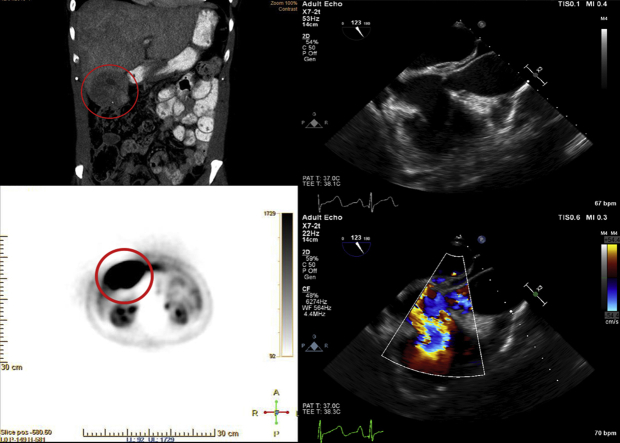
Graphical abstract
Keywords: Carcinoid, Neuroendocrine, Tricuspid regurgitation, Pulmonic regurgitation, Transesophageal echocardiography
Highlights
-
•
Carcinoid heart disease typically involves the right-sided valves of the heart.
-
•
Carcinoid valves are fixed and retracted with limited mobility.
-
•
Pathognomonic appearance of valves increases carcinoid likelihood.
-
•
Carcinoid diagnosis has intraoperative implications.
Introduction
“Carcinoid” has historically referred to well-differentiated neuroendocrine tumors (NETs) that originate in the digestive tract, lungs, or rarely kidneys and ovaries. Active carcinoid tumors are well known for secreting vasoactive hormones and peptides. They can present in several different ways, including carcinoid syndrome, which consists of diarrhea and flushing, effects of tumor growth such as abdominal pain or hepatomegaly, or as an incidental finding on radiographic studies.1
We report the case of a previously healthy, active woman with a recent history of shortness of breath and lower extremity edema who was to undergo tricuspid annuloplasty for isolated tricuspid regurgitation (TR) but was found to have cardiac manifestations of advanced carcinoid diagnosed intraoperatively.
Case Presentation
A 50-year-old woman with no significant medical history presented to an outside emergency department with a history of increasing shortness of breath and fatigue for several weeks, bilateral pedal edema, and new chest pain with orthopnea. She was diagnosed with acute right heart failure, promptly treated with diuretics, and eventually evaluated by a community cardiologist in the outpatient setting. Subsequent transthoracic echocardiography, which was performed at an outside facility (images unavailable), reported right ventricular (RV) dilatation, right atrial enlargement, and severe TR with mild thickening of the tricuspid leaflets and a normal pulmonic valve. In addition, left ventricular size and function were normal, and no other significant left-sided valvular disease was noted. The patient had also undergone a nuclear stress test that revealed mild anteroapical ischemia, but subsequent heart catheterization demonstrated normal coronary arteries. At this time, the patient was diagnosed with isolated TR and referred to our facility by her cardiologist for minimally invasive tricuspid repair given the patient's wishes to avoid a sternotomy. On the basis of the information provided from the outside facility, the patient's age, and the lack of comorbidities, she was deemed to be of low surgical risk for durable tricuspid repair using a right mini-thoracotomy approach.
On the day of surgery, the patient was brought to the operating room, and after placement of invasive monitors, general endotracheal anesthesia was induced. During induction and just after muscle relaxant administration, the patient developed an erythematous rash and flushing localized to the chest, but no evidence of bronchospasm or significant hypotension followed. Because of expected tricuspid pathology, pulmonary artery catheterization was deferred for this procedure, but a standard transesophageal echocardiographic probe (X2-7t 3D Matrix; Philips Medical Systems, Andover, MA) was placed. Comprehensive intraoperative TEE was performed on the patient before any surgical incision.
