Abstract
Background: The addition of ezetimibe to statin therapy has been reported to result in increased efficacy for reduction of LDL-C levels and achievement of lipid targets, compared with monotherapy.
Objective: This study was designed to demonstrate the noninferiority of therapy with fixed-dose rosuvastatin plus ezetimibe formulations versus fixed dose simvastatin and ezetimibe formulations for reduction of LDL-C levels in Brazilian patients with hypercholesterolemia or mixed dyslipidemia.
Methods: Phase III, multicenter, randomized, parallel, open-label, noninferiority study that included male and female participants (aged 21–80 years) with hypercholesterolemia or mixed dyslipidemia. After a 1-week screening period with washout of lipid-lowering medications when needed, patients were treated with simvastatin 20 mg/d for 5 weeks. Participants with LDL-C levels ≥100 mg/dL after the initial treatment were submitted to a 1-week washout period, and then randomized 1:1 to receive either combined rosuvastatin 10 mg + ezetimibe 10 mg (R/E) or simvastatin 20 mg + ezetimibe 10 mg (S/E) for 4 weeks and, if they still did not achieve the stipulated target, doses were readjusted to rosuvastatin 20 mg + ezetimibe 10 mg or simvastatin 40 mg + ezetimibe 10 mg, respectively, for 4 weeks.
Results: One hundred twenty-nine participants were enrolled, including 66 in R/E and 63 in S/E. At the end of simvastatin 20 mg treatment period, mean LDL-C values were 124.79 mg/dL and 121.27 mg/dL for participants randomized to R/E and S/E arms, respectively. After 4 weeks of R/E 10 mg + 10 mg or S/E 20 mg + 10 mg combined treatments, adjusted mean LDL-C values were 74.21 mg/dL and 85.58 mg/dL, respectively (P = 0.0005), and after 9 weeks, with dose adjustment to R/E 20 mg + 10 mg in 6 patients and to S/E 40 mg +10 mg in 19 patients, LDL-C adjusted mean values were 75.29 mg/dL and 86.62 mg/dL, respectively (P = 0.0006). There was a statistically significant difference between the association R/E and S/E (P = 0.0013) in percentage change of LDL-C after 9 weeks of combined treatments. The adjusted mean difference was estimated at –10.32% (95% CI, –16.94% to –3.70%). The LDL-C <100 mg/dL target was achieved in a significantly greater proportion of participants at week 4 in the R/E compared with the S/E arm (84.8% vs 68.2%; P = .0257), and at week 9, the proportion was 81.2% versus 73.0%, respectively (P = 0.23). LDLC <70 mg/dL was achieved at a significantly greater proportion in the R/E arm, both at week 4 (45.4% vs 15.9%; P = 0.003) and week 9 (40.9% vs 15.9%; P = 0.0017). A statistically significant difference at week 9 (P = 0.0106) was observed in fasting blood glucose in the R/E arm, but the overall incidence of adverse events was not significantly different between groups.
Conclusions: Rosuvastatin and ezetimibe fixed dose combination in both 10 mg/10 mg and 20 mg/10 mg doses, respectively, provided significantly lower levels of LDL-C compared with simvastatin and ezetimibe in doses of 20 mg/10 mg and 40 mg/10 mg, respectively. The fixed-dose combinations were both effective and well tolerated in this Brazilian study population. ClinicalTrials.gov identifier: NCT01420549. (Curr Ther Res Clin Exp. 2020; 81:XXX–XXX)
Keywords: Hypercholesterolemia, Rosuvastatin plus ezetimibe, Single-pill combination, Sinvastatin plus ezetimibe
Introduction
The relationship between elevated LDL-C plasma levels and cardiovascular diseases has been demonstrated in multiple clinical trials.1, 2, 3, 4 The use of statins to reduce LDL-C levels in individuals who are at increased risk of major cardiovascular events is an effective therapeutic strategy that leads to a significant reduction in the risk of coronary and cerebrovascular events. Rosuvastatin acts by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase, which converts 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate by preventing the substrate from binding, blocking a crucial step in the biosynthesis of cholesterol, upregulating LDL receptors in the liver, and resulting in decreased levels of circulating cholesterol.5 The intensity of the reduction in LDL-C levels and the targets to be achieved are set forth in treatment guidelines; however, it is not always possible to achieve these objectives with statin monotherapy regimens.6, 7, 8, 9
Ezetimibe lowers lipids levels by inhibiting the intestinal absorption of cholesterol,10 and its combined use with statins results in an additional reduction in LDL-C levels, thus enhancing the possibility of achieving lipid targets.11, 12, 13, 14, 15
The purpose of the present study was to evaluate the efficacy and safety profiles of a fixed-dose combination containing either 10 or 20 mg rosuvastatin combined with 10 mg ezetimibe, compared with a fixed-dose combination containing either 20 or 40 mg simvastatin with 10 mg ezetimibe for the reduction of LDL-C levels in patients with primary hypercholesterolemia or mixed dyslipidemia.
Patients and Methods
Trial design and participants
This was a 14-week, Phase III, multicenter, parallel, open-label, controlled study conducted at 7 Brazilian study centers (Supplemental Table 1 in the online version at doi:XXXXXXX), and samples were processed in a central laboratory. The study protocol and the informed consent form were approved by each participating institution and the study protocol was evaluated and approved by the Brazilian Health Surveillance Agency.
The inclusion criteria were male or female participants aged 21 to 80 years, diagnosed with primary hypercholesterolemia or mixed dyslipidemia. Participants were eligible if they were receiving prior treatment with statins and had a LDL-C level ≥130 mg/dL but ≤220 mg/dL with triglyceride (TG) level ≤350 mg/dL or if they were not receiving prior treatment with statins and had an LDL-C level ≥160 mg/dL and ≤220 mg/dL, with TG level ≤350 mg/dL. Patients could not have any other clinically significant comorbidities in addition to hyperlipidemia that could interfere with the study evaluations. Participants had to agree to maintain a low-cholesterol diet throughout the study and be able to understand and accept the instructions received during the study visits.
