Abstract
The D-dimer test is a component of the modified scoring criteria for periprosthetic joint infection (PJI). The performance of the D-dimer test varies greatly among laboratories because of the lack of standardization. Laboratories may use different assays and will produce widely varying results for the same sample. This study used published proficiency testing data from 3903 laboratories to demonstrate the variability in D-dimer results and estimate the misclassification rate of patients using the proposed cutoff for the test as a component of PJI criteria. Given the variability in D-dimer results, a clinically significant percentage of patients are likely to be misclassified. The data illustrate that a universal cutoff for this marker in the context of assessment for PJI is not appropriate. Each site must conduct a study to determine an appropriate cutoff for their unique testing platform.
Keywords: D-dimer, Periprosthetic joint infection
Introduction
D-dimer is formed when cross-linked fibrin is degraded by plasmin. It is elevated not only in patients with venous thromboembolism or with disseminated intravascular coagulation but also in patients with recent trauma or surgery. D-dimer is measured in the clinical laboratory to aid in diagnosis of venous thromboembolism/pulmonary embolism or disseminated intravascular coagulation. In brief, D-dimer is a challenging analyte to measure. D-dimer is reported in 2 unit types (D-dimer units and fibrinogen equivalent unit [FEU], which differ from each other by a factor of approximately 2), as well as multiple unit magnitudes (eg, ng/mL, μg/mL). This leads to potential confusion when comparing results between laboratories [1]. Further complicating D-dimer interpretation is the lack of standardization between different D-dimer assays, which is related to differing antibody specificity and the lack of a common assay calibrator, among other factors [[1], [2], [3]].
The D-dimer test is a component of the proposed modified scoring criteria for diagnosis of periprosthetic joint infection (PJI) [4]. Some authors claim that newer markers for PJIs have not been sufficiently studied, despite prior claims of sufficient data to include them in the scoring-based definition [5]. The lack of standardization of this test among laboratories leads to significant variability in results, which will impact the external validity of the proposed PJI criteria across institutions [[1], [2], [3]]. The objective of this study is to demonstrate variation in D-dimer results obtained from laboratories across the country and to estimate the misclassification rate of the proposed PJI criteria cutoff of 860 FEU ng/mL [4]. Of note, the unit type (D-dimer units or FEU) for the proposed PJI criteria was not stated by the authors [4].
Methods
Monte Carlo simulation was used to estimate the distribution of D-dimer results that would be obtained across the country from a single sample with a particular D-dimer concentration. We did this in 2 steps. First, we randomly selected an instrument/reagent combination (testing platform). The testing platform was weighted by the prevalence of a particular platform in the College of American Pathologists (CAP) 2019 CGL-C proficiency testing survey. For example, if platform X accounted for 50 of 1000 instruments in the survey, the probability of selecting that platform was 5 percent (50/1000). Then, assuming the result for each platform followed a truncated normal distribution (mean ± 2 standard deviations), we randomly generated a D-dimer result using the mean and standard deviation for that platform. Because different platforms report results in different units according to individual manufacturer specifications, we converted all results in this simulation to a common set of units (FEU ng/ml units). This procedure was repeated 100,000 times to simulate the distribution of results that would be obtained from sending 100,000 identical samples to laboratories across the country. We then used the simulated distribution to determine the percentage of results would be misclassified relative to the proposed PJI cutoff of 860 FEU ng/mL, given a particular true concentration of D-dimer standardized to a single unit type.
