Abstract
Superoxide dismutases, which catalytically remove intracellular superoxide radicals by the disproportionation of molecular oxygen and hydrogen peroxide, are encoded by the sod-1 to -5 genes in the nematode C. elegans. Expression of the sod genes is mutually compensatory for the modulation of intracellular oxidative stress during aging. Interestingly, several-fold higher expression of the sod-1 to -4 was induced in a sod-5 deletion mutant, despite the low expression levels of sod-5 in wild-type animals. Consequently, this molecular compensation facilitated recovery of lifespan in the sod-5 mutant. In previous reports, two transcription factors DAF-16 and SKN-1 are associated with the compensatory expression of sod genes, which are downstream targets of the ins/IGF-1 and p38 MAPK signaling pathways activated under oxidative and heavy metal stresses, respectively. Here, we show that p38 MAPK signaling regulates induction of not only the direct expression of sod-1, -2 and -4 but also the indirect modulation of DAF-16 targets, such as sod-3 and -5 genes. Moreover, a SKN-1 target, the insulin peptide gene ins-5, partially mediates the expression of DAF-16 targets via p38 MAPK signaling. These findings suggest that the interaction of ins/IGF-1 and p38 MAPK signaling pathways plays an important role in the fine-tuning of molecular compensation among sod genes to protect against mitochondrial oxidative damage during aging.
Keywords: C. elegans, sod genes, p38 MAPK signaling, ins/IGF-1 signaling, Molecular compensation, Insulin-like peptide
Highlights
-
•
Mitochondrial ROS is removed by SODs during aging.
-
•
Expression of sod genes in C. elegans related to lifespan maintenance.
-
•
Interaction of the ins/IGF-1 and p38 MAPK signalings regulates the fine-tuning of sod genes expression.
-
•
ins-5 of SKN-1 target encodes an agonist of ins/IGF-1 signaling.
1. Introduction
Mitochondria are the primary source of reactive oxygen species (ROS), including superoxide radicals (O2.-), in cells of aerobic organisms. Almost all mitochondrial oxygen consumption is efficiently coupled to the production of ATP; however, a small percentage (<0.1%) of molecular oxygen is reduced by electrons that mechanistically leak in mitochondria and subsequently produce toxic ROS [1]. Superoxide dismutase (SOD) is an important enzyme that catalytically removes O2.- and protects organisms from the oxidative damage during aging [2,3]. However, SOD results in further production of hydrogen peroxide (H2O2), which is a type of ROS, in cells by the disproportionation reaction [2,4]. Therefore, the activation of SOD in cells is vital and critically balanced for controlling intracellular ROS.
In the Caenorhabditis elegans (C. elegans) genome, the sod-1 to -5 genes encode distinct SOD isozymes; sod-1 and -5 encode Cu/Zn SODs, sod-2 and -3 encode mitochondrial Mn SODs, and sod-4 encodes the homolog of an extracellular Cu/Zn SOD in mammals [[5], [6], [7], [8], [9]]. Disruption of the expression of sod genes increases mitochondrial O2.- levels and directly affects the lifespan of C. elegans [9,10]. Indeed, experimental estimates of the effect of each sod gene on lifespan have varied because the expression of these sod genes is changed in a mutually compensatory manner to maintain catalytic function in cells [[9], [10], [11], [12]].
We previously revealed the compensatory expression of sod-5 in sod-1 deletion mutants and its regulation via an insulin/insulin-like growth factor-1 (ins/IGF-1) signaling pathway [9]. The mammalian forkhead transcription factor FOXO ortholog DAF-16 functions downstream of the ins/IGF-1 signaling pathway and regulates the expression of target genes such as sod-3 and -5 in C. elegans. In fact, the consensus sequence of the DAF-16 binding element (DBE) is present in the promoter regions of sod-2, -3 and -5 genes [9,[13], [14], [15]]. In addition to DBE, a binding site for the mammalian NF-E2-related factor (Nrf) ortholog SKN-1, which is upregulated by the p38 mitogen-activated protein kinase (MAPK) signaling pathway, is found in the promoter regions of all sod genes [[15], [16], [17], [18]]. Thus, recent studies have shown that the ins/IGF-1 and other signaling pathways including the p38 MAPK cascade, are associated with the regulation of SOD expression in response to oxidative stress and immunity during normal aging in C. elegans. However, this interaction between the ins/IGF-1 and p38 MAPK signaling pathways for the compensatory expression of sod genes has been only partly understood except for the participation of several insulin peptides [[19], [20], [21]]. Determining the molecular mechanisms of this interaction could contribute to the control of ROS levels in aged cells and animals by regulating the expression of antioxidant genes such as sod genes. In this study, we examined whether sod-1 expression levels were also increased in a sod-5 deletion mutant, and attempted to clarify whether the mammalian MAPK kinase (MAPKK) homolog SEK-1 and downstream transcription factor SKN-1, which are predicted to regulate the transcription of all sod genes, are required for modulating their compensatory expression. Consequently, we report the molecular mechanisms for fine-tuning the expression of sod genes via switching between the ins/IGF-1 and p38 MAPK signalings by an insulin peptide [15,19].
