Abstract
[Purpose]
Blood glucose and insulin resistance were lower following hypoxic exposure in previous studies. However, the effect of hypoxia as therapy in obese model has not been unknown.
[Methods]
Six-week-old mice were randomly divided into chow diet (n=10) and high-fat diet (HFD) groups (n=20). The chow diet group received a non-purified commercial diet (65 % carbohydrate, 21 % protein, and 14 % fat) and water ad libitum. The HFD group was fed an HFD (Research Diet, #D12492; 60% kcal from fat, 5.24 kcal/g). Both groups consumed their respective diet for 7 weeks. Subsequently, HFD-induced mice (12-weeks-old) were randomly divided into two treatment groups : HFD-Normoxia (HFD; n=10) and HFD-Hypoxia (HYP; n=10, fraction of inspired=14.6%). After treatment for 4 weeks, serum glucose, insulin and oral glucose tolerance tests (OGTT) were performed.
[Results]
Homeostatic model assessment values for insulin resistance (HOMA-IR) of the HYP group tended to be lower than the HFD group. Regarding the OGTT, the area under the curve was 13% lower for the HYP group than the HFD group.
[Conclusion]
Insulin resistance tended to be lower and glucose uptake capacity was significantly augmented under hypoxia. From a clinical perspective, exposure to hypoxia may be a practical method of treating obesity.
Keywords: Hypoxia, OGTT, insulin, diabetes, HOMA-IR, blood glucose, obesity
INTRODUCTION
Worldwide more than 1.4 billion adults are overweight, of which more than 400 million are obese1. Obesity represents a major health burden because it is accompanied by an increased risk of insulin resistance (IR) and diabetes.
Recent data points to an important role of hypoxia in glucose metabolism disorders2-3. Hypoxia is a state of low oxygen consumption and can be classified into four stages based on altitude. Low hypoxia is defined as an altitude of 500-2,000 m (inspired oxygen fraction (FIO2) = 16.7-19.8%), moderate hypoxia is 2,000-3,000 m (FIO2 = 14.8-16.7%), high hypoxia is 3,000-5,500 m (FIO2 = 10.9-14.8%) and extreme hypoxia as >5,500 m (FIO2 < 10.9%)4.
High altitude populations have lower blood glucose concentrations and a lower incidence of type 2 diabetes. Hill et al.5 demonstrated that blood glucose concentration and insulin resistance were lower following gradual ascent in altitude (3,600-5,120 m). Woolcott et al.6 reported that prolonged exposure to high hypoxia (altitude 3,500 m) might decrease concentration. Wang et al.2 and Mackenzie et al.7 reported that exposure to high-intermittent hypoxia (inspired oxygen fraction=14.6-14.7%) improved glycemic control. Along with glycemic control, the capacity for glucose uptake is important. Hypoxia itself stimulates glucose uptake mediated by AMP-activated protein kinase (AMPK) and glucose transporter 4 (GLUT4) translocation8-9.
High hypoxia, which is effective in regulating blood glucose concentration, has the potential to be a therapeutic method for obesity-related glucose metabolic disorders. However, in the case of obese model studies, most studies have used hypoxia with extreme intensity as a method to induce obstructive sleep apnea (OSA)10-11.
Taken together, it is necessary to confirm the effect of high hypoxia on an obese model, which has a higher risk of glucose metabolic disorders. Therefore, the purpose of this study is to investigate the effects of high hypoxia on high-fat diet (HFD)-induced obese mice.
METHODS
Animal Subjects
Five-week-old male ICR mice were purchased from Orient Bio Inc. (Seongnam, Korea). All mice were housed in standard plastic cages under controlled humidity (50%) and temperature (23 ± 1℃) conditions in a 12 h/12 h dark/light cycle. Mice were acclimatized to the laboratory housing condition for 1 week. The animal experiments were conducted according to protocols reviewed and approved by the Konkuk University Institutional Animal Care and Use Committee (permit number: KU19187). The chow diet mice received a non-purified commercial diet (65% carbohydrate, 21% 86 protein, and 14% fat) and water ad libitum. The HFD mice were fed with an HFD (Research Diet, #D12492; 60% kcal from fat, 5.24 kcal/g) from 5 weeks old.
