selection in future studies. Power calculations used a log-normal distribution of leukotriene E4 levels with expected inhibition of 96% for AZD5718 and 0% for placebo (both with a standard deviation of <4%), based on a phase I multiple ascending dose study in healthy volunteers [22]. A 30% coefficient of variation was assumed for change in CFVR power calculations, based on data from an observational study in patients with heart failure [30].
2.10.2. Control for multiple comparisons
A hierarchical testing procedure was pre-specified to preserve the overall false positive rate at 0.05 or below when performing multiple comparisons of AZD5718 200 mg with placebo for the primary and key secondary endpoints only (Table 1). These will be tested in the following sequence: first, change in urine leukotriene E4 levels to week 4; secondly, change in urine leukotriene E4 levels to week 12; thirdly, change in CFVR at 12 weeks; and finally, change in CFVR at 4 weeks. This hierarchy reflects the original 4-week design of the study and anticipated rapid change in the primary efficacy outcome (leukotriene E4 levels) but prioritizes the 12-week extension for CFVR outcomes, in which more gradual changes are anticipated. Testing will stop as soon as a non-significant result is observed (p > 0.05) and all subsequent comparisons will be declared non-significant. Comparisons of AZD5718 50 mg with placebo will be performed in parallel, but no adjustment for multiplicity is required because the results will not be used to judge efficacy. Other secondary and exploratory endpoints are not included in hierarchical testing procedure, including other echocardiographic outcomes.
2.10.3. Inferential analyses
A mixed model repeated measures analysis will be used to test the change from baseline in urine leukotriene E4 levels in each AZD5718 group versus placebo (one-sided α = 0.05), with treatment, visit, and treatment–visit interaction as fixed factors and type of myocardial infarction and baseline leukotriene E4 value as covariates. The model will use an unequally spaced variance–covariance matrix, if possible. An analysis of covariance will be used to test the change in CFVR from baseline in each AZD5718 group versus placebo (one-sided α = 0.05), with treatment as a fixed factor and type of myocardial infarction and baseline CFVR value as covariates. Similar analyses will be used for other secondary and exploratory efficacy outcomes (two-sided α = 0.05), with no corrections for multiplicity.
2.10.4. Subgroup analyses
Descriptive efficacy results will be stratified by type of myocardial infarction (with or without ST-segment elevation) and protocol cohort (4-week or 12-week treatment). The study is not powered for comparisons in subgroups. No statistical analyses are pre-specified in these or other subgroups of patients (e.g., baseline level of inflammatory biomarkers, baseline CFVR impairment or site of enrolment). Any post hoc subgroup analysis will be non-inferential.
2.11. Ethics and dissemination
FLAVOUR is taking place at three sites in Denmark, two sites in Finland, and four sites in Sweden. The study is registered on ClinicalTrials.gov (identifier: NCT03317002) and EudraCT (2017-001582-25), and conforms to the principles of the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice, and all applicable regulatory requirements. An ethical review board in each country reviewed and approved the study protocol and its amendments. All participants give their written informed consent before study enrolment.
2.12. Current status
As of November 2019, we have enrolled 150 participants and randomized 120 participants in FLAVOUR (those not randomized did not pass screening). A total of 100 participants are planned for randomization to 12 weeks of treatment with AZD5718 or placebo. An additional 38 patients were enrolled under the original 4-week protocol. The first patient was enrolled in October 2017 and the first dose was taken in November 2017. We expect to enrol the last patient in December 2019 and complete the last follow-up visit in April 2020.
2.13. Patient and public involvement
Neither patients nor the public were involved in this research, except as participants in the study. Participants are recruited at the study centres and advertising is approved by the ethics committee.
