Abstract
Low childhood socioeconomic status (SES) predisposes individuals to altered trajectories of brain development and increased rates of mental illness. Brain connectivity at birth is associated with psychiatric outcomes. We sought to investigate whether SES at birth is associated with neonatal brain connectivity and if these differences account for socioeconomic disparities in infant symptoms at age 2 years that are predictive of psychopathology. Resting state functional MRI was performed on 75 full-term and 37 term-equivalent preterm newborns (n = 112). SES was characterized by insurance type, the Area Deprivation Index, and a composite score. Seed-based voxelwise linear regression related SES to whole-brain functional connectivity of five brain regions representing functional networks implicated in psychiatric illnesses and affected by socioeconomic disadvantage: striatum, medial prefrontal cortex (mPFC), ventrolateral prefrontal cortex (vlPFC), and dorsal anterior cingulate cortex. Lower SES was associated with differences in striatum and vlPFC connectivity. Striatum connectivity with frontopolar and medial PFC mediated the relationship between SES and behavioral inhibition at age 2 measured by the Infant-Toddler Social Emotional Assessment (n = 46). Striatum-frontopolar connectivity mediated the relationship between SES and externalizing symptoms. These results, convergent across three SES metrics, suggest that neurodevelopmental trajectories linking SES and mental illness may begin as early as birth.
Keywords: Neonatal neuroimaging, Functional connectivity, Socioeconomic status, Externalizing, Striatum, Prefrontal cortex
1. Introduction
The effects of socioeconomic status (SES) on mental health and cognitive development are numerous and profound (Yoshikawa et al., 2012; McLaughlin et al., 2011). Low SES during childhood and its associated stressors have been linked to the development and persistence of both externalizing disorders, such as attention-deficit hyperactivity disorder (ADHD), and internalizing disorders, such as depression (Najman et al., 2010). The impact of SES on mental health during childhood may be a consequence of altered brain development, as low SES during childhood has been associated with reduced gray matter volume, reduced cortical surface area, slower brain growth (Blair and Raver, 2016), and altered functional network development (Tooley et al., 2019; Gao et al., 2015). While these prior results underscore the impact of SES on brain development in early childhood and later life, accumulating evidence indicates that the brain changes associated with developing many psychiatric illnesses are already present near birth (Graham et al., 2019, 2018; Rogers et al., 2017; Sylvester et al., 2018). Yet, very little is known about the impact of SES on brain development at the time of birth and whether any neonatal SES-related brain changes are associated with subsequent risk for development of psychiatric symptoms. Such effects would have major public health implications and may inform how best to reduce risk for mental illness associated with low SES.
Childhood socioeconomic disadvantage predisposes individuals to the development and persistence of many psychiatric disorders including anxiety, depression, and attention-deficit hyperactivity disorder (ADHD) (Yoshikawa et al., 2012; McLaughlin et al., 2011; Najman et al., 2010). Some studies have shown that the alleviation of socioeconomic disadvantage reduces psychiatric symptoms, suggesting a causative pathway (Costello et al., 2003). Specific factors intimately tied to low socioeconomic status such as parental stress, food insecurity, and overall family adversity are also associated with psychiatric symptoms and may explain the mechanisms by which SES impacts risk (Ackerman et al., 2001; Slopen et al., 2010).
Converging lines of evidence suggest that lower SES is associated with altered functional brain development that results in psychiatric disorders. First, independent studies indicate that the brain systems most impacted by low SES are the same brain systems that are altered in psychiatric disorders. For example, low SES (Tooley et al., 2019; Gao et al., 2015; Gianaros et al., 2011; Marshall et al., 2018; Sripada et al., 2014) and psychiatric symptoms (Cortese et al., 2012; Padmanabhan and Luna, 2014; Holz et al., 2017; Tomasi and Volkow, 2012; Castellanos et al., 2008; Fair et al., 2010) have both independently been linked to alterations in functional connectivity of the striatum and regions of the default mode (DMN), cingulo-opercular (CON), and ventral attention networks (VAN). These regions include the medial prefrontal cortex (mPFC) from the DMN, the dorsal anterior cingulate cortex (dACC) from the CON, and the ventrolateral prefrontal cortex (vlPFC) from the VAN. In addition, studies in children and adolescents have specifically demonstrated in longitudinal designs that functional connectivity mediates the association between low SES and subsequent psychopathology (Fareri et al., 2017; Barch et al., 2016), though further longitudinal work is required to understand the full developmental trajectory of these links.
Recent work indicates that many of the brain changes associated with risk for developing a psychiatric disorder in later life are already present at birth. Resting state functional connectivity (Rogers et al., 2017; Sylvester et al., 2018) and diffusion tensor imaging (Rogers et al., 2012) during the neonatal period have been linked to psychiatric symptoms in later childhood. These brain changes present at birth likely represent a combination of genetic risk for psychiatric illnesses as well as consequences of prenatal maternal environment and stress. For example, maternal immune activation and glucocorticoids during pregnancy have been associated with alterations in amygdala and salience network functional connectivity and subsequent behavioral outcomes (Graham et al., 2019, 2018; Spann et al., 2018). Additionally, an extensive animal literature demonstrates that prenatal stress leads to alterations in offspring brain development and behavior (Harris and Seckl, 2011).
A fundamental outstanding issue is the extent to which SES has already impacted the human brain by the time of birth, and whether any SES-related brain changes are associated with risk for subsequent psychiatric symptoms. Such an association would indicate that preventative efforts should encompass the period before birth and would also elucidate the developmental neurobiology underlying mental illness more generally. One recent study has linked prenatal SES with neonatal cortical volumes; (Spann et al., 2020) however, whether neonatal resting state functional connectivity varies with SES remains unknown. To address this issue, we longitudinally examined associations between socioeconomic status at birth, brain connectivity at birth, and psychiatric symptoms at age 2 years. We focused on the whole-brain functional connectivity of the left and right striatum and the medial prefrontal (mPFC), the dorsal anterior cingulate (dACC), and the ventrolateral prefrontal cortices (vLPFC), because of the extensive prior work cited above that links these specific regions and their associated brain networks to variation in SES and psychiatric symptoms (Tooley et al., 2019; Gao et al., 2015; Sylvester et al., 2018; Gianaros et al., 2011; Marshall et al., 2018; Sripada et al., 2014; Cortese et al., 2012; Padmanabhan and Luna, 2014; Holz et al., 2017; Tomasi and Volkow, 2012; Castellanos et al., 2008; Fair et al., 2010; Sylvester et al., 2013, 2012). We addressed two primary questions: (1) how does neonatal resting state functional connectivity relate to socioeconomic disadvantage and (2) do SES-related differences in neonatal connectivity mediate the relationship between lower SES and psychiatric symptoms at age two years? Results underscore several neurodevelopmental pathways by which exposure to socioeconomic disadvantage results in psychiatric symptoms.