Animal Model Preparation
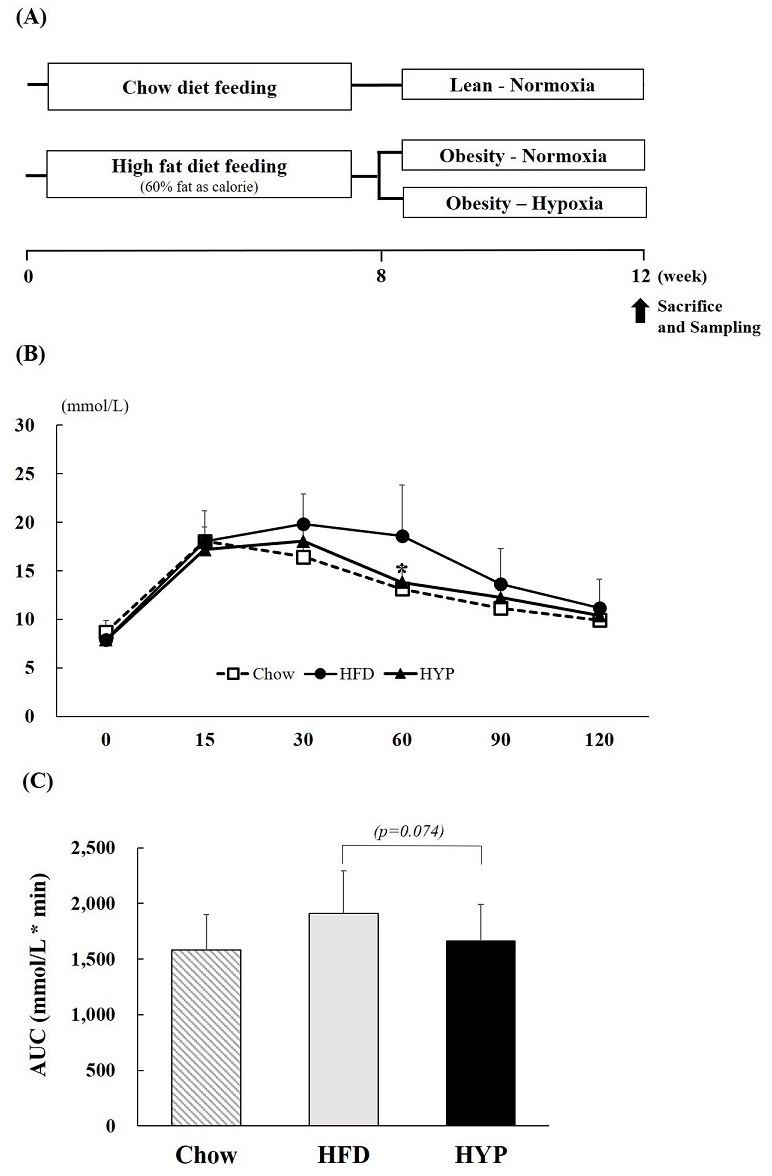
Six-week-old mice were randomly divided into two groups: chow diet (n=10) and HFD groups (n=20). Both groups consumed each diet for 7 weeks. After the 7 weeks, the HFD-induced mice (12-weeks-old) were randomly divided into two treatment groups: HFD-Normoxia (HFD; n=10), HFD-Hypoxia (HYP; n=10). In addition, the chow diet group consumed a chow diet for the experiment period (Chow; n=10) (Fig. 1A).
Figure 1. (A) Experiment design (B) OGTT and (C) the area under the curve responses to an oral glucose challenge (2 g/kg) after 12 h of food deprivation at week 12. Values represent the mean ± S.D. (n = 10). Chow (□), mice consuming regular diet; HFD (●), mice consuming high-fat diet; HYP (▲), mice fed with high-fat diet in exposure of hypoxia. *p < 0.05 vs. HFD.
Hypoxic exposure
The HYP group was placed in identical commercially designed chambers (model HCC-550, SFET, Korea) and exposed to hypoxic conditions during only the light phase (from 19:00 hours to 07:00 hours) to coincide with the mouse sleep cycle. Oxygen concentration was set at 14.6% (although the actual oxygen concentration raged from 14.4-14.7% during the experiment). The use of the high-altitude intervention in our study was with reference to previous reports in the literature2,7. After the 4-week exposure, all tissue harvesting was performed. Food was withdrawn for 4 h during the day of tissue harvesting. Mice were anesthetized with avertin.
Oral glucose tolerance test (OGTT)
An oral glucose tolerance test (OGTT) was performed at the end of the treatment. On the test day, animals were fasted for 12 h, after which glucose (2 g/kg) was orally administered. Blood samples were taken from the tail at 0, 15, 30, 60, 90, and 120 min after glucose administration. The level of glucose was measured using a glucose meter (ACCU Check, France). The areas under the curve (AUCs) were calculated using trapezoidal integration.
