Main Text
Chromosomes have a complex spatial organization in the cell nucleus (1,2), as revealed by new high-throughput technologies (3,4). Such an organization serves vital functional purposes as genes have to establish physical contacts with their distal DNA regulators to control transcriptional activities. Mutations producing architectural rearrangements can rewire those regulatory interactions and induce severe human diseases (5). However, the physical and molecular mechanisms whereby those contacts are formed and controlled across genomic scales remain to be understood.
To explain the complexity of chromosomes’ architectural data, two main models from polymer physics have been introduced to date that are supported by growing experimental evidence. The loop-extrusion model envisages that a molecular complex acts as an active motor extruding DNA loops between cognate anchor points in a nonequilibrium process requiring energy influx by, e.g., ATP molecule consumption (6). In another scenario, recapitulated by the Strings and Binders model (7), the interactions between DNA sites are mediated by diffusing cognate binding molecules that can bridge those sites. DNA-molecule interactions cause thermodynamics phase transitions, resulting in structural changes of the DNA chain, such as coil-to-globule or phase-separation transitions. The system architectural reorganization, in turn, spontaneously establishes the contact or segregation between specific distal DNA sites, such as genes, enhancers, or insulators (8, 9, 10).
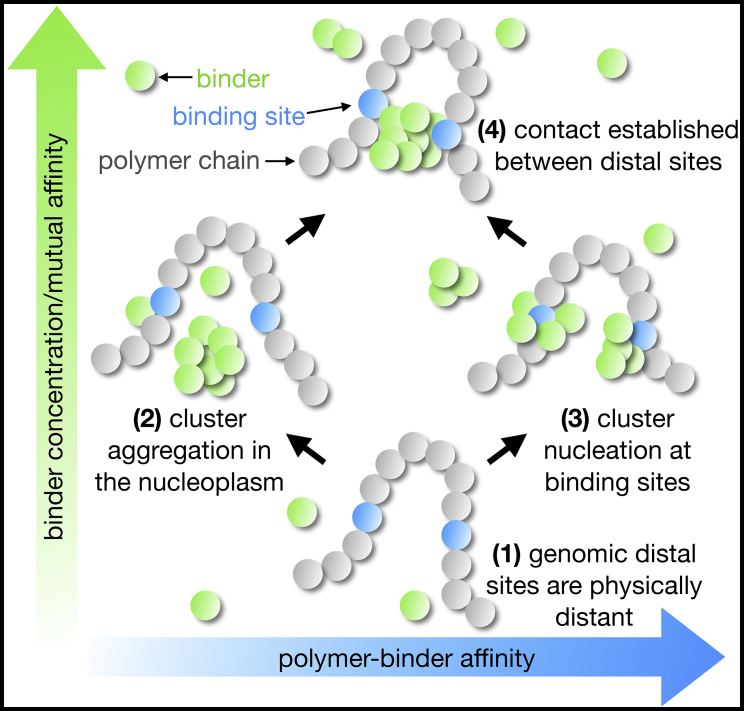
In this issue of Biophysical Journal, Chiariello and co-workers (11) make an important step within the latter framework. They investigate how a system of mutually interacting molecules, which can also bind to sites along the chromatin fiber, can self-assemble in aggregates by phase separation, hence producing stable, robust bridges between distant regulatory elements, such as gene promoters and enhancers. An interesting aspect is that binding sites along the DNA chain can locally nucleate cluster growth. Those molecular clusters emerge under suitable conditions of molecules concentration, DNA binding sites abundance, and molecule-molecule and molecule-DNA interactions. In particular, as predicted by the theory of phase separation, the clusters can form, within different dynamical regimes, either by nucleation at DNA binding sites or by spontaneous aggregation in the nucleoplasm, next bridging cognate DNA sites (see Fig. 1). Interestingly, those mechanisms could also play a role in other biological processes, such as in the symmetry breaking events, leading to X chromosome inactivation (12).