2. Materials and methods
2.1. Participants
Participants were recruited as part of a large longitudinal study of the impact of preterm birth on development (Rogers et al., 2017; Sylvester et al., 2018). Preterm infants (PT; N = 106; gestational age <30 weeks) were recruited from St. Louis Children’s Hospital Neonatal Intensive Care Unit. Full term infants (FT; N = 94; GA > 36 weeks) were recruited from Barnes-Jewish Hospital. Parents provided written informed consent and the study was approved by the Washington University Human Studies Committee. FT infants were scanned within 4 days of birth (mean postmenstrual age: 39.3 weeks, SD = 1.2) and PT infants were scanned at term-equivalent (mean postmenstrual age: 37.5 weeks, SD = 1.4).
Exclusion criteria are detailed in Supplemental Methods. Of the 200 total subjects included in any analysis, 112 had neonatal MRI of sufficient quality, 134 subjects had follow-up behavioral data at age 2 years, and 46 had both high-quality neonatal MRI data and follow-up behavioral data at age 2 years.
2.2. MRI data acquisition
Scanning occurred during natural sleep or at rest without the use of sedating medications using well-established guidelines (Mathur et al., 2008). Imaging was performed on a Siemens Trio 3 T scanner (Erlangen, Germany) using an infant-specific, quadrature head coil (Advanced Imaging Research, Cleveland, OH). Structural images were collected using a T2-weighted sequence (TR 8600 ms; TE 161 ms; voxel size 1 × 1 × 1 mm). Resting state fMRI data were collected using a gradient echo, echo-planar-image (EPI) sequence sensitized to T2* blood oxygen level dependent (BOLD) contrast (TR 2910 ms; TE 28 ms; voxel size 2.4 × 2.4 × 2.4 mm; flip angle 90°; FOV 151 mm; and matrix size was of 64 × 64). Each fMRI run included 200 volumes (frames). A minimum of one run (9.6 min) was obtained in each infant, with additional runs acquired in a subset of participants depending upon tolerance.
2.3. Socioeconomic status at birth
Sociodemographic data were collected at birth through parent survey, and socioeconomic status was measured in three ways. Our primary index for SES was insurance type; individuals with public health insurance were considered lower SES and individuals with private health insurance were considered higher SES. Insurance type was used for main analyses because it is associated with other key SES variables in our data (Table S1), it is directly determined by household income poverty guidelines (https://dss.mo.gov/fsd/povlev.htm), and is easily replicable. Insurance type was transformed into a binary variable with values of -1 (private; higher SES) or 1 (public; lower SES). Although we did not have family income or income-to-needs ratio for our participants in the perinatal period because it was not collected until age 5 years, public insurance eligibility is directly determined by family economic disadvantage, is a common measure of socioeconomic status (Marcin et al., 2003; Kachmar et al., 2019), and has been linked with perinatal health disparities (Bengiamin et al., 2010; Jarlenski et al., 2014; Oberg et al., 1990). To confirm that we were precisely measuring socioeconomic status, we used two other measures: neighborhood disadvantage and a principal component analysis-based social risk. Neighborhood level disadvantage was measured using the Area Deprivation Index (ADI) (Kind and Buckingham, 2018; Singh, 2003; Lantos et al., 2018; Kind et al., 2014). This metric calculates census block group-level disadvantage using characteristics such as average home value, educational attainment, poverty prevalence, and household crowding and is reported as a national percentile. As such, the ADI encapsulates several domains of socioeconomic status, and has been linked with a variety of health disparities (Kind and Buckingham, 2018; Singh, 2003; Lantos et al., 2018; Kind et al., 2014). A higher percentile corresponds to greater disadvantage. Principal component analysis (PCA) was employed to construct a composite social risk that weighs each constituent variable appropriately, a method widely used to calculate SES (Noble et al., 2006; Vyas and Kumaranayake, 2006). PCA was performed on five SES variables used in our previous studies: insurance type, maternal race, maternal age, single-parent status, and maternal education (Rogers et al., 2017; Sylvester et al., 2018; Lean et al., 2018). Individual scores on the first component (which explained 43.5 % of variance) were used as a PCA-based social risk score, with higher scores corresponding to greater disadvantage (R Studio version 1.0.143; R version 3.4.0; Fig. S1) (Noble et al., 2006).
2.4. Follow-up at age 2 years
A subsample of the original neonatal neuroimaging cohort was recruited at age two years to collect behavioral follow-up. As previously described (Rogers et al., 2017), this subsample was specifically designed to have equal numbers of term and preterm, as well as lower and higher SES toddlers; as a result, the 2-year follow up subsample had different characteristics than the neonatal imaging cohort (described in Section 3.1). Child symptoms at age two years were measured by parent report using the Infant-Toddler Social Emotional Assessment (ITSEA) which has four domains: internalizing, externalizing, dysregulation, and competence, which are reported as T-scores with a mean of 50 and standard deviation of 10 (Carter et al., 2003). The ITSEA has good psychometric properties, correlates with metrics based on direct behavioral observation (Carter et al., 2003), and has been used to predict later behavioral outcomes (Treyvaud et al., 2012). Subscales of the ITSEA externalizing domain include activity/impulsivity, aggression/defiance, and peer aggression, while subscales of the internalizing domain include behavioral inhibition, depression/withdrawal, general anxiety, and separation distress, all ranging from 0 to 2. To examine the influence of clinically significant maternal mental illness and the heritable components of psychopathology, maternal affective symptoms were analyzed as a dichotomous variable as previously done (Rogers et al., 2017; Sylvester et al., 2018), based on whether mothers had a history of anxiety or depression gathered by the Family History Assessment Module (Rice et al., 1995) or had moderate/severe anxiety or depression symptoms at follow-up as ascertained by the Hospital Anxiety and Depression Scale (Snaith and Zigmond, 1994) (HADS; anxiety score ≥ 11) and the Beck Depression Index-II (BDI-II; depression score ≥ 20).
2.5. MRI data preprocessing
MRI data preprocessing was performed as previously described (Rogers et al., 2017; Sylvester et al., 2018). Resting state fMRI (rs-fMRI) data were preprocessed using in-house software (https://readthedocs.org/projects/4dfp/). Magnetization inhomogeneity-related distortions were corrected using a mean field map (Gholipour et al., 2008). Atlas transformation was computed using infant templates (Smyser et al., 2010). Volumetric time series in adult Talairach atlas space (3 × 3 × 3 mm voxels) were generated, combining motion correction and atlas transformation in a single resampling step. Affine registration to Talairach atlas space was performed to facilitate comparison with adult neuroimaging literature and has been done successfully in prior studies (Rogers et al., 2017; Sylvester et al., 2018; Smyser et al., 2010). Additional preprocessing included regression of nuisance waveforms derived from rigid body motion correction, cerebrospinal fluid and white matter signal, plus whole brain global signal. The data were low-pass filtered (<0.08 Hz) and spatially smoothed (6 mm FWHM). Frames affected by sudden change in head position (volume-to-volume head displacement ≥.25 mm) were excluded from the rs-fMRI computations (“scrubbing”). A minimum of 5 min of rs-fMRI data, excluding censored frames, was required for inclusion in the analysis.