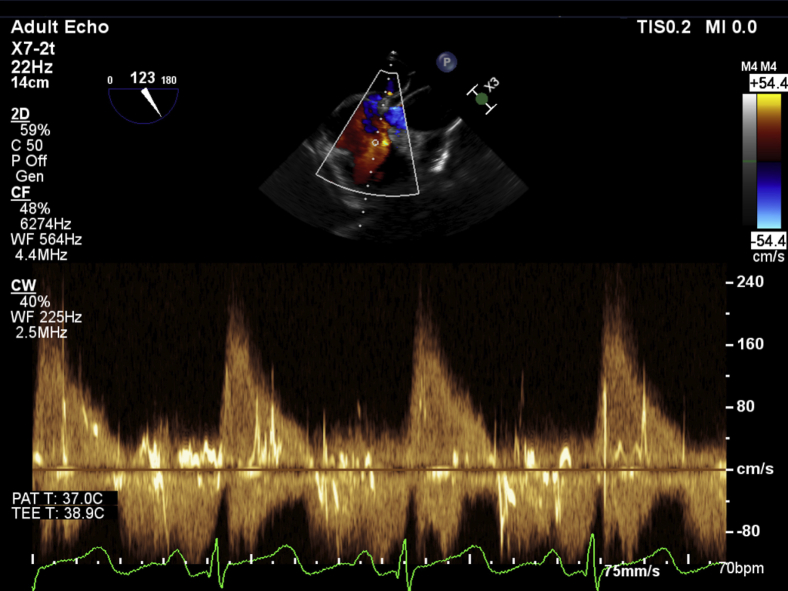
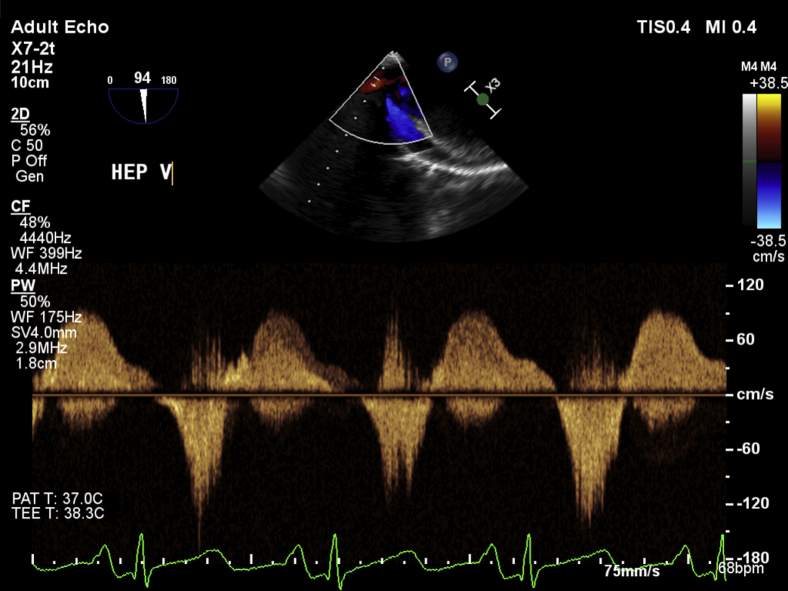
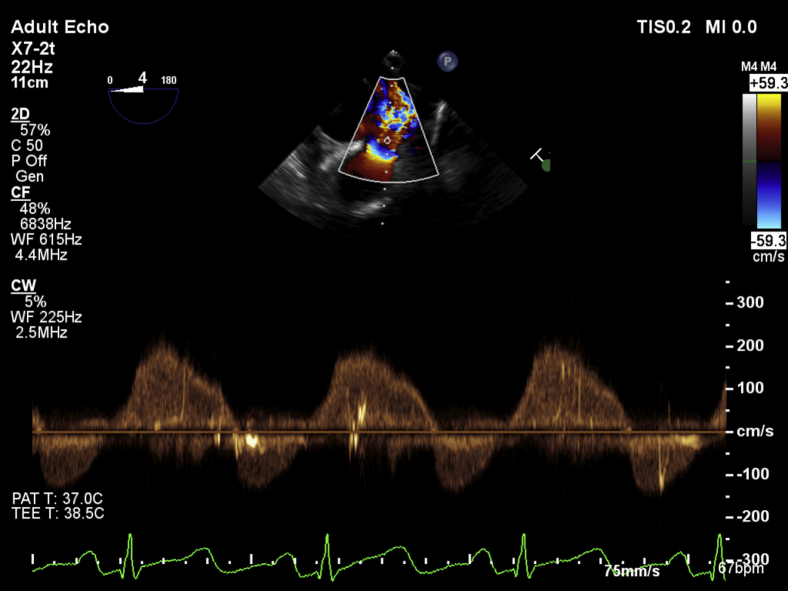
The midesophageal four-chamber view at a multiplane angle of 0° to 12° demonstrated a severely dilated right ventricle with good systolic function assessed by RV fractional area of change of 49% and a severely dilated right atrium with severe tricuspid annular dilatation, fixed tricuspid leaflets, and severe regurgitation. The RV free wall was of normal thickness. Significant bowing of the interatrial septum into the left atrium suggested the presence of elevated right-sided filling pressures. Bubble contrast seen in the images arose from rapid intravenous fluid administration during intraoperative care of the patient (Video 1). Color flow Doppler (CFD) assessment of the interatrial septum did not demonstrate any evidence of intra-atrial shunting or the presence of a patent foramen ovale. In the midesophageal RV inflow-outflow (70°–90°) view and the modified bicaval view (110°–120°), the tricuspid leaflets were found to be fixed and retracted without any mobility throughout the cardiac cycle (Video 2). There was also severe thickening isolated to the posterior leaflet and posterior annulus of the tricuspid valve, particularly noticeable in the modified bicaval view (Video 3). CFD interrogation of the tricuspid valve again demonstrated severe regurgitation of low velocity and laminar flow. Continuous-wave Doppler interrogation revealed a dense triangular jet (“sail sign”) in systole representing the rapid equilibration of chamber pressures (Figure 1). RV systolic pressure measured from the TR jet was 34 mm Hg (on the basis of an invasive central venous pressure of 12 mm Hg) but was likely to be an underestimate because of the severe insufficiency of the tricuspid valve. TEE also revealed a dilated inferior vena cava and flow reversal in the hepatic vein on CFD (Video 4, Figure 2). The midesophageal RV inflow-outflow view revealed pulmonic valve involvement. The pulmonic valve leaflets were found to be fixed, retracted, and immobile. CFD and continuous-wave Doppler assessment of the RV outflow tract revealed severe pulmonic regurgitation (Video 5, Figure 3). Imaging of the left heart noted a normal mitral valve, a trileaflet aortic valve with mild central regurgitation, and a normally functioning left ventricle. In the transgastric midpapillary view, septal flattening was noted in diastole consistent with RV volume overload and a small circumferential pericardial effusion. In a modified transgastric midpapillary view with counterclockwise rotation, the spleen could be clearly visualized, surrounded by considerable abdominal ascites (Video 6). These findings on TEE were most consistent with the cardiac sequelae of carcinoid syndrome. Before any surgical incision, the operative team, including the cardiac anesthesiologist, cardiac surgeon, and operating room staff members, discussed options for this patient, as two heart valves were affected. Further physical examination of the patient's abdomen under anesthesia noted a hard mass in the right upper quadrant below the liver edge. The new findings on TEE of two affected right-sided heart valves, the previous episode of flushing linked with the development of a rash, and a new right upper quadrant mass not noted previously increased the surgical team's suspicion of an undiagnosed carcinoid malignancy. These findings led to a decision to abort the start of the surgical procedure and arrange for the patient to be further evaluated by a medical team. Urinary 5-hydroxyindoleacetic acid and metanephrine were sent for suspicion of a carcinoid tumor intraoperatively.
Figure 1.
Continuous-wave Doppler through the tricuspid valve shows a dense triangular jet, the “sail sign,” characteristic of severe TR.