3. Discussion
FLAVOUR is the first study to assess the effect of a FLAP antagonist in patients with coronary artery disease. The primary efficacy outcome is pharmacodynamic and is expected to confirm the rapid and sustained 90% or greater inhibition of leukotriene biosynthesis observed in healthy volunteers receiving AZD5718 [22,23]. We also designed the study to encompass multiple cardiovascular surrogate efficacy measures, including assessment of coronary microvascular function in pre-specified, multiplicity-controlled analyses of echocardiographic CFVR.
Leukotriene E4 levels were assessed non-invasively in the urine for the primary outcome measure. Blood leukotriene levels are typically very low, and assays involve technically challenging ex vivo stimulation of lymphocyte leukotriene B4 secretion. AZD5718 reduced urine leukotriene E4 and blood leukotriene B4 levels in the phase I study in human volunteers, demonstrating target engagement using both assays [22]. Leukotriene levels were therefore assessed only in urine in the present study. Atherosclerosis involves both large-vessel occlusive disease and microvascular dysfunction, and leukotrienes are involved in both of these pathologies [11]. FLAVOUR includes a comprehensive panel of echocardiographic parameters assessed at rest and under stress as secondary or exploratory outcomes, together with CFVR as the key secondary outcome. These will provide a thorough assessment of the participants’ cardiac function before and during treatment with AZD5718. We hypothesize that not only myocardial contractility (e.g., left ventricular ejection fraction), but also coronary flow and cardiac reserve may be improved with AZD5718 treatment. The extended 12-week duration of FLAVOUR will enable potential enhancements in CFVR and other surrogate efficacy parameters to be assessed over a suitably extended period of treatment with AZD5718. Any potential effects of AZD5718 will need to be detected in addition to spontaneous improvement in CFVR in the weeks and months following myocardial infarction, and in addition to improvements resulting from standard care. Improvements in CFVR over an 8-week period have been reported in patients with myocardial ischaemia but without occlusive coronary artery disease in a small double-blind placebo-controlled study of ranolazine (N = 58) [31].
CFVR is an appealing surrogate outcome measure for detecting potentially beneficial therapeutic effects of AZD5718 in a phase II study for several reasons. First, coronary microvascular dysfunction is common in patients with obstructive coronary artery disease [32]. CFVR provides an integrated measure of blood flow through the large epicardial arteries and the coronary microcirculation [33]. When stenosis of the coronary arteries is below 50%, as in the present study, CFVR is a marker of coronary microvascular function [34,35]. Second, in echocardiographic studies, CFVR is prognostic of maximal exercise performance and represents a composite of systolic and diastolic function in patients with chest pain and patients with coronary artery disease [34,35]. Third, independent of other risk factors, impaired CFVR predicts mortality in patients with heart failure [36]; and known or suspected coronary artery disease [37]; predicts cardiovascular death, myocardial infarction, or acute revascularization in patients with suspected myocardial ischaemia [38]; and predicts death, myocardial infarction, or hospitalization for unstable angina in patients with known or suspected coronary artery disease [39]. In risk modelling studies, CFVR was a significant independent predictor of cardiovascular mortality in patients with diabetes and patients with chronic kidney disease [40,41]. Fourth, CFVR also reflects basal myocardial oxygen demand [42,43], suggesting that improved or unchanged CFVR might indicate that any enhancement in resting ventricular contractility is energy neutral and therefore potentially beneficial. Finally, echocardiographic measurement of CFVR is highly reproducible and has low variability among trained operators at a single experienced study site [44]. The design of the present study reduces variability between sites by using standardized cardiac sonography protocols and equipment, centralized image reading, and a hands-on sonographer training and certification procedure (S Svedlund et al., manuscript in preparation).