2.6. Statistical analyses
Relations between SES and both internalizing and externalizing symptoms were investigated using t-tests for the purpose of informing brain-behavior analyses. Because subscales of the ITSEA have distinct developmental trajectories (Carter et al., 2010) and distinct neural correlates (Rogers et al., 2017), we examined both domain T-scores, as well as subscales scores. Relations between variables of interest (SES, internalizing symptoms, externalizing symptoms) and potential confounding variables (preterm birth, sex, maternal affective symptoms) were identified by t-tests, correlation tests, and chi squared tests, all performed in R Studio version 1.0.143 (R version 3.4.0; Table S5).
Whole-brain functional connectivity maps consisting of Fisher z-transformed correlation coefficients were created for each of the following seeds representing regions or networks implicated in externalizing disorders and socioeconomic disadvantage: right and left striatum (±13.5, +12, +3 in Talairach coordinates; 8 mm in diameter), right medial prefrontal cortex (MPFC; +7, +37, 0; 10 mm), right ventrolateral prefrontal cortex (VLPFC; +50, +27, +6; 10 mm), and right dorsal anterior cingulate cortex (DACC; +4, +18, +39; 10 mm) (Gao et al., 2015; Sylvester et al., 2018; Gianaros et al., 2011; Marshall et al., 2018; Sripada et al., 2014; Cortese et al., 2012; Padmanabhan and Luna, 2014; Holz et al., 2017; Tomasi and Volkow, 2012; Castellanos et al., 2008; Fair et al., 2010; Sylvester et al., 2013, 2012). Because left and right regions have high homotopic connectivity, as well as the right lateralized nature of the ventral attention network, right cortical seeds were chosen. Each functional connectivity seed map served as the dependent variable in a regression analysis against socioeconomic status, controlling for preterm birth as a dichotomous variable (n = 112). Preterm birth was treated as a dichotomous variable as opposed to using continuous gestational age because there were no participants with gestational ages between 30 and 35 weeks. Preterm birth was included as a covariate in our analyses given its known association with brain development (Rogers et al., 2017; Smyser et al., 2016; Thompson et al., 2020). These analyses were implemented using in-house software written in IDL (Harris Geospatial, Broomfield, CO) and Matlab (The Mathworks Inc., Natick, MA). This in-house software using IDL has routinely been used at Washington University for decades, including in recent manuscripts that utilize the same linear models we currently implement (Rogers et al., 2017; Sylvester et al., 2018). To address the pervasive issue of high false positive rates in whole-brain fMRI analyses, we employed a stringent threshold for identifying significant clusters according to current recommendations (Cox et al., 2017; Eklund et al., 2016). Following the computation of study-specific autocorrelation parameters using 3dFWHMx from AFNI, simulations were implemented using 3dClustSim within the gray matter mask to calculate family-wise cluster-based error rates based on a voxelwise Z threshold of 3 (p < .0027) (Cox et al., 2017). Accordingly, multiple comparisons correction was performed conservatively with a cluster size of 37 voxels (999 mm3) to attain a whole-brain false positive rate of 0.05. To correct for the five seeds tested, Bonferroni correction was implemented by using an alpha of 0.01 as determined by 3dClustSim (Z > 3, cluster size>61 voxels/1647 mm3). This analysis identified clusters of voxels whose functional connectivity with a seed region was significantly related to insurance type. Functional connectivity values between seed regions and significant clusters were extracted for each subject. Given previous evidence that brain development varies with socioeconomic status as measured by parental education (Farah, 2017), we used maternal high school graduation as the predictor of interest in supplementary analyses, as previously done (Rogers et al., 2017; Sylvester et al., 2018; Lean et al., 2018, 2020).
Mediation analysis was performed using the PROCESS Macro in SPSS (version 25.0.0.0) to determine whether or not SES-related functional connectivity mediated the relationship between SES and symptoms at age 2 years, controlling for preterm birth (Hayes, 2013). The PROCESS Macro tests the mediation effect as the product of two regression coefficients: (a) the effect of the independent variable (SES) on the mediator (functional connectivity) and (b) the effect of the mediator on the outcome (symptoms), controlling for the independent variable. It then tests whether the mediation, or indirect effect (a*b) is different from zero based on its null distribution generated by 10,000 resamples of the data (Hayes and Rockwood, 2017). This analysis reports the meditation effect as a two-sided 95 % confidence interval.
3. Results
3.1. Sample characteristics and symptoms
Sample demographics are detailed in Table 1 and perinatal clinical characteristics are in Table S2. Analyses comparing newborns with and without follow-up data are in Table S3, while analyses comparing the neonatal imaging sample and the follow-up subset are in Table S4. As described above, participants in the follow-up subsample were selectively recruited to have equal numbers of toddlers born full term and preterm, as well as higher and lower SES. Therefore, by design the follow-up sample had a smaller proportion of toddlers born preterm (x2 = 7.5, p = .006) and a smaller proportion with public insurance (x2 = 7.85, p = .005) compared to the full sample. Post-hoc analyses described below verify that reported results were not related to this sampling procedure.
Table 1.
Neonatal neuroimaging sample characteristics.
|
Neonatal imaging sample (n = 112) | |
| Preterm Birth, n (%) | 37 (33) |
| Male, n (%) | 46 (41) |
| Race, n (%) | |
| African-American | 70 (62) |
| Caucasian | 35 (31) |
| Asian | 3 (3) |
| Biracial | 4 (4) |
| Public Insurance, n (%) | 84 (75) |
| Mother Not a High School Graduate, n (%) | 23 (21) |
| Single Parent Household, n (%) | 53 (47) |
| ADI National Percentile, mean (SD) | 71 (25) |
| Framewise displacement after censoring, mm (SD) | 0.12 (0.03) |
|
Subset of imaged neonates with follow-up at age 2 (n = 46) | |
| Preterm Birth, n (%) | 26 (57) |
| Male, n (%) | 21 (46) |
| Race, n (%) | |
| African-American | 22 (8) |
| Caucasian | 20 (43) |
| Asian | 3 (7) |
| Biracial | 1 (2) |
| Public Insurance, n (%) | 24 (52) |
| Mother Not a High School Graduate, n (%) | 5 (11) |
| Single Parent Household, n (%) | 15 (33) |
| ADI National Percentile, mean (SD) | 61 (30) |
| ITSEA | |
| Externalizing T-score, mean (SD) | 54.26 (13.7) |
| Activity/Impulsivity, mean (SD) | 0.89 (0.43) |
| Aggression/Defiance, mean (SD) | 0.52 (0.34) |
| Peer Aggression, mean (SD) | 0.34 (0.50) |
| Internalizing T-score, mean (SD) | 49.85 (11.35) |
| Behavioral Inhibition, mean (SD) | 0.93 (0.58) |
| Depression/Withdrawal, mean (SD) | 0.10 (0.15) |
| General Anxiety, mean (SD) | 0.33 (0.26) |
| Separation Distress, mean (SD) | 0.87 (0.41) |
| Maternal Affective Symptoms | |
| HADS Anxiety ≥ 11, n (%) | 17 (37) |
| BDI Depression ≥ 20, n (%) | 5 (11) |
| Family History of Anxiety/Depression, n (%) | 4 (9) |
ADI = Area Deprivation Index; ITSEA = Infant-Toddler Social Emotional Assessment; HADS= Hospital Anxiety and Depression Scale; BDI = Beck Depression Index.