Results
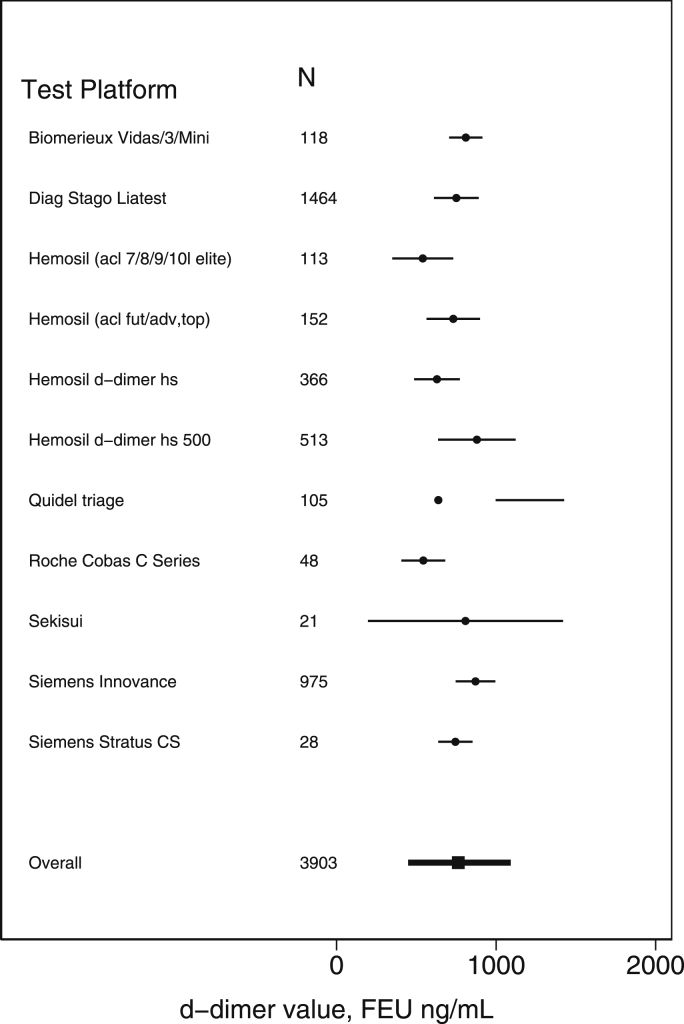
The CAP proficiency testing survey obtained results from 3903 different laboratories using 11 different platforms (Fig. 1). Given identical samples, the mean D-dimer value varied from 540 to 880 FEU ng/mL depending on the platform. In addition, each platform showed considerable variability across sites. Over all platforms, the estimated standard deviation ranged from 32 to 312 FEU ng/mL.
Figure 1.
Distribution of D-dimer results. The figure shows the distribution of D-dimer results that were obtained from a survey conducted by the College of American Pathologists. The distribution reflects results from 3903 sites using 11 different platforms. N, the number of sites using the platform.
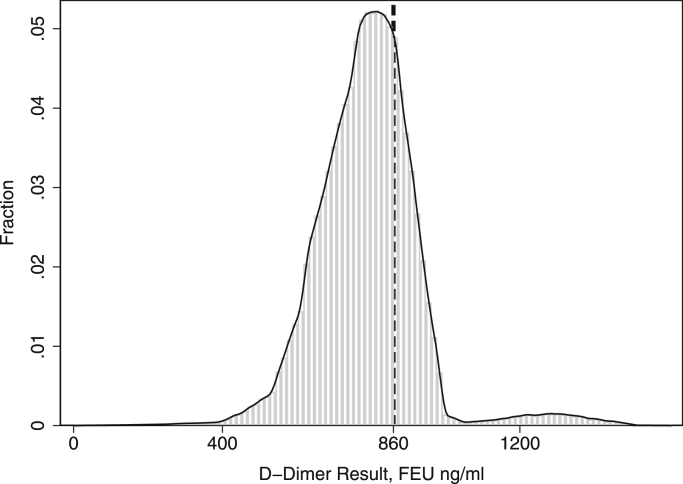
We simulated the distribution of results that would be obtained nationally based on the results of the CAP survey (Fig. 2). The distribution was multimodal. Results ranged from 294 to 1321 FEU ng/mL (mean: 763, SD: 157). The wide variability leads to significant misclassification, even when the underlying true D-dimer value is relatively far from the 860 ng/mL decision limit. For example, the results suggest that 18% of results would exceed the 860 ng/mL cutoff when the true value was 760 ng/mL and that 24% of results would be less than the 860 ng/mL cutoff when the true value was 960 ng/mL.
Figure 2.
Histogram of D-dimer results. The figure illustrates the results of a simulation showing the distribution of results that would be obtained if 100,000 identical samples with a true value of 760 FEU ng/mL had been sent to laboratories across the United States. The figure shows a histogram of D-dimer results along with the smoothed probability density function that was obtained by kernel density estimation. The dashed line shows the cutoff concentration (860 FEU ng/mL) for D-dimer used for diagnosis of periprosthetic joint infection.
