Abstract
Management of oxaliplatin-induced peripheral neuropathy (OIPN) has proven challenging owing to the concern that any OIPN-preventing agents may also decrease the efficacy of the chemotherapeutic agent and fail to reverse established neuronal damage. Nevertheless, targeting redox signaling pathways constitutes a promising therapy in OIPN and we have previously demonstrated the protective role of nuclear factor erythroid-2 related factor 2 (NRF2) in this disorder. Here, we investigated the protective properties of formononetin (FN), a clinical preparation extract, in OIPN. RNA interference experiments revealed that FN protects against OIPN directly through activation of the NRF2 pathway. Further expression profile sequencing showed that FN exerts its protective effect via the NRF2 downstream-oxaliplatin metabolism enzyme, GSTP1. We also demonstrated that FN does not influence the chemotherapeutic function of oxaliplatin, as NRF2 exhibits a different drug metabolic enzyme activation state downstream in colorectal cell lines than that in neurons. Following synthesis of Bio-FN to screen the target binding proteins, we found that FN selectively binds to His129 and Lys131 in the BTB domain of KEAP1. In vivo experiments revealed that FN-induced activation of the NRF2 signaling pathway alleviated the nociceptive sensations in mice. Our findings highlight a new binding mechanism between KEAP1 and isoflavones for activation of the NRF2 system and suggest that pharmacological or therapeutic activation of the NRF2-GSTP1 axis may serve as an effective strategy to prevent or attenuate the progression of OIPN.
Keywords: Formononetin, Oxaliplatin-induced peripheral neuropathy, NRF2, KEAP1, GSTP1
Graphical abstract
Highlights
-
•
FN prevents oxaliplatin-induced peripheral neuropathy via KEAP1-NRF2-GSTP1 axis.
-
•
FN retains oxaliplatin in vitro antitumor activity in cancer cells.
-
•
FN selectively binds His129 and Lys131 in the Keap1 BTB domain.
Abbreviations:
- AHR
Aryl hydrocarbon receptor
- ARE
Antioxidant-responsive element
- DHE
dihydroethidium
- DAB
3,3′-diaminobenzidine-tetrahydrochloride-dihydrate
- DRG
dorsal root ganglion
- FN
formononetin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase modifier subunit
- GSTP1
glutathione S-transferase pi 1
- HE
hematoxylin-eosin
- HMOX1
heme oxygenase 1
- HPLC
High Performance Liquid Chromatography
- HUVEC
Human umbilical vein endothelial cells
- IHC
immunohistochemical
- KEAP1
keleh-like ECH-associated protein-1
- HC
immunohistochemistry
- NQO1
NAD(P)H quinone dehydrogenase 1
- NRF2
NF-E2 p45-related factor 2
- OHP
Oxaliplatin
- OIPN
oxaliplatin-induced peripheral neuropathy
- ROS
reactive oxygen species
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SFN
sulforaphane
- TB
toluidine blue
- TRP
transient receptor potential
- TRPA1
TRP ankyrin 1
- TRPV1
TRP vanilloid 1
- TRPM8
TRP melastatin 8
- ΔΨm
mitochondrial membrane potential
1. Introduction
Peripheral neuropathy constitutes a major dose-limiting adverse effect of anticancer drugs [1]. Oxaliplatin (OHP), a platinum-based chemotherapeutic agent, is used as the standard of care treatment for metastatic colorectal cancer. However, clinical use of OHP may be limited owing to the development of severe peripheral neuropathy [2]. Approximately 80% of patients with colorectal cancer treated with OHP alone, or in combination with other chemotherapeutics, experience neurotoxicity with the impairment potentially being permanent [3]. Although the pathomechanisms underlying OHP-induced neuropathy remain unclear, altered Na+-K+ channel activity consequent to mitochondrial dysfunction and bioenergetic crisis are involved in acute neuropathy, whereas neurotoxicity resulting from accumulation of platinum adducts in dorsal root ganglia (DRGs) is implicated in chronic neuropathy [4,5]. However, current therapeutic options are limited to drugs approved for the relief of neuropathic pain and have no impact on the underlying neuronal damage caused by chemotherapy-induced peripheral neuropathy (CIPN) [6]. Thus, therapies to limit the neurotoxicity induced by platinum compounds while maintaining their anticancer activity are being actively sought [7,8].
Oxidative stress in peripheral nerves and DRGs may represent an important underlying pathomechanism leading to neurodegeneration, interconnected with mitochondrial dysfunction, inflammation, and apoptosis. The mitotoxicity hypothesis posits that OHP causes mitochondrial injury along with swelling and vacuolation, leading to abnormal spontaneous discharge, and compartment degeneration in somatosensory primary afferent neurons [9,10]. However, although several antioxidant treatments for chemotherapy-induced neuropathy, including n-acetylcysteine, n-acetyl carnitine, glutathione, alpha-lipoic acid, and vitamin E have been tested, they have exhibited limited efficacy owing to their inability to modify redox signaling pathways [11,12]. The efficacy of antioxidant administration in oxaliplatin-induced peripheral neuropathy (OIPN) is also debatable, as promising preliminary results for numerous antioxidants have not been confirmed in larger clinical trials [13]. Hence, therapeutic interventions that simultaneously prevent the oxidative stress associated with mitochondrial dysfunction while maintaining the bioenergetic status of the neuron may have therapeutic potential in the treatment of OHP-induced neuropathy.
The transcription factor NF-E2 p45-related factor 2 (NRF2) participates in the adaptation and survival of cells under conditions of stress through regulation of mitochondrial function in diverse networks of cytoprotective proteins [14]. Pharmacological NRF2 activators, such as the naturally occurring sulforaphane (SFN), resveratrol, and luteolin, reverse the deficits associated with neuropathy and protect rat DRGs by increasing NRF2 pathway activation [13,[15], [16], [17]]. Thus, NRF2 plays a prominent role in supporting the structural and functional integrity of mitochondria, with this role being particularly crucial under conditions of stress in OIPN [13]. However, as NRF2 activation is associated with cancer progression and chemoresistance [18], the protective role of NRF2 in OIPN requires further clarification.
The use of natural products and dietary agents in the clinical setting has gained enormous popularity over recent years [19]. In particular, formononetin (7-hydroxy-3-(4-methoxyphenyl)chromen-4-one; FN), an isoflavone predominantly isolated from the roots of Astragalus radix, Trifolium pratense, Glycyrrhiza glabra, and Pueraria lobate, can affect various important hallmarks of cancer in different malignant cells through diverse molecular mechanism(s) [[20], [21], [22], [23]]. Recent studies have also shown that FN attenuates cisplatin-mediated apoptosis in LLC-PK1 pig kidney cells in addition to cisplatin-induced acute kidney injury [24,25]. Furthermore, protective effects of different Astragali radix extracts have been reported in a cellular model of OHP-induced neurotoxicity, whereas none of the tested extracts interfered with the toxicity elicited by OHP in HT-29 human colon adenocarcinoma cells, suggesting the potential of these extracts as an adjuvant for OHP therapy [26].
Nevertheless, the protective mechanism of FN, as a main compound in Astragali radix extracts, remains unknown. Recent studies have associated its protective effect with activation of the NRF2 pathway [27,28]; however the mechanism is not fully understood. In the present study, we therefore aimed to determine the effects of FN on OIPN and OHP chemotherapy and explore its potential mechanisms in vitro and in vivo.
2. Materials and methods
2.1. Cell culture
Mouse ND7/23 neuron cells, colon cancer cells (CT-26), human colorectal carcinoma cells (Caco-2, DLD-1, and HCT-116), human lung adenocarcinoma cells (PC9, A649, H1975, and HCC8827), human lung squamous cell carcinoma cells (H520), and human pancreatic cancer cells (BxPC3 and Panc1) were purchased from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology (China). Human umbilical vein endothelial cells (HUVECs) were purchased from Sciencell (USA). Cells were cultured in DMEM (ND7/23, HCT-116, PC9, A649, H1975, HCC8827, H520, and BxPC3 cells), RMPI 1640 medium (CT-26, DLD-1, and Panc1 cells), MEM (Caco-2 cells), and ECM (HUVECs) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (all purchased from Invitrogen, Grand Island, NY, USA). All cell lines were cultured in a humidified 5% CO2 incubator at 37 °C.