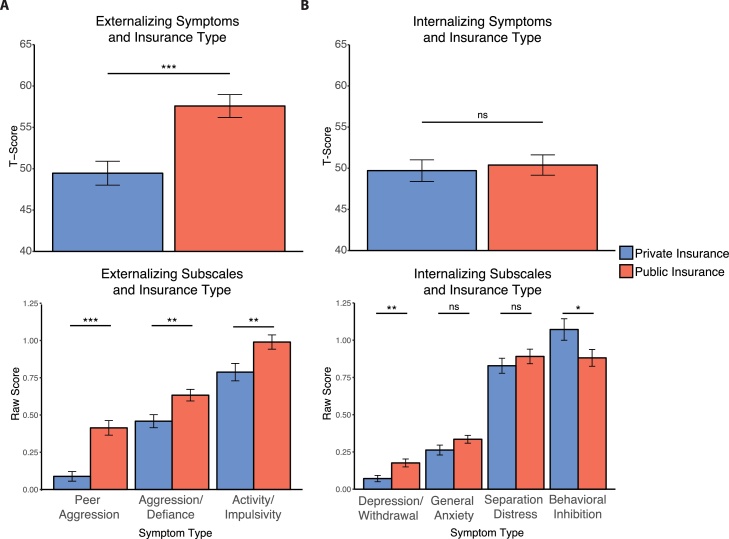
Newborns with public insurance developed increased externalizing symptoms at age 2 years relative to those with private insurance (mean difference, 8.12 points; t(130) = 4.1, p < .001; Fig. 1A; Table 2). There was no group difference in overall internalizing symptoms (mean difference, 0.68 points; t = .38, p = .71; Fig. 1B), though the public insurance group showed increased depression/withdrawal (mean difference, 0.10; t = 3.08, p = .003) and decreased behavioral inhibition (mean difference, 0.19; t=-2.08, p = .04), which did not pass Bonferroni correction. Subsequent analyses focused on overall externalizing score and behavioral inhibition because these symptoms were present at more impairing levels overall. We also continued to analyze these two scales because both contribute to behavioral exuberance, which refers to a child’s tendency to approach novelty and has been shown to predict later externalizing symptoms (Stifter et al., 2008; Degnan et al., 2011). In supplementary analyses, we tested whether sex, preterm birth, or maternal anxiety and depression were associated with symptoms (Table S5), and verified that SES remained a strong predictor when controlling for variables related to symptoms (Table S6).
Fig. 1.
Relations between insurance type at birth and child behaviors at age two years. Two year-olds with public insurance at birth show A) elevated externalizing symptoms and B) decreased behavioral inhibition, and increased depression/withdrawal. *p < .05 **p < .01 ***p < .001.
Table 2.
Child behavior at age two years in toddlers with public versus private insurance at birth.
| ITSEA Scale | Private Insurance Mean | Public Insurance Mean | t(130) | p |
|---|---|---|---|---|
| Externalizing T-Score | 49.45 | 57.58 | 4.07 | <.001* |
| Peer Aggression | 0.09 | 0.41 | 5.54 | <.001* |
| Aggression/Defiance | 0.46 | 0.63 | 2.98 | .004* |
| Activity/Impulsivity | 0.79 | 0.99 | 2.67 | .009 |
| Internalizing T-Score | 49.71 | 50.39 | 0.38 | .71 |
| Depression/Withdrawal | 0.07 | 0.18 | 3.08 | .003* |
| General Anxiety | 0.26 | 0.34 | 1.71 | .09 |
| Separation Anxiety | 0.83 | 0.89 | 0.89 | .38 |
| Behavioral Inhibition | 1.07 | 0.88 | 2.08 | .04 |
ITSEA = Infant-Toddler Social Emotional Assessment.
p < .05 following Bonferroni correction for 9 tests (uncorrected p < .0056).
3.2. Neonatal resting state functional connectivity and SES
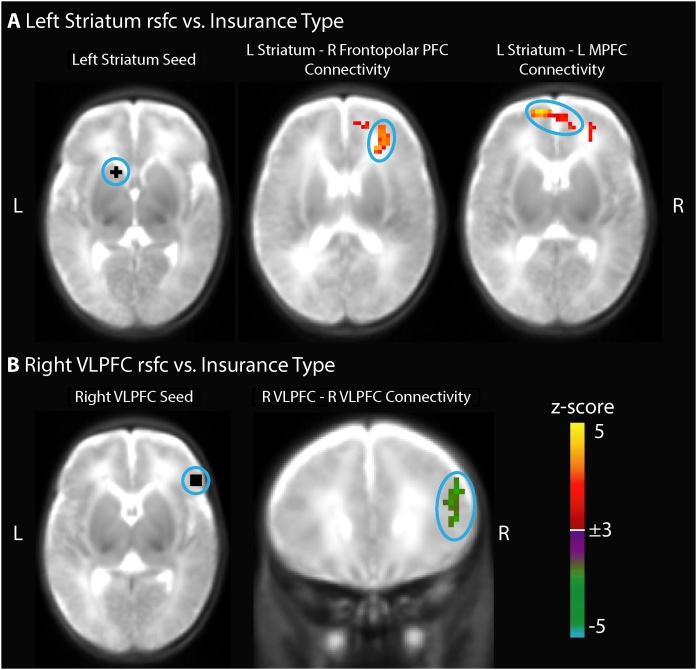
Neonatal functional connectivity between the left striatum seed and two clusters in the prefrontal cortex was more positive in newborns with public health insurance relative to newborns with private insurance, controlling for preterm birth (Fig. 2A). One cluster passed Bonferroni correction (p < .01) and was located in the right frontopolar prefrontal cortex (peak z-score = 3.8, cluster size = 2079 mm3, with all voxels |z|>3.0, centered at [+27 + 35 + 19] in Talairach coordinates); another cluster that did not pass Bonferroni correction (uncorrected p < .05) was located in the left anterior medial prefrontal cortex (peak z-score = 4.3, cluster size = 1593 mm3, centered at [-2 + 49 + 12]). In consonance, left striatal functional connectivity with the right frontopolar prefrontal cortex was also more positive in newborns residing in more disadvantaged neighborhoods (Fig. S2). Functional connectivity between the right ventrolateral prefrontal cortex (VLPFC) seed and a nearby region in the right VLPFC was decreased in newborns with public health insurance, controlling for preterm birth, though this cluster did not pass Bonferroni correction (uncorrected p < .05, peak z-score= -3.4, cluster size = 1161 mm3, centered at [+46 + 23 + 21]; Fig. 2B). All of these connections were also associated with both Area Deprivation and PCA-based measures of SES (Table S7). Supplementary post-hoc analyses stratified by preterm birth confirmed that associations between SES at birth and functional connectivity were present in both preterm and full term newborns (Table S8). Additional analyses verified that these results were not due to motion (Tables S9 and S10), postmenstrual age at scan (Table S10), or sex (Table S10). Given associations between maternal mental health and child externalizing symptoms both in our sample (Table S5) and previously documented (Madigan et al., 2018), we confirmed that our findings linking SES and neonatal functional connectivity remained significant when controlling for maternal affective symptoms (Table S11). Finally, we confirmed that inclusion of preterm neonates with low-grade intraventricular hemorrhage and punctate white matter injury did not drive our results (Table S12). Insurance type was not associated with functional connectivity of the right striatum, right medial prefrontal cortex, or right dorsal anterior cingulate cortex seeds. Results using maternal educational attainment as the independent variable are presented in Supplemental Results.