National Cholesterol Education Program Adult Treatment Program (ATP) III guidelines16 define high cardiovascular risk by the presence of clinical atherosclerotic disease that confers high risk for coronary heart disease (CHD) events (CHD risk equivalent): clinical CHD, symptomatic carotid artery disease, peripheral arterial disease, abdominal aortic aneurysm, and also the presence of major risk factors (other than LDL-C levels) like cigarette smoking, hypertension (blood pressure >140/90 mm Hg or taking antihypertension medication), low HDL-C, and age 45 years [women >55 years]. These data were collected, together with results of complementary laboratory tests. Metabolic syndrome is recognized by ATP III as a secondary target of risk-reduction therapy, after the primary target; that is, LDL-C level.16
Exclusion criteria included participants with heart failure determined to be New York Heart Association class III or IV,16 blood dyscrasia, unstable angina pectoris, myocardial infarction during the past 3 months, planned myocardial bypass, peripheral or carotid percutaneous intervention during the past 90 days, renal insufficiency (estimated glomerular filtration rate <30 mL/min/m2), history of alcoholism that could compromise compliance with drug treatment, comorbidities that might hinder the interpretation of results or contraindicate the lipid-lowering therapy (eg, uncontrolled hypothyroidism determined by thyroid-stimulating hormone >8 µIU/mL), uncontrolled diabetes (eg, glycated hemoglobin >8%), active liver disease, antiretroviral therapy for HIV, neoplasm (except for adequately treated skin cancer within the past 5 years), concomitant immunosuppressive therapy (eg, transplant recipients and rheumatic disease), uncontrolled systemic arterial hypertension, hypersensitivity to any component of the investigational product, aspartate transaminase or alanine aminotransferase more than twice the normal upper limit of the central laboratory reference range or creatine phosphokinase more than 3 times the normal upper limit of the central laboratory reference range. Concomitant use of prohibited drugs was considered as a discontinuation criterion for the trial, based on its potential to interfere with treatment efficacy, and safety profile of the participants. Prohibited drugs were macrolides, antifungals, fibrates, digoxin, warfarin, tacrolimus, niacin, cyclosporine, fusidic acid, nefazodone, chlorzoxazone, and antiviral and antituberculosis drugs (eg, rifampicin, isoniazid, pyrazinamide, or ethambutol). All other concomitant medications were recorded, evaluated, and considered to have no potential influence on the efficacy of the study drugs.
The study's enrollment phase occurred from March 2013 to July 2014 and the final patient visit was in November 2014, so the data analysis was carried out between December 2014 and March 2015, when the final study report was issued. For data analysis, the clinical criteria for the definition of patients at high cardiovascular risk were all reviewed considering both the ATP III as well as the new guidelines for the treatment of dyslipidemia published after the conclusion of the study, to verify exactly which type of risk profile was included in the study.8,17
To confirm the degree of cardiovascular risk, we used the Global Risk Score, and also the criteria published in the 2013 American College of Cardiology/American Heart Association Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults.17,18
Randomization and procedures
Eligible participants were randomized at a ratio of 1 to 1 to each arm of the study. Randomization was determined by a computer program using a randomization list generated by a computer algorithm per a permutation regimen with a block size of 4 performed by an independent statistics company, and allocated via electronic case report form to research centers per demand for inclusion. Although the treatment was open label, employees of the sponsor did not participate in randomization, nor did they participate in the clinical and laboratory assessments performed during the study.
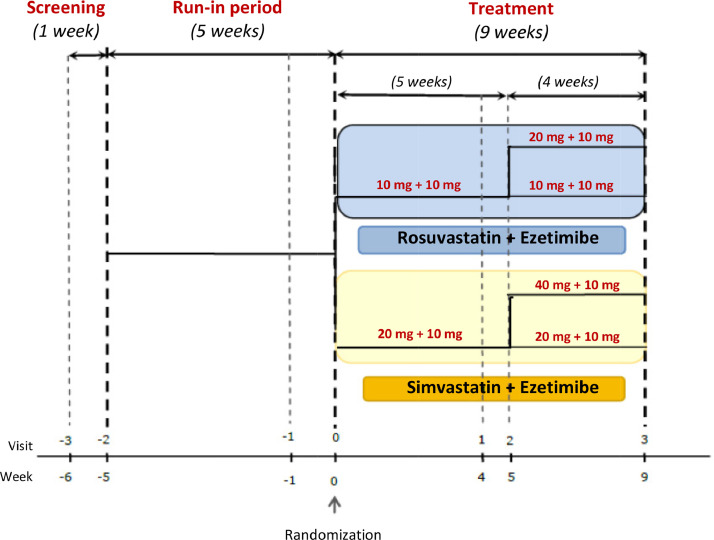
The study was conducted over 15 weeks and comprised 7 study visits divided into the screening period to confirm the eligibility (week –6 to –5); the run-in period during which eligible participants after a 1-week washout period were treated with simvastatin 20 mg for 5 weeks (week –5 to –1); and the combined treatment period (week 0 to 9) during which participants who maintained LDL-C ≥100 mg/dL after the run-in period were randomized to 1 of the study treatments: rosuvastatin 10 mg + ezetimibe 10 mg (Trezete; Aché Laboratórios Farmacêuticos SA, Guarulhos, São Paulo, Brazil) or simvastatin 20 mg + ezetimibe 10 mg (Vytorin; Merck and Co, Inc, Whitehouse Station, NJ) for 9 weeks (weeks 0–9). At week 4, participants’ LDL-C levels were assessed and, from week 5, those with LDL-C <100 mg/dL had the medication dose maintained for another 4 weeks, whereas those who presented with LDL-C levels ≥100 mg/dL had their doses readjusted to rosuvastatin 20 mg + ezetimibe 10 mg or simvastatin 40 mg + ezetimibe 10 mg, for 4 weeks (Fig. 1).
Fig. 1.
Flow chart for the randomization process of the treatment arms.
Primary and secondary objectives
The primary objective of this study was to evaluate the efficacy of a fixed-dose combination containing either 10 or 20 mg rosuvastatin combined with 10 mg ezetimibe in a noninferiority comparison with a fixed-dose combination either 20 or 40 mg simvastatin with 10 mg ezetimibe for the reduction of LDL-C levels compared with a stipulated baseline in participants with primary hypercholesterolemia or mixed dyslipidemia.
The primary end point was the percentage of LDL-C decrease at the end of 9 weeks of treatment, compared with baseline (prerandomization), wherein participants who achieved LDL-C level <100 mg/dL were considered to have been successfully treated.
The secondary end points were the decrease in HDLC, TG, C-reactive protein (CRP), and apolipoprotein B (Apo-B) levels after 9 weeks of treatment, compared with baseline levels; and the rate of success at 9 weeks of treatment in achieving LDL-C <70 mg/dL and non-HDL-C <100 mg/dL. The safety profile outcomes included adverse events and changes in laboratory tests and vital signs.
Statistical analysis
Demographic characteristics as well as clinical and laboratory data were evaluated prerandomization and summarized by descriptive statistics, considering study participants from the intent-to-treat and per-protocol populations.
The 1-sided hypothesis test (noninferiority study) with a significance level (α) of 5%, testing power (1 – β) of 80%, 52% success rate among the control participants, and test sensitivity (minimum detectable difference) of 20% were considered for sample calculation.