Measurement of blood glucose and insulin
Serum was harvested from ICR mice at the end of the experiment and stored at −80°C. Insulin levels were measured with a mouse enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (Alpco, Salem, NH). Glucose levels were determined using a glucose meter (ACCU Check, Penzberg, Germany).
Statistical analysis
Significant differences between groups were determined using independent t-tests (SPSS for Windows, version 24.0, Chicago, IL, USA). All values are reported as a mean ± S.D. A p-value of < 0.05 was considered statistically significant.
RESULTS
Body weights and food consumption
The body weights and food consumption are shown in Table 1. There was a significant difference between the initial and final body weight in the HYP group. The food consumption was not different between the HYP and HFD groups.
Table 1. The change of body weight, food intake and blood variables.
| Chow | HFD | HYP | |
|---|---|---|---|
| Initial body weight(g) | 39.7 ± 2.9 | 50.1 ± 5.2 | 51.2 ± 3.8 |
| Final body weight(g) | 38.1 ±1.6 | 48.2 ± 3.6 | 48.3 ± 2.3† |
| Body weight gain(g) | -1.6 ± 1.6 | -1.9 ± 3.6 | -3.1 ± 2.3 |
| Food intake(g/day) | 4.4 ± 0.4 | 3.2 ± 0.4 | 3.3 ± 0.6 |
| Serum glucose(mmol/L) | 10.7 ± 2.1 | 12.7 ± 1.4 | 13.0 ± 1.3 |
| Serum Insulin(μU/mL) | 1.9 ± 0.0 | 2.0 ± 0.1 | 1.9 ± 0.0 |
| HOMA-IR | 0.9 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 |
Values represent the mean ± S.D. (n = 10). Homeostasis Model Assessment was used to calculate an index of insulin resistance as insulin (μU/mL) × glucose (mmol/L)/22.5. † p < 0.05 vs. initial.
Serum glucose, insulin, and insulin resistance index
Fasting serum glucose and insulin levels were measured at the end of the treatment. Hyperglycemia did not develop in any of the groups over the 4-week trial (Table 1). Serum insulin levels tended to be lower in the HYP group compared to the HFD group (p=0.057). The homeostatic model assessment values for insulin resistance (HOMA-IR), calculated by insulin (μU/ml) × glucose (mM)/22.512, of the HYP group tended to be lower than the HFD group.
Oral glucose tolerance test (OGTT)
After 4-weeks of hypoxic exposure, the OGTT was performed. Glucose challenge dramatically increased the blood glucose levels in HFD fed mice compared to those in the HYP group. HYP treated groups were seen to have significantly reduced rising blood glucose levels, especially at 60 min time points, which were at similar levels to the chow diet-fed group (Fig. 1B). When the area under the curve (AUC) was compared between groups, HYP treated groups showed a 13% lower AUC compared to the HFD control group (Fig. 1C).
DISCUSSION
We investigated the effects of hypoxic exposure on glycemic control in HFD-induced obese mice. We found that fasting blood insulin tended to be lower in the hypoxic exposed group (HYP) than the normoxia group (HFD). Insulin sensitivity, determined according to the HOMA-IR, tended to be higher in the HYP group than in the HFD group.
In addition, as shown in the results related to the OGTT, blood glucose concentration was lower in the HYP group and was a similar level to the chow diet-fed group. Therefore, these results implied that hypoxic exposure could improve glycemic control.
As control factors of glycemic control, changes in glucose transporters and its translocation impact glucose homeostasis. Hypoxia causes an increase in glucose transporters into the skeletal muscle. Primarily, insulin-dependent glucose uptake in skeletal muscle is mediated by the translocation of GLUT-4 transporter because of increased activation of the PI3K/Akt pathway13. Wang et al.14 reported that GLUT-4 protein expression was 33% higher in the skeletal muscle following hypoxic exposure (fractional inspired O2 of 15%) for 4 weeks in mice. Besides, a previous study reported that blood glucose concentration and insulin resistance were lower following gradual ascent in altitude (3,600-5,120 m)5. In the present study, blood insulin tended to be lower, and glucose concentration was lower in the hypoxic exposure group. These results might be due to mediation by a glucose transporter. Further studies on protein expression of glucose transporters and glucose uptake are required.
In addition to hypoxic therapy, previous studies suggest that regular exercise reduces the risk of developing type 2 diabetes and significantly improves glycemic control15-20. This might be due to the translocation of glucose trans¬porters (e.g., GLUT-4)21-22. In¬deed, studies have demonstrated increased GLUT-4 concentrations with aerobic training, which is accompanied by an increase in insulin-mediated glucose uptake23-25.