2. Materials and methods
2.1. Nematode strains and maintenance
The wild-type N2 strain var. Bristol and other strains of C. elegans, namely daf-16(mgDf50) and sek-1(km4), were obtained from the Caenorhabditis Genetics Center at the University of Minnesota (Minneapolis, MN). The daf-16(mgDf50) strain has a deficiency of the daf-16 gene-coding region including almost all daf-16 transcripts alternatively spliced [22]. Exons 4–6 of sek-1 gene are deleted in the sek-1(km4) strain [23]. The sod-1(tm776), sod-5(tm1146) and ins-5(tm2560) deletion mutants were supplied by the National Bioresource Project for the Experimental Animal “Nematode C. elegans” [9]. A mutant strain, sod-1(tm776);sod-5(tm1146), was obtained from Dr. T. Sakamoto of Kitasato University. In addition, we isolated sod-1(tm776);daf-16(mgDf50) and sod-1(tm776);sek-1(km4) double mutants by outbreeding. Worms were grown at 20 °C on nematode growth medium (NGM) agar plates with Escherichia coli (E. coli) OP50, a uracil-requiring strain [24].
2.2. Measurement of lifespan
The lifespan of hermaphrodites at 20 °C was measured using 100 worms per trial in at least three independent experiments [24]. To prevent reproduction, 5-fluoro-2′-deoxyuridine, FUdR (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was added to the NGM agar plate to a final concentration of 40 μM after the animals had reached adulthood. Means were compared using Student's t-test. Data are expressed as means ± standard deviation (SD). A two-tailed p-value of <0.05 was considered statistically significant.
2.3. Real-time PCR analysis of gene expression
Poly(A)+ RNA from 5-day-old animals was prepared for reverse transcription PCR (RT-PCR), and complimentary DNA (cDNA) was synthesized as described previously [24]. Quantitative measurement using a TaqMan gene expression assay in 5-day-old animals was performed using a 7500 Fast Real-Time PCR System (Applied Biosystems by Thermo Fisher Scientific, Waltham, MA). The results were normalized to the transcript level of act-4 using the wild-type strain as a control. The means of at least three measurements were compared using Student's t-test. Data are expressed as means ± standard error of the mean (SEM). A two-tailed p-value of <0.05 was considered statistically significant.
2.4. Measurement of mitochondrial and sub-mitochondrial particle O2.- production
To isolate the mitochondrial fraction, 5-day-old animals were washed with S-buffer and mannitol-sucrose buffer and homogenized with 60 strokes of a Teflon homogenizer, Eyela Mazela Z (Tokyo Rikakikai Co., Ltd., Tokyo, Japan) at 1300 rpm on ice. The intact mitochondrial fraction was isolated as described previously [9,26]. After storage at −80 °C for 2 days, the mitochondrial fraction was sonicated using an ultrasonic homogenizer UH-50 (SMT Co., Ltd., Tokyo, Japan) to isolate the sub-mitochondrial particle (SMP) fraction, which is the characteristic mitochondrial inner membrane fragments reformed into inside-out vesicles. The SMP fraction includes little or no mitochondrial matrix, which contains soluble enzymes such as Mn SODs [4,25]. The SMP fraction was resuspended in Tris-EDTA (TE) buffer [26]. The protein content of each fraction was determined using a BCA Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL). Mitochondrial and SMP O2.- production was measured using a specific chemiluminescent probe, 2-methyl-6-p-methoxyphenylethynyl-imidazopyrazinone, MPEC (Atto Co., Tokyo, Japan), in an AccuFLEX Lumi 400 luminometer (Aloka Co., Ltd., Tokyo, Japan) [9,26,27].