Figure 2.
Continuous-wave Doppler shows reversal of flow in hepatic vein characteristic of severe TR.
Figure 3.
Continuous-wave Doppler echocardiography of the pulmonic valve shows a triangular-shaped jet in diastole, characteristic of severe regurgitation.
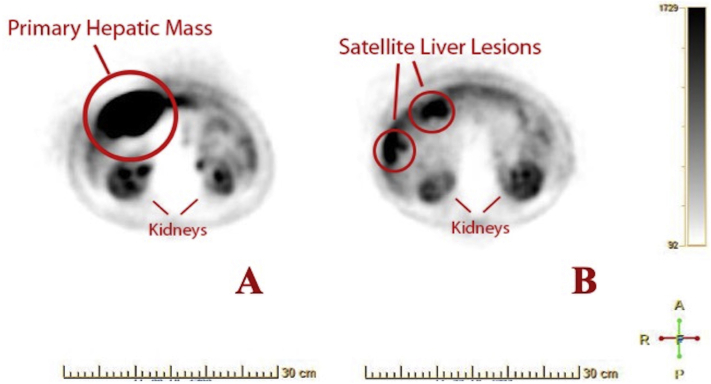
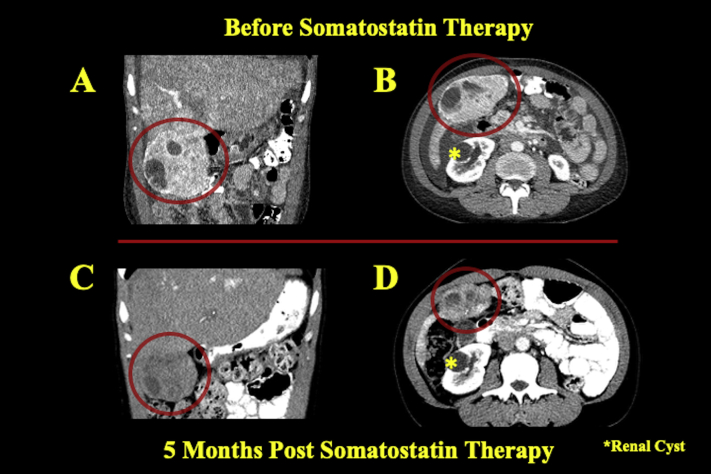
After cancellation of surgery, the patient was referred to the hematology-oncology service for further diagnostic workup and medical management. Diagnostic biochemical markers, 5-hydroxyindoleacetic acid, and serum chromogranin were elevated at 277 mg/24 h and 19,950 ng/mL, respectively. Imaging studies including positron emission tomography demonstrated a highly active subhepatic mass with satellite lesions of the liver (Figures 4A and 4B). Abdominal contrast computed tomography showed a 9.6-cm exophytic mass in the right lower hepatic lobe in cross and coronal sections (Figures 5A and 5B). Tumor biopsy samples confirmed a well-differentiated NET with tumor marker Ki-67 index of 0.6%, indicating histologic grade 1 cancer. Consequently, the patient was placed on lanreotide, a long-acting somatostatin analog. Repeat computed tomography 5 months later demonstrated a decrease in the size of the liver lesions corresponding to response to somatostatin therapy (Figures 5C and 5D). The patient's chromogranin A levels accordingly fell significantly to just 760 ng/mL. Six months after somatostatin therapy initiation, follow-up transthoracic echocardiography revealed mild to moderate RV dilatation but normal function. The tricuspid leaflets still appeared fixed and retracted, with severe TR. CFD imaging of the inferior vena cava and hepatic vein spectral Doppler demonstrated persistent flow reversal (Video 7). On a modified apical five-chamber view, the pulmonic valve exhibited reduced mobility of the pulmonic leaflets without stenosis, but there has been a marked improvement in pulmonic insufficiency noted by the reduced regurgitant flow from CFD imaging and the significantly reduced density of the regurgitant jet profile on continuous-wave Doppler (Video 8).
Figure 4.