Moreover, insulin-mediated glucose uptake is inversely correlated with body fat mass26-27. In a previous study, hypoxic exposure (altitude 2,200-4,000 m) combined with aerobic exercise decreased body weight and fat mass28-30. Therefore, it could be used as a therapy for obesity with glucose control disorders. In the present study, we confirmed that hypoxic exposure decreased body weight and blood glucose levels. We implied that this was induced by reduced body fat mass. Therefore, exercise, in combination with hypoxic exposure, could have a synergic effect on glucose control within a shorter period than exercise alone.
In conclusion, insulin resistance tended to be lower, and glucose uptake capacity was significantly augmented under high hypoxia. From a clinical perspective, intermittent exposure to high hypoxia (14.6 FIO2) may be a practical method for treating obesity. Further, a combination of exercise could have a synergic effect. However, the current study did not confirm the protein expression of glucose metabolism. Therefore, future research should be conducted to elucidate the molecular mechanism of glucose metabolism.
Acknowledgments
KL(corresponding author) and HYP contributed to the design of all experiments and interpreted the results of the data. HYP and JK interpreted the results of the data and revised the paper. YP collected the data, undertook the statistical analyses, and wrote the manuscript. IJ carried out the studies and provided technical support. All of the authors read and approved the final manuscript. The authors would like to thank the Exercise & Nutrition Laboratory for their excellent laboratory work. This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2019S1A5A2A03034583).
References
- 1. [World Health Organization. Obesity and Overweight – Fact Sheet 311. 2006.] [Google Scholar]
- 2. doi: 10.3389/fendo.2019.00527. [Wang R, Guo S, Tian H, Huang Y, Yang Q, Zhao K, Kuo C-H, Hong S, Chen P and Liu T. Hypoxic training in obese mice improves metabolic disorder. Front Endocrinol (Lausanne). 2019;10:527.] [DOI] [Google Scholar]
- 3. doi: 10.1371/journal.pone.0203551. [Wang Y, Wen L, Zhou S, Zhang Y, Wang XH, He YY, Davie A, Broadbent S. Effects of four weeks intermittent hypoxia intervention on glucose homeostasis, insulin sensitivity, GLUT4 translocation, insulin receptor phosphorylation, and Akt activity in skeletal muscle of obese mice with type 2 diabetes. PLoS One. 2018;13:e0203551.] [DOI] [Google Scholar]
- 4. doi: 10.24985/kjss.2018.29.4.737. [Park HY, Kim JS, Lim KW. Exercise physiology basis and necessity of hypoxic training to improve exercise performance in elite athletes. Korean J Sport Science. 2018;29:737-52.] [DOI] [Google Scholar]
- 5. doi: 10.1249/MSS.0000000000001624. [Hill NE, Deighton K, Matu J, Misra S, Oliver NS, Newman C, Mellor A, O’Hara J, Woods D. Continuous glucose monitoring at high altitude-effects on glucose homeostasis. Med Sci Sports Exerc. 2018;50:1679-86.] [DOI] [Google Scholar]
- 6. doi: 10.1002/oby.20800. [Woolcott OO, Castillo OA, Gutierrez C, Elashoff RM, Stefanovski D, Bergman RN. Inverse association between diabetes and altitude: A cross-sectional study in the adult population of the united states. Obesity (Silver Spring). 2014;22:2080-90.] [DOI] [Google Scholar]
- 7. doi: 10.1002/dmrr.1156. [Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (SI2*) in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2011;27:94-101.] [DOI] [Google Scholar]
- 8. doi: 10.1152/jappl.1991.70.4.1593. [Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol (1985). 1991;70:1593-600.] [DOI] [Google Scholar]
- 9. doi: 10.2337/diabetes.49.4.527. [Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527-31.] [DOI] [Google Scholar]
- 10. doi: 10.1164/ajrccm.165.5.2104087. [Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677-82.] [DOI] [Google Scholar]
- 11. doi: 10.1016/j.amjmed.2009.04.026. [Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122-7.] [DOI] [Google Scholar]
- 12. doi: 10.1007/BF00280883. [Matthews DR, Hosker JP, Rudenky AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412-9.] [DOI] [Google Scholar]