2.5. Subcellular localization of DAF-16::GFP
To detect the expression and subcellular localization of DAF-16, we used a pGP30 plasmid, which includes green fluorescent protein (GFP) gene fused to the daf-16a2 gene containing a 6-kb upstream region. pGP30 was microinjected into the gonads of several strains at 100 ng/μL with pRF4 containing the rol-6(su1006) gene [9]. DAF-16:GFP localization in the nuclei of 5-day-old animals was observed using a fluorescence microscope with a digital imaging system BX51TRF (Olympus Co., Tokyo, Japan).
3. Results
3.1. Lifespan and compensatory expression of other sod genes in the sod-5 mutant
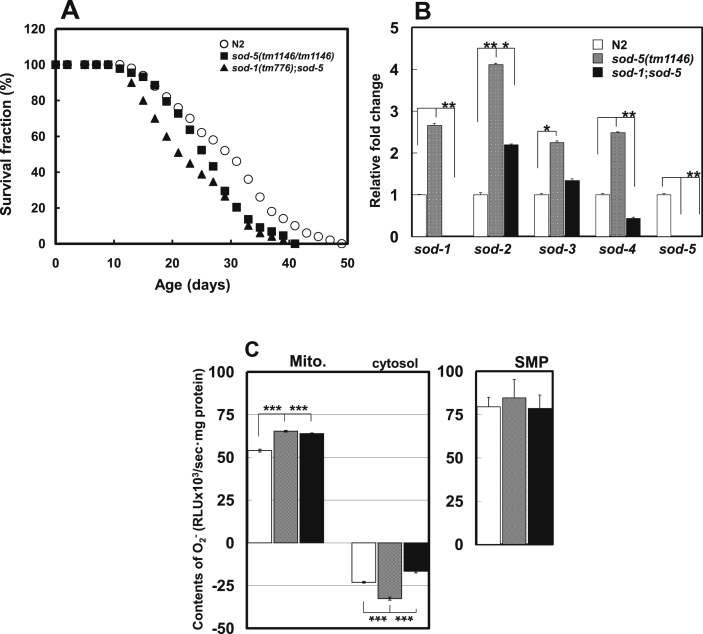
Means and maximum lifespans of the sod-5 mutant of C. elegans were moderately decreased compared with the wild-type strain, and those of the sod-1;sod-5 mutant was further decreased (Fig. 1a). The mRNA levels of sod-1 to -4 genes were enhanced in the sod-5 mutant, but the compensatory expression of other sod genes was diminished in the sod-1;sod-5 mutant (Fig. 1b). These results show that defects in both sod-1 and -5 have more deleterious effects on lifespan compared with the sod-5 mutant.
Fig. 1.
(A) Lifespan of the sod-5-related mutants at 20 °C. Mean lifespan ±SD was as follows; 29.8 ± 9.1 days in the wild-type strain, 26.4 ± 7.0 days in the sod-5 mutant, and 23.4 ± 7.9 days in the sod-1;sod-5 mutant. The mean lifespans of the sod-5 and sod-1;sod-5 mutants were shorter compared with that of the wild-type strain (P < 0.05 and < 0.0001, respectively). Means were conducted by Student's t-test. (B) Real-time PCR data of sod genes expression in the strains. Data are shown as means ± SEM. Means were compared by Student's t-test (*P < 0.05, **P < 0.001). (C) O2.- production in mitochondria, cytosol and SMP of the strains. Open, shaded and closed columns indicate O2.- levels of the wild-type strain, sod-5 and sod-1;sod-5 mutants, respectively. Data are shown as means ± SD. Means were compared by Student's t-test (***P < 0.0001).
3.2. Changes in the mitochondrial and SMP O2.- levels in the sod-5 mutant
We found significant increases in the O2.- levels of mitochondria but not SMP fraction, which includes little or no mitochondrial matrix and the soluble enzymes such as Mn SODs [25], in the sod-5 and sod-1;sod-5 mutants compared with the wild-type strain. Generally, the O2.- levels in SMP are unaffected by the Mn SODs and indicating a net side-product capable of activating the electron transport chain (ETC) and oxidative phosphorylation (OxPhos) in the SMP fraction itself. That is, the increases in mitochondrial O2.- levels in the sod-5 and sod-1:sod-5 mutants involved mainly the deficient activity of mitochondrial SODs, which include not only Mn SODs in the matrix but also Cu/Zn SODs in the mitochondrial intermembrane space [9,25]. In contrast, cytosolic O2.- levels were increased in the sod-1;sod-5 mutant, but not in the sod-5 mutant, compared with the wild-type strain (Fig. 1c). This shows that deletion of both sod-1 and -5 does not affect the net production of mitochondrial O2.-, however, it results in the impaired removal of O2.- from mitochondria by SODs. Probably, the compensatory expression of other sod genes in the sod-5 mutant contributed to the cytosolic decrease of O2.- levels in cytoplasm and prevented mitochondrial O2.- leakage (Fig. 1b and c).