(A) Fluorine-18 fluorodeoxyglucose positron emission tomographic image shows high glucose uptake in the hepatic lesion consistent with cancerous activity. (B) High-uptake areas in more superior portions of the liver revealed the presence of metastatic satellite lesions. Kidneys are labeled in both images for anterior-posterior reference.
Figure 5.
Coronal (A) and axial (B) computed tomographic images show a large hypoattenuating hepatic mass in the right lower lobe representing the primary tumor before somatostatin analog therapy. After 5 months on therapy, repeat coronal (C) and axial (D) computed tomography shows considerable reduction in size of the mass. An incidental renal cyst is labeled (asterisk) as a marker of axial level.
As the patient reported significant improvement of her cardiac symptoms, valve replacement surgery is no longer anticipated in the near future. Given the favorable response of the liver mass and satellite lesions to long-acting somatostatin analog therapy, hepatic resection surgery is no longer in consideration. The patient will remain on lanreotide lifelong. Radio-immunotherapy with 177Lu peptide receptor radionuclide therapy may be considered if the tumor becomes refractory to somatostatin analogs.
Discussion
Carcinoid syndrome is caused by the secretion of hormones and vasoactive peptides such as serotonin, histamines, and bradykinins from NETs.1 NETs often arise in the gastrointestinal tract or bronchi, and the liver inactivates the secretions, causing most patients to be asymptomatic.1 However, when liver metastases occur, NET secretions enter the bloodstream, producing clinical manifestations known as carcinoid syndrome, which consists of flushing, bronchospasm, rash, diarrhea, and/or hypotension.1 Ovarian and lung tumors are exceptions. Because their venous drainage bypasses the liver, NET secretions can cause carcinoid symptoms before the occurrence of liver metastases.2
A late complication of carcinoid syndrome is carcinoid heart disease, also called Hedinger syndrome.3 Carcinoid heart disease classically involves the right heart, because the bronchoalveolar tissue inactivates serotonin.4 Fibrous plaques become deposited onto the right sided heart valves, papillary muscles, and chordae tendineae.3 Isolated TR is the classic lesion, with the appearance of thickened and retracted valve leaflets.3 Leaflet thickening may present diffusely in all three leaflets or can be isolated to a single leaflet, as seen in the posterior leaflet of our patient.5 The valve may eventually become immobile, causing it to be in a fixed position, which may cause tricuspid stenosis. In addition, the pulmonic valve often develops the same characteristic leaflets with the potential of developing regurgitation and/or stenosis,3 which was a new finding in our patient not seen on preoperative transthoracic echocardiography by report. In many patients with carcinoid syndrome, plaque deposition in the RV free wall can create the appearance of RV hypertrophy, but this was not evident in our patient.5
Carcinoid heart disease greatly decreases survival, and surgery is indicated for patients with symptoms of congestive heart failure uncontrolled by medical management.2 Valvular surgery improves survival time for patients with carcinoid heart disease. Only 10% of medically managed patients with carcinoid heart disease with symptoms greater than New York Heart Association functional class II survive >2.5 years. Comparatively, patients with carcinoid heart disease in New York Heart Association functional classes III and IV who undergo valvular surgery have survival rates of 69%, 35%, and 24% at 1, 5, and 10 years, respectively.6 Surgery provides the majority of patients with carcinoid heart disease with a functional improvement in symptoms. Furthermore, the valvular surgery should include a multidisciplinary approach with experienced physicians in order to provide decreased operative mortality.6 Fortunately, our patient responded favorably to somatostatin therapy and will not currently require cardiac surgery.