2.2. RNA extraction, library construction, and sequencing
Total RNA was extracted using the TRIzol reagent kit (Invitrogen) according to the manufacturer's protocol. RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and confirmed using RNase-free agarose gel electrophoresis. Then, the enriched mRNA was fragmented into short fragments using fragmentation buffer and reverse transcribed into cDNA with random primers. Second-strand cDNA was synthesized using DNA polymerase I, RNase H, dNTPs, and buffer. The cDNA fragments were purified using the QiaQuick PCR extraction kit (Qiagen, Venlo, The Netherlands), end repaired, poly(A)-tailed, and ligated to Illumina sequencing adapters. The ligation products were size-selected using agarose gel electrophoresis, PCR-amplified, and sequenced using an Illumina HiSeq2500 by Gene Denovo Biotechnology Co. (Guangzhou, China). Primers used for real-time RT-PCR are listed in Table S3.
2.3. Small interfering (si)RNA-mediated knockdown
Inhibition of NRF2 and GSTP1 expression in ND7/23 cells was performed using directed siRNA reagents. GSTP1-specific siRNA (5′-CCCTCATCTACACTAACTA-3′), NRF2-specific siRNA (5′-GCAAGAAGCCAGATACAAA-3′), and AHR-specific siRNA (5′- GGACCAGUGUAGAGCACAATT-3′) obtained from RIBOBIO (GuangZhou, China) were used. ND7/23 cells were transfected with the 21-nt duplexes using Lipofectamine® 3000 Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. RIBOBIO negative control siRNAs were purchased and used as controls. Cells were rinsed and harvested for Western blot analysis 24–48 h after transfection.
2.4. Dual-luciferase reporter gene assay
We investigated the NRF2-mediated transcriptional activity of NRF2 by using the dual-luciferase reporter assay. First, pGL3 promoter vector (Promega, UK) was used to produce eight copies of the antioxidant-responsive element (ARE)-luciferase report plasmids. After the plasmid was generated, the DNA sequence of the insert was verified. For the dual-luciferase reporter gene assay, ND7/23 cells were cultured in 10-cm cell culture dishes to 70–80% density. The cells were co-transfected with 10.5 μg of the expression vectors for both an ARE-firefly luciferase and a TK-Renilla luciferase (8*ARE-Luc/PRL-TK = 20:1) by using LipofectamineTM 3000 transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. At 24 h post-transfection, the transfected cells were treated with the compounds at the indicated concentrations for different treatment times, and Firefly and Renilla luciferase activities were then measured using the dual-luciferase reporter gene assay system (Promega, UK). The firefly luciferase activity was normalized to Renilla luciferase activity.
2.5. Evaluation of oxidative stress markers and mitochondrial function
Following OHP treatment, the ROS content of cells was measured by incubating them with dihydroethidium (DHE) (Beyotime, China) for 30 min (5 μM for ND7/23 cells and 20 μM for DRG neurons and HUVECs), and the total superoxide anion levels were determined. Mitochondrial superoxide anion levels were measured by incubating cells with 0.5 μM MitoSOX™ Red (Invitrogen, USA) for 45 min for further analysis. The JC-1 probe (Beyotime, China) was applied to measure mitochondrial oxidative stress damage following the manufacturer's instructions. All cellular fluorescence intensities were measured using a fluorescence spectrophotometer (Thermo Fisher Scientific).
2.6. Synthesis of biotin-conjugated FN (Bio-FN)
The coupling reaction was performed using biotin NN′-dicyclohexylcarbodiimide (1.0 eq) (Sigma-Aldrich, St. Louis, MO, USA) in tetrahydrofuran and dimethylformamide solution (Sigma-Aldrich). DCC (1.2 eq) and 4-Dimethylaminopyridine (1%) (Sigma-Aldrich) were added to the solution and stirred for 24 h at room temperature. Bio-FN was purified using high-performance liquid chromatography and analyzed by mass spectrometry.
2.7. Immunofluorescence staining and immunohistochemical (IHC) analysis
ND7/23 cells were seeded into 24-well chamber slides at the density of 70–80%. After treatment with test samples for the indicated times, cells were analyzed using immunofluorescence staining as previously described [29]. For biotin-conjugated FN immunofluorescence analysis, ND7/23 cells were fixed with 4% (w/v) paraformaldehyde, rehydrated in PBS, and permeabilized in 0.1% (w/v) Triton X-100 at room temperature. After being washed with PBS, endogenous biotin was removed using an Endogenous Biotin-Blocking kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Cells were then treated with biotin-conjugated FN and co-localization was detected via immunofluorescence staining according to a previously published protocol [29]. Finally, slides were mounted and examined under a Fluoview FV10i confocal laser scanning microscope system (Olympus, Japan). Negative controls were processed in the presence of biotin. For IHC analysis, deparaffinized DRG sections were boiled in sodium citrate buffer and incubated with primary antibodies at 4 °C overnight. Immunostaining was visualized using 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate and the samples were counterstained with hematoxylin. Negative controls were processed in the absence of the primary antibody. The nuclei were blue after hematoxylin staining and the positive expression of DAB was brownish yellow.
2.8. Pull-down assay and relative mass spectrometry analysis
Protein were isolated by IP lysis buffer (Thermo Fisher Scientific, USA) and then the cleared lysate was incubated with Bio-FN overnight at 4 °C. Proteins associated with Bio-FN were precipitated using Dynabeads™ MyOne™ Streptavidin T1 (Thermo Fisher Scientific, USA). After three washes in washing buffer (50 mM HEPES, pH 7.5, 50 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N'-tetraacetic acid, 0.1% Tween-20, 10% (v/v) glycerol, 1 mM NaF, 0.1 mM Na3VO4, and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA)), the beads were eluted with elution buffer (0.1 M glycine-HCl, pH 2.8). Bio–FN–IP Samples were digested with trypsin at 37 °C for 2 h in buffer containing 2 M urea and 50 mM ammonium bicarbonate, acidified with glacial acetic acid to a final concentration of 2%, and desalted using ZipTips (Millipore Corp., Billerica, MA, USA). Tryptic peptides were analyzed by highly sensitive nanospray liquid chromatography-coupled tandem mass spectrometry using an LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific). Confident peptide identifications were determined using the stringent filter criteria for database match scoring followed by manual evaluation of the results. The other samples were boiled in SDS-PAGE sample buffer and separated by 12% PAGE.
2.9. Molecular docking
The crystal structure of KEAP1 in complex with FN was obtained from the protein data bank (PDB ID: 3VNG, 5CGJ, 5DAD). The protein structure was determined using Protein Preparation Tool (ProPrep) in Schrödinger 2015-3 suite software (New York, NY, USA). Hydrogen atoms were added at pH 7.0 using the PROPKA tool in Maestro with an optimized hydrogen bond network. The OPLS3 force field was used for restrained minimization with convergence of heavy atoms to 0.30 Å. The ligand was sketched in Maestro and evaluated with the Ligand Preparation Tool (LigPrep) using OPLS3 at pH 7.0 to generate the low-energy conformation. Molecular docking was performed using the Glide module.
2.10. DNA constructs
pcDNA3.1-Flag-KEAP1, pcDNA3.1-Flag-KEAP1(H129A), pcDNA3.1-Flag-KEAP1(K131A), pcDNA3.1-Flag-KEAP1 (R380A), pcDNA3.1-Flag-KEAP1(N382A), pcDNA3.1-Flag-KEAP1 (R415A), pcDNA3.1-Flag-KEAP1(F478A), pcDNA3.1-Flag-KEAP1(H129A, K131A) and pcDNA3.1-Flag-KEAP1(C151S) were constructed using standard techniques. Briefly, DNA fragments encoding wild type (WT), Flag-tagged KEAP1 were generated by high-fidelity PCR and cloned into the pcDNA3.1 vector. All of the constructs were confirmed by DNA sequencing and purified using the Endofree Plasmid Preparation Kit (Qiagen, Valencia, CA, USA).