3.3. Predicted binding sites of the DAF-16 and SKN-1 transcription factors
According to previous reports, each of sod-2, -3 and -5 has one or two predicted DBEs, and all sod genes have one or two predicted SKN-1 binding sites in their promoter regions [9,[13], [14], [15], [16], [17]]. Here, we found two novel predicted SKN-1 binding sites in the upstream region of sod-4 gene and a novel predicted DBE in the upstream region of sod-2 gene. As a result, at least one predicted DAF-16 and/or SKN-1 binding site is present in the promoter regions of all sod genes (Fig. 2).
Fig. 2.
Putative DBE sequences and SKN-1 binding sites located upstream of the first exon of the sod genes. Lower and upper case letters show parts of the promoter and exon regions in the sod genes, respectively. The DBE and SKN-1 binding sites are represented as closed and shaded boxes, respectively.
3.4. Changes in lifespan and compensatory expression of sod genes under DAF-16 activation
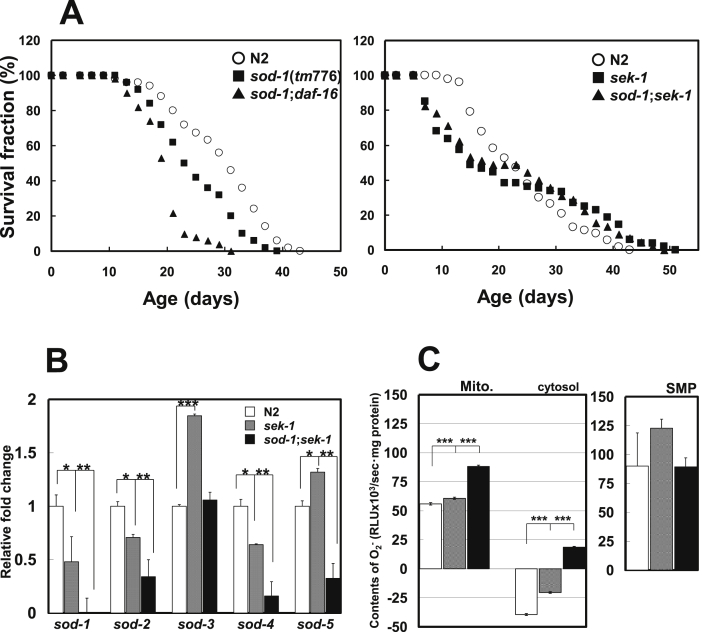
Based on the predicted binding sites, we examined the effects of the transcription factors DAF-16 and SKN-1, which are involved in the compensatory expression of sod genes, on the lifespan of C. elegans. As a result, we found that DAF-16 and SKN-1 had different effects on the lifespan of sod-1 mutant. Deletion of daf-16 further shortened lifespan, however, deletion of sek-1, which is associated with the activation of SKN-1 through the p38 MAPK signaling [17], had almost no effect on the lifespan of sod-1 mutant (Fig. 3a). According to our previous data, intrinsic DAF-16 activation in the sod-1 mutants leads to the compensatory expression of sod-5 [9]. We confirmed the compensatory expression of sod-3 and -5 in the sek-1 mutant, but this effect was suppressed in the sod-1;sek-1 mutant (Fig. 3b).
Fig. 3.
(A) Effects of impaired signaling on lifespan at 20 °C in the sod-1 mutant. Mean lifespan ±SD was as follows; 29.8 ± 7.7 days in the wild-type strain, 25.1 ± 6.8 days in the sod-1 mutant, and 19.9 ± 4.2 days in the sod-1;daf-16 mutant. Mean lifespan ±SD was as follows; 29.9 ± 7.3 days in the wild-type strain, 19.5 ± 17.0 days in the sek-1 mutant, and 19.7 ± 13.6 days in the sod-1;sek-1 mutant. Mean lifespan was different between the sod-1 and sod-1;daf-16 mutants (P < 0.0001), but not between the sek-1 and sod-1;sek-1 mutants. Means were compared by Student's t-test was performed. (B) Real-time PCR data of the sod genes expression in the strains. Data shown represent the means ± SEM. Means were compared by Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.001). (C) O2.- production in mitochondria, cytosol and SMP of the strains. Open, shaded and closed columns indicate O2.- levels of the wild-type strain, sek-1 and sod-1;sek-1 mutants, respectively. Data are shown as means ± SD. Means were compared by Student's t-test (***P < 0.0001).