- 13. doi: 10.1016/S0014-5793(03)00562-3. [Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003;546:108-12.] [DOI] [Google Scholar]
- 14. doi: 10.1371/journal.pone.0203551. [Wang Y, Wen L, Zhou S, Zhang Y, Wang XH, He YY, Davie A, Broadbent S. Effects of four weeks intermittent hypoxia intervention on glucose homeostasis, insulin sensitivity, GLUT4 translocation, insulin receptor phosphorylation, and Akt activity in skeletal muscle of obese mice with type 2 diabetes. PLoS ONE. 2018;13:e0203551.] [DOI] [Google Scholar]
- 15. doi: 10.1056/NEJM199107183250302. [Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non-insulin-de¬pendent diabetes mellitus. N Engl J Med. 1991;325:147-52.] [DOI] [Google Scholar]
- 16. doi: 10.1001/archinte.1996.00440110073010. [Lynch J, Helmrich SP, Lakka TA, Kaplan GA, Cohen RD, Sa¬lonen R, Salonen JT. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med. 1996;156:1307-14.] [DOI] [Google Scholar]
- 17. doi: 10.1056/NEJMoa012512. [Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, La¬chin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 di¬abetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.] [DOI] [Google Scholar]
- 18. doi: 10.7326/0003-4819-147-6-200709180-00005. [Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357-69.] [DOI] [Google Scholar]
- 19. doi: 10.2337/diacare.26.6.1706. [Gan SK, Kriketos AD, Ellis BA, Thompson CH, Kraegen EW, Chisholm DJ. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care. 2003;26:1706-13.] [DOI] [Google Scholar]
- 20. doi: 10.2337/diacare.26.3.557. [Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26:557-62.] [DOI] [Google Scholar]
- 21. doi: 10.1152/ajpendo.1997.273.6.E1039. [Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol-Endoc M. 1997;273:E1039-51.] [DOI] [Google Scholar]
- 22. doi: 10.1152/ajpendo.1990.258.4.E667. [Goodyear LJ, King PA, Hirshman MF, Thompson CM, Horton ED, Horton ES. Contractile activity increases plasma mem¬brane glucose transporters in absence of insulin. Am J Physiol-Endoc M. 1990;258:E667-72.] [DOI] [Google Scholar]
- 23. doi: 10.1016/j.metabol.2004.03.022. [Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Kashyap S, Mandarino LJ. Exercise training in¬creases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 di¬abetic subjects. Metabolism. 2004;53:1233-42.] [DOI] [Google Scholar]
- 24. doi: 10.1152/jappl.1996.81.3.1162. [Houmard JA, Tyndall GL, Midyette JB, Hickey MS, Dolan PL, Gavigan KE, Weidner ML, Dohm GL. Effect of reduced train¬ing and training cessation on insulin action and muscle GLUT-4. J Appl Physiol (1985). 1996;81:1162-8.] [DOI] [Google Scholar]
- 25. doi: 10.1152/ajpendo.1993.264.6.E896. [Houmard JA, Shinebarger MH, Dolan PL, Leggett-Frazier N, Bruner RK, McCammon MR, Israel RG, Dohm GL. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol. 1993;264:E896-901.] [DOI] [Google Scholar]
- 26. doi: 10.1371/journal.pone.0056928. [Ruiz-Alcaraz AJ, Lipina C, Petrie JR, Murphy MJ, Morris AD, Sutherland C, Cuthbertson DJ. Obesity-induced insulin resistance in human skeletal muscle is characterised by defective activation of p42/p44 MAP Kinase. PLoS One. 2013;8:e56928.] [DOI] [Google Scholar]
- 27. doi: 10.1210/jcem.86.11.7992. [Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy asian indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366-71.] [DOI] [Google Scholar]
- 28. doi: 10.1080/02701367.2010.10599708. [Chen MT, Lee WC, Chen SC, Chen CC, Chen CY, Lee SD, Jensen J, Kuo CH. Effect of a prolonged altitude expedition on glucose tolerance and abdominal fatness. Res Q Exerc Sport. 2010;81:472-7.] [DOI] [Google Scholar]
- 29. doi: 10.4077/CJP.2013.BAA071. [Chia M, Liao CA, Huang CY, Lee WC, Hou CW, Yu SH, Harris MB, Hsu TS, Lee SD, Kuo CH. Reducing body fat with altitude hypoxia training in swimmers: role of blood perfusion to skeletal muscles. Chin J Physiol. 2013;56:18-25.] [DOI] [Google Scholar]
- 30. doi: 10.1007/BF00243503. [Schena F, Guerrini F, Tregnaghi P, Kayser B. Branched-chain amino acid supplementation during trekking at high altitude. The effects on loss of body mass, body composition, and muscle power. Eur J Appl Physio Occup Physiol. 1992;65:394-8.] [DOI] [Google Scholar]