2.11. Animal experiments
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Jiangsu Province Institute of Traditional Chinese Medicine and procedural details were drafted following the ARRIVE guidelines. Experiments were performed in accordance with published National Institutes of Health guidelines. C57BL/6 male mice (8–10 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (China), and mice weighted 20–22 g at study initiation. After habituation to the test environment and baseline measurements of pain sensitivity, mice were randomized into four groups as follows: Control, OIPN, FN, and SFN (n = 7 per group). In the control group, the mice were only injected intraperitoneally with solvent (5% glucose solution and 2% dimethylsulfoxide in corn oil). In the OIPN group, the mice were intraperitoneally injected with OHP (3.0 mg/kg, dissolved in 5% glucose solution). Using injection volumes of 5 mL/kg, the mice were administered daily with an intraperitoneal injection for 5 days, followed by 5 days of rest, for two weekly cycles. A total cumulative dose of 30 mg/kg OHP was used over a total of ten injections. In the FN and SFN groups, mice were pretreated with FN (10.0 mg/kg, dissolved in corn oil) or SFN (10.0 mg/kg, dissolved in corn oil), then after 2 h they were administered with an intraperitoneal injection of OHP as for the OIPN group. The FN and SFN groups were separately administered with FN or SFN using daily intraperitoneal injections throughout the entire cycle. All the behavioral tests were performed by an observer blind to the drug administration.
2.12. Statistical analysis
The experimental data obtained from cultured cells and mice were analyzed using the Student's t-test and one way ANOVA to determine the significance of difference between two groups and are presented as the means ± SD from independent experiments. Western blotting analyses were repeated with three independent experiments and the results were quantified using ImageJ software. Statistical analysis was carried out using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered significant.
2.13. Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information File or from the corresponding authors upon request.
3. Results
3.1. FN exhibits protective effects against OHP-induced peripheral nerve damage by activating the NRF2 pathway in neuron cells
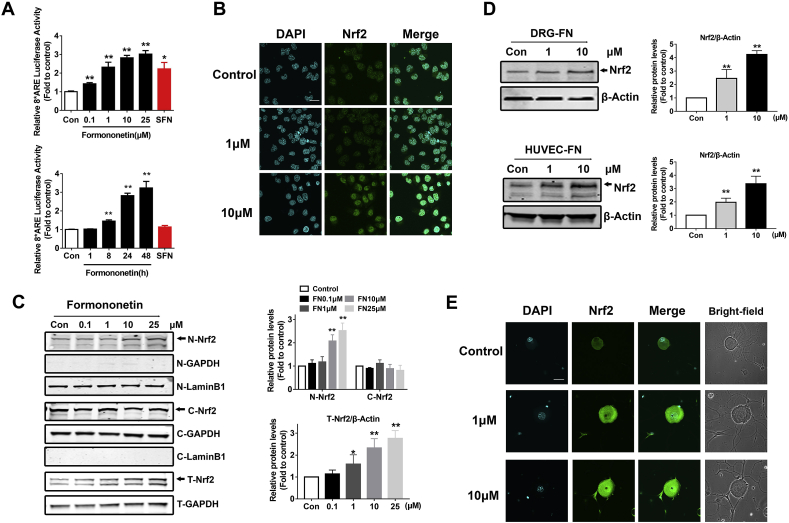
In our previous clinical study, we found that AC591 could prevent the neuropathy caused by OHP without reducing its anti-tumor activity [30]. Through screening the components in AC591, we determined that FN not only increased the activity of NRF2 but also exhibited a recovery effect on the cell damage caused by OHP (Supplementary Fig. 1). We also demonstrated that NRF2 may inhibit OIPN by protecting mitochondrial function [13]. To analyze FN-induced NRF2 transcriptional activity, we performed a dual-luciferase reporter gene assay in ND7/23 cells and found that FN induced ARE-luciferase reporter gene activity in a dose and time dependent manner. Moreover, FN showed a more significant effect in activating ARE-luciferase than did SFN (Fig. 1A). In addition, immunofluorescence experiments demonstrated that NRF2 was markedly increased following FN stimulation (Fig. 1B). The data also showed that FN increased the level of NRF2 protein in both the nuclear and total protein fractions, suggesting impaired NRF2 degradation upon FN treatment (Fig. 1C). Meanwhile, FN was found to stabilize the level of NRF2 in DRG neurons and HUVECs (Fig. 1D and E).
Fig. 1.
FN activates the NRF2 pathway in neurons. (A) FN induces ARE-luciferase activity in dose and time dependent manner. ND7/23 cells were transfected with the ARE-firefly luciferase and TK-Renilla luciferase expression plasmids for 24 h. ND7/23 cells were then stimulated with FN for the indicated concentrations (24 h, SFN for 10 μM) and for different treatment time (0–48 h, SFN for 48 h), and the cells were then subjected to the dual-luciferase assay. SFN was set as a positive control. (B) Immunofluorescence staining showed the relative abundance of NRF2 in ND7/23 cells (scale bar = 20 μm). (C) The level of NRF2 protein in cytoplasm and nucleus was detected using the nuclear protein and cytoplasmic protein separation assay following application of different concentrations of FN in ND7/23 cells. Protein grayscale analysis was performed using Image J. (D) The level of NRF2 in the primary DRG neurons of mice and the human umbilical vein endothelial cells (HUVECs). (E) Immunofluorescence assay showed the relative abundance of NRF2 in primary DRG neurons of mice (scale bar = 20 μm). The data are expressed as the means ± standard deviation (Data in A–E, n = 3). Statistical differences between groups were analyzed using the unpaired Student's t-test, *P < 0.05, **P < 0.01, compared to control.
As Astragalus extracts exert neuroprotective effects in rats subchronically treated with OHP [31], we next evaluated whether pre-treatment with the main compound FN afforded similar benefits. We found that FN activated the NRF2 signaling pathway when administered concurrently with OHP (Fig. 2A). Moreover, NRF2 was markedly accumulated in the nucleus upon FN treatment as shown by immunofluorescence and western bolt analyses (Fig. 2B and C). As shown in Fig. 2D, the cell viability was significantly decreased following OHP treatment, yet was increased upon pre-treatment with FN. As NRF2 plays a crucial role in maintaining cellular redox homeostasis by regulating mitochondrial function in OIPN [32], we used DHE and MitoSOX™ Red probes to investigate the changes in reactive oxygen species (ROS) levels, and JC-1 to measure mitochondrial damage owing to oxidative stress. Results showed that OHP increased the levels of total superoxide anion and mitochondrial superoxide anion, whereas these were reduced by FN pre-treatment (Fig. 2E and F). Moreover, OHP induced a significant change in mitochondrial membrane potential in ND7/23 cells whereas FN pre-treatment reduced this effect (Fig. 2G). In addition, following knockdown of NRF2 expression via specific siRNA we found that ND7/23 cells were more vulnerable to OHP-induced insult (Fig. S2A). Furthermore, the cell viability decreased more obviously, and ROS levels increased more markedly following treating with OHP. Notably, the FN-mediated protection was lost in neurons transfected with si-NRF2 (Fig. 2H–K), suggesting that FN protects against OIPN directly through activation of the NRF2 signaling pathway.
Fig. 2.