3.5. Changes in mitochondrial and SMP O2.- levels under DAF-16 activation
We confirmed significant increases in the O2.- levels of mitochondria, but not in the SMP fraction, in the sek-1 and sod-1;sek-1 mutants compared with the wild-type strain. Likewise, cytosolic O2.- levels were higher in the sek-1 and sod-1;sek-1 mutants than in the wild-type strain (Fig. 3c). These findings show that deletion of both sek-1 and sod-1 has almost no effect on the net production levels of mitochondrial O2.-, however, it decreases remarkably the catalytic removal of mitochondrial O2.- in the sod-1;sek-1 mutant.
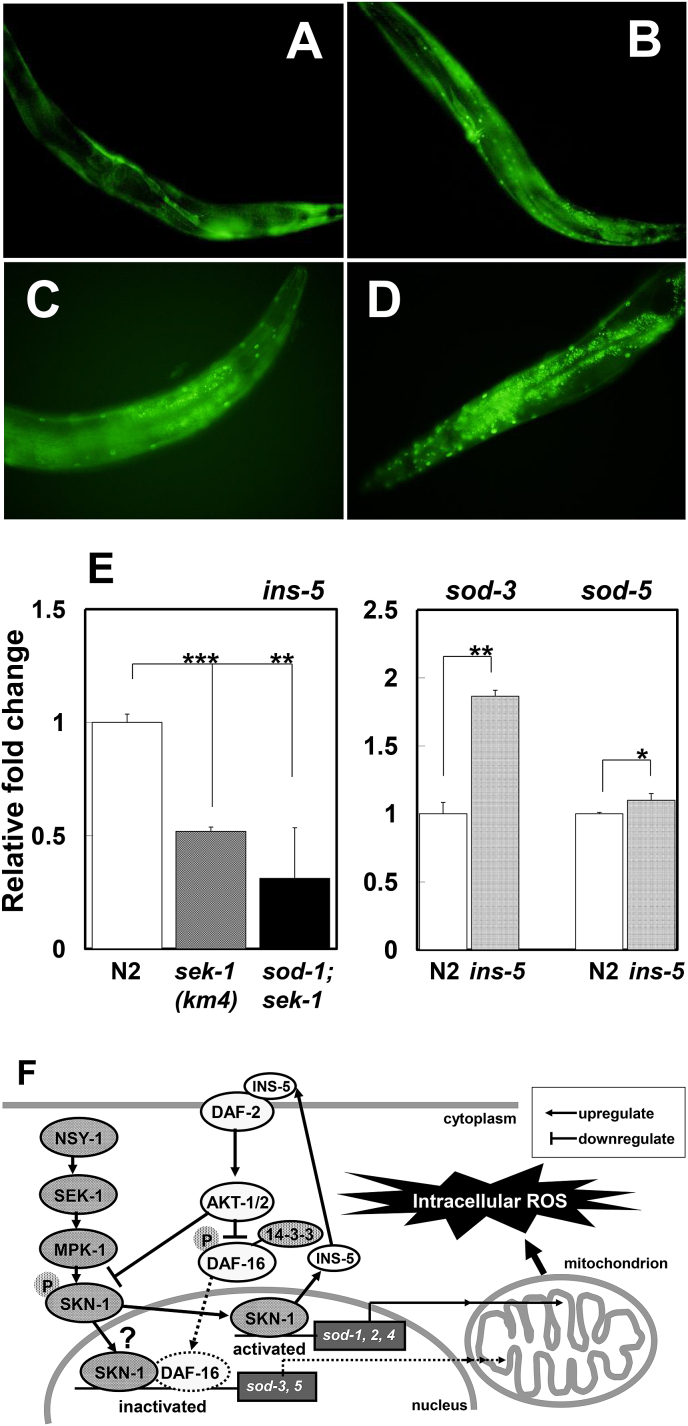
3.6. Reduction of DAF-16::GFP nuclear translocalization via p38 MAPK signaling
In 5-day-old wild-type animals, almost all DAF-16a2 protein was observed in the cytoplasm and not in the nucleus (Fig. 4a). However, in the 5-day-old sod-1, sek-1 and sod-1;sek-1 mutant animals, most DAF-16 protein was translocated into the nucleus (Fig. 4b–d).