We specified an interval of 1–4 weeks from patients having a myocardial infarction to starting treatment with AZD5718 or placebo in FLAVOUR. This time interval offers the best therapeutic window between feasibility in a clinical trial and early intervention, and eligible patients represent the most likely potential target population for AZD5718. Rates of recurrent myocardial infarction are highest soon after an initial myocardial infarction and decline thereafter [45,46]. Inflammatory responses to necrotic cells in a myocardial infarction begin immediately with leukocyte infiltration and sustained upregulation of pro-inflammatory cytokines [47]. This is followed by proliferation of reparative immune cells in a remodelling and reperfusion phase lasting for a few weeks [47]. CFVR may improve spontaneously in all patients during the remodelling phase following myocardial infarction. Nevertheless, interventions able to improve microvascular function may be more beneficial in this remodelling stage than in the subsequent resolution and scar maturation stage, when the majority of reparative immune cells undergo apoptosis [47].
A potential limitation of the study design is that we did not require participants to have high levels of leukotrienes or other inflammatory biomarkers, or to have impaired CFVR at study entry. In CANTOS, eligible patients had high C-reactive protein levels indicative of high residual inflammatory risk [7]. Furthermore, in a subgroup analysis, participants whose high-sensitivity C-reactive protein levels remained below 2 mg/L after 3 months of anti-interleukin-1β treatment with canakinumab had a lower risk of major adverse cardiovascular events than those with higher levels (≥2 mg/L) [48]. Exploratory outcomes in the present study include comprehensive inflammation and cardiovascular biomarker panels.
In conclusion, FLAVOUR will provide information on potentially clinically meaningful effects of FLAP inhibition with AZD5718 in patients with recent myocardial infarction receiving standard secondary preventive care. Any potentially beneficial effects of AZD5718 on surrogate efficacy outcomes would require further investigation, for example in a phase 3 cardiovascular outcomes trial.
Author contributions
Conceptualization: ML-F, L-MG; Data curation and formal analysis: MK; Investigation: AA, OA, DE, ELG, MH, LOJ, EP, JP, AS, SS; Methodology: ML-F, L-MG, ELG, MK, EP, SS; Reviewing, editing and approving manuscript: all authors.
Funding statement
This study is funded by AstraZeneca. AstraZeneca develops and markets treatments for cardiovascular disease. AZD5718 is an investigational medical product with no approved indication. Under the direction of the authors, Dr Matt Cottingham of Oxford PharmaGenesis provided medical writing support funded by AstraZeneca. AstraZeneca participates in study design, data collection, data analysis, data interpretation, and writing the study report. AstraZeneca reviewed this publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy, and to protect intellectual property. The corresponding author had access to all study protocols and had the final responsibility for the decision to submit the manuscript for publication.
Declaration of competing interest
AA, OA, DE and EP have received speaker honoraria and/or consultancy fees from AstraZeneca. ELG has received speaker honoraria and/or consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, MSD, Mundipharma, Portola Pharmaceuticals and Roche, and research grants from Boehringer Ingelheim. AS has received speaker honoraria and/or consultancy fees from AstraZeneca Bayer, Amgen and Novartis, and research grants from AstraZeneca. L-MG is an employee of AstraZeneca and has received research grants from AstraZeneca. MK and ML-F are employees of AstraZeneca. MH, LOJ, JP and SS have nothing to disclose.
Acknowledgments
We thank the patients and study site staff who are taking part in the study.