The primary efficacy evaluation was performed using a quantitative approach to LDL-C levels, established by the difference between the value at the end of treatment (week 9 or last measurement after randomization for participants who discontinued treatment early) and baseline (week –1), compared with baseline ((LDLfinal – LDLbaseline) / LDLbaseline) × 100).
For noninferiority assessment, the bilateral 95% CI for the ratio of the mean LDLfinal / LDLbaseline were compared between the test rosuvastatin plus ezetimibe (R/E) and comparator simvastatin plus ezetimibe (S/E) treatments and the bilateral 95% CI for the difference between the means of the LDL-C percentage variations of the treatments [((LDLfinal – LDLbaseline) / LDLbaseline)) × 100) R + E] – [((LDLfinal – LDLbaseline)/LDLbaseline)) × 100) S + E] was calculated.
Supplementary, qualitative, and quantitative analyses were performed for LDL-C levels, as follows: proportion of participants who achieved the effective response rate, defined as ≥50% of the LDL-C level after 9 weeks of treatment, compared with baseline; proportion of participants who achieved an LDL-C level <100 mg/dL after 9 weeks of treatment; and proportion of participants who achieved an LDL-C level <70 mg/dL after 9 weeks of treatment. The treatment arms were compared using the exact 95% CI for the difference between the proportions and Pearson χ2 test. The generalized estimating equations method was employed for the adjustment of the linear model. The last observation carried forward data imputation method was used in the statistical analyses. The incidence of adverse events was calculated by dividing the number of study participants who reported at least 1 adverse event episode by the total number of participants evaluated in the safety population in each treatment arm.
Results
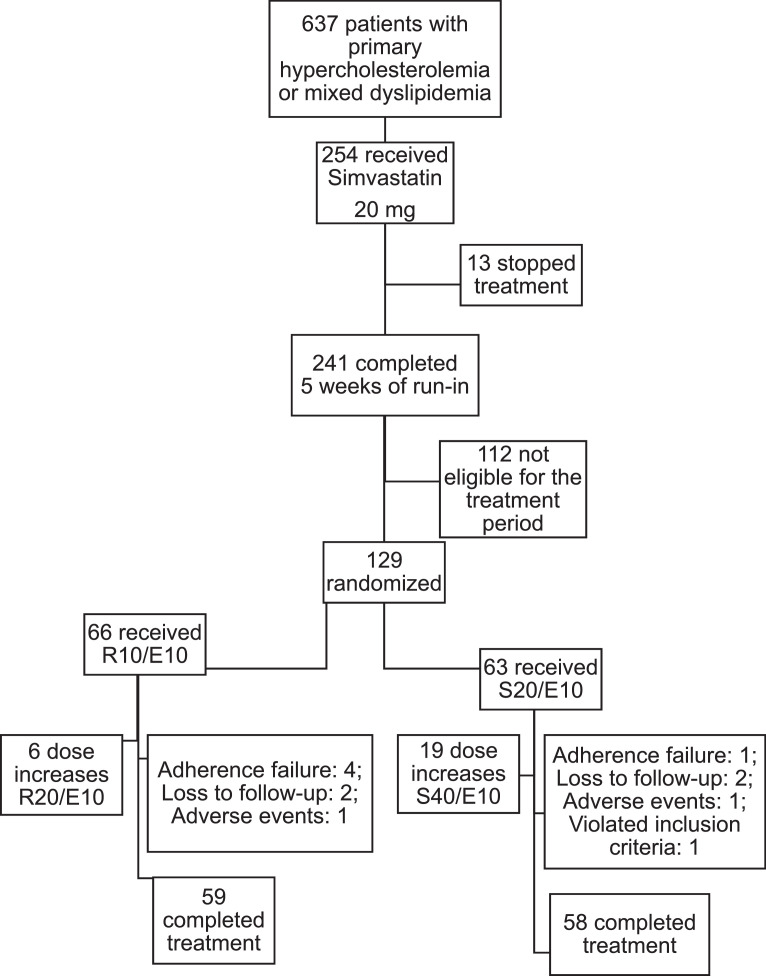
The study enrollment phase occurred from March 2013 to July 2014 and the final patient visit was in November 2014. Of 254 participants enrolled in the run-in phase, 129 participants were eligible for the treatment phase and randomized at the ratio of 1 to 1 for treatment with rosuvastatin 10 mg + ezetimibe 10 mg (R/E arm n = 66) fixed-dose combination, or simvastatin 20 mg + ezetimibe 10 mg (S/E arm n = 63) fixed-dose combination, for the first 5 weeks.
The intent-to-treat population had 129 participants randomized, including 66 in the R/E arm and 63 in the S/E arm. After 5 weeks, 119 participants remained in treatment, including 59 (89.4%) in the R/E arm, of whom 6 (10.2%) had their doses increased, and 60 (95.2%) in the S/E arm, of whom 19 (31.7%) had their doses increased. Treatment was concluded by 59 out of 66 (89.4%) and 58 out of 63 (92.1%) participants in the R/E and S/E arms, respectively. Two participants discontinued the study treatment due to adverse effects concerning other comorbidities not related to the study medication (pulmonary tuberculosis in 1 case and pneumonia in the other case, and 8 were lost to follow-up or had no compliance; that is, participants who did not receive at least 1 dose of the product under investigation). The safety population comprised 129 participants, including 66 (100%) in the R/E arm and 63 (100%) in the S/E arm (100%) (Fig. 2).
Fig. 2.
Study flow chart. R10E10 = rosuvastatin 10 mg + ezetimibe 10 mg; R20E10 = rosuvastatin 20 mg + ezetimibe 10 mg; S20E10 = simvastatin 20 mg + ezetimibe 10 mg; S40E10 = simvastatin 40 mg + ezetimibe 10 mg.
Baseline characteristics
The mean (SD) age of participants in the study was 59.3 (9.1) years, and they were predominantly women (83.7%). No patients younger than age 21 years were included in the study. Regarding diagnosis, most of the patients enrolled presented with primary hypercholesterolemia (63.6%), whereas mixed dyslipidemia was observed in 36.4%. Associated comorbidities were present in both study arms. The most frequent conditions in the R/E and S/E arms, respectively, were arterial hypertension (75.8% and 66.7%), type 2 diabetes (34.8% and 33.3%), ischemic heart disease (3.0% and 3.2%), and metabolic syndrome (72.7% and 60.3%). The baseline characteristics of the patients enrolled in the study are summarized in Table 1.
Table 1.