Fig. 4.
DAF-16a2:GFP localization in the (A) wild-type strain, (B) sod-1 mutant, (C) sek-1 mutant and (D) sod-1;sek-1 mutant. (E) Left panel indicates a partial loss of ins-5 gene expression in the sek-1 deletion mutants. The predicted SKN-1 binding site was located 84 bp upstream of the first exon in ins-5 (data not shown). Right panel indicates the real-time PCR data of sod-3 and -5 in the ins-5 mutant. Data shown represent the means ± SEM. Means were compared by Student's t-test (*P < 0.05, **P < 0.0005, ***P < 0.0001). (F) Schematic diagram of sod gene regulation via the ins/IGF-1 and p38 MAPK signaling pathways.
3.7. Decreased expression of ins-5 gene with impaired p38 MAPK signaling
The mRNA levels of a gene encoding an insulin peptide, ins-5, were decreased in the sek-1 mutants compared with the wild-type strain (Fig. 4e). In the promoter region of ins-5, a SKN-1 binding site is located at 84 base pairs (bp) upstream of the first exon [18]. These findings support the view that ins-5 is a target gene of SKN-1 and its expression is upregulated via the p38 MAPK signaling.
4. Discussion
We previously reported that the compensatory expression of sod-5, which encodes a Cu/Zn SOD, in sod-1 mutants of C. elegans is regulated through the ins/IGF-1 signaling [9]. Expression of sod-1 and -5, which encode distinct Cu/Zn SOD isozymes, is tightly controlled and localized in the cytoplasm, lysosomes and mitochondrial intermembrane space [9,25]. However, it is unclear whether the compensatory expression of other sod genes likewise occurs in a sod-5 mutant. Here, we showed that several-fold higher expression of not only sod-1 encoding another Cu/Zn SOD isozyme, but also sod-2 and -3 encoding Mn SOD isozymes, and the sod-4 encoding an extracellular Cu/Zn SOD was induced in the sod-5 mutant. Despite the low expression levels of sod-5 in the wild-type strain [9], its transcriptional disruption was remarkably compensated for other sod genes. Therefore, we inferred that this molecular compensation was responsible for the only slight decrease in lifespan of the sod-5 mutant compared with the wild-type strain (but not the sod-1 mutants). The compensatory expression in the sod-5 and sod-1;sod-5 mutants did not affect net mitochondrial O2.- production, while it increased mitochondrial O2.- levels compared with the wild-type strain. These results indicate that the compensatory expression of other sod genes plays a role in the maintenance of longevity in the mutants through the removal of intracellular ROS including mitochondrial O2.- in the mutants; however, lifespan does not completely recover to that of the wild-type strain.
Both sod-3 and -5 are regulated via the ins/IGF-1 signaling pathway, which is associated with longevity and stress resistance in C. elegans [9,14]. DAF-16 is activated downstream of ins/IGF-1 signaling. We discovered putative DBE sequences, which the DAF-16 transcription factor binds to, in the promoter regions of sod-2, -3 and -5; however, the two DBE sequences in the promoter region of sod-2 do not seem to function according to previous studies using a daf-16 null mutant [9,14,15]. SKN-1 also has putative binding sites in the promoter regions of all sod genes, and is associated with longevity through the regulation of intracellular ROS levels during aging and caloric restriction in C. elegans [15,16,18,28]. We propose that sod-2 expression is predominantly upregulated by the SKN-1 transcription factor, and it is also associated with longevity in C. elegans under stressful intracellular conditions in which sod-1 and -5 are both deleted. In contrast, sod-3 and -5 expression is mainly upregulated by the DAF-16 transcription factor in response to reduced ins/IGF-1 signaling under stressful condition [16,26].