In our particular patient, the intraoperative echocardiographic findings ultimately changed the patient's diagnosis from isolated severe TR to possible carcinoid heart disease given the appearance of the valves as described. We can only speculate that the outside transthoracic echocardiography was performed early in her condition. Aside from dyspnea with exertion, she did not initially report any other typical carcinoid symptoms (flushing, diarrhea). The medical workup at the outside facility was >2 months earlier, before surgery. The disease may have progressed during this time to involve the pulmonic valve. Our case clearly illustrates the benefits of comprehensive intraoperative TEE before surgical incision by an experienced echocardiographer, particularly in cardiac valve surgery. A previous study of cardiac surgical patients showed that prebypass TEE influenced surgical decisions during isolated valvular procedures in 6.3% of patients.7 Hence, the American Society of Anesthesiologists guidelines recommend intraoperative TEE in all cardiac surgical procedures to help refine the preoperative echocardiographic reading, diagnose new pathology, assist in the surgical and anesthetic plan, and assess postprocedural results.8
The change in our diagnosis had important implications in our patient's care. Her new diagnosis of a NET with carcinoid heart disease would have changed the planned surgical procedure from a minimally invasive approach to a full sternotomy for a double valve replacement. The type of valve (bioprosthetic vs mechanical) also needed to be discussed, as each valve type had new clinical implications.
The use of bioprosthetic versus mechanical valves has been a topic of controversy. Mechanical valves require lifelong anticoagulation, which may pose an increased risk for patients undergoing additional oncologic surgery or chemotherapy.9 Although bioprosthetic valves allow avoidance of permanent anticoagulation, new fibrous plaques may deposit on bioprosthetic valves because of continual circulation of NET secretions and lead to eventual degeneration of the valve.10 Although rare, plaque deposition may occur at 3 to 8 years postoperatively.11 Median survival time after valve replacement has been estimated to be 6 to 11 years.3
Patients with carcinoid syndrome often require surgery for primary tumor removal. Medical optimization before anesthesia is essential for patients with carcinoid tumors to prevent an intraoperative carcinoid crisis. Carcinoid crisis is an exaggerated form of carcinoid syndrome with symptoms such as flushing, bronchospasm, tachycardia, and hypotension that may be unresponsive to typical inotropic or vasoactive therapy.11 Stressors such as the induction of general anesthesia, tumor manipulation, and/or certain drugs can provoke a crisis.4 Clinicians should avoid histamine-releasing drugs such as morphine, meperidine, and atracurium.4 Additionally, succinylcholine, β2 agonists, and drugs that promote excessive catecholamine release may lead to NET secretion.12 To optimize hemodynamics, the somatostatin analog octreotide is recommended before surgery. Somatostatin analogs inhibit secretions of growth hormone, insulin, somatostatin, thyrotropin, and likely all intrinsic and extrinsic gut hormones. Multiple different preoperative regimens exist, but sources agree that the preoperative dose should be modified to minimize carcinoid symptoms.4,13 Typically, the patient will be started on subcutaneous octreotide with thrice-daily dosing for initial stabilization and then switched to long-acting depot octreotide injections.13 Additionally, a preinduction depot dose is recommended to minimize risk for carcinoid crisis.13 Intraoperatively, episodes of flushing, bronchospasm, or hypotension may be treated with 500- to 1,000-μg intravenous boluses of octreotide.14 If an intraoperative bolus dose is required, a postoperative infusion of 50 to 200 μg/h should be continued for 24 hours.13 Phenylephrine and vasopressin have also proved safe for use in conjunction with octreotide for hypotension. Research has shown that the operative course cannot be predicted by the severity of preoperative carcinoid symptoms or the level of elevation of urinary 5-hydroxyindoleacetic acid.12 Therefore, similar precautions should be taken with all patients undergoing surgery who have carcinoid tumors.
The optimal timing of cardiac surgery for carcinoid heart disease has yet to be determined.9 However, surgery is the only definitive treatment for patients with severe symptomatic carcinoid heart disease and has been shown to decrease mortality. Most patients with well-controlled carcinoid and life expectancy of >1 year are considered for surgery if they are symptomatic or have evidence of ventricular dysfunction.15 Medically, carcinoid heart disease may be managed with loop diuretic therapy and fluid restriction to help minimize edema and volume overload.9 A somatostatin analog may help prevent carcinoid symptoms, but it has not been shown to slow progression of carcinoid heart disease.15
Conclusion
NETs such as carcinoid can present in a multitude of different ways. It is important to use the available imaging resources to further investigate what may seem like an isolated finding in an otherwise healthy patient. The findings of a fixed, retracted tricuspid valve leaflets, severe TR, RV dilatation, and/or hypertrophy on echocardiography in an otherwise healthy young patient should prompt close evaluation of all the other valves as well as a thorough physical examination looking for pathology.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.05.003.