FN protects ND7/23 cells against OHP-induced neuron damage by activating the NRF2 pathway. ND7/23 cells were pretreated with FN for 2 h and then treated with OHP (5 μM) for 24 h. (A) The dual-luciferase reporter system was used to detect activation of the NRF2-ARE signaling pathway in ND7/23 cells. (B) Immunofluorescence staining showed NRF2 relative abundance induced by FN (10 μM) and OHP stimulation in ND7/23 cells (scale bar = 20 μm). (C) Western blot analysis showed the comparison of NRF2 levels between total, cytoplasmic, and nuclear protein fractions. Protein grayscale analysis was performed using Image J. (D) Cell viability was measured using the CCK-8 assay. (E) Total superoxide anion was detected using the fluorescent probe DHE. (F) Mitochondrial superoxide anion was detected using MitoSOX™ Red probes. (G) The mitochondrial membrane potential (ΔΨm) was measured using a JC-1 probe. (H–K) Cell viability, total superoxide anion, mitochondrial superoxide anion, and mitochondrial membrane potential were determined following transfection with siNC and siNRF2 plasmids in ND7/23 cells. The data are expressed as the mean ± standard deviation (Data in A–K, n = 3). Data in (A–G) were analyzed using one way ANOVA followed by Tukey's post-hoc test comparisons. Data in (H–K) were analyzed using two-way ANOVA followed by Tukey's post-hoc test comparisons.*P < 0.05, **P < 0.01 compared to the Control group; #P < 0.05, ##P < 0.01 compared to the OHP group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. FN selectively activates the NRF2-GSTP1 pathway
We further screened the downstream target genes of the NRF2 signaling pathway, and subsequent expression profile sequencing revealed that FN exerts its protective effect through the NRF2-GSTP1 pathway (Fig. 3A). The mRNA and protein levels of GSTP1 were both increased following FN treatment as shown by quantitative reverse transcription-polymerase chain reaction (PCR) and Western blot assays (Fig. 3B and C). Furthermore, these data showed that FN selectively activated the NRF2-GSTP1 pathway. Following OHP treatment, FN was also found to activate the GSTP1 signaling pathway in an NRF2-dependent manner (Fig. 3D and Fig. S2B). Since FN is a planar molecule, it likely induces the Aryl hydrocarbon receptor (AHR), which may cause subsequent induction of GSTP1; however, si-AHR transfection showed that FN retained GSTP1 activation in the absence of AHR, demonstrating that AHR is not required for GTSP1 activation. Furthermore, si-NRF2 transfection as well as GSTP1-ARE mutation revealed that intact ARE is required for FN-mediated enhanced GSTP1 expression, and thus, FN-induced NRF2 activation is required for GSTP1 induction (Fig. S2A, S2C and S3). We next transfected si-GSTP1 into ND7/23 cells to verify whether FN retained its efficacy in the case of GSTP1 knockdown (Fig. S2B). Notably, no protective effects were observed to be elicited by FN against peripheral nerve damage caused by OHP following si-GSTP1 transfection. As shown in Fig. 3E–H, regardless of FN pre-treatment, low cellular activity, high ROS levels, and significantly altered mitochondrial membrane potential were observed upon OHP treatment in conjunction with GSTP1 knockdown.
Fig. 3.
FN selectively activates the NRF2-GSTP1 pathway. (A) The mRNA expression profile of ND7/23 cells was used to screen the level of expression of NRF2-ARE signaling pathway-related genes following treatment with FN. (B) Real-time quantitative fluorescence PCR assay was used to investigate the mRNA levels of Nqo1, Hmox1, Gclc, Gclm, and Gstp1. The data were analyzed using the unpaired Student's t-test; *P < 0.05, **P < 0.01, compared to control. (C–D) Western blot showed the protein levels of NRF2 and its downstream protein. (E–H) Following transfection with si-GSTP1, reverse verification was performed by cell viability, mitochondrial membrane potential, total superoxide anion, and mitochondrial superoxide anion analyses. Data are expressed as the means ± SDs (Data in B–K, n = 3). Data in (E–H) were analyzed using two-way ANOVA followed by Tukey's post-hoc test.*P < 0.05, **P < 0.01 compared to the Control group; #P < 0.05, ##P < 0.01 compared to the OHP group.
Furthermore, we found that FN activated the NRF2-GSTP1 pathway in DRGs, as evidenced by the increased mRNA and protein expression following FN treatment in DRGs (Fig. 4A–C). Immunofluorescence experiments demonstrated that FN and OHP could increase the stability of NRF2 (Fig. 4D). Additionally, consistent with the results in ND7/23 cells, FN protected DRGs against OHP-induced peripheral nerve damage by activating the NRF2-GSTP1 pathway. As shown in Fig. 4E–H, OHP indeed led to a significant decrease in cell activity and mitochondrial membrane potential, while also inducing increased levels of ROS; however, FN effectively reversed these changes. We also observed a protective role for FN in HUVECs upon OHP stimulation (Fig. S4).
Fig. 4.
FN protects against OHP-induced neuron damage by activating the NRF2-GSTP1 axis in DRG neurons. DRGs were dissected from the spinal column of mice. (A) Real-time quantitative fluorescence PCR was used to investigate the mRNA levels of Nqo1, Hmox1, Gclc, Gclm, and Gstp1. (B) Western blot showed the protein levels of GSTP1 in the DRGs of mice. Protein grayscale analysis was performed using Image J. (C–H) The DRGs were pretreated with FN for 2 h and then treated with OHP (5 μM) for 24 h. (C) Western blot analysis showed the protein levels of NRF2 in the DRGs of mice. Protein grayscale analysis was performed using Image J. (D) Immunofluorescence assays showed the relative abundance of NRF2 in DRGs of mice (FN = 10 μM, scale bar = 20 μm). (E) Cell viability was measured using the CCK-8 assay. (F) Total superoxide anion was detected using the fluorescent probe DHE. (G) Mitochondrial superoxide anion was detected using MitoSOX™ Red probes. (H) The mitochondrial membrane potential (ΔΨm) was measured using a JC-1 probe. The data are expressed as the mean ± standard deviation (Data in A–H, n = 3). Data in (A–B) were analyzed using the unpaired Student's t-test; *P < 0.05, **P < 0.01, compared to control. Data in (C–H) were analyzed using two-way ANOVA followed by Tukey's post-hoc test comparisons.*P < 0.05, **P < 0.01 compared to the Control group; #P < 0.05, ##P < 0.01 compared to the OHP group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. FN does not interfere with OHP toxicity toward colon cancer cells
To confirm whether FN affects the anti-tumor efficacy of OHP, in an effort to gauge its potential for use as clinical supportive care in chemotherapy, we conducted preliminary experiments in mouse and human colon cancer cell lines: CT-26 (mouse), Caco2 (human), HCT116 (human), and DLD1 (human). As shown in Fig. 5A and S5A-B, FN also activated ARE-luciferase in these cells. Notably, although the levels of NRF2 activity increased following FN treatment in all cell lines, it did not activate the NRF2-GSTP1 pathway in these colon cancer cells, which differed from what was observed in DRG neurons (Fig. 5B-C and S5 C-F). Similarly, when we applied an accepted NRF2-inducer (sulforaphane) to DRG neurons and colon cancer cell lines we found that it induced high levels of NRF2-dependent genes in DRG neurons but not in cancer cell lines (Fig. S6). Taken together, these results suggest that the drug metabolic enzyme activation state induced by FN downstream of NRF2 differs according to cell type. To evaluate cytotoxicity against cancer cells, we administered different concentrations of OHP (10, 20, and 40 μM) for 24 h following FN pre-treatment. Subsequent cell viability analysis showed that FN administration did not diminish the anti-tumor effects of OHP in this treatment protocol (Fig. 5D and S5 G-H, S7).
Fig. 5.
FN does not interfere with the toxicity of OHP in colon cancer cells. (A) Activation of the NRF2-ARE signaling pathway following FN stimulation in Caco2 cells and CT-26 cells was detected using the dual-luciferase reporter gene system. (B) The mRNA expression profiles of the Caco2 cells and CT-26 cells to screen the level of expression of NRF2-ARE signaling pathway-related genes following treatment with FN. (C) Real-time quantitative fluorescence PCR assay was used to investigate the mRNA levels of Nqo1, Hmox1, Gclc, Gclm, and Gstp1 in Caco2 cells and CT-26 cells. (D) The cells were pretreated with FN (1 and 10 μM) for 2 h and then treated with OHP (10, 20, and 40 μM) for 24 h. The cell viability was measured using the CCK-8 assay. SFN (10 μM) was set as a positive control. Data are expressed as the mean ± SDs (Data in A, C–D, n = 3). Data in (A and C) were analyzed using unpaired Student's t-tests. *P < 0.05; **P < 0.01, compared to control. Data in (D) were analyzed using two-way ANOVA followed by Tukey's post-hoc test.*P < 0.05; **P < 0.01 compared to the Control group; #P < 0.05; ##P < 0.01 compared to the OHP group.