Demographic and baseline clinical characteristics in the study's intention to treat (ITT) population.
| Variable | Rosuvastatin + Ezetimibe (n = 66) | Simvastatin + Ezetimibe (n = 63) | Total (n = 129) |
||
|---|---|---|---|---|---|
| Demographic | |||||
| Gender | Female | 55 (83.3%) | 53 (84.1%) | 108 (83.7%) | |
| Male | 11 (16.7%) | 10 (15.9%) | 21 (16.3%) | ||
| Age, mean (SD), y | Mean (SD) | 59.39 (8.7%) | 59.16 (9.6%) | 59.28 (9.1%) | |
| Race | White | 51 (77.3%) | 45 (71.4%) | 96 (74.4%) | |
| Non-white | 15 (22.7%) | 18 (28.5%) | 33 (25.6%) | ||
| BMI, mean (SD), kg/m2 | Overweight (25-30) | 25 (37.9%) | 25 (39.7%) | 50 (38.8%) | |
| Obesity (>30) | 29 (43.9%) | 25 (39.7%) | 54 (41.9%) | ||
| Normal (<25) | 12 (18.2%) | 13 (20.6%) | 25 (19.4%) | ||
| Medical history | |||||
| Diagnosis | Mixed dyslipidemia | 26 (39.4%) | 21 (33.3%) | 47 (36.4%) | |
| Primary hypercholesterolemia | 40 (60.6%) | 42 (66.7%) | 82 (63.6%) | ||
| Tobacco use | Never smoked/ex-smoker | 55 (83.3%) | 59 (93.7%) | 114 (88.4%) | |
| Smoker | 11 (16.7%) | 4 (6.3%) | 15 (11.6%) | ||
| Comorbidities | Controlled hypertension | 37 (56.1%) | 31 (49.2%) | 68 (52.7%) | |
| Uncontrolled hypertension | 13 (19.7%) | 11 (17.5%) | 26 (20.2%) | ||
| Diabetes | 23 (34.8%) | 21 (33.3%) | 44 (34.1%) | ||
| Ischemic heart disease | 2 (3.0%) | 2 (3.2%) | 4 (3.1%) | ||
| Chronic kidney disease | 0 (0.0%) | 1 (1.6%) | 1 (0.8%) | ||
| Metabolic syndrome | 48 (72.7%) | 38 (60.3%) | 86 (66.7%) | ||
| Blood Pressure, mean (SD) | |||||
| SBP (mmHg) | 129,14 (15,64) | 128,06 (15,82) | 128,61 (15,68) | ||
| DBP (mmHg) | 78,02 (10,13) | 78,86 (9,02) | 78,43 (9,58) | ||
| Lipid profiles, mean (SD) | |||||
| HDL-C (mg/dL) | 54,50 (12,20) | 55,03 (10,29) | 54,76 (11,27) | ||
| Non-HDL-C (mg/dL) | 194,58 (33,32) | 191,57 (27,16) | 193,11 (30,39) | ||
| TC (mg/dL) | 249,08 (34,72) | 246,62 (29,00) | 247,88 (31,95) | ||
| LDL-C (mg/dL) | 163,62 (29,56) | 160,89 (24,48) | 162,29 (27,12) | ||
| TG (mg/dL) | 154,71 (61,89) | 148,49 (51,75) | 151,67 (57,02) |
SD = Standard Deviation; BMI = Body Mass Index; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; TC = Total Cholesterol; TG = Triglycerides
The patients’ baseline characteristics were further evaluated for cardiovascular risk assessment according to Brazilian,18 North American,8 and European19 guidelines for blood cholesterol management, and 108 patients (83.7%) were considered at high cardiovascular risk, whereas 21 patients (16.3%) were at intermediate or low risk.
Primary efficacy variables
Mean (SD) total cholesterol and LDL-C values before the start of the run-in period with simvastatin were 248 (32) mg/dL and 162 (27) mg/dL, respectively. Baseline values at the beginning of the treatment period with the R/E (10/10 mg) and S/E (20/10 mg) combinations were considered from the end of the run-in period with the use of simvastatin 20 mg, and mean (SD) of the LDL-C values were 125 (20) mg/dL for the group randomized to R/E and 121 (17) mg/dL for S/E.
Treatment with the fixed-dose combinations were used for 9 weeks, wherein during the first 4 weeks, rosuvastatin 10 mg + ezetimibe 10 mg or simvastatin 20 mg + ezetimibe 10 mg doses were used, administered once daily.
After 4 weeks of R/E 10 mg + 10 mg or S/E 20 mg + 10 mg combined treatments, adjusted mean LDL-C values were 74.21 mg/dL and 85.58 mg/dL, respectively (P = 0.0005). Dose increases due to failure to achieve the LDL-C target of <100 mg/mL were required in 6 patients in the R/E arm and in 19 patients in the S/E arm, with increases in the combination doses to rosuvastatin 20 mg + ezetimibe 10 mg and simvastatin 40 mg + ezetimibe 10 mg for another 4 weeks. At the end of 9 weeks, LDL-C adjusted mean values were 75.29 mg/dL and 86.62 mg/dL, respectively (P = 0.0006) (see Supplemental Table 2 in the online version at doi:XXXXXX)
In the assessment of mean absolute percentages of LDL-C variation, including the monotherapy phase with simvastatin 20 mg/d and the combined therapy phase (with rosuvastatin + ezetimibe or simvastatin + ezetimibe), the total variations were –51.3% and –43.3% for R/E and S/E combinations, respectively (see Supplemental Figure in the online version at doi: XXXXX).
The least square estimates of percentage variation of the LDL-C levels indicated a mean reduction in LDL-C of –39.5% (95% CI, –44% to –35%) for the R/E arm, and –29.1% (95% CI, –34% to –24%)] for the S/E arm. The adjusted mean difference was estimated at –10.32% (95% CI, –16.94% to –3.70%). At study week 4, it was observed that the mean LDL-C level for the R/E arm was 13% lower compared with the mean LDL-C level for the S/E arm (P = 0.0005), and a similar result was observed at week 9 (P = 0.0006). (Supplemental Table 3 in the online version at doi:XXXXXX).
Secondary efficacy variables
The mean variations of HDL-C, TG, non-HDL-C, CRP, Apo-B plasma levels, and the percentage of LDL-C target levels achievement throughout the study were analyzed as secondary efficacy variables.
Comparison of the mean (SE) HDL-C from week –6 to week –1, evaluated for both arms, indicated a significant reduction (P = 0.0015), estimated at –3% (1%). However, there was no statistically significant difference between weeks –1 and 9 (P = 0.9265; 95% CI, 0.97–1.03).
Mean (SE) TG levels from week –6 to week –1, evaluated for both arms, indicated a statistically significant (P = 0.0014) reduction, estimated at –9% (3%). Furthermore, there was a significant difference between weeks –1 and 9 (P = 0.0012; 95% CI, 1.05–1.21), when a mean (SE) reduction of 13% (4%) was observed in TG levels.