References
- 1.Ridker P.M. How common is residual inflammatory risk? Circ. Res. 2017;120:617–619. doi: 10.1161/CIRCRESAHA.116.310527. [DOI] [PubMed] [Google Scholar]
- 2.Fiechter M., Ghadri J.R., Jaguszewski M. Impact of inflammation on adverse cardiovascular events in patients with acute coronary syndromes. J Cardiovasc Med (Hagerstown) 2013;14:807–814. doi: 10.2459/JCM.0b013e3283609350. [DOI] [PubMed] [Google Scholar]
- 3.Task Force Members. Montalescot G., Sechtem U. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; 2017. Cardiovascular Diseases Geneva.http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds Accessed 16 January 2019. [Google Scholar]
- 6.Aday A.W., Ridker P.M. Antiinflammatory therapy in clinical care: the CANTOS trial and beyond. Front. Cardiovasc. Med. 2018;5:62. doi: 10.3389/fcvm.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 8.Tardif J.C., Kouz S., Waters D.D. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 9.Ridker P.M., Everett B.M., Pradhan A. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2018 doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker P.M. Anti-inflammatory therapy for atherosclerosis: interpreting divergent results from the CANTOS and CIRT clinical trials. J. Intern. Med. 2018 doi: 10.1111/joim.12862. [DOI] [PubMed] [Google Scholar]
- 11.Ridker P.M., Luscher T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014;35:1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bäck M., Weber C., Lutgens E. Regulation of atherosclerotic plaque inflammation. J. Intern. Med. 2015;278:462–482. doi: 10.1111/joim.12367. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Golden M., Henderson W.R., Leukotrienes N. Engl. J. Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 14.Bäck M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc. Drugs Ther. 2009;23:41–48. doi: 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettersen D., Davidsson O., Whatling C. Recent advances for FLAP inhibitors. Bioorg. Med. Chem. Lett. 2015;25:2607–2612. doi: 10.1016/j.bmcl.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 16.Balendran C., Ericsson N., Whatling C. P3247 – polymorphisms of the ALOX5AP gene (5-lipoxygenase activating protein) are associated with increased cardiovascular risk in patients with acute coronary syndrome: analysis of PLATO genetics sub-study. Eur. Heart J. 2017;38:P3247. doi: 10.1093/eurheartj/ehx504.P3247. ehx504. [DOI] [Google Scholar]
- 17.Tardif J.C., L'Allier P.L., Ibrahim R. Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ. Cardiovasc. Imag. 2010;3:298–307. doi: 10.1161/CIRCIMAGING.110.937169. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S., Ibrahim R., Gregoire J.C. Effect of treatment with 5-lipoxygenase inhibitor VIA-2291 (atreleuton) on coronary plaque progression: a serial CT angiography study. Clin. Cardiol. 2017;40:210–215. doi: 10.1002/clc.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M.C., Dube L.M., Lancaster J., Zileuton Study Group Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: a 6-month randomized multicenter trial. J. Allergy Clin. Immunol. 1996;98:859–871. doi: 10.1016/s0091-6749(96)80002-9. [DOI] [PubMed] [Google Scholar]
- 20.Israel E., Cohn J., Dube L., Drazen J.M., Zileuton Clinical Trial Group Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma: a randomized controlled trial. J. Am. Med. Assoc. 1996;275:931–936. [PubMed] [Google Scholar]
- 21.Patel R.S., Syed H., Blanco P.R. The 5-lipoxygenase inhibitor zileuton improves endothelial function in carriers of coronary heart disease risk haplotypes in the ALOX5A and LTA4H leukotriene pathway genes. Circulation. 2011;124(21_MeetingAbstracts) [Google Scholar]
- 22.Ericsson H., Nelander K., Lagerstrom-Fermer M. Initial clinical experience with AZD5718, a novel once daily oral 5-lipoxygenase activating protein inhibitor. Clin. Transl. Sci. 2018;11:330–338. doi: 10.1111/cts.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ericsson H., Nelander K., Kjaer M. Phase 1 pharmacokinetic study of AZD5718 in healthy volunteers: effects of co-administration with rosuvastatin, formulation and food on oral bioavailability. J. Clin. Pharm. 2019 doi: 10.1002/cpdd.756. [MS in preparation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 25.TIMI Study Group The Thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N. Engl. J. Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 26.Janssen M.F., Pickard A.S., Golicki D. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual. Life Res. 2013;22:1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware J.E. Quality Metric, Inc.; Lincoln, RI, USA: 2007. User's Manual for the SF-36v2 Health Survey. [Google Scholar]