Supplementary Data
Midesophageal four-chamber view shows a severely dilated right ventricle and severely dilated right atrium with bowing of the interatrial septum. The tricuspid valve leaflets are fixed and thickened. CFD depicts severe TR. Also note that the mitral valve appears normal.
Midesophageal RV inflow-outflow view shows CFD of the tricuspid valve with a large regurgitant orifice. Tricuspid valve leaflets are retracted and lack mobility throughout the cardiac cycle.
Modified bicaval view shows CFD over the tricuspid valve depicting severe TR with low velocity and laminar flow. The tricuspid valve leaflets are fixed without coaptation. Note that leaflet thickening appears isolated to the posterior leaflet and posterior tricuspid annulus.
TEE shows that the inferior vena cava is dilated with minimal inspiratory collapse.
CFD of the RV inflow-outflow tract shows severe pulmonic valve regurgitation. The pulmonic valve leaflets are thick, fixed, and immobile. Note that the aortic valve appears normal.
Transgastric midpapillary view demonstrates a small pericardial effusion and diastolic septal flattening. Counterclockwise rotation reveals abdominal ascites surrounding the spleen.
Transthoracic echocardiographic apical four-chamber view of the right heart with color flow comparison shows fixed tricuspid leaflets and severe TR at 6 months after medical therapy. CFD and spectral Doppler of the inferior vena cava and hepatic vein demonstrate flow reversal consistent with severe TR.
Modified transthoracic echocardiographic apical five-chamber view focusing on the pulmonic valve with CFD comparison reveals decreased pulmonic regurgitation (PR). Continuous-wave Doppler (CWD) interrogation of the pulmonic valve demonstrates a shortened regurgitation time in diastole and diminished density of the spectral Doppler profile, also indicating decreased PR. The triangular shape of the jet is due to overlap with tricuspid E-wave CWD velocity profile using this modified five-chamber view.
References
- 1.Rubin de Celis Ferrari A.C., Glasberg J., Riechelmann R.P. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics. 2018;73(suppl 1):e490s. doi: 10.6061/clinics/2018/e490s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ockert D.B.M., White R.D. Anesthetic management of patients with carcinoid heart disease undergoing cardiac surgery: two case reports and a review of previous experience. J Card Anesth. 1988;2:658–665. doi: 10.1016/0888-6296(88)90060-9. [DOI] [PubMed] [Google Scholar]
- 3.Ram P., Penalver J.L., Lo K.B.U., Rangaswami J., Pressman G.S. Carcinoid heart disease: review of current knowledge. Texas Heart Inst J. 2019;46:21–27. doi: 10.14503/THIJ-17-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalal A., Stone M. Intraoperative management of carcinoid heart disease for valvular surgery. J Cardiothorac Vasc Anesth. 2016;30:1046–1049. doi: 10.1053/j.jvca.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya S., Toumpanakis C., Burke M., Taylor A.M., Caplin M.E., Davar J. Features of carcinoid heart disease identified by 2- and 3-dimensional echocardiography and cardiac MRI. Circ Cardiovasc Imaging. 2010;3:103–111. doi: 10.1161/CIRCIMAGING.109.886846. [DOI] [PubMed] [Google Scholar]
- 6.Connolly H.M., Schaff H.V., Abel M.D., Rubin J., Askew J.W., Li Z. Early and late outcomes of surgical treatment in carcinoid heart disease. J Am Coll Cardiol. 2015;66:2189–2196. doi: 10.1016/j.jacc.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Eltzschig H.K., Rosenberger P., Löffler M., Fox J.A., Aranki S.F., Shernan S.K. Impact of intraoperative transesophageal echocardiography on surgical decisions in 12,566 patients undergoing cardiac surgery. Ann Thorac Surg. 2008;85:845–852. doi: 10.1016/j.athoracsur.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Thys D.M., Brooker R.F., Cahalan M.K., Connis R.T., Duke P.G., Nickinovich D.G. Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084–1096. doi: 10.1097/ALN.0b013e3181c51e90. [DOI] [PubMed] [Google Scholar]