The lifespans in the sod-1 mutants were shortened compared with the wild-type strain [9,10]. This effect was enhanced by dysfunction of DAF-16; however, a deficiency of the p38 MAPK signaling had no effect on lifespan. In the sod-1 mutant, DAF-16 targets such as sod-3 and -5 play an important role in the maintenance of lifespan [9]. These findings indicate that sod-1 expression requires p38 MAPK signaling, and is almost entirely upregulated by downstream SKN-1 via a DAF-16-independent pathway. According to previous reports, the MAPK signaling cascade extends lifespan through the nuclear localization of SKN–I and downregulation of the ins/IGF-1 signaling during aging [17,19,20]. SKN-1 activated by its nuclear accumulation induces the transcriptional downregulation of insulin-like peptide genes, such as ins-7 and -39, and the consequent downregulation of the ins/IGF-1 signaling [20,21]. The C. elegans genome encodes 40 insulin-like peptides, which play distinct roles as agonists or antagonists of the ins/IGF-1-like receptor DAF-2 [29]. Furthermore, we found that the insulin peptide gene, ins-5, which has a SKN-1 binding site in its promoter region, functions as a putative agonist of DAF-2, at least in part, and is transcriptionally regulated via the p38 MAPK signaling [29]. Therefore, these results are consistent with the nuclear localization of DAF-16:GFP and the higher expression levels of the DAF-16 targets in the sek-1 mutant.
In addition to sod-1, we found that sod-2 and -4 are transcriptionally regulated as targets of SKN-1, which is a downstream component of the p38 MAPK signaling. A deficiency of the p38 MAPK signaling leads to the decreased expression of sod-1, -2 and -4, and instead, sod-3 and -5 expression is activated by the nuclear localization of DAF-16 under inactive ins/IGF-1 signaling. Unexpectedly, a simultaneous deficiency of SEK-1 in the sod-1 mutant did not lead to increased expression of sod-3 and -5 despite the nuclear localization of DAF-16. The reduced expression of these DAF-16 target sod genes may be associated with damage to transcription factors as a result of higher levels of intracellular oxidative stress due to increased levels of mitochondrial O2.-. Otherwise, other DAF-16 isoforms may have a role in the transcriptional regulation of target sod genes under more stressful conditions. In this study, we observed the subcellular localization of only DAF-16a2, but not other isoforms such as DAF-16b and DAF-16df [30]. A previous report showed the different effects of DAF-16a, including the DAF-16a2 isoform, on sod-3 and -5 expression [30]. We might be unable to observe the nuclear localization, which indicates activity of not DAF-16a but other DAF-16 isoforms, such as DAF-16b and DAF-16df, under more stressful conditions. However, this suggests a need to examine the roles of factors other than DAF-16a, including the DAF-16b and DAF-16df isoforms, in the systematic regulation of sod expression under conditions of more severe intracellular oxidative stress.
Authors' contributions
Sumino Yanase designed the study, and wrote the initial draft of the manuscript. Kayo Yasuda and Naoaki Ishii contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. All authors have contributed to data collection and interpretation, and critically reviewed the manuscript. Ultimately, all authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of competing interest
Nothing.
Acknowledgements
We are grateful to Dr. T. E. Johnson and P. Tedesco of the University of Colorado at Boulder for the kindly supplying the pGP30 plasmid; and Dr. S. Oshiro of Daito Bunka University for use of a luminometer. This study was financially supported by a grant-in-aid for scientific research to S.Y. from the Japanese Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 2.McCord J.M., Fridovich I. Superoxide dismutase. J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 3.Tolmasoff J.M., Ono T., Cutler R.G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc. Natl. Acad. Sci. U.S.A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loschen G., Azzi A., Richter C., Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 5.Larsen P.L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki N., Inokuma K., Yasuda K., Ishii N. Cloning, sequencing and mapping of a manganese superoxide dismutase gene of the nematode Caenorhabditis elagans. DNA Res. 1996;3:171–174. doi: 10.1093/dnares/3.3.171. [DOI] [PubMed] [Google Scholar]
- 7.Giglio M.-P., Hunter T., Bannister J.V., Bannister W.H., Hunter G.J. The manganese superoxide dismutase gene of Caenorhabditis elegans. Biochem. Mol. Biol. Int. 1994;33:37–40. [PubMed] [Google Scholar]