Fig. 2.
Neonatal resting state functional connectivity (rsfc) is related to insurance type at birth, controlling for preterm birth (n = 112). Newborns with public insurance have A) increased left striatum functional connectivity with right frontopolar prefrontal cortex and left medial prefrontal cortex as well as B) decreased local right ventrolateral prefrontal cortex (VLPFC) functional connectivity. Whole brain false positive rate of 0.05 was achieved with a cluster size of 37 voxels (999 mm3) and |Z|>3.
3.3. Functional connectivity links SES and psychiatric symptoms at age 2
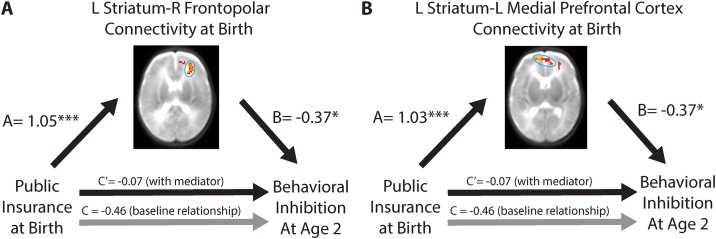
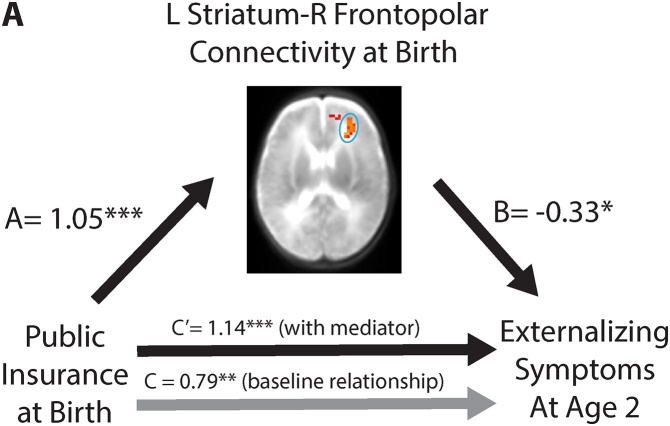
First, we verified that associations between SES and neonatal resting-state functional connectivity found in our neonatal sample (n = 112) were also present our follow-up subsample despite differences in sampling (n = 46; left striatum – right frontopolar PFC beta = 1.05, p < .001; left striatum – left medial PFC beta = 1.03, p < .001; R VLPFC – R VLPFC beta=-1.08, p < .001). Next, we tested whether any of the three SES-related functional connections mediated the relation between lower SES at birth and decreased behavioral inhibition or increased externalizing symptoms at age 2. As above, these analyses controlled for preterm birth. Striatum connectivity with the Bonferroni-corrected cluster in the right frontopolar PFC mediated the association between public health insurance at birth and decreased behavioral inhibition at age 2 years (indirect effect 95 % confidence interval: -0.81, -0.06, Fig. 3A); striatum-left medial PFC functional connectivity also served as a mediator (indirect effect 95 % CI: -0.81, -0.06, Fig. 3B). Furthermore, the relationship between public health insurance and externalizing symptoms was also mediated by functional connectivity between the left striatum and right frontopolar PFC (indirect effect 95 % confidence interval: -0.76, -0.07, Fig. 4A), but through suppression. That is, although lower SES was associated with both increased striatum-frontopolar PFC functional connectivity at birth as well as increased externalizing symptoms at age 2 years, the increased functional connectivity actually served to reduce externalizing symptoms. In other words, after controlling for the effects of neonatal functional connectivity on externalizing symptoms, the positive relation between public health insurance and externalizing symptoms at age two years became stronger. These mediation results were corroborated using Area Deprivation Index and PCA-based SES measures (Fig. S4).
Fig. 3.
Relations among insurance type, striatal functional connectivity at birth, and behavioral inhibition at age 2 years. The association between insurance type at birth and behavioral inhibition at age 2 years is explained by left striatum functional connectivity with A) right frontopolar prefrontal cortex and B) left medial prefrontal cortex (n = 46). *p < .05 **p < .01 ***p < .001.
Fig. 4.
Relations among insurance type, striatal functional connectivity at birth, and externalizing symptoms at age 2 years. Public insurance is associated with increased functional connectivity between the left striatum and the right frontopolar prefrontal cortex at birth; public insurance is also associated with increased externalizing symptoms at age 2 years (C = 0.79). After accounting for functional connectivity, the relationship between public health insurance and externalizing symptoms strengthened (C = 1.14). *p < .05 **p < .01 ***p < .001.
4. Discussion
4.1. Summary of results
The first goal of this study was to investigate whether lower SES is associated with differences in early postnatal resting state functional connectivity. The second goal was to ascertain whether SES-associated differences in neonatal functional connectivity were associated with subsequent psychiatric symptoms at age 2 years. We found that lower SES newborns developed decreased behavioral inhibition and increased externalizing symptoms by age 2 years. Lower SES neonates also had decreased functional connectivity between the right ventrolateral prefrontal cortex (VLPFC) and a nearby cluster in the right VLPFC, as well as increased functional connectivity between the left striatum and clusters in both the left medial and right frontopolar PFC. These associations could not be explained by preterm birth or maternal depression and anxiety. Finally, we found that SES-associated increases in frontostriatal functional connectivity mediated the relationship between lower SES and both increased externalizing symptoms and decreased behavioral inhibition. Results using both categorical SES (public vs. private health insurance) as well as continuous SES (Area Deprivation Index and PCA-based social risk score) were largely convergent.