Figure 1.
Thermodynamic mechanisms of recognition at a distance. Contacts between genomically distal polymer sites (1) can be established by different thermodynamics routes. Clusters of bridging molecules (binders), according to their concentrations and affinities, can spontaneously aggregate in the nucleoplasm (2) or nucleate at binding sites along the polymer chain (3), eventually producing stable contacts between those sites (4). Such thermodynamic phase transition mechanisms could explain how distal recognition and physical proximity are established and controlled between, e.g., gene promoters and their enhancers. To see this figure in color, go online.
Chiariello and co-workers considered a minimal toy model of molecule-DNA interactions, based on the concept of universality in phase transitions. More complex molecular systems can exhibit a variety of thermodynamics phases and related structures, which could be relevant to different biological situations. It also remains to be clarified under which circumstances near equilibrium can be reached in the nucleoplasm. Nevertheless, thermodynamics phase transitions and molecular self-assembling are reliable and reversible organizational mechanisms, requiring no fine-tuning of, e.g., concentrations and affinities, and no energy inputs beyond the thermal bath. They can be implemented in the cell via simple biological processes, such as upregulation of genes transcribing for binding molecules or epigenetic modifications of DNA sites, to transit the system across the threshold point in a different structural phase.
Editor: Tamar Schlick.
References
- 1.Dekker J., Mirny L. The 3D genome as moderator of chromosomal communication. Cell. 2016;164:1110–1121. doi: 10.1016/j.cell.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon J.R., Gorkin D.U., Ren B. Chromatin domains: the unit of chromosome organization. Mol. Cell. 2016;62:668–680. doi: 10.1016/j.molcel.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigal Y.M., Zhou R., Zhuang X. Visualizing and discovering cellular structures with super-resolution microscopy. Science. 2018;361:880–887. doi: 10.1126/science.aau1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempfer R., Pombo A. Methods for mapping 3D chromosome architecture. Nat. Rev. Genet. 2020;21:207–226. doi: 10.1038/s41576-019-0195-2. [DOI] [PubMed] [Google Scholar]
- 5.Spielmann M., Lupiáñez D.G., Mundlos S. Structural variation in the 3D genome. Nat. Rev. Genet. 2018;19:453–467. doi: 10.1038/s41576-018-0007-0. [DOI] [PubMed] [Google Scholar]
- 6.Banigan E.J., Mirny L.A. Loop extrusion: theory meets single-molecule experiments. Curr. Opin. Cell Biol. 2020;64:124–138. doi: 10.1016/j.ceb.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Nicodemi M., Prisco A. Thermodynamic pathways to genome spatial organization in the cell nucleus. Biophys. J. 2009;96:2168–2177. doi: 10.1016/j.bpj.2008.12.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco S., Chiariello A.M., Nicodemi M. Computational approaches from polymer physics to investigate chromatin folding. Curr. Opin. Cell Biol. 2020;64:10–17. doi: 10.1016/j.ceb.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Conte M., Fiorillo L., Nicodemi M. Polymer physics indicates chromatin folding variability across single-cells results from state degeneracy in phase separation. Nat. Commun. 2020;11:3289. doi: 10.1038/s41467-020-17141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu, J.-K., C. Bouchoux, …, C. Dekker. Phase separation induced by cohesin SMC protein complexes. bioRxiv doi: 10.1101/2020.06.13.149716. [DOI] [PMC free article] [PubMed]
- 11.Chiariello A.M., Corberi F., Salerno M. The interplay between phase-separation and gene-enhancer communication: a theoretical study. Biophys. J. 2020;119:873–883. doi: 10.1016/j.bpj.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicodemi M., Prisco A. Symmetry-breaking model for X-chromosome inactivation. Phys. Rev. Lett. 2007;98:108104. doi: 10.1103/PhysRevLett.98.108104. [DOI] [PubMed] [Google Scholar]