Discussion
The simulation data illustrate the significant variation in D-dimer results across laboratories, even for identical samples. The results vary by platform with considerable variation among the same platform at different sites. Therefore, a cutoff obtained at one site using a particular platform is only valid for that site. All results in the simulation were converted to a common set of units (FEU ng/mL), so none of the variation can be attributed to differences in units reported by different laboratories.
There are 5 primary research publications that studied the application of the D-dimer test to evaluation for PJIs [[6], [7], [8], [9], [10]]. Only 1 of the 5 studies included information about the testing platform and reagent used [9]. This information is important for readers to determine the applicability of the findings to external institutions. The study by Qin et al [7] proposes a cutoff for D-dimer that is different than that of the Musculoskeletal Infection Society criteria [4], further illustrating the point that a cutoff developed at one site may not be suitable for broad application to results generated by different D-dimer assays.
Our results show that a universal cutoff for D-dimer would most likely result in clinically significant misclassification of patients across all categories of the modified PJI scoring criteria (infected, possibly infected, or not infected). D-Dimer may be useful for the diagnosis of PJIs, but given the lack of standardization of the test among laboratories, it is impossible to safely integrate this marker into the current PJI scoring criteria for diagnosis without further validation. Validation of cutoffs specific for each D-dimer assay would be required to understand whether or not D-dimer is a useful biomarker for PJIs globally, and not just with the D-dimer assay(s) included in the initial study leading to proposed PJI criteria. Given the information available at this time, we do not currently recommend the use of D-dimer results in PJI diagnostic criteria.
Conflict of interest
The authors declare there are no conflicts of interest.
Supplementary data
References
- 1.Olson J.D., Cunningham M.T., Higgins R.A., Eby C.S., Brandt J.T. D-dimer: simple test, tough problems. Arch Pathol Lab Med. 2013;137(8):1030. doi: 10.5858/arpa.2012-0296-CP. [DOI] [PubMed] [Google Scholar]
- 2.Longstaff C., Adcock D., Olson J.D. Harmonisation of D-dimer—a call for action. Thromb Res. 2016;137:219. doi: 10.1016/j.thromres.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G., Tripodi A., Simundic A.-M., Favaloro E.J. International survey on D-dimer test reporting: a call for standardization. Semin Thromb Hemost. 2015;41:287. doi: 10.1055/s-0035-1549092. [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J., Tan T.L., Goswami K. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 5.Shohat N., Tan T.L., Della Valle C.J. Development and validation of an evidence-based algorithm for diagnosing periprosthetic joint infection. J Arthroplasty. 2019;34(11):2730. doi: 10.1016/j.arth.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Xu H., Xie J., Huang Q., Lei Y., Zhang S., Pei F. Plasma fibrin degradation product and D-dimer are of limited value for diagnosing periprosthetic joint infection. J Arthroplasty. 2019;34(10):2454. doi: 10.1016/j.arth.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Qin L., Li F., Gong X., Wang J., Huang W., Hu N. Combined measurement of D-dimer and C-reactive protein levels: highly accurate for diagnosing chronic periprosthetic joint infection. J Arthroplasty. 2020;35(1):229. doi: 10.1016/j.arth.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Shahi A., Kheir M.M., Tarabichi M., Hosseinzadeh H.R., Tan T.L., Parvizi J. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am. 2017;99(17):1419. doi: 10.2106/JBJS.16.01395. [DOI] [PubMed] [Google Scholar]
- 9.Xiong L., Li S., Dai M. Comparison of D-dimer with CRP and ESR for diagnosis of periprosthetic joint infection. J Orthop Surg Res. 2019;14(1):240. doi: 10.1186/s13018-019-1282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannu T.S., Villa J.M., Patel P.D., Riesgo A.M., Barsoum W.K., Higuera C.A. The utility of serum d-dimer for the diagnosis of periprosthetic joint infection in revision total hip and knee arthroplasty. J Arthroplasty. 2020;35(6):1692–1695. doi: 10.1016/j.arth.2020.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.