- 9.Hayes A.R., Davar J., Caplin M.E. Carcinoid heart disease: a review. Endocrinol Metab Clin North Am. 2018;47:671–682. doi: 10.1016/j.ecl.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Hollander K.N., Joshi B.L. Bioprosthetic valve thrombosis in carcinoid heart disease. Ann Card Anaesth. 2019:79. doi: 10.4103/aca.ACA_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen A., Schaff H.V., Abel M.D., Luis S.A., Lahr B.D., Halfdanarson T.R. Improving outcome of valve replacement for carcinoid heart disease. J Thorac Cardiovasc Surg. 2019;158:99–107.e2. doi: 10.1016/j.jtcvs.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Kromas M.L., Passi Y., Kuzumi C., Shikhar S. Intra-operative carcinoid crisis: revised anaesthesia management. Ind J Anaesth. 2017;61:443–444. doi: 10.4103/ija.IJA_161_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borna R.M., Jahr J.S., Kmiecik S., Mancuso K.F., Kaye A.D. Pharmacology of octreotide: clinical implications for anesthesiologists and associated risks. Anesthesiol Clin. 2017;35:327–339. doi: 10.1016/j.anclin.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Rebêlo I., Maurício S., Coelho D., Gaspar A. Anesthetic management of carcinoid syndrome: is octreotide enough? A case report. Rev Mex Anestesiol. 2019;42:133–136. [Google Scholar]
- 15.Møller J.E., Connolly H.M., Rubin J., Seward J.B., Modesto K., Pellikka P.A. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348:1005–1015. doi: 10.1056/NEJMoa021451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Midesophageal four-chamber view shows a severely dilated right ventricle and severely dilated right atrium with bowing of the interatrial septum. The tricuspid valve leaflets are fixed and thickened. CFD depicts severe TR. Also note that the mitral valve appears normal.
Midesophageal RV inflow-outflow view shows CFD of the tricuspid valve with a large regurgitant orifice. Tricuspid valve leaflets are retracted and lack mobility throughout the cardiac cycle.
Modified bicaval view shows CFD over the tricuspid valve depicting severe TR with low velocity and laminar flow. The tricuspid valve leaflets are fixed without coaptation. Note that leaflet thickening appears isolated to the posterior leaflet and posterior tricuspid annulus.
TEE shows that the inferior vena cava is dilated with minimal inspiratory collapse.
CFD of the RV inflow-outflow tract shows severe pulmonic valve regurgitation. The pulmonic valve leaflets are thick, fixed, and immobile. Note that the aortic valve appears normal.
Transgastric midpapillary view demonstrates a small pericardial effusion and diastolic septal flattening. Counterclockwise rotation reveals abdominal ascites surrounding the spleen.
Transthoracic echocardiographic apical four-chamber view of the right heart with color flow comparison shows fixed tricuspid leaflets and severe TR at 6 months after medical therapy. CFD and spectral Doppler of the inferior vena cava and hepatic vein demonstrate flow reversal consistent with severe TR.
Modified transthoracic echocardiographic apical five-chamber view focusing on the pulmonic valve with CFD comparison reveals decreased pulmonic regurgitation (PR). Continuous-wave Doppler (CWD) interrogation of the pulmonic valve demonstrates a shortened regurgitation time in diastole and diminished density of the spectral Doppler profile, also indicating decreased PR. The triangular shape of the jet is due to overlap with tricuspid E-wave CWD velocity profile using this modified five-chamber view.