4.2. Socioeconomic status impacts early brain development
The current study indicates that the impact of socioeconomic disadvantage on brain development is already evident at birth, extending previous work demonstrating SES-related brain changes in infants (Gao et al., 2015), children (Blair and Raver, 2016; Tooley et al., 2019), and adults (Sripada et al., 2014). There are several mechanisms by which lower SES might impact in utero brain development. Socioeconomic disadvantage is associated with higher stress, which in turn is associated with higher circulating maternal stress hormones such as cortisol (McEwen and Gianaros, 2010). Cortisol may enter fetal circulation and directly impact brain development (Harris and Seckl, 2011). Consistent with this hypothesis, a prior study linked maternal prenatal cortisol to variation in neonatal default mode network functional connectivity (Graham et al., 2019). Animal models have shown that prenatal maternal stress causes reduced spine density, length, and complexity in the prefrontal cortex of offspring (Murmu et al., 2006). Additional mechanisms by which lower SES might impact in utero brain development include poor maternal nutrition and poor maternal physical health, given their association with child temperament (Slopen et al., 2010; Kramer et al., 2000; Lipton et al., 2017; Gustafsson et al., 2016; Mina et al., 2017). Recent research provides evidence that maternal physical characteristics have a measurable effect on early brain development. For example, both maternal obesity and inflammatory signaling measured by interleukin-6 and C-reactive protein, have been linked with neonatal functional connectivity (Graham et al., 2018; Brydon et al., 2004; Owen et al., 2003; Spann et al., 2018; Li et al., 2016).
As the neonates in this study were imaged shortly after birth, early postnatal environmental factors may have also contributed to brain differences. Additionally, genetic factors associated with parental mental health may also affect neonatal brain function, suggested by the finding that SES-associated right VLPFC functional connectivity varied similarly in newborns of mothers with histories of affective symptoms. Notably, previous studies have linked SES and maternal prenatal stress with preterm birth, a known risk factor for abnormal neurodevelopment (Zeka et al., 2008). However, lower SES and risk for preterm birth were not related to each other in the current study, likely because subjects were recruited from a hospital serving a low SES community (and thus the full term infants were also disproportionately lower SES relative to the United States as a whole). Determining the exact mechanisms by which low SES impacts brain development is imperative, because many or most of these SES-related factors are modifiable and may provide targets for preventative measures.
4.3. Brain alterations associated with lower SES are also associated with psychiatric symptoms at age 2 years
The SES-related brain functional connectivity alterations present near birth in the current study were associated with psychiatric symptoms at age 2 years. Consistent with previous work, lower SES at birth was specifically associated with increased externalizing symptoms and decreased behavioral inhibition at age two years (McLaughlin et al., 2011). Decreased behavioral inhibition, which has been described as behavioral exuberance, refers to the tendency of an infant to approach novelty and may be a risk factor for externalizing conditions (Stifter et al., 2008; Degnan et al., 2011). Importantly, externalizing symptoms during early childhood have been linked to the later development of externalizing psychopathology (Reef et al., 2011). Thus, the SES-related brain changes detected in the current study may signal the onset of an abnormal neurodevelopmental cascade that increases risk for psychopathology across the lifespan.
The SES-related functional connectivity alterations that were associated with externalizing and behavioral inhibition symptoms at age 2 years included brain systems that have previously been implicated in externalizing symptoms in later childhood. Specifically, the current study identified two distinct frontostriatal connections in which lower SES-related increases in functional connectivity at birth were associated with externalizing symptoms at age 2 years. Disruptions in the striatum (Holz et al., 2017; Tomasi and Volkow, 2012) and specifically in frontostriatal functional connectivity (Padmanabhan and Luna, 2014; Fareri et al., 2017) have been robustly linked to the expression of externalizing behaviors and ADHD. A prior study specifically linked early childhood adversity to increased frontostriatal functional connectivity and social problems in adolescence (Fareri et al., 2017). The current study therefore parallels this prior work and indicates that even variation in brain functional connectivity at birth is related to development of psychiatric symptoms.
The current study suggests that socioeconomic disadvantage during pregnancy has protracted adverse effects on development. Given the immense plasticity of the developing brain, the prenatal and postnatal periods may be opportune times for intervention. Indeed, several studies have found that alleviation of socioeconomic disadvantage through income augmentation can have beneficial ramifications for pediatric emotional development (Yoshikawa et al., 2012; Costello et al., 2003). Further studies should investigate the effects of such interventions during and after pregnancy on both maternal wellbeing and child development. Considering nearly one fifth of children in the United States live in poverty, standardizing and implementing effective ways to support healthy child development at all stages remains an urgent task (Fontenot et al., 2018).
4.4. A potential protective mechanism
An unexpected finding was that the lower SES-related increase in functional connectivity between the left striatum and right frontopolar cortex was related to decreased externalizing symptoms at age 2 years. This result is counterintuitive because children with lower SES at birth had higher externalizing symptoms at age 2 years. This finding suggests a possible mechanism wherein lower SES leads to increased frontostriatal functional connectivity which tempers the development of externalizing symptoms. A similar phenomenon has been observed wherein amygdala functional connectivity linked to childhood adversity protects against the later development of internalizing symptoms (Herringa et al., 2016; Gee et al., 2013). Notably, the observed increase in frontostriatal functional connectivity in lower SES neonates was simultaneously associated with decreased externalizing symptoms and increased behavioral exuberance. This supports the notion that although behavioral exuberance may be a risk factor for externalizing symptoms, other factors such as self-regulatory capacity may modify this association (Stifter et al., 2008). Further examination of this connection’s function and modulators may offer a possible route for alleviating the effects of poverty on psychiatric risk.
4.5. Limitations
Our primary measure of socioeconomic status (insurance type), while not commonly used, is directly determined by the poverty threshold and is related to perinatal health disparities; thus it is particularly apt for this study of neonatal and early life outcomes (Bengiamin et al., 2010; Jarlenski et al., 2014; Oberg et al., 1990). Furthermore, our analyses using a composite social risk score and the Area Deprivation Index, a fine grained measure of census-block level socioeconomic characteristics, implicated the same frontostriatal circuit. This high level of convergence suggests that we are precisely indexing socioeconomic status and its impact on the neonatal brain despite not using more widely used measures such as income-to-needs ratio (which was not collected in our sample until age 5).
Additional studies are warranted to further explore the relations demonstrated here. While our sample size of 112 is large for a neonatal functional connectivity study, our behavioral follow-up subsample had considerably less power. Nevertheless, associations between SES and functional connectivity shown in the larger sample were corroborated in the smaller subsample. Additional studies with follow-up data at later ages would provide a better sense of the precise psychopathological trajectories that may have commenced. In addition to parent report, observational behavioral data might be used. The effects of potential moderating factors such as parenting style should also be investigated as possible sites of intervention. Finally, although our inclusion of term-equivalent preterm infants enhanced our power to detect effects of SES and we confirmed that our findings hold within preterm and full-term subsamples, future studies should investigate shared and unique effects of SES on these groups of infants.