At week 4, it was observed that the mean (SE) non-HDL-C level for the R/E arm was 12% (3%) lower, compared with the level for the S/E arm (P = 0.0008). The difference between the arms observed at week 4 prevailed at week 9 (P = 0.0018; 95% CI, 0.83–0.96).
Comparison of the mean (SE) CRP levels from week –6 to week –1 indicated a statistically significant (P = 0.0085), reduction, estimated at –20% (8%). Furthermore, there was a statistically significant difference between weeks –1 and 9 (P = 0.0114; 95% CI, 0.05–0.41), when a mean (SE) reduction of 26% (12%) was observed in CRP levels.
At week 4, it was observed that the mean (SE) level of Apo-B for the R/E arm was 13% (3%) lower compared with the level for the S/E arm (P = 0.0005). The difference between the arms observed at week 4 prevailed at week 9 (P = 0.0063; 95% CI, 0.83–0.97).
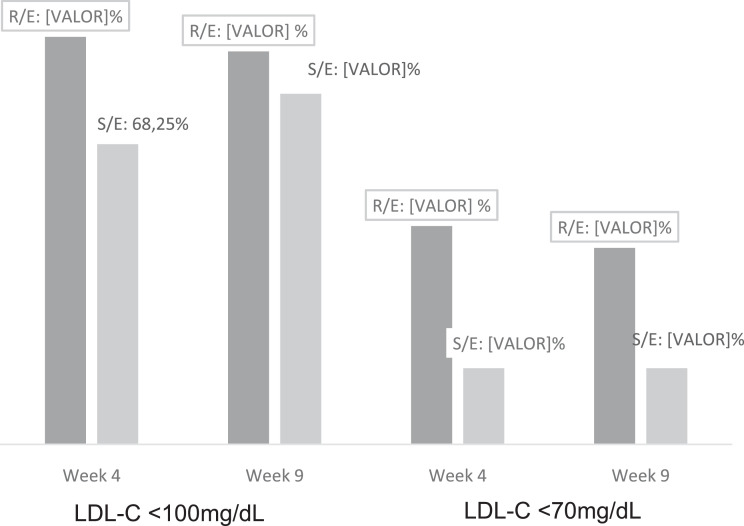
The LDL-C <100 mg/dL target was achieved by 84.8% of patients in the R/E arm, compared with 68.2% in the S/E arm at week 4 (P = 0.0257), and by 81.2% versus 73.0%, respectively, at week 9 (P = 0.23). Achievement of the LDL-C <70 mg/dL target was significantly greater for the R/E arm, both at week 4 (45.4% vs 15.9%; P = 0.003) and at week 9 (40.9% vs 15.9%; P = 0.0017), with a difference between the arms for a response considered effective of 20.85% at week 4 (P = 0.017) and of 17.75% at week 9 (P = 0.043) ((Fig. 3).
Fig. 3.
Percentage of low-density lipoprotein cholesterol targets achievement(LDL-C >100mg/dL and LDL-C >70mg/dL) per treatment group and study week, intention-to-treat population.
Safety profile and tolerability
The incidence of adverse events was calculated by dividing the number of research participants who reported at least 1 adverse event episode by the total number of participants evaluated. A total of 93 adverse events were reported, including 40 (43%) in the R/E arm and 53 (57.0%) in the S/E arm. The most frequent adverse events were increased fasting plasma glucose, defined as fasting plasma glucose levels between 100 and 125 mg/dL (4.5%), myalgia (3.0%), increased creatine phosphokinase (3.0%), and back pain (3.0%) in the R/E arm, and increased fasting plasma glucose (4.5%) and myalgia (6.1%) in the S/E arm. The creatine kinase levels assessed were considered normal in 82.5% of patients at the end of the study and in the remaining 17.5% of patients, these changes were not considered clinically significant (see Table 2).
Table 2.
Summary and frequency (n [%]) of adverse events (AEs) experienced by ≥1.0% of participants per treatment group and protocol period.
| AE | Total (N = 129) | Treatment |
|||
|---|---|---|---|---|---|
| Rosuvastatin + Ezetimibe (n = 66) | Simvastatin + Ezetimibe (n = 63) | ||||
| Run-in* | Treatment | Run-in* | Treatment | ||
| Increased fasting plasma glucose | 15 (11.6) | 3 (4.5) | 3 (4.5) | 6 (9.1) | 3 (4.5) |
| Myalgia | 8 (6.2) | 1 (1.5) | 2 (3.0) | 1 (1.5) | 4 (6.1) |
| Elevation of creatine phosphokinase | 4 (3.1) | 0 (0.0) | 2 (3.0) | 1 (1.5) | 1 (1.5) |
| Urinary tract infection | 3 (2.3) | 1 (1.5) | 1 (1.5) | 0 (0.0) | 1 (1.5) |
| Low back pain | 3 (2.3) | 0 (0.0) | 2 (3.0) | 0 (0.0) | 1 (1.5) |
| Headache | 3 (2.3) | 1 (1.5) | 1 (1.5) | 0 (0.0) | 1 (1.5) |
| Diarrhea | 2 (1.6) | 1 (1.5) | 0 (0.0) | 1 (1.5) | 0 (0.0) |
| Tonsillitis | 2 (1.6) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| Reduction of bone mineral density | 2 (1.6) | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (1.5) |
| Hypertriglyceridemia | 2 (1.6) | 0(0.0) | 0 (0.0) | 0 (0.0) | 2 (3.0) |
| Hypertension | 2 (1.6) | 0(0.0) | 1 (1.5) | 1 (1.5) | 0 (0.0) |
5-week period of simvastatin (20 mg/d) treatment.
Liver enzymes (alanine aminotransferase and aspartate aminotransferase) were considered normal in 97% of patients and in the remaining 3%, this variation did not affect the safety profile of the study. Quantitative analysis of blood glucose levels revealed means of 97 mg/dL at the beginning of the study and 102 mg/dL at the end of the 9 weeks of the study in the R/E group, and the mean difference observed between the run-in period and the first 4 weeks of combined treatment was 3.43 mg/dL. The total difference throughout the study, including the run-in period, was 5.24 mg/dL. There were no statistically significant differences between the R/E and S/E study arms at study weeks –1 (P = 0.7381) and 4 (P = 0.9359). However, there was a statistically significant difference at week 9 (P = 0.0106) New cases of diabetes (patients who had fasting glucose levels <126 mg/dL or glycated hemoglobin ≥6.5% at the beginning of the study and exceeded these values at the end of treatment) were not observed after use of the R/E combination, and 1 patient (1.5%) occurred after use of the S/E combination.
Only 2 patients (1 in each arm) discontinued the study medication due to adverse events that were related to other comorbidities not related to the study medication (pulmonary tuberculosis in 1 patient and pneumonia in the other patient), and no adverse event with the R/E combination was considered severe.
Discussion
The use of statins in a monotherapy regimen has proven to be effective in lowering LDL-C levels; however, many high-risk patients fail to achieve LDL-C level targets, even with the use of high doses.7,9 Rosuvastatin appears to be at least twice and 4 times more potent than atorvastatin and simvastatin, respectively, and at least 8 times more potent than pravastatin and lovastatin.20 The Comparison of the Efficacy and Safety of Rosuvastatin versus Atorvastatin, Simvastatin, and Pravastatin Across Doses trial reported that 10 to 80 mg rosuvastatin significantly reduced LDL-C by a mean of 8.2% more than atorvastatin 10 to 80 mg, 26% more than pravastatin 10 to 40 mg, and 12% to 18% more than simvastatin 10 to 80 mg.21
In our study, treatment with a fixed-dose combination containing either 10 or 20 mg rosuvastatin combined with 10 mg ezetimibe treatment resulted in a reduction of LDL-C levels that was significantly greater than those obtained with a fixed-dose combination of either 20 or 40 mg simvastatin combined with 10 mg ezetimibe, without increasing the risk of adverse events. The rosuvastatin + ezetimibe combination in the doses that were used in this study also allowed more patients to reach LDL-C level targets.
Several studies have demonstrated that the addition of ezetimibe to treatment with statins provides important synergistic decrease in LDLC concentrations.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 In a previous study that included participants with coronary heart disease or type 2 diabetes, the addition of ezetimibe 10 mg to run-in therapy with simvastatin 20 mg proved to be a more effective strategy than doubling the simvastatin dose to 40 mg/d, resulting in more intense reduction of LDL-C, total cholesterol, and total cholesterol to HDL-C ratio, as well as significantly increasing the likelihood of achieving LDL-C levels <100 mg/dL, regardless of the level of LDL-C at the beginning of treatment.33
In a combined analysis of 3 identical, prospective, Phase III, double-blind, controlled studies, patients with primary hypercholesterolemia were randomized to receive placebo, a combination of simvastatin + ezetimibe at doses of 10 mg/10 mg, 10 mg/20 mg, 10 mg/40 mg, or 10 mg/80 mg; ezetimibe 10 mg; or simvastatin 10 mg, 20 mg, 40 mg, or 80 mg, for 12 weeks. The simvastatin + ezetimibe combination proved to be significantly more effective both for lowering LDL-C (52.5% vs 38.0%, respectively) and to decrease CRP levels (31.0% vs 14.3%, respectively) than simvastatin alone, when evaluated at equivalent doses.34
When compared with rosuvastatin 40 mg, the combined use of the same dose with ezetimibe 10 mg for 6 weeks led to a significantly greater number of patients with LDL-C <100 mg/dL (79.1% vs 94.0%; P < 0.001) at the end of treatment, as well a significantly lower LDL-C levels (81.5 mg/dL vs 56.9 mg/dL).35
In another study with higher doses of simvastatin, the addition of ezetimibe 10 mg/d after a 6-week run-in treatment period with either rosuvastatin 10 mg or 20 mg, or simvastatin 40 mg or 80 mg, also led to a greater reduction in LDL-C levels when used in combination with rosuvastatin (63.5% vs 57.4%; P < 0.001), and a greater achievement of LDL-C level <100 mg/dL (95.6%) and LDL-C level <70 mg/dL (77.0%).36 The superiority of the use of ezetimibe combined with statins, compared with progressive titration of statins alone in the reduction of LDL-C levels, as well as in the achievement of targets below 100 mg/dL and 70 mg/dL, were also confirmed by a systematic review.37
This study was the first we are aware of to evaluate the efficacy of the rosuvastatin + ezetimibe fixed-dose combination, compared with simvastatin + ezetimibe in a Brazilian population. The study included Brazilian men and women aged 21 to 80 years with hypercholesterolemia uncontrolled by statin monotherapy, a number of whom also had common comorbidities. This suggests the results may be applicable to other, similar adult populations.
As opposed to the GRAVITY38 study, run-in treatment comprised solely simvastatin 20 mg/d for 5 weeks, and simvastatin + ezetimibe combination doses did not exceed 40 mg due to the greater risk of myopathy observed with use of simvastatin 80 mg and guidelines pertaining to use of this dose.38 We found a greater total absolute percentage reduction of LDL-C levels with rosuvastatin + ezetimibe combination compared with simvastatin + ezetimibe combination with a significantly greater rate of achievement of the LDLC <70 mg/dL targets after 4 and 9 weeks of treatment.
A small decrease in HDL-C levels was observed in our study and have been previously reported following the use of lipid-lowering drugs.39,40 Although increased HDL-C levels are desirable, the benefits of significant reduction in LDL-C, Apo-B, non-HDL-C, and CRP outweigh, in terms of cardiovascular risk prevention, the decrease in HDL-C levels observed.
The majority of our study population had associated comorbidities, including a high prevalence (66.7%) of patients with metabolic syndrome. These characteristics enabled a comparative evaluation of the treatment outcomes in a patient population for whom more intense reductions in LDL-C levels are both necessary and recommended.
Comparison of the outcomes found here with studies of the use of single-agent rosuvastatin in patients with metabolic syndrome or type 2 diabetes, although this specific assessment was not part of the initial study hypothesis, suggests that adding ezetimibe to rosuvastatin could represent an efficient strategy for a more intense lipid level reduction recommended in these patient populations.41, 42, 43
The fact that the protocol for this study was approved based on the National Cholesterol Education Program ATP III16 treatment guidelines should be considered a limitation because considerable changes were made to these recommendations in subsequent guidelines.9,15 However, the need for intense reduction in LDL-C levels in patients with high cardiovascular risk continues to be considered a fundamental principle in the treatment of high cardiovascular risk patients, as seen in a recent report from the American College of Cardiology/American Heart Association that recommends this for patients at very-high-risk for atherosclerotic cardiovascular disease. It is reasonable to add ezetimibe to maximally tolerated statin therapy when LDL-C levels remain ≥70 mg/dL (≥1.8 mmol/L).44,45
Results of this study can be considered applicable to patient populations at high risk for cardiovascular disease because the majority of patients had this profile. However, this may also be applicable to patients with intermediate or low cardiovascular disease risk because a number of such patients were also included.
Although the results suggest superiority of the R/E combination compared with the S/E combination, this study was not designed with sufficient statistical power to prove this superiority, and longer-term studies in larger groups of patients are needed.
Conclusions
Rosuvastatin and ezetimibe fixed-dose combination in both 10 mg/10 mg and 20 mg/10 mg doses, respectively, provided significantly lower levels of LDL-C compared with simvastatin + ezetimibe in doses of 20 mg/10 mg and 40 mg/10 mg, respectively. The fixed-dose combinations were both effective and well tolerated in this Brazilian study population.
Aknowledgments
A. Sapienza contributed to data analysis; C. Goulart Peron, M. Mathias, and J. Macedo participated in the study design and implantation of the study; P. Bonilha contributed to final report elaboration; E. Magaton, A. B. Soares Pires, L. Augusto Furlan, R. Herrera, and E. Cardoso Rorato contributed to startup and collecting data; R. H. Antonelli Cardoso contributed to statistical analysis; O. Kohlmann contibuted as an investigator (Hospital do Rim e Hipertensão); Fernando Augusto Alves da Costa contributed as an investigator (Centro Paulista de Doenças Cardiovasculares); T. C. Piscitelli Bonansea contributed as an investigator (Centro Paulista de Investigação Clínica); L. Henrique de Gregorio contributed as an investigator (CCBR Brasil); F. A Bandeira e Farias contributed as an investigator (Centro Pesquisas Médicas Básica e Clínica); and M. Nasser Hissa contributed as an investigator (Centro de Pesquisa em Diabetes e Doenças Endocrino metabólicas).
Conflicts of Interest
This research was financially supported by Aché Laboratórios Farmacêuticos SA. The sponsor, Aché Laboratórios Farmacêuticos SA, participated in study design, interpretation of data, writing of the report, and in the decision to submit the article for publication. The sponsor had no participation in the collection and analysis of study data.
A. Vattimo, D. C. Morais, L, Fontes-Generoso, R. Herrera, C. M. Barbosa, and S Zung are employees at Aché Laboratórios Farmacêuticos SA. F. A. H. Fonseca received grants from by Aché Laboratórios Farmacêuticos SA during the conduct of the study and personal fees from Amgen, Abbott, Libbs, Novo Nordisk, Takeda, and Sandoz, outside the submitted work. M. C. O. Izar received grants from Aché Laboratórios Farmacêuticos SA during the conduct of the study and personal fees from Amgen, Abbott, Libbs, and Sanofi outside the submitted work. R. Antonelli Cardoso received grants outside this study from Ipsen, EuroFarma, EMS, and Abbott.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2020.100595.
Appendix. Supplementary materials
References
- 1.Klag MJ, Ford DE, Mead LA. Serum cholesterol in young men and subsequent cardiovascular disease. New England Journal of Medicine. 1993;328:313–318. doi: 10.1056/NEJM199302043280504. [DOI] [PubMed] [Google Scholar]
- 2.Lemieux L, Lamarche B, Couillard C. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Archives of internal medicine. 2001;161:2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 3.Sniderman AD, Williams K, Contois JH. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Cardiovascular Quality and Outcomes. 2011;4:337–345. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 4.Verschuren WM, Jacobs DR, Bloemberg BP. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274:131–136. [PubMed] [Google Scholar]
- 5.Istvan E. Statin inhibition of HMG-CoA reductase: a 3-dimensional view. Atherosclerosis Supplements. 2003;4:3–8. doi: 10.1016/s1567-5688(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 6.Mihaylova B, Emberson J, Blackwell L. Cholesterol Treatment Trialists (CTT). The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. The Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cholesterol Education Program, National Heart, Lung, and Blood Institute National Institutes of Health NIH. 2001; 01-3670
- 8.Stone NJ, Robinson JG, Lichtenstein AH. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Circulation. 2013;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 9.The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) ESC/EAS Guidelines for the management of dyslipidaemias. European Heart Journal. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 10.Faludi AA, Izar MCO, Saraiva JFK. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose – 2017. Arq Bras Cardiol. 2017;109:1–76. doi: 10.5935/abc.20170121. [DOI] [PubMed] [Google Scholar]
- 11.Kosoglou T, Statkevich P, Johnson-Levonas AO. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44:467–494. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Cannon CP, Blazing MA, Giugliano RP. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372 doi: 10.1056/NEJMoa1410489. 2387:2397. [DOI] [PubMed] [Google Scholar]
- 13.Gagné C, Bays HE, Weiss SR. Efficacy and Safety of Ezetimibe Added to Ongoing Statin Therapy for Treatment of Patients with Primary Hypercholesterolemia. The American Journal of Cardiology. 2002;90:1084–1091. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 14.Feldman T, Koren M, Insull W. Treatment of High-Risk patients with Ezetimibe plus Simvastatin Co-Administration versus Simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III Low-Density lipoprotein cholesterol goals. The American Journal of Cardiology. 2004;93:1481–1486. doi: 10.1016/j.amjcard.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne CM, Houri J, Notarbartolo A. Effect of Ezetimibe Coadministered With Atorvastatin in 628 Patients with Primary Hypercholesterolemia A Prospective, Randomized, Double-Blind Trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 17.New York Heart Association (NYHA). Classification for heart failure. Available at:https://manual.jointcommission.org/releases/TJC2018A/DataElem0439.html. 2019.
- 18.Xavier HT, Izar MC, JR Faria Neto. V Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq Bras Cardiol. 2013;101(4):S1. doi: 10.5935/abc.2013S010. [DOI] [PubMed] [Google Scholar]
- 19.Catapano AL, Graham I, De Backer G, Wiklund O. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Rev Esp Cardiol (Engl Ed). 2017 Feb;70(2):115. doi: 10.1016/j.rec.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Kendrach MG, Kelly-Freedman M. Approximate Equivalent Rosuvastatin Doses for Temporary Statin Interchange Programs. Annals of Pharmacotherapy. 2004;38(7-8):1286–1292. doi: 10.1345/aph.1D391. [DOI] [PubMed] [Google Scholar]
- 21.Jones PH. Comparison of the Efficacy and Safety of Rosuvastatin Versus Atorvastatin, Simvastatin, and Pravastatin Across Doses (STELLAR* Trial) The American Journal of Cardiology. July 15, 2003;92 doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamaoka-Tojo M, Tojo T, Kosugi R. Effects of Ezetimibe add-on therapy for high-risk patients with dyslipidemia. Lipids Health Dis. 2009;12:41. doi: 10.1186/1476-511X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissonnette S, Habib R, Sampalis F. Efficacy and tolerability of Ezetimibe 10 mg/day coadministered with statins in patients with primary hypercholesterolemia who do not achieve target LDL-C while on statin monotherapy: A Canadian, multicentre, prospective study–the Ezetrol Add-On Study. Can J Cardiol. 2006;22:1035–1044. doi: 10.1016/s0828-282x(06)70319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González CA, Rubio-Guerra AF, Pavía A. Effectiveness and safety of Ezetimibe added to statin therapy in patients with primary dyslipidaemia not achieving the LDL-C treatment goal on statin monotherapy. Clin Drug Investig. 2007;27:333–337. doi: 10.2165/00044011-200727050-00004. [DOI] [PubMed] [Google Scholar]
- 25.Simons L, Tonkon M, Masana L. Effects of ezetimibe added to on-going statin therapy on the lipid profile of hypercholesterolemic patients with diabetes mellitus or metabolic syndrome. Current medical research and opinion. 2004;20:1437–1445. doi: 10.1185/030079904x2321. [DOI] [PubMed] [Google Scholar]
- 26.Pearson T, Denke M, McBride P. Effectiveness of the addition of ezetimibe to ongoing statin therapy in modifying lipid profiles and attaining low-density lipoprotein cholesterol goals in older and elderly patients: subanalyses of data from a randomized, double-blind, placebo-controlled trial. Am J Geriatr Pharmacother. 2005;3:218–228. [PubMed] [Google Scholar]
- 27.Chirinos JA, Williams MM, Bregman DB. Efficacy of cholesterol uptake inhibition added to statin therapy among subjects following a low-carbohydrate diet: a randomized controlled trial. Am Heart J. 2010;159:918 e 1-6. doi: 10.1016/j.ahj.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Bennett S, Sager P, Lipka L. Consistency in efficacy and safety of Ezetimibe coadministered with statins for treatment of hypercholesterolemia in women and men. J Womens Health (Larchmt) 2004;13:1101–1107. doi: 10.1089/jwh.2004.13.1101. [DOI] [PubMed] [Google Scholar]
- 29.Toth PP, Foody JM, Tomassini JE. Therapeutic practice patterns related to statin potency and Ezetimibe/Simvastatin combination therapies in lowering LDL-C in patients with high-risk cardiovascular disease. J Clin Lipidol. 2014;8:107–116. doi: 10.1016/j.jacl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Maron DJ, Hartigan PM, Neff DR. Impact of adding Ezetimibe to statin to achieve low-density lipoprotein cholesterol goal (from the Clinical Outcomes utilizing revascularization and aggressive drug evaluation trial. Am J Cardiol. 2013;111:1557–1562. doi: 10.1016/j.amjcard.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Bardini G, Giorda CB, Pontiroli AE. Ezetimibe + simvastatin versus doubling the dose of simvastatin in high cardiovascular risk diabetics: a multicenter, randomized trial (the LEAD study) Cardiovasc Diabetol. 2010;9:20. doi: 10.1186/1475-2840-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derosa G, D'Angelo A, Franzetti IG. Efficacy and safety of Ezetimibe/Simvastatin association on non-diabetic and diabetic patients with polygenic hypercholesterolemia or combined hyperlipidemia and previously intolerant to standard statin treatment. Journal of Clinical Pharmacy and Therapeutics. 2009;34:267–276. doi: 10.1111/j.1365-2710.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 33.Rotella CM, Zaninelli A, Grazie CL. Ezetimibe/Simvastatin vs Simvastatin in coronary heart disease patients with or without diabetes. Lipids Health Dis. 2010;9:80. doi: 10.1186/1476-511X-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson T, Ballantyne C, Sisk C. Comparison of effects of Ezetimibe/Simvastatin versus Simvastatin versus atorvastatin in reducing C-reactive protein and low-density lipoprotein cholesterol levels. Am J Cardiol. 2007;99:1706–1713. doi: 10.1016/j.amjcard.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 35.Ballantyne CM, Weiss R, Moccetti T. Efficacy and safety of Rosuvastatin 40 mg alone or in combination with Ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study) Am J Cardiol. 2007;99:673–680. doi: 10.1016/j.amjcard.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Catapano AL, Davidson MH, Ballantyne CM, Brady WE, Gazzara RA, Tomassini JE. Lipid-altering efficacy of the ezetimibe/simvastatin single tablet versus rosuvastatin in hypercholesterolemic patients. Curr Med Res Opin. 2006;22(10):2041–2053. doi: 10.1185/030079906X132721. [DOI] [PubMed] [Google Scholar]
- 37.Mikhailidis DP, Lawson RW, McCormick AL. Comparative efficacy of the addition of Ezetimibe to statin vs statin titration in patients with hypercholesterolaemia: systematic review and meta-analysis. Curr Med Res Opin. 2011;27:1191–1210. doi: 10.1185/03007995.2011.571239. [DOI] [PubMed] [Google Scholar]
- 38.Ballantyne CM, Hoogeveen RC, Raya JL. Efficacy, safety and effect on biomarkers related to cholesterol and lipoprotein metabolism of rosuvastatin 10 or 20 mg plus ezetimibe10 mg vs. simvastatin 40 or 80 mg plus ezetimibe 10 mg in high-risk patients: Results of the GRAVITY randomized study. Atherosclerosis. 2014;232:86–93. doi: 10.1016/j.atherosclerosis.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Barakat L, Jayyousi A, Bener A, Zuby B, Zirie M. Comparison of Efficacy and Safety of Rosuvastatin, Atorvastatin and Pravastatin among Dyslipidemic Diabetic Patients. ISRN Pharmacology. 2013;2013:146579. doi: 10.1155/2013/146579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyton JR, Betteridge DJ, Farnier M. Achievement of recommended lipid and lipoprotein levels with combined Ezetimibe/statin therapy versus statin alone in patients with and without diabetes. Diab Vasc Dis Res. 2011;8:160–172. doi: 10.1177/1479164111406457. [DOI] [PubMed] [Google Scholar]
- 41.Kim W, Yoon YE, Shin SH. Efficacy and Safety of Ezetimibe and Rosuvastatin Combination Therapy Versus Those of Rosuvastatin Monotherapy in Patients With Primary Hypercholesterolemia. Clin Ther. 2018;40(6):993–1013. doi: 10.1016/j.clinthera.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Torimoto K, Okada Y, Mori H. Efficacy of combination of Ezetimibe 10 mg and rosuvastatin 2.5 mg versus rosuvastatin 5 mg monotherapy for hypercholesterolemia in patients with type 2 diabetes. Lipids Health Dis. 2013;12:137. doi: 10.1186/1476-511X-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betteridge DJ, Gibson JM, Sager PT. Comparison of effectiveness of Rosuvastatin versus atorvastatin on the achievement of combined C-Reactive Protein (<2mg/L) and Low-Density Lipoprotein Cholesterol (<70 mg/dL) targets in patients with Type 2 Diabetes Mellitus (from the ANDROMEDA Study) American Journal of Cardiology. 2007;100:1245–1248. doi: 10.1016/j.amjcard.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 44.Stone NJ, Robinson JG, Lichtenstein AH. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.