- 28.Rose G.A., Blackburn H. Cardiovascular survey methods. Monogr. Ser. World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 29.Webster K., Cella D., Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual. Life Outcome. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah S.J., Lam C.S.P., Svedlund S. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018;39:3439–3450. doi: 10.1093/eurheartj/ehy531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tagliamonte E., Rigo F., Cirillo T. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography. 2015;32:516–521. doi: 10.1111/echo.12674. [DOI] [PubMed] [Google Scholar]
- 32.Taqueti V.R., Di Carli M.F. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72:2625–2641. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camici P.G., d'Amati G., Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat. Rev. Cardiol. 2015;12:48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 34.Blomster J.I., Svedlund S., Uw H., Gan L.M. Coronary flow reserve as a link between exercise capacity, cardiac systolic and diastolic function. Int. J. Cardiol. 2016;217:161–166. doi: 10.1016/j.ijcard.2016.04.179. [DOI] [PubMed] [Google Scholar]
- 35.Snoer M., Monk-Hansen T., Olsen R.H. Coronary flow reserve as a link between diastolic and systolic function and exercise capacity in heart failure. Eur. Heart J. Cardiovasc. Imag. 2013;14:677–683. doi: 10.1093/ehjci/jes269. [DOI] [PubMed] [Google Scholar]
- 36.Anantharam B., Janardhanan R., Hayat S. Coronary flow reserve assessed by myocardial contrast echocardiography predicts mortality in patients with heart failure. Eur. J. Echocardiogr. 2011;12:69–75. doi: 10.1093/ejechocard/jeq109. [DOI] [PubMed] [Google Scholar]
- 37.Cortigiani L., Rigo F., Gherardi S. Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. JACC Cardiovasc. Imag. 2012;5:1079–1085. doi: 10.1016/j.jcmg.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Gan L.M., Svedlund S., Wittfeldt A. Incremental value of transthoracic Doppler echocardiography-assessed coronary flow reserve in patients with suspected myocardial ischemia undergoing myocardial perfusion scintigraphy. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigo F., Sicari R., Gherardi S. The additive prognostic value of wall motion abnormalities and coronary flow reserve during dipyridamole stress echo. Eur. Heart J. 2008;29:79–88. doi: 10.1093/eurheartj/ehm527. [DOI] [PubMed] [Google Scholar]
- 40.Murthy V.L., Naya M., Foster C.R. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc. Imag. 2012;5:1025–1034. doi: 10.1016/j.jcmg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murthy V.L., Naya M., Foster C.R. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koepfli P., Wyss C.A., Namdar M. Beta-adrenergic blockade and myocardial perfusion in coronary artery disease: differential effects in stenotic versus remote myocardial segments. J. Nucl. Med. 2004;45:1626–1631. [PubMed] [Google Scholar]
- 43.Porenta G., Cherry S., Czernin J. Noninvasive determination of myocardial blood flow, oxygen consumption and efficiency in normal humans by carbon-11 acetate positron emission tomography imaging. Eur. J. Nucl. Med. 1999;26:1465–1474. doi: 10.1007/s002590050480. [DOI] [PubMed] [Google Scholar]
- 44.Wittfeldt A., Emanuelsson H., Brandrup-Wognsen G. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J. Am. Coll. Cardiol. 2013;61:723–727. doi: 10.1016/j.jacc.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 45.Smolina K., Wright F.L., Rayner M., Goldacre M.J. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ. Cardiovasc. Qual Outcomes. 2012;5:532–540. doi: 10.1161/CIRCOUTCOMES.111.964700. [DOI] [PubMed] [Google Scholar]
- 46.Thune J.J., Signorovitch J.E., Kober L. Predictors and prognostic impact of recurrent myocardial infarction in patients with left ventricular dysfunction, heart failure, or both following a first myocardial infarction. Eur. J. Heart Fail. 2011;13:148–153. doi: 10.1093/eurjhf/hfq194. [DOI] [PubMed] [Google Scholar]
- 47.Frangogiannis N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridker P.M., MacFadyen J.G., Everett B.M. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]