4.6. Conclusions
The current study demonstrates that lower socioeconomic status has a measurable impact on brain development already at birth. These lower SES-related brain changes, furthermore, are associated with risk for psychiatric symptoms at age 2 years. This work suggests that preventative measures aimed at reducing psychopathology in high risk children might have to begin prior to birth. This work further underscores that the neurodevelopmental trajectory resulting in psychopathology may onset for some individuals near or prior to birth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Joshua Shimony, M.D., Ph.D. and Abraham Snyder, M.D., Ph.D. for assistance with data collection and analysis. This work was supported by the National Institutes of Health [grant numbers K23MH109983 and R01MH122389 to CMS, R01 MH113570 to CDS and CER, K02 NS089852 to CDS, K23MH105179 to CER, K23 MH118426 to DJW, R01 HD061619, R01 HD057098, U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University, P30 NS048056 to the Neuroinformatics Research Group at Washington University], NARSAD Young Investigator Grant #26735 (to CMS), Taylor Family Institute Award (to CMS), the Parker Fund (to CMS), Cerebral Palsy International Research Foundation (to CDS), The Dana Foundation (to CDS), Child Neurology Foundation (to CDS), and March of Dimes (to CDS).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100811.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ackerman B.P., D’Eramo K.S., Umylny L., Schultz D., Izard C.E. Family structure and the externalizing behavior of children from economically disadvantaged families. J. Fam. Psychol. 2001;15(2):288–300. doi: 10.1037//0893-3200.15.2.288. [DOI] [PubMed] [Google Scholar]
- Barch D., Pagliaccio D., Belden A. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am. J. Psychiatry. 2016;173(6):625–634. doi: 10.1176/appi.ajp.2015.15081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengiamin M.I., Capitman J.A., Ruwe M.B. Disparities in initiation and adherence to prenatal care: impact of insurance, race-ethnicity and nativity. Matern. Child Health J. 2010;14(4):618–624. doi: 10.1007/s10995-009-0485-y. [DOI] [PubMed] [Google Scholar]
- Blair C., Raver C.C. Poverty, stress, and brain development: new directions for prevention and intervention. Acad. Pediatr. 2016;16(3 Suppl):S30–36. doi: 10.1016/j.acap.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L., Edwards S., Mohamed-Ali V., Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav. Immun. 2004;18(3):281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Carter A.S., Briggs-Gowan M.J., Jones S.M., Little T.D. The Infant-Toddler Social and Emotional Assessment (ITSEA): factor structure, reliability, and validity. J. Abnorm. Child Psychol. 2003;31(5):495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- Carter A.S., Godoy L., Wagmiller R.L., Veliz P., Marakovitz S., Briggs-Gowan M.J. Internalizing trajectories in young boys and girls: the whole is not a simple sum of its parts. J. Abnorm. Child Psychol. 2010;38(1):19–31. doi: 10.1007/s10802-009-9342-0. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly C. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012;169(10):1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Compton S.N., Keeler G., Angold A. Relationships between poverty and psychopathology: a natural experiment. JAMA. 2003;290(15):2023–2029. doi: 10.1001/jama.290.15.2023. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7(3):152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan K.A., Hane A.A., Henderson H.A., Moas O.L., Reeb-Sutherland B.C., Fox N.A. Longitudinal stability of temperamental exuberance and social-emotional outcomes in early childhood. Dev. Psychol. 2011;47(3):765–780. doi: 10.1037/a0021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Posner J., Nagel B.J. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68(12):1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M.J. The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron. 2017;96(1):56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B. Altered ventral striatal-medial prefrontal cortex resting-state connectivity mediates adolescent social problems after early institutional care. Dev. Psychopathol. 2017;29(5):1865–1876. doi: 10.1017/S0954579417001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot K., Semega J., Kollar M. United States Census Bureau; Washington DC: 2018. Income and Poverty in the United States: 2017. [Google Scholar]
- Gao W., Alcauter S., Elton A. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb. Cortex. 2015;25(9):2919–2928. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A., Kehtarnavaz N., Gopinath K., Briggs R., Panahi I. Average field map image template for echo-planar image analysis. Conf Proc IEEE Eng Med Biol Soc. 2008. 2008:94–97. doi: 10.1109/IEMBS.2008.4649099. [DOI] [PubMed] [Google Scholar]
- Gianaros P.J., Manuck S.B., Sheu L.K. Parental education predicts corticostriatal functionality in adulthood. Cereb. Cortex. 2011;21(4):896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Rudolph M.D. Maternal systemic Interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry. 2018;83(2):109–119. doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Entringer S. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry. 2019;85(2):172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson H.C., Kuzava S.E., Werner E.A., Monk C. Maternal dietary fat intake during pregnancy is associated with infant temperament. Dev. Psychobiol. 2016;58(4):528–535. doi: 10.1002/dev.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A., Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011;59(3):279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. The Guilford Press; New York: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- Hayes A.F., Rockwood N.J. Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav. Res. Ther. 2017;98:39–57. doi: 10.1016/j.brat.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Herringa R.J., Burghy C.A., Stodola D.E., Fox M.E., Davidson R.J., Essex M.J. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1(4):326–334. doi: 10.1016/j.bpsc.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz N.E., Boecker-Schlier R., Buchmann A.F. Ventral striatum and amygdala activity as convergence sites for early adversity and conduct disorder. Soc. Cogn. Affect. Neurosci. 2017;12(2):261–272. doi: 10.1093/scan/nsw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlenski M.P., Bennett W.L., Barry C.L., Bleich S.N. Insurance coverage and prenatal care among low-income pregnant women: an assessment of states’ adoption of the “Unborn Child” option in Medicaid and CHIP. Med. Care. 2014;52(1):10–19. doi: 10.1097/MLR.0000000000000020. [DOI] [PubMed] [Google Scholar]
- Kachmar A.G., Connolly C.A., Wolf S., Curley M.A.Q. Socioeconomic status in pediatric health research: a scoping review. J. Pediatr. 2019;213:163–170. doi: 10.1016/j.jpeds.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Kind A.J.H., Buckingham W.R. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N. Engl. J. Med. 2018;378(26):2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind A.J., Jencks S., Brock J. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann. Intern. Med. 2014;161(11):765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M.S., Seguin L., Lydon J., Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr. Perinat. Epidemiol. 2000;14(3):194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- Lantos P.M., Hoffman K., Permar S.R. Neighborhood disadvantage is associated with high cytomegalovirus seroprevalence in pregnancy. J. Racial Ethn. Health Disparities. 2018;5(4):782–786. doi: 10.1007/s40615-017-0423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean R.E., Paul R.A., Smyser T.A., Smyser C.D., Rogers C.E. Social adversity and cognitive, language, and motor development of very preterm children from 2 to 5 years of age. J. Pediatr. 2018;203:177–184. doi: 10.1016/j.jpeds.2018.07.110. e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean R.E., Lessov-Shlaggar C.N., Gerstein E.D. Maternal and family factors differentiate profiles of psychiatric impairments in very preterm children at age 5-years. J. Child Psychol. Psychiatry. 2020;61(2):157–166. doi: 10.1111/jcpp.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Andres A., Shankar K. Differences in brain functional connectivity at resting state in neonates born to healthy obese or normal-weight mothers. Int. J. Obes. 2016;40(12):1931–1934. doi: 10.1038/ijo.2016.166. [DOI] [PubMed] [Google Scholar]