- 8.Fujii M., Ishii N., Joguchi A., Yasuda K., Ayusawa D. A novel superoxide dismutase gene encoding membrane-bound and extracellular isoforms by alternative splicing in Caenorhabditis elegans. DNA Res. 1998;5:25–30. doi: 10.1093/dnares/5.1.25. [DOI] [PubMed] [Google Scholar]
- 9.Yanase S., Onodera A., Tedesco P., Johnson T.E., Ishii N. SOD-1 deletions in Caenorhabditis elegans alter localization of intracellular reactive oxygen species and show molecular compensation. J. Gerontol. 2009;64A:530–539. doi: 10.1093/gerona/glp020. [DOI] [PubMed] [Google Scholar]
- 10.Yen K., Patel H.B., Lublin A.L., Mobbs C.V. SOD isoforms play no role in lifespan in ad lib or dietary restricted conditions, but mutational inactivation of SOD-1 reduces life extension by cold. Mech. Ageing Dev. 2009;130:173–178. doi: 10.1016/j.mad.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Doonan R., McElwee J.J., Marthijssens F., Walker G.A., Houthoofd K., Back P., Matscheski A., Vanfleteren J.R., Gems D. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raamsdonk J.M.V., Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:1–13. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuyama T., Nakazawa T., Nakano I., Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda Y., Honda S. Life span extensions associated with upregulation of gene expression of antioxidant enzymes in Caenorhabditis elegans; Studies of mutation in the AGE-1, PI3 kinase homologue and short-term exposure to hyperoxia. J. Amer. Aging Assoc. 2002;24:21–25. doi: 10.1007/s11357-002-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Back P., Matthijssens F., Vlaeminck C., Braeckman B.P., Vanfleteren J.R. Effects of sod gene overexpression and deletion mutation on the expression profiles of reporter genes of major detoxification pathways in Caenorhabditis elegans. Exp. Gerontol. 2010;45:603–610. doi: 10.1016/j.exger.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Panowski S.H., Wolff S., Aguilaniu H., Durieux J., Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 17.Inoue H., Hisamoto N., An J.H., Oliveira R.P., Nishida E., Blackwell T.K., Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An J.H., Blackwell T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M., Yanase S., Ishii T., Hartman P.S., Matsumoto K., Ishii N. The p38 signal transduction pathway participates in the oxidative stress-mediated translocation of DAF-16 to Caenorhabditis elegans nuclei. Mech. Ageing Dev. 2005;126:642–647. doi: 10.1016/j.mad.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Okuyama T., Inoue H., Ookuma S., Satoh T., Kano K., Honjoh S., Hisamoto N., Matsumoto K., Nishida E. The ERK MAPK pathway regulates longevity through SKN-1 and Insulin-like signaling in C. elegans. J. Biol. Chem. 2010;285:30274–30281. doi: 10.1074/jbc.M110.146274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira R.P., Abate J.P., Dilks K., Landis J., Ashraf J., Murphy C.T., Blackwell T.K. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell. 2009;8:524–541. doi: 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogg S., Paradis S., Gottlieb S., Patterson G.I., Lee L., Tissenbaum H.A., Ruvkun G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka-Hino M., Sagasti A., Hisamoto N., Kawasaki M., Nakano S., Ninomiya-Tsuji J., Bargmann C.I., Matsumoto K. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 2002;3:56–62. doi: 10.1093/embo-reports/kvf001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanase S., Yasuda K., Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech. Ageing Dev. 2002;123:1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- 25.Okado-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 26.Yanase S., Ishii N. Hyperoxia exposure induced hormesis decreases mitochondrial superoxide radical levels via Ins/IGF-1 signaling pathway in a long-lived age-1 mutant of Caenorhabditis elegans. J. Radiat. Res. 2008;49:211–218. doi: 10.1269/jrr.07043. [DOI] [PubMed] [Google Scholar]
- 27.Shimomura O., Wu C., Murai A., Nakamura H. Evaluation of five imidazopyrazinone-type chemiluminescent superoxide probes and their application to the measurement of superoxide anion generated by Listeria monocytogenes. Anal. Biochem. 1998;258:230–235. doi: 10.1006/abio.1998.2607. [DOI] [PubMed] [Google Scholar]
- 28.Bishop N.A., Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan R.E.W., Maxwell C.S., Codd N.K., Baugh L.R. Pervasive positive and negative feedback regulation of insulin-like signaling in Caenorhabditis elegans. Genetics. 2019;211:349–361. doi: 10.1534/genetics.118.301702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon E.-S., Narasimhan S.D., Yen K., Tissenbaum H.A. A new DAF-16 isoform regulates longevity. Nature. 2010;466:498–502. doi: 10.1038/nature09184. [DOI] [PMC free article] [PubMed] [Google Scholar]