3.4. FN activates the NRF2 signaling pathway by binding to KEAP1 at His129 and Lys131
Ononin and sodium formononetin-3′-sulfonate are water-soluble derivatives of FN, and we found that both ononin and FN activated the NRF2 signaling pathway to a similar degree, whereas sodium formononetin-3′-sulfonate had no effect (Fig. 6A–C). These results indicate that modification of the FN A ring might not affect its NRF2 activation capacity. We, therefore, synthesized a biotin-labeled probe in which a linker was attached to the A ring of FN and used the ARE-luciferase reporter gene assay to confirm that the probe retained the ability to induce NRF2 pathway genes (Fig. 6D). Furthermore, the differential protein KEAP1 was identified by mass spectrometry of Bio-FN and biotin binding targets obtained through immunoprecipitation (Fig. 6E). Immunoprecipitation followed by SDS-PAGE confirmed that, whereas biotin and streptavidin alone did not pull-down KEAP1, Bio-FN was found to bind KEAP1 (Fig. 6F). Immunofluorescence analysis confirmed these results (Fig. 6G). Meanwhile, KEAP1 dimerization was reduced and the relative level of KEAP1 monomer increased following FN treatment (Fig. 6H). Furthermore, immunoprecipitation confirmed that FN interfered with the contact between KEAP1 and CUL3 and decreased the binding of NRF2 to KEAP1. It is, therefore, reasonable to postulate that the binding of FN to KEAP1 leads to a decrease in KEAP1-dimer formation; and that FN inhibits KEAP1-mediated ubiquitination of NRF2 by interfering with the interaction between CUL3 and the KEAP1-dimer.
Fig. 6.
Molecular mechanism of FN-induced NRF2 activation. (A) Formononetin; (B) Ononin; (C) Sodium formononetin-3′-sulfonate; (D) Formononetin linked with biotin probe (Bio-FN). (A–D) Chemical structures of the four compounds and the dual-luciferase assay in ND7/23 cells. (E) Immunoprecipitation and liquid chromatography-tandem mass spectrometry were performed to screen the possible binding targets of Bio-FN (Left: biotin; Right: Bio-FN). The proteomic data for Biotin-FN showed the result excluded from the Biotin group, and the list of protein ranked by the relative score. (F) For immunoprecipitation, the cell lysates were incubated with Bio-FN (10 and 25 μM) at 37 °C for 2 h, followed by a Dynabeads-streptavidin pull-down. Then, mixed samples were separated by SDS-PAGE and the KEAP1 protein was detected. (G) Immunofluorescence assay showed the combination of Bio-FN and KEAP1 in ND7/23 cells (scale bar = 20 μm). (H) ND7/23 cells were treated with FN, and the cell lysates were then subjected to electrophoresis on non-denaturing gels to investigate the effects on KEAP1 dimerization. Relative dimerization of KEAP1 was determined by immunoblots. (I) ND7/23 cells were stimulated with different concentrations of FN. Immunoprecipitation analysis the conjugated CUL3, NRF2 with KEAP1 by immunoblotting. Data are expressed as the mean ± SD (Data in A–D, F–I, n = 3). The data were analyzed using the unpaired Student's t-test (*P < 0.05, **P < 0.01, compared to control).
To further evaluate whether FN activates the NRF2 signaling pathway by binding to KEAP1, we conducted a protein molecular simulation of the binding modes of KEAP1 with FN (Fig. 7A). Based on molecular docking, we designed different mutant plasmids of KEAP1 (with a Flag label) to investigate the possible binding sites with FN. Dual-luciferase reporter gene assays in ND7/23 cells transfected with WT- or mutant KEAP1 plasmids showed that compared with the WT-KEAP1-transfected group, the effect of FN on NRF2 activation was markedly weakened following transfection of H129A and K131A mutant plasmids (Fig. 7B). Among these mutants, H129A and K131A significantly reduced KEAP1 binding capacity to FN in ND7/23 cells, whereas other tested mutants retained their high FN binding affinity (Fig. 7C). Additionally, the endogenous FN-KEAP1 complex was analyzed by MALDI-TOF/TOF MS, which revealed two definitive peaks representing m/z 1652.85 and m/z 1921.12 corresponding to KEAP1 peptide E117-K131 and FN-bound KEAP1 peptide E117-K131, respectively (Fig. S8 and Tables S1–S2). Moreover, the MS/MS analysis of the precursor ion (m/z 1921.12) revealed a specific neutral loss of 268.27 Da, where the Mw of FN was 268.26 Da. This indicated that FN may non-covalently interact with KEAP1, in a manner that is dependent on the E117-K131 region of KEAP1. We then designed co-mutated-KEAP1 plasmids at the two sites (H129A and K131A) for verification. As shown in Fig. 7D and F, after transfection with the co-plasmids, NRF2 activation induced by FN was decreased, and the co-localization between Bio-FN and KEAP1 was significantly weakened. Additionally, since Cys151 in KEAP1 is the main cysteine sensor for the cyanoenone class of NRF2 activators, it was necessary to verify whether Cys151 was the binding site of FN and KEAP1. However, in attempting to conduct the molecular docking experiment, we determined that FN may not be able to form a covalent bond with the Cys151 of KEAP1 donating the oxygen atoms. Following transfection of the C151S plasmid, little effect was observed on the KEAP1 activation induced by FN, indicating that this site did not significantly affect the binding of FN and KEAP1 (Fig. 7E and G).
Fig. 7.
Binding site analysis of the FN-KEAP1 complex. (A) The possible binding sites of FN and KEAP1 were predicted using computer molecular docking analysis. (B) ND7/23 cells were transfected with the WT-KEAP1 or mutant -KEAP1 plasmids for 24 h, together with the ARE-firefly luciferase and TK-Renilla luciferase expression plasmids. Following treatment with FN for an additional 24 h, cells were subjected to the dual-luciferase assay. Activation of the NRF2-ARE signaling pathway by FN treatment following mutant-KEAP1 plasmid transfection was evaluated. Data were analyzed using two-way ANOVA followed by Tukey's post-hoc test comparisons (*, compared with the control group; #, compared with the KEAP1 group). (C) ND7/23 cells were transfected with the WT-KEAP1 or mutant -KEAP1 plasmids for 24 h. The cell lysates were selectively treated with Dynabeads-streptavidin for immunoprecipitation, and the level of Flag-KEAP1 was measured using an anti-Flag antibody. The data were analyzed using the unpaired Student's t-test (*, compared with the KEAP1 group). (D and E) ND7/23 cells were transfected with WT-KEAP1, the H129A/K131A co-mutant, or C151S mutant plasmids for 24 h. The cell lysates were selectively treated with Dynabeads-streptavidin for immunoprecipitation, and the level of Flag-KEAP1 was measured using an anti-Flag antibody. (F and G) ND7/23 cells were transfected with plasmids encoding the Flag-tagged WT, H129A and K131A co-mutant, or C151S mutant of KEAP1, and co-transfected with an ARE-luciferase reporter. Following treatment with FN for an additional 24 h, cells were subjected to the dual-luciferase assay. Data were analyzed using two-way ANOVA followed by Tukey's post-hoc test comparisons (*, compared with the control group; #, compared with the WT-KEAP1 group). All data are expressed as the mean ± SD (Data in A–G, n = 3).
3.5. FN exerts protective effects against the peripheral nerve injury induced by OHP in mice
We used an OIPN mouse model to explore whether FN afforded protection against OHP-induced peripheral nerve damage. The mice were intraperitoneally injected with FN (1.0 and 10.0 mg/kg) or SFN (10.0 mg/kg) for 24 and 48 h. DRGs were then dissected from the spinal column of mice [33]. FN (10.0 mg/kg) and SFN (10.0 mg/kg) induced an increase in NRF2 and GSTP1 protein expression in the DRG of mice at 24 and 48 h (Fig. 8A). We, therefore, selected FN (10.0 mg/kg) as the dose for subsequent experiments. We next performed in vivo experiments to evaluate the effects of FN on OHP-induced neuropathy in mice. The effects of OHP administration on behavioral sensitivity to mechanical and cold stimuli were assessed using von Frey filament and cold-plate tests, respectively [34]. During the two cycles of OHP administration, the mice exhibited severe weight loss along with a lower paw withdrawal threshold and higher cold escape behavior scores (Fig. 8B–D). The FN and SFN groups were then separately treated with FN or SFN via daily intraperitoneal injections throughout the entire cycle. The data indicated that FN and SFN reduced the OHP-induced mechanical and cold sensitivities of mice, although none of the groups regained weight during the administration period. Morphometric analysis of DRG sensory neurons using hematoxylin eosin (HE) and Nissl body staining assays [35] revealed severe DRG neuron nucleolar area shrinkage, obvious cytoplasmic vacuolization, and Nissl stain fragmentation and reduction in the OIPN animal model. Less serious morphological damage were observed in DRG neurons from the FN and SFN groups than in those from OIPN groups (Fig. 8E). Additionally, the average nucleolar area of the OIPN group was much smaller than that of the control group, whereas the nucleolar area was larger following FN or SFN treatment (Fig. 8F).
Fig. 8.
FN exerts protective effect against peripheral nerve injury induced by OHP in mice. (A) Western blot showed the protein levels of NRF2 and GSTP1 in the DRGs of mice. The mice were intraperitoneally injected with FN (1.0, 10.0 mg/kg) and SFN (10.0 mg/kg) for 24 and 48 h. Protein grayscale analysis was performed using Image J. The data were analyzed using the unpaired Student's t-test (*, compared with the control group). (B–D) At the time points indicated, body weights, paw withdrawal thresholds to mechanical stimulation (von Frey filament), and escape behaviors in response to cold stimulation (5 °C; cold-plate) were evaluated. (E) DRG sections of each mouse were subjected to HE and Nissl staining (scale bar = 20 μm) and (F) the nuclear areas of the DRG neurons were measured. DRG sections were labeled with anti-NRF2 and anti-GSTP1 (scale bar = 20 μm). Data are expressed as the means ± SD (Data in A, n = 3; B–E, n = 7; F, n = 100). The data were analyzed using two-way ANOVA followed by Tukey's post-hoc test. *P < 0.05, **P < 0.01 compared to the Control group; #P < 0.05, ##P < 0.01 compared to the OHP group.
4. Discussion
Management of CIPN has proven challenging. Opioids have been, and continue to be, prescribed despite a lack of evidence supporting the practice. The National Cancer Institute has sponsored 15 CIPN-directed clinical trials that studied its prevention (e.g., using alpha-lipoic acid, intravenous calcium/magnesium, vitamin E, acetyl-l-carnitine, or glutathione) and symptomatic treatment (e.g., via nortriptyline, gabapentin, lamotrigine, amifostine, topical amitriptyline/ketamine, topical baclofen,/amitriptyline/ketamine, or duloxetine) [36]. Among these studies, only duloxetine was shown to relieve neuropathic pain in established CIPN [37]. In addition, numerous other medications (e.g., gabapentin or topical preparations) are used in an off-label fashion. Although novel electrostimulation techniques have shown early promise [38], the benefits need to be confirmed in ongoing larger randomized and controlled trials. Moreover, with the advent of newer and more targeted chemotherapies, there was hope that CIPN would wane as a significant clinical problem. However, many of the older agents associated with CIPN continue to be mainstays of cancer therapy. Furthermore, many novel agents also exhibit CIPN as a dose-limiting side-effect, whether directly or as a secondary toxicity consequent to immune-mediated processes. Thus, the delayed effects of CIPN continue to produce a significant burden of suffering for cancer survivors with disease prevalence likely to increase with improved cancer treatments and longer survival in the absence of a treatment breakthrough.
Given the pathological role of oxidative/nitrosative stress-mediated mitochondrial dysfunction in peripheral neuropathy, pharmacological agents that may maintain mitochondrial homeostasis have attracted considerable interest [9]. Previous reports have shown that markers of oxidative/nitrosative stress including increased lipid peroxidation, nitrite levels, oxidatively damaged proteins, and DNA are increased in the systemic circulation, peripheral nerves, and spinal cord of OHP-induced neuropathic rats [39,40]. Oxidative stress-mediated abnormalities in mitochondrial structure and function in peripheral nerve fibers and DRGs have been postulated as a key underpinning mechanism and appear to be correlated directly to pain behavior [41]. However, although the therapeutic benefits of antioxidants and agents that improve mitochondrial function for the treatment/prevention of OIPN have been shown in multiple preclinical and clinical studies, none of the agents have yet been approved for treatment [42]. Hence, it is worth exploring the mechanisms underlying the disruption of mitochondrial homeostasis to enable the development of novel therapeutic agents that may treat OHP-evoked neuropathic pain.
Increasing evidence suggests that NRF2, which plays a crucial role in the maintenance of mitochondrial homeostasis, and its downstream phase II mediators are promising targets for the treatment of neurological diseases owing to their powerful ability to detoxify harmful compounds, combat ROS, and alleviate mitochondrial dysfunction [43]. GSTP1 is an important phase II metabolism enzyme downstream of NRF2 that is also mainly responsible for the drug metabolism of a wide range of chemotherapeutic agents. GSTP1 constitutes an important isozyme of the GST enzyme family, which is primarily known for the ability to catalyze the conjugation of the reduced form of glutathione to xenobiotic substrates (e.g., OHP) for the purpose of detoxification [44,45]. Furthermore, genetic variation in GSTP1 is associated with OIPN in colorectal cancer, and, therefore, serves as a predictive marker [46]. Thus, as GSTP1 is a critical enzyme for detoxifying OHP in patients with OIPN, pharmacological or therapeutic activation of GSTP1 may serve as a strategy to prevent or attenuate OIPN progression.
Our previous work revealed that Nrf2−/− mice display severe mechanical allodynia and cold sensitivity, and thus experience more peripheral nervous system injury than Nrf2+/+ mice. Furthermore, Nrf2 knockout aggravated OHP-induced ROS production, decreased the mitochondrial membrane potential, led to abnormal intracellular calcium levels, and induced cytochrome c-related apoptosis and overexpression of members of the TRP family. Conversely, NRF2 overexpression via lentivirus or SFN administration in DRG neurons attenuated OHP-induced mitochondrial damage and apoptosis [13]. Thus, elucidation of the NRF2-mediated defense system as a protective mechanism against OIPN will likely facilitate the development of novel therapeutic interventions targeting NRF2 to also prevent or attenuate OIPN progression.
In the present study, FN exerted its neuroprotective effect through NRF2 activation. Conversely, evidence has shown that KEAP1/NRF2 mutation or unbalanced regulation leads to NRF2 overexpression or hyperactivation, which may contribute to tumorigenesis and chemoresistance [47]. In addition, the NRF2 activator SFN increases the chemotherapeutic effect of OHP on mouse and human colorectal cancer cell lines [13]. Moreover, NRF2 activation can inhibit cell growth or induce apoptosis in cancer lines [48]. Herein, we determined that the activation of NRF2 by FN in colorectal cancer cell lines did not influence the drug metabolism enzymes (e.g., GSTP1, ABCC2), and further did not reduce the chemotherapeutic efficacy of OHP. The observed difference in NRF2-dependent gene activation induced by FN between colon cancer cell lines and DRG neurons may be related to the associated oxidative stress level. Colon cancer cell lines possess a high ROS level and a correspondingly high level of antioxidant enzymes [49], whereas the oxidative stress and antioxidant levels are relative low in DRG neurons [7]. Thus NRF2-inducers (formononetin) may induce high levels of NRF2-dependent genes in DRG neurons but not in cancer cell lines. We also analyzed the effect on NRF2-dependent genes in mouse liver and small intestine, and found that FN slightly induced GSH-related genes, such as Gstp1, compared to other antioxidant enzymes (Fig. S9). This result may be related to the high expression level of GSH-related genes in these organs [50,51]. However, this mild activation might still influence the antitumor activity of oxaliplatin in vivo, and further study is needed to evaluate the drug interval of FN and oxaliplatin which might reduce the impact of FN on oxaliplatin in vivo antitumor activity.
Chemical inducers of NRF2 that block KEAP1 function have proven an effective mechanism to exploit the antioxidant response in the fight against human disease [52,53]. In the present study, comparison of three in silico molecular docking studies suggested that FN specifically binds to His129 and Lys131 in the BTB domain of KEAP1, potentially interfering with the binding of NRF2 and leading to its activation. The BTB domain mediates the homodimerization of KEAP1 and additionally contributes to its interaction with CUL3, which mediates NRF2 degradation [53]. Cysteine residues in the human KEAP1 protein are particularly important for the physiological function of KEAP1 [54]. The covalent interaction between NRF2 activators and Cys151 has recently been validated by a crystal structure of the KEAP1 BTB domain in complex with CDDO [55]. However unlike Chalcones and other Michael acceptors that could activate the KEAP1/NRF2 pathway through covalent bonds with the cysteines of KEAP1 [56,57], FN may not be able to form a covalent bond with the Cys151 of KEAP1 as the oxygen atoms and the conjugate system may weaken its electrophilicity, although KEAP1 C151 is required for NRF2 activation, interactions with other KEAP1 residues are critical for the stereospecific recognition and potency of these ligands [54]. Interestingly, we observed that specific KEAP1 mutated proteins (R380A, N382A and R415A, F478A) exhibited increased pull-down efficiency in the IP experiment, leading us to hypothesize that these KEAP1 mutations may induce structural changes in the protein and result in increased affinity between FN and KEAP1. Overall, our data highlights a new binding mechanism between KEAP1 and isoflavones for activation of the NRF2 system.
Taken together, our findings provide novel insights into the mechanisms underlying OHP-induced neurotoxicity and reveal that FN has beneficial roles in preventing OHP-induced mitochondrial dysfunction and apoptosis by enhancing cell survival through the NRF2-GSTP1 axis. Moreover, these data demonstrate that screening therapeutic drugs for their effects on the NRF2-GSTP1 signaling pathway might provide a new method for identifying OIPN therapeutics. Finally, our experimental findings provide a robust foundation for clinical studies exploring the role of FN in OHP-induced neuropathy.
Authors’ contributions
YY: Procurement of funding, conception and design, acquisition of data, analysis and interpretation of data, writing of the manuscript. YF, JY, BZ: Conception and design, interpretation of data, revision of the manuscript. JS, XC, LR: Analysis and interpretation of data. NG, XC, JC, WZ: Acquisition of data, analysis and interpretation of data, proof-reading of the manuscript. PC: Procurement of funding, conception and design, study supervision, revision of the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China [grant numbers 81973498, 81602733, 81774283]; Project of the Major National Science and Technology Program of China for Innovative Drug [grant number 2019ZX09301-145]; Key R&D Program of Jiangsu Province [grant number BE2018756]; and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Integration of Chinese and Western Medicine) grant. Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX19_0373).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101677.
Contributor Information
Yang Yang, Email: young1570@126.com.
Peng Cao, Email: cao_peng@njucm.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kim J.H., Dougherty P.M., Abdi S. Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol. Oncol. 2015;136:453–459. doi: 10.1016/j.ygyno.2015.01.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Canc. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 3.Yehia R., Saleh S., El Abhar H., Saad A.S., Schaalan M. L-Carnosine protects against Oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a perspective on targeting Nrf-2 and NF-kappaB pathways. Toxicol. Appl. Pharmacol. 2019;365:41–50. doi: 10.1016/j.taap.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Muthuraman A., Singh N., Jaggi A.S., Ramesh M. Drug therapy of neuropathic pain: current developments and future perspectives. Curr. Drug Targets. 2014;15:210–253. [PubMed] [Google Scholar]
- 5.Jamieson S.M., Liu J., Connor B., McKeage M.J. Oxaliplatin causes selective atrophy of a subpopulation of dorsal root ganglion neurons without inducing cell loss. Canc. Chemother. Pharmacol. 2005;56:391–399. doi: 10.1007/s00280-004-0953-4. [DOI] [PubMed] [Google Scholar]
- 6.Pachman D.R., Barton D.L., Watson J.C., Loprinzi C.L. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin. Pharmacol. Ther. 2011;90:377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 7.Areti A., Yerra V.G., Naidu V., Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox biology. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett G.J., Doyle T., Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 2014;10:326–336. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Areti A., Yerra V.G., Komirishetty P., Kumar A. Potential therapeutic benefits of maintaining mitochondrial Health in peripheral neuropathies. Curr. Neuropharmacol. 2016;14:593–609. doi: 10.2174/1570159X14666151126215358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaggi A.S., Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Schloss J.M., Colosimo M., Airey C., Masci P.P., Linnane A.W., Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review. Clin. Nutr. 2013;32:888–893. doi: 10.1016/j.clnu.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.T., Chung Y.H., Lee H.S., Chung S.J., Lee J.H., Sohn U.D., Shin Y.K., Park E.S., Kim H.C., Bang J.S., Jeong J.H. Protective effects of phosphatidylcholine on oxaliplatin-induced neuropathy in rats. Life Sci. 2015;130:81–87. doi: 10.1016/j.lfs.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y., Luo L., Cai X., Fang Y., Wang J., Chen G., Yang J., Zhou Q., Sun X., Cheng X., Yan H., Lu W., Hu C., Cao P. Nrf2 inhibits oxaliplatin-induced peripheral neuropathy via protection of mitochondrial function. Free Radic. Biol. Med. 2018;120:13–24. doi: 10.1016/j.freeradbiomed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 15.Negi G., Kumar A., Sharma S.S. Nrf2 and NF-kappaB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovascular Res. 2011;8:294–304. doi: 10.2174/156720211798120972. [DOI] [PubMed] [Google Scholar]
- 16.Li M., Li Q., Zhao Q., Zhang J., Lin J. Luteolin improves the impaired nerve functions in diabetic neuropathy: behavioral and biochemical evidences. Int. J. Clin. Exp. Pathol. 2015;8:10112–10120. [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., Liang X.C., Zhang H., Wu Q.L., Qu L., Sun Q. Quercetin protects rat dorsal root ganglion neurons against high glucose-induced injury in vitro through Nrf-2/HO-1 activation and NF-kappaB inhibition. Acta Pharmacol. Sin. 2013;34:1140–1148. doi: 10.1038/aps.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the hallmarks of cancer. Canc. Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong S.K.L., Shanmugam M.K., Fan L., Fraser S.E., Arfuso F., Ahn K.S., Sethi G., Bishayee A. Focus on formononetin: anticancer potential and molecular targets. Cancers. 2019;11 doi: 10.3390/cancers11050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou R., Xu L., Ye M., Liao M., Du H., Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm. Metab. Res. 2014;46:753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y., Zhang X., Li Z., Yan H., Qin J., Li T. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food Funct. 2017;8:1061–1066. doi: 10.1039/c6fo01535b. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Zhao Y., Ai X., Cheng B., Lu S. Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int. J. Clin. Exp. Pathol. 2014;7:8453–8461. [PMC free article] [PubMed] [Google Scholar]
- 23.Auyeung K.K., Law P.C., Ko J.K. Novel anti-angiogenic effects of formononetin in human colon cancer cells and tumor xenograft. Oncol. Rep. 2012;28:2188–2194. doi: 10.3892/or.2012.2056. [DOI] [PubMed] [Google Scholar]
- 24.Huang D., Wang C., Duan Y., Meng Q., Liu Z., Huo X., Sun H., Ma X., Liu K. Targeting Oct2 and P53: formononetin prevents cisplatin-induced acute kidney injury. Toxicol. Appl. Pharmacol. 2017;326:15–24. doi: 10.1016/j.taap.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Lee H., Lee D., Kang K.S., Song J.H., Choi Y.K. Inhibition of intracellular ROS accumulation by formononetin attenuates cisplatin-mediated apoptosis in LLC-PK1 cells. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Cesare Mannelli L., Zanardelli M., Bartolucci G., Karioti A., Bilia A.R., Vannacci A., Mugelli A., Ghelardini C. In vitro evidence for the use of Astragali radix extracts as adjuvant against oxaliplatin-induced neurotoxicity. Planta Med. 2015;81:1045–1055. doi: 10.1055/s-0035-1546117. [DOI] [PubMed] [Google Scholar]
- 27.Li Z., Dong X., Zhang J., Zeng G., Zhao H., Liu Y., Qiu R., Mo L., Ye Y. Formononetin protects TBI rats against neurological lesions and the underlying mechanism. J. Neurol. Sci. 2014;338:112–117. doi: 10.1016/j.jns.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Jin F., Wan C., Li W., Yao L., Zhao H., Zou Y., Peng D., Huang W. Formononetin protects against acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. PloS One. 2017;12 doi: 10.1371/journal.pone.0170900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y., Chen G., Cheng X., Teng Z., Cai X., Yang J., Sun X., Lu W., Wang X., Yao Y., Hu C., Cao P. Therapeutic potential of digitoflavone on diabetic nephropathy: nuclear factor erythroid 2-related factor 2-dependent anti-oxidant and anti-inflammatory effect. Sci. Rep. 2015;5:12377. doi: 10.1038/srep12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X., Huo J., Wang D., Cai X., Sun X., Lu W., Yang Y., Hu C., Wang X., Cao P. Herbal medicine AC591 prevents oxaliplatin-induced peripheral neuropathy in animal model and cancer patients. Front. Pharmacol. 2017;8:344. doi: 10.3389/fphar.2017.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Cesare Mannelli L., Pacini A., Micheli L., Femia A.P., Maresca M., Zanardelli M., Vannacci A., Gallo E., Bilia A.R., Caderni G., Firenzuoli F., Mugelli A., Ghelardini C. Astragali radix: could it be an adjuvant for oxaliplatin-induced neuropathy? Sci. Rep. 2017;7:42021. doi: 10.1038/srep42021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Boutten A., Goven D., Boczkowski J., Bonay M. Oxidative stress targets in pulmonary emphysema: focus on the Nrf2 pathway. Expert Opin. Ther. Targets. 2010;14:329–346. doi: 10.1517/14728221003629750. [DOI] [PubMed] [Google Scholar]
- 33.Sleigh J.N., Weir G.A., Schiavo G. A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Res. Notes. 2016;9:82. doi: 10.1186/s13104-016-1915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng Zhao K.I., Nakamura Saki, nakagawa TakayuK1, Kaneko Shuji. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol. Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H., Ren N.T., Zhou F.Q., Li J., Lei W., Liu N., Bi L., Wu Z.X., Zhang R., Zhang Y.G., Cui G. Effects of hindlimb unweighting on MBP and GDNF expression and morphology in rat dorsal root ganglia neurons. Neurochem. Res. 2016;41:2433–2442. doi: 10.1007/s11064-016-1956-3. [DOI] [PubMed] [Google Scholar]
- 36.Majithia N., Temkin S.M., Ruddy K.J., Beutler A.S., Hershman D.L., Loprinzi C.L. National Cancer Institute-supported chemotherapy-induced peripheral neuropathy trials: outcomes and lessons. Support. Care Canc. 2016;24:1439–1447. doi: 10.1007/s00520-015-3063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith E.M., Pang H., Cirrincione C., Fleishman S., Paskett E.D., Ahles T., Bressler L.R., Fadul C.E., Knox C., Le-Lindqwister N., Gilman P.B., Shapiro C.L., Alliance for Clinical Trials in O. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majithia N., Smith T.J., Coyne P.J., Abdi S., Pachman D.R., Lachance D., Shelerud R., Cheville A., Basford J.R., Farley D., O'Neill C., Ruddy K.J., Sparadeo F., Beutler A., Loprinzi C.L. Scrambler Therapy for the management of chronic pain. Support. Care Canc. 2016;24:2807–2814. doi: 10.1007/s00520-016-3177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McQuade R.M., Carbone S.E., Stojanovska V., Rahman A., Gwynne R.M., Robinson A.M., Goodman C.A., Bornstein J.C., Nurgali K. Role of oxidative stress in oxaliplatin-induced enteric neuropathy and colonic dysmotility in mice. Br. J. Pharmacol. 2016;173:3502–3521. doi: 10.1111/bph.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., O W., Li W., Jiang Z.G., Ghanbari H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y., Smith M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN) Front. Pharmacol. 2013;4:156. doi: 10.3389/fphar.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur. J. Canc. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M., An C., Gao Y., Leak R.K., Chen J., Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong X., Yang Y., Zhou Y., Bi X., Zhao N., Zhang Z., Li L., Hang Q., Zhang R., Chen D., Cao P., Yin Z., Luo L. Glutathione S-transferases P1 protects breast cancer cell from adriamycin-induced cell death through promoting autophagy. Cell Death Differ. 2019;26:2086–2099. doi: 10.1038/s41418-019-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tew K.D. Glutathione-associated enzymes in anticancer drug resistance. Canc. Res. 2016;76:7–9. doi: 10.1158/0008-5472.CAN-15-3143. [DOI] [PubMed] [Google Scholar]
- 46.Zajaczkowska R., Kocot-Kepska M., Leppert W., Wrzosek A., Mika J., Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20061451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganan-Gomez I., Wei Y., Yang H., Boyano-Adanez M.C., Garcia-Manero G. Oncogenic functions of the transcription factor Nrf2. Free Radic. Biol. Med. 2013;65:750–764. doi: 10.1016/j.freeradbiomed.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 48.Chen D., Dai F., Chen Z., Wang S., Cheng X., Sheng Q., Lin J., Chen W. Dimethoxy curcumin induces apoptosis by suppressing survivin and inhibits invasion by enhancing E-cadherin in colon cancer cells. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2016;22:3215–3222. doi: 10.12659/MSM.900802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chio I.I.C., Tuveson D.A. ROS in cancer: the burning question. Trends Mol. Med. 2017;23:411–429. doi: 10.1016/j.molmed.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaminsky L.S., Zhang Q.Y. The small intestine as a xenobiotic-metabolizing organ. Drug Metabol. Dispos.: the biological fate of chemicals. 2003;31:1520–1525. doi: 10.1124/dmd.31.12.1520. [DOI] [PubMed] [Google Scholar]
- 52.Huerta C., Jiang X., Trevino I., Bender C.F., Ferguson D.A., Probst B., Swinger K.K., Stoll V.S., Thomas P.J., Dulubova I., Visnick M., Wigley W.C. Characterization of novel small-molecule NRF2 activators: structural and biochemical validation of stereospecific KEAP1 binding. Biochim. Biophys. Acta. 2016;1860:2537–2552. doi: 10.1016/j.bbagen.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 53.Canning P., Sorrell F.J., Bullock A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015;88:101–107. doi: 10.1016/j.freeradbiomed.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto T., Suzuki T., Kobayashi A., Wakabayashi J., Maher J., Motohashi H., Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cleasby A., Yon J., Day P.J., Richardson C., Tickle I.J., Williams P.A., Callahan J.F., Carr R., Concha N., Kerns J.K., Qi H., Sweitzer T., Ward P., Davies T.G. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PloS One. 2014;9 doi: 10.1371/journal.pone.0098896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhuang C., Zhang W., Sheng C., Zhang W., Xing C., Miao Z. Chalcone: a privileged structure in medicinal chemistry. Chem. Rev. 2017;117:7762–7810. doi: 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Freitas Silva M., Pruccoli L., Morroni F., Sita G., Seghetti F., Viegas C., Tarozzi A. The keap1/Nrf2-ARE pathway as a pharmacological target for Chalcones. Molecules. 2018;23 doi: 10.3390/molecules23071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information File or from the corresponding authors upon request.