- Lipton L.R., Brunst K.J., Kannan S. Associations among prenatal stress, maternal antioxidant intakes in pregnancy, and child temperament at age 30 months. J. Dev. Orig. Health Dis. 2017;8(6):638–648. doi: 10.1017/S2040174417000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan S., Oatley H., Racine N. A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57(9):645–657. doi: 10.1016/j.jaac.2018.06.012. e648. [DOI] [PubMed] [Google Scholar]
- Marcin J.P., Schembri M.S., He J., Romano P.S. A population-based analysis of socioeconomic status and insurance status and their relationship with pediatric trauma hospitalization and mortality rates. Am. J. Public Health. 2003;93(3):461–466. doi: 10.2105/ajph.93.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.A., Marusak H.A., Sala-Hamrick K.J., Crespo L.M., Rabinak C.A., Thomason M.E. Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Hum. Brain Mapp. 2018;39(5):1982–1994. doi: 10.1002/hbm.23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A.M., Neil J.J., McKinstry R.C., Inder T.E. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr. Radiol. 2008;38(3):260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Breslau J., Green J.G. Childhood socio-economic status and the onset, persistence, and severity of DSM-IV mental disorders in a US national sample. Soc. Sci. Med. 2011;73(7):1088–1096. doi: 10.1016/j.socscimed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina T.H., Lahti M., Drake A.J. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol. Med. 2017;47(2):353–362. doi: 10.1017/S0033291716002452. [DOI] [PubMed] [Google Scholar]
- Murmu M.S., Salomon S., Biala Y., Weinstock M., Braun K., Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur. J. Neurosci. 2006;24(5):1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Najman J.M., Hayatbakhsh M.R., Clavarino A., Bor W., O’Callaghan M.J., Williams G.M. Family poverty over the early life course and recurrent adolescent and young adult anxiety and depression: a longitudinal study. Am. J. Public Health. 2010;100(9):1719–1723. doi: 10.2105/AJPH.2009.180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Farah M.J., McCandliss B.D. Socioeconomic background modulates cognition-achievement relationships in reading. Cogn. Dev. 2006;21(3):349–368. doi: 10.1016/j.cogdev.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg C.N., Lia-Hoagberg B., Hodkinson E., Skovholt C., Vanman R. Prenatal care comparisons among privately insured, uninsured, and Medicaid-enrolled women. Public Health Rep. 1990;105(5):533–535. [PMC free article] [PubMed] [Google Scholar]
- Owen N., Poulton T., Hay F.C., Mohamed-Ali V., Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav. Immun. 2003;17(4):286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A., Luna B. Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain Cogn. 2014;89:27–38. doi: 10.1016/j.bandc.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reef J., Diamantopoulou S., van Meurs I., Verhulst F.C., van der Ende J. Developmental trajectories of child to adolescent externalizing behavior and adult DSM-IV disorder: results of a 24-year longitudinal study. Soc. Psychiatry Psychiatr. Epidemiol. 2011;46(12):1233–1241. doi: 10.1007/s00127-010-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J.P., Reich T., Bucholz K.K. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol. Clin. Exp. Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rogers C.E., Anderson P.J., Thompson D.K. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(2):181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C.E., Sylvester C.M., Mintz C. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(2):157–166. doi: 10.1016/j.jaac.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G.K. Area deprivation and widening inequalities in US mortality, 1969-1998. Am. J. Public Health. 2003;93(7):1137–1143. doi: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., Fitzmaurice G., Williams D.R., Gilman S.E. Poverty, food insecurity, and the behavior for childhood internalizing and externalizing disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(5):444–452. doi: 10.1097/00004583-201005000-00005. [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser T.A., Smyser C.D., Rogers C.E., Gillespie S.K., Inder T.E., Neil J.J. Cortical Gray and adjacent white matter demonstrate synchronous maturation in very preterm infants. Cereb. Cortex. 2016;26(8):3370–3378. doi: 10.1093/cercor/bhv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R., Zigmond A. InferNelson Publishing; London: 1994. The Hospital Anxiety and Depression Scale Manual. [Google Scholar]
- Spann M.N., Monk C., Scheinost D., Peterson B.S. Maternal immune activation during the third trimester is associated with neonatal functional connectivity of the salience network and fetal to toddler behavior. J. Neurosci. 2018;38(11):2877–2886. doi: 10.1523/JNEUROSCI.2272-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann M.N., Bansal R., Hao X., Rosen T.S., Peterson B.S. Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychol. 2020;26(2):170–188. doi: 10.1080/09297049.2019.1648641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., Swain J.E., Evans G.W., Welsh R.C., Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39(9):2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter C.A., Putnam S., Jahromi L. Exuberant and inhibited toddlers: stability of temperament and risk for problem behavior. Dev. Psychopathol. 2008;20(2):401–421. doi: 10.1017/S0954579408000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Corbetta M., Raichle M.E. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35(9):527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Barch D.M., Corbetta M., Power J.D., Schlaggar B.L., Luby J.L. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(12):1326–1336. doi: 10.1016/j.jaac.2013.10.001. e1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Smyser C.D., Smyser T. Cortical functional connectivity evident after birth and behavioral inhibition at age 2. Am. J. Psychiatry. 2018;175(2):180–187. doi: 10.1176/appi.ajp.2017.17010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Matthews L.G., Alexander B. Tracking regional brain growth up to age 13 in children born term and very preterm. Nat. Commun. 2020;11(1):696. doi: 10.1038/s41467-020-14334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2012;71(5):443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley U.A., Mackey A.P., Ciric R. Associations between neighborhood SES and functional brain network development. Cereb. Cortex. 2019 doi: 10.1093/cercor/bhz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyvaud K., Doyle L.W., Lee K.J. Social-emotional difficulties in very preterm and term 2 year olds predict specific social-emotional problems at the age of 5 years. J. Pediatr. Psychol. 2012;37(7):779–785. doi: 10.1093/jpepsy/jss042. [DOI] [PubMed] [Google Scholar]
- Vyas S., Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21(6):459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Aber J.L., Beardslee W.R. The effects of poverty on the mental, emotional, and behavioral health of children and youth: implications for prevention. Am. Psychol. 2012;67(4):272–284. doi: 10.1037/a0028015. [DOI] [PubMed] [Google Scholar]
- Zeka A., Melly S.J., Schwartz J. The effects of socioeconomic status and indices of physical environment on reduced birth weight and preterm births in Eastern Massachusetts. Environ. Health. 2008;7:60. doi: 10.1186/